Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Laccase Clarification and Amination
2.3. Enzyme Activity Estimation
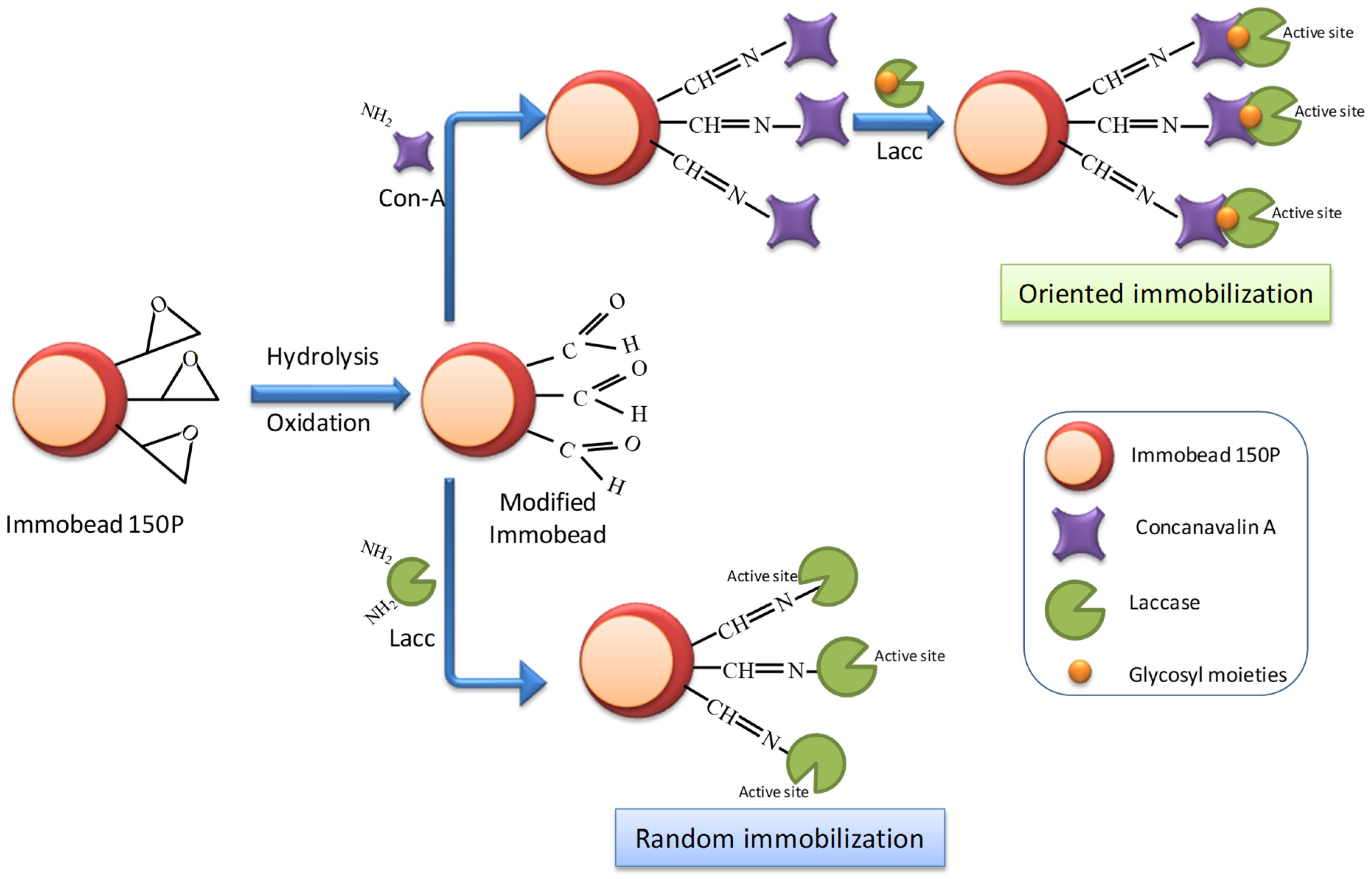
2.4. Immobead 150P Modification
2.5. Random and Oriented Laccase Immobilization
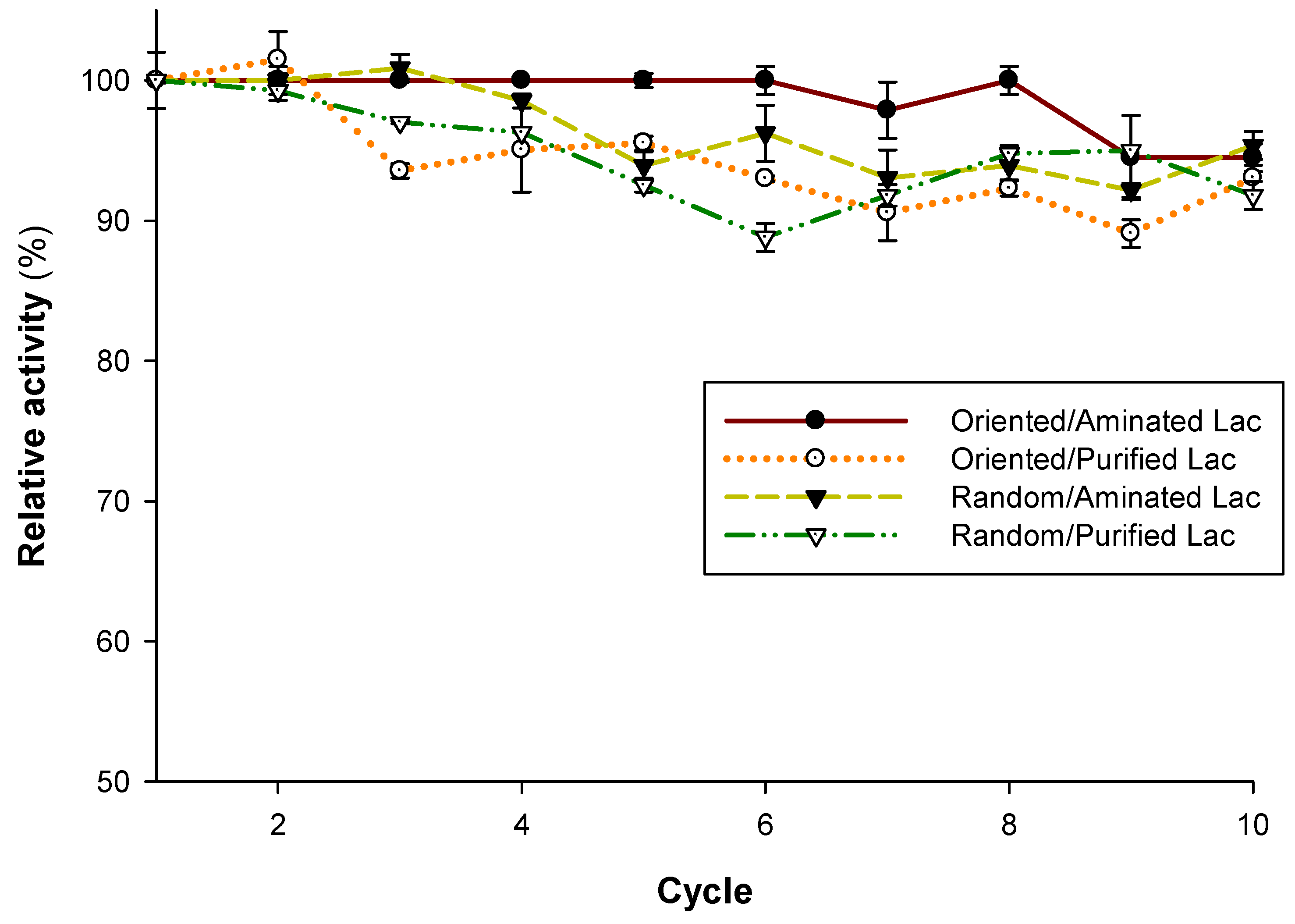
2.6. Operational Stability of Random and Oriented Immobilized Laccase
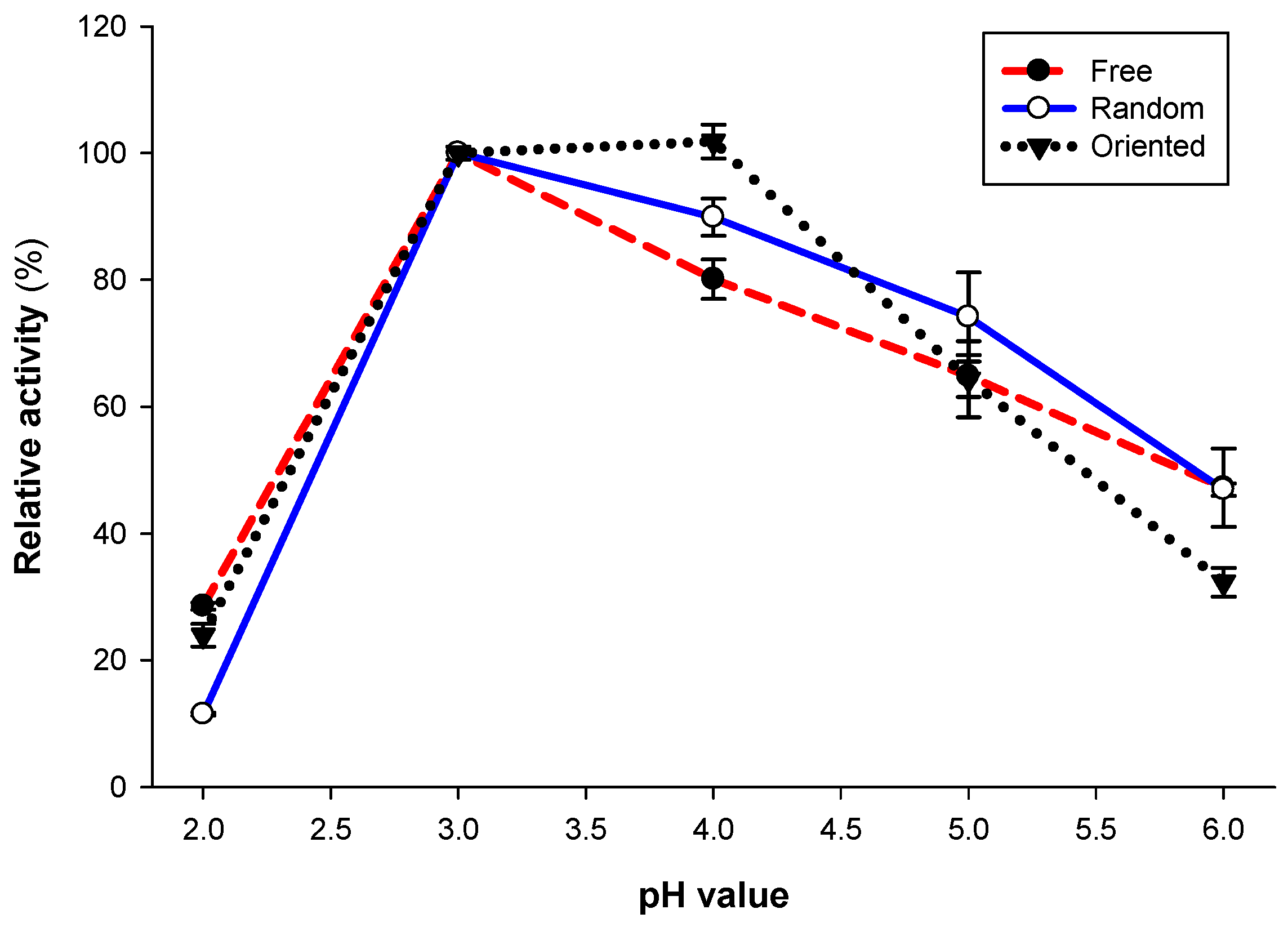
2.7. Temperature and pH Profiles
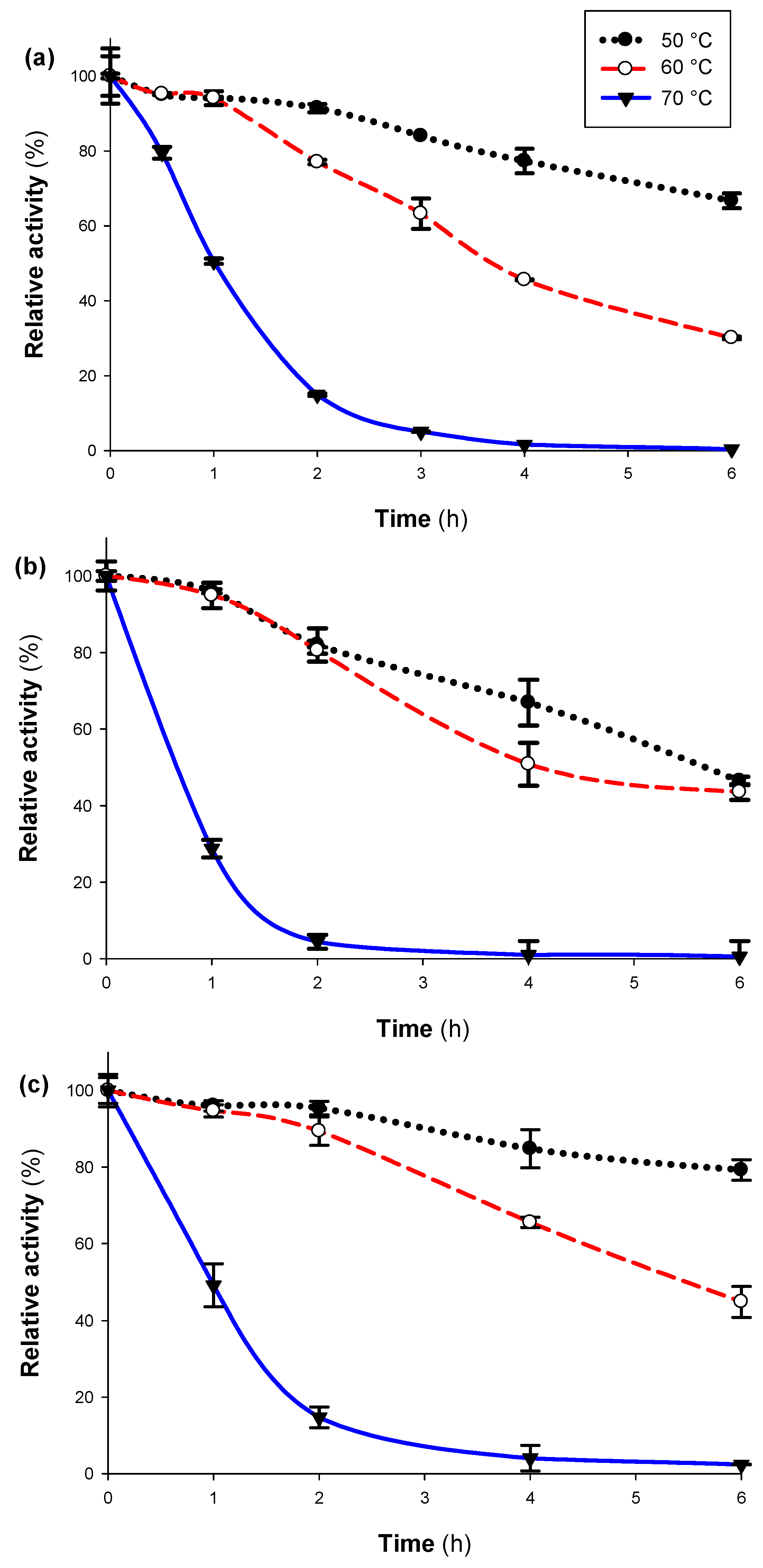
2.8. Thermal and pH Stability
2.9. Thermal Kinetics
2.10. Kinetic Constants
2.11. Biodegradation of Some Phenolic Compounds Using Immobilized Laccase
3. Results
3.1. Bioaffinity Immobilization of Myceliophthora thermophila Laccase Utilizing Con-A onto Immobead 150P
3.2. Reusability of the Immobilized Laccase Preparations
3.3. Optimum Temperature of Different Laccase Preparations
3.4. Thermal Stability and Thermodynamic Parameters
3.5. Optimum pH and pH Stability of Laccase Preparations
3.6. Kinetic Constants
3.7. Biodegradation of Some Compounds Using Random and Oriented Immobilized Laccase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maryskova, M.; Vrsanska, M.; Sevcu, A.; Novotny, V.; Blahutova, A.; Voberkova, S. Laminated PAA Nanofibers as a Practical Support for Crude Laccase: A New Perspective for Biocatalytic Treatment of Micropollutants in Wastewaters. Environ. Technol. Innov. 2022, 26, 102316. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A Review on Emerging Contaminants in Wastewaters and the Environment: Current Knowledge, Understudied Areas and Recommendations for Future Monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Zwart, N.; Jonker, W.; ten Broek, R.; de Boer, J.; Somsen, G.; Kool, J.; Hamers, T.; Houtman, C.J.; Lamoree, M.H. Identification of Mutagenic and Endocrine Disrupting Compounds in Surface Water and Wastewater Treatment Plant Effluents Using High-Resolution Effect-Directed Analysis. Water Res. 2020, 168, 115204. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of Pharmaceutical Compounds in Urban Wastewater: Removal, Mass Load and Environmental Risk after a Secondary Treatment—A Review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Bilal, M.; Noreen, S.; Asgher, M.; Parveen, S. Development and Characterization of Cross-Linked Laccase Aggregates (Lac-CLEAs) from Trametes Versicolor IBL-04 as Ecofriendly Biocatalyst for Degradation of Dye-Based Environmental Pollutants. Environ. Technol. Innov. 2021, 21, 101364. [Google Scholar] [CrossRef]
- Rodríguez Couto, S.; Toca Herrera, J.L. Industrial and Biotechnological Applications of Laccases: A Review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Solomon, E.I. Electron Transfer and Reaction Mechanism of Laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Mani, P.; Fidal Kumar, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. The Role of Natural Laccase Redox Mediators in Simultaneous Dye Decolorization and Power Production in Microbial Fuel Cells. Energies 2018, 11, 3455. [Google Scholar] [CrossRef] [Green Version]
- Torres-Duarte, C.; Roman, R.; Tinoco, R.; Vazquez-Duhalt, R. Halogenated Pesticide Transformation by a Laccase–Mediator System. Chemosphere 2009, 77, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key Fungal Degradation Patterns, Enzymes and Their Applications for the Removal of Aliphatic Hydrocarbons in Polluted Soils: A Review. Int. Biodeterior. Biodegrad. 2020, 147, 104866. [Google Scholar] [CrossRef]
- Othman, A.M.; González-Domínguez, E.; Sanromán, Á.; Correa-Duarte, M.; Moldes, D. Immobilization of Laccase on Functionalized Multiwalled Carbon Nanotube Membranes and Application for Dye Decolorization. RSC Adv. 2016, 6, 114690–114697. [Google Scholar] [CrossRef]
- Othman, A.M.; Wollenberger, U. Amperometric Biosensor Based on Coupling Aminated Laccase to Functionalized Carbon Nanotubes for Phenolics Detection. Int. J. Biol. Macromol. 2020, 153, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent Developments and Applications of Immobilized Laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ma, F.; Han, Y.; Zhang, X.; Yu, H. Removal of Sulfonamide Antibiotics by Oriented Immobilized Laccase on Fe3O4 Nanoparticles with Natural Mediators. J. Hazard. Mater. 2014, 279, 203–211. [Google Scholar] [CrossRef]
- Kunamneni, A.; Ghazi, I.; Camarero, S.; Ballesteros, A.; Plou, F.J.; Alcalde, M. Decolorization of Synthetic Dyes by Laccase Immobilized on Epoxy-Activated Carriers. Process Biochem. 2008, 43, 169–178. [Google Scholar] [CrossRef] [Green Version]
- González-Domínguez, E.; Comesaña-Hermo, M.; Mariño-Fernández, R.; Rodríguez-González, B.; Arenal, R.; Salgueiriño, V.; Moldes, D.; Othman, A.M.; Pérez-Lorenzo, M.; Correa-Duarte, M.A. Hierarchical Nanoplatforms for High-Performance Enzyme Biocatalysis under Denaturing Conditions. ChemCatChem 2016, 8, 1264–1268. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef]
- Othman, A.M. Maria Ángeles Sanromán; Diego Moldes Kinetic and Thermodynamic Study of Laccase Cross-Linked onto Glyoxyl Immobead 150P Carrier: Characterization and Application for Beechwood Biografting. Enzyme Microb. Technol. 2021, 150, 109865. [Google Scholar] [CrossRef]
- Andreescu, S.; Njagi, J.; Ispas, C. Chapter 7—Nanostructured Materials for Enzyme Immobilization and Biosensors. In The New Frontiers of Organic and Composite Nanotechnology; Erokhin, V., Ram, M.K., Yavuz, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 355–394. ISBN 9780080450520. [Google Scholar]
- Andreescu, S.; Barthelmebs, L.; Marty, J.-L. Immobilization of Acetylcholinesterase on Screen-Printed Electrodes: Comparative Study between Three Immobilization Methods and Applications to the Detection of Organophosphorus Insecticides. Anal. Chim. Acta 2002, 464, 171–180. [Google Scholar] [CrossRef]
- Bonnet, C.; Andreescu, S.; Marty, J.-L. Adsorption: An Easy and Efficient Immobilisation of Acetylcholinesterase on Screen-Printed Electrodes. Anal. Chim. Acta 2003, 481, 209–211. [Google Scholar] [CrossRef]
- Gao, Y.; Kyratzis, I.; Taylor, R.; Huynh, C.; Hickey, M. Immobilization of Acetylcholinesterase onto Carbon Nanotubes Utilizing Streptavidin-Biotin Interaction for the Construction of Amperometric Biosensors for Pesticides. Anal. Lett. 2009, 42, 2711–2727. [Google Scholar] [CrossRef]
- Anzai, J.; Kobayashi, Y.; Nakamura, N. Alternate Deposition of Concanavalin A and Mannose-Labelled Enzymes on a Solid Surface to Prepare Catalytically Active Enzyme Thin Films. J. Chem. Soc. Perkin Trans. 1998, 2, 461–462. [Google Scholar] [CrossRef]
- Bucur, B.; Andreescu, S.; Marty, J.-L. Affinity Methods to Immobilize Acetylcholinesterases for Manufacturing Biosensors. Anal. Lett. 2004, 37, 1571–1588. [Google Scholar] [CrossRef]
- Ganesana, M.; Istarnboulie, G.; Marty, J.-L.; Noguer, T.; Andreescu, S. Site-Specific Immobilization of a (His)6-Tagged Acetylcholinesterase on Nickel Nanoparticles for Highly Sensitive Toxicity Biosensors. Biosens. Bioelectron. 2011, 30, 43–48. [Google Scholar] [CrossRef]
- Opitz, L.; Salaklang, J.; Büttner, H.; Reichl, U.; Wolff, M.W. Lectin-Affinity Chromatography for Downstream Processing of MDCK Cell Culture Derived Human Influenza A Viruses. Vaccine 2007, 25, 939–947. [Google Scholar] [CrossRef]
- Krenkova, J.; Cesla, P.; Foret, F. Macroporous Cryogel Based Spin Column with Immobilized Concanavalin A for Isolation of Glycoproteins: Liquid Phase Separations. Electrophoresis 2015, 36, 1344–1348. [Google Scholar] [CrossRef]
- Miller, J.N.; Nwokedi, G.I.C. The Luminescence Properties of Concanavalin A. Biochim. Biophys. Acta BBA—Protein Struct. 1975, 393, 426–434. [Google Scholar] [CrossRef]
- Powell, A.E.; Leon, M.A. Reversible Interaction of Human Lymphocytes with the Mitogen Concanavalin A. Exp. Cell Res. 1970, 62, 315–325. [Google Scholar] [CrossRef]
- Shoham, J.; Inbar, M.; Sachs, L. Differential Toxicity on Normal and Transformed Cells in Vitro and Inhibition of Tumour Development In Vivo by Concanavalin A. Nature 1970, 227, 1244–1246. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Hollerman, C.E.; Merrick, J.M. Protein-Carbohydrate Interaction I. The Interaction of Polysaccharides with Concanavalin A. Biochim. Biophys. Acta BBA—Gen. Subj. 1965, 97, 68–76. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Hollerman, C.E.; Smith, E.E. Protein-Carbohydrate Interaction. II. Inhibition Studies on the Interaction of Concanavalin A with Polysaccharides. Biochemistry 1965, 4, 876–883. [Google Scholar] [CrossRef]
- Kalb, A.J.; Levitzki, A. Metal-Binding Sites of Concanavalin A and Their Role in the Binding of Alpha-Methyl d-Glucopyranoside. Biochem. J. 1968, 109, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Kalb, A.J.; Lustig, A. The Molecular Weight of Concanavalin A. Biochim. Biophys. Acta 1968, 168, 366–367. [Google Scholar] [CrossRef]
- Becker, J.W.; Reeke, G.N.; Cunningham, B.A.; Edelman, G.M. New Evidence on the Location of the Saccharide-Binding Site of Concanavalin A. Nature 1976, 259, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Matto, M.; Husain, Q. Entrapment of Porous and Stable Concanavalin A–Peroxidase Complex into Hybrid Calcium Alginate–Pectin Gel. J. Chem. Technol. Biotechnol. 2006, 81, 1316–1323. [Google Scholar] [CrossRef]
- Xue, Y.; Ding, L.; Lei, J.; Yan, F.; Ju, H. In Situ Electrochemical Imaging of Membrane Glycan Expression on Micropatterned Adherent Single Cells. Anal. Chem. 2010, 82, 7112–7118. [Google Scholar] [CrossRef]
- Altunbaş, C.; Uygun, M.; Uygun, D.A.; Akgöl, S.; Denizli, A. Immobilization of Inulinase on Concanavalin A-Attached Super Macroporous Cryogel for Production of High-Fructose Syrup. Appl. Biochem. Biotechnol. 2013, 170, 1909–1921. [Google Scholar] [CrossRef]
- Franco Fraguas, L.; Batista-Viera, F.; Carlsson, J. Preparation of High-Density Concanavalin A Adsorbent and Its Use for Rapid, High-Yield Purification of Peroxidase from Horseradish Roots. J. Chromatogr. B 2004, 803, 237–241. [Google Scholar] [CrossRef]
- Hashizume, H.; Tanase, K.; Shiratake, K.; Mori, H.; Yamaki, S. Purification and Characterization of Two Soluble Acid Invertase Isozymes from Japanese Pear Fruit. Phytochemistry 2003, 63, 125–129. [Google Scholar] [CrossRef]
- Yavuz, H.; Akgöl, S.; Arica, Y.; Denizli, A. Concanavalin A Immobilized Affinity Adsorbents for Reversible Use in Yeast Invertase Adsorption. Macromol. Biosci. 2004, 4, 674–679. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Matte, C.R.; Bussamara, R.; Dupont, J.; Rodrigues, R.C.; Hertz, P.F.; Ayub, M.A.Z. Immobilization of Thermomyces Lanuginosus Lipase by Different Techniques on Immobead 150 Support: Characterization and Applications. Appl. Biochem. Biotechnol. 2014, 172, 2507–2520. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Qu, Y. A Strategy for Efficient Immobilization of Laccase and Horseradish Peroxidase on Single-Walled Carbon Nanotubes: Immobilize Glycoproteins on SWNTs. J. Chem. Technol. Biotechnol. 2013, 88, 2227–2232. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Z.; Zeng, G.; Tang, L.; Pang, Y.; Li, Z.; Liu, C.; Lei, X.; Wu, M.; Ren, P.; et al. Immobilization of Laccase on Magnetic Bimodal Mesoporous Carbon and the Application in the Removal of Phenolic Compounds. Bioresour. Technol. 2012, 115, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ladero, M.; Ruiz, G.; Pessela, B.C.C.; Vian, A.; Santos, A.; Garcia-Ochoa, F. Thermal and PH Inactivation of an Immobilized Thermostable β-Galactosidase from Thermus sp. Strain T2: Comparison to the Free Enzyme. Biochem. Eng. J. 2006, 31, 14–24. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Rodríguez, O.; Fernández-Fernández, M.; Domínguez, A.; Moldes, D.; Sanromán, M.A.; Macedo, E.A. Immobilization of Laccase on Modified Silica: Stabilization, Thermal Inactivation and Kinetic Behaviour in 1-Ethyl-3-Methylimidazolium Ethylsulfate Ionic Liquid. Bioresour. Technol. 2013, 131, 405–412. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Lin, Y.; Ng, T.B.; Ye, X.; Lin, J. Immobilized Cerrena sp. Laccase: Preparation, Thermal Inactivation, and Operational Stability in Malachite Green Decolorization. Sci. Rep. 2017, 7, 16429. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.P.; Sant’Ana, V.; Hertz, P.F.; Rodrigues, R.C.; Ninow, J.L.; Klein, M.P.; Sant’Ana, V.; Hertz, P.F.; Rodrigues, R.C.; Ninow, J.L. Kinetics and Thermodynamics of Thermal Inactivation of β-Galactosidase from Aspergillus Oryzae. Braz. Arch. Biol. Technol. 2018, 61, 489. [Google Scholar] [CrossRef] [Green Version]
- Ansari, S.A.; Husain, Q. Immobilization of Kluyveromyces Lactis β Galactosidase on Concanavalin A Layered Aluminium Oxide Nanoparticles—Its Future Aspects in Biosensor Applications. J. Mol. Catal. B Enzym. 2011, 70, 119–126. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization Strategies to Develop Enzymatic Biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, Y.; Gao, J.; Zhao, X.; Ma, L.; Zhou, Q. Oriented Immobilization of Glucose Oxidase on Graphene Oxide. Biochem. Eng. J. 2012, 69, 28–31. [Google Scholar] [CrossRef]
- Spinelli, D.; Fatarella, E.; Di Michele, A.; Pogni, R. Immobilization of Fungal (Trametes Versicolor) Laccase onto Amberlite IR-120 H Beads: Optimization and Characterization. Process Biochem. 2013, 48, 218–223. [Google Scholar] [CrossRef]
- Davis, S.; Burns, R.G. Covalent Immobilization of Laccase on Activated Carbon for Phenolic Effluent Treatment. Appl. Microbiol. Biotechnol. 1992, 37, 972. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Purification and Biochemical Characterization of Two Isolated Laccase Isoforms from Agaricus Bisporus CU13 and Their Potency in Dye Decolorization. Int. J. Biol. Macromol. 2018, 113, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; El-Bindary, A.A.; Ismail, A.; Morshidy, A.M. Magnetic Chitosan Grafted with Polymerized Thiourea for Remazol Brilliant Blue R Recovery: Effects of Uptake Conditions. J. Dispers. Sci. Technol. 2017, 38, 943–952. [Google Scholar] [CrossRef]
- Krajewska, B.; Brindell, M. Thermodynamic Study of Competitive Inhibitors’ Binding to Urease. J. Therm. Anal. Calorim. 2016, 123, 2427–2439. [Google Scholar] [CrossRef] [Green Version]
- Arcus, V.L.; Prentice, E.J.; Hobbs, J.K.; Mulholland, A.J.; Van der Kamp, M.W.; Pudney, C.R.; Parker, E.J.; Schipper, L.A. On the Temperature Dependence of Enzyme-Catalyzed Rates. Biochemistry 2016, 55, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Saldin, D.K. Enzyme Transient State Kinetics in Crystal and Solution from the Perspective of a Time-Resolved Crystallographer. Struct. Dyn. 2014, 1, 024701. [Google Scholar] [CrossRef]
- Schückel, J.; Matura, A.; van Pée, K.-H. One-Copper Laccase-Related Enzyme from Marasmius sp.: Purification, Characterization and Bleaching of Textile Dyes. Enzyme Microb. Technol. 2011, 48, 278–284. [Google Scholar] [CrossRef]
- Neelkant, K.S.; Shankar, K.; Jayalakshmi, S.K.; Sreeramulu, K. Purification, Biochemical Characterization, and Facile Immobilization of Laccase from Sphingobacterium Ksn-11 and Its Application in Transformation of Diclofenac. Appl. Biochem. Biotechnol. 2020, 192, 831–844. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, D.; Jiang, S.; Yu, H.; Cheng, Y.; Zhang, S.; Zhang, X.; Chen, W. The Study of Laccase Immobilization Optimization and Stability Improvement on CTAB-KOH Modified Biochar. BMC Biotechnol. 2021, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.M.; Twarda-Clapa, A.; Białkowska, A.M. Screening of Novel Laccase Producers—Isolation and Characterization of Cold-Adapted Laccase from Kabatiella Bupleuri G3 Capable of Synthetic Dye Decolorization. Biomolecules 2021, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal Laccases—Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demkiv, O.M.; Gayda, G.Z.; Broda, D.; Gonchar, M.V. Extracellular Laccase from Monilinia Fructicola: Isolation, Primary Characterization and Application. Cell Biol. Int. 2021, 45, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Mtibaà, R.; Barriuso, J.; de Eugenio, L.; Aranda, E.; Belbahri, L.; Nasri, M.; Martínez, M.J.; Mechichi, T. Purification and Characterization of a Fungal Laccase from the Ascomycete Thielavia sp. and Its Role in the Decolorization of a Recalcitrant Dye. Int. J. Biol. Macromol. 2018, 120, 1744–1751. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yan, Y.; Tian, Y.; Zhao, W.; Li, Z.; Gao, J.; Peng, R.; Yao, Q. Heterologous Expression and Characterisation of a Laccase from Colletotrichum Lagenarium and Decolourisation of Different Synthetic Dyes. World J. Microbiol. Biotechnol. 2016, 32, 40. [Google Scholar] [CrossRef]
- Daâssi, D.; Zouari-Mechichi, H.; Prieto, A.; Martínez, M.J.; Nasri, M.; Mechichi, T. Purification and Biochemical Characterization of a New Alkali-Stable Laccase from Trametes sp. Isolated in Tunisia: Role of the Enzyme in Olive Mill Waste Water Treatment. World J. Microbiol. Biotechnol. 2013, 29, 2145–2155. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Liao, X.; Cai, Y. Purification and Characterization of a New Laccase from Shiraia sp.SUPER-H168. Process Biochem. 2013, 48, 351–357. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, Y.; Liao, X.; Zhang, F.; Zhang, D.; Li, Z. Production and Characterization of a Novel Laccase with Cold Adaptation and High Thermal Stability from an Isolated Fungus. Appl. Biochem. Biotechnol. 2010, 162, 280–294. [Google Scholar] [CrossRef]
- Mtibaà, R.; de Eugenio, L.; Ghariani, B.; Louati, I.; Belbahri, L.; Nasri, M.; Mechichi, T. A Halotolerant Laccase from Chaetomium Strain Isolated from Desert Soil and Its Ability for Dye Decolourization. 3 Biotech 2017, 7, 329. [Google Scholar] [CrossRef]
- Sondhi, S.; Kaur, R.; Madan, J. Purification and Characterization of a Novel White Highly Thermo Stable Laccase from a Novel Bacillus sp. MSK-01 Having Potential to Be Used as Anticancer Agent. Int. J. Biol. Macromol. 2021, 170, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Choudhary, G.; Thakur, I.S. Characterization of Laccase Activity Produced by Cryptococcus Albidus. Prep. Biochem. Biotechnol. 2012, 42, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yong, Y.; Wang, Z.; Jiang, G.; Wu, J.; Liu, Z. Concanavalin A Coated Activated Carbon for High Performance Enzymatic Catalysis. ACS Sustain. Chem. Eng. 2017, 5, 90–96. [Google Scholar] [CrossRef]



| Immobilization Technique | Enzyme Type | Immobilized Activity (U/g) | Protein Loading (mg/g) | Immobilization Yield (%) |
|---|---|---|---|---|
| Oriented | Aminated | 1.25 ± 0.05 | 4.26 ± 0.01 | 93.57 ± 2.75 |
| Purified | 1.17 ± 0.15 | 3.20 ± 0.08 | 80.97 ± 3.07 | |
| Random | Aminated | 1.13 ± 0.03 | 3.69 ± 0.16 | 79.42 ± 1.75 |
| Purified | 0.68 ± 0.00 | 2.14 ± 0.02 | 55.31 ± 5.02 |
| Temperature (°C) | Half-Life (h) | k (h−1) | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Free | Random | Oriented | Free | Random | Oriented | Free | Random | Oriented | |
| 50 | 18.01 ± 0.25 | 11.20 ± 0.13 | 28.90 ± 1.33 | 0.038 | 0.062 | 0.024 | 0.765 | 0.990 | 0.976 |
| 60 | 8.58 ± 0.73 | 10.63 ± 0.85 | 10.87 ± 0.76 | 0.081 | 0.065 | 0.064 | 0.935 | 0.964 | 0.982 |
| 70 | 6.02 ± 0.08 | 6.03 ± 0.23 | 6.14 ± 0.55 | 0.115 | 0.152 | 0.113 | 0.972 | 0.998 | 0.993 |
| Parameter | T (K) | Form of Laccase Enzyme | ||
|---|---|---|---|---|
| Free | Random Immobilized | Oriented Immobilized | ||
| ΔG (kJ mol−1) | 323.15 | 110.14 | 108.83 | 111.38 |
| 333.15 | 111.54 | 112.15 | 112.19 | |
| 343.15 | 113.97 | 113.18 | 114.02 | |
| ΔH (kJ mol−1) | 323.15 | 47.99 | 38.35 | 68.88 |
| 333.15 | 47.91 | 38.27 | 68.80 | |
| 343.15 | 47.83 | 38.19 | 68.72 | |
| ΔS (J mol−1 K−1) | 323.15 | −192.34 | −218.09 | −131.51 |
| 333.15 | −191.00 | −221.76 | −130.25 | |
| 343.15 | −192.77 | −218.53 | −132.03 | |
| Ea (kJ mol−1) | 50.678 | 28.296 | 71.570 | |
| Km (mM) | 0.040 | 0.280 | 1.951 | |
| Vmax (μmol min−1) | 1.986 | 1.723 | 10.74 | |
| Vmax/km | 49.650 | 6.154 | 5.505 | |
| Compound | Structural Formula | Molecular Weight | EC Number | Water Solubility (g/L) | Treatment Type | Residual Contaminants (%) | ||
|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | ||||||
| m-Cresol |  | 108.14 | 203-577-9 | 23.5 g/L at 20 °C | Random | 100.00 | 11.53 ± 1.26 | 11.68 ± 0.76 |
| Oriented | 100.00 | 12.23 ± 0.08 | 12.40 ± 0.15 | |||||
| 4-Nitrophenol | 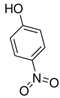 | 139.11 | 202-811-7 | 11.6 g/L at 20 °C | Random | 100.00 | 19.35 ± 2.13 | 21.22 ± 1.75 |
| Oriented | 100.00 | 19.23 ± 0.09 | 19.00 ± 1.33 | |||||
| Pyrimethanil |  | 199.25 | 414-220-3 | 0.121 g/L at 25 °C | Random | 100.00 | 2.13 ± 0.08 | 1.99 ± 0.15 |
| Oriented | 100.00 | 2.47 ± 0.33 | 2.10 ± 0.00 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, A.M.; Sanroman, A.; Moldes, D. Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics. Sustainability 2022, 14, 13306. https://doi.org/10.3390/su142013306
Othman AM, Sanroman A, Moldes D. Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics. Sustainability. 2022; 14(20):13306. https://doi.org/10.3390/su142013306
Chicago/Turabian StyleOthman, Abdelmageed M., Angeles Sanroman, and Diego Moldes. 2022. "Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics" Sustainability 14, no. 20: 13306. https://doi.org/10.3390/su142013306
APA StyleOthman, A. M., Sanroman, A., & Moldes, D. (2022). Laccase-Oriented Immobilization Using Concanavalin A as an Approach for Efficient Glycoproteins Immobilization and Its Application to the Removal of Aqueous Phenolics. Sustainability, 14(20), 13306. https://doi.org/10.3390/su142013306








