Removal of Diclofenac Sodium from Wastewater in Microbial Fuel Cell by Anode Modified with MnCo2O4
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Preparation Method of the MnCo2O4@CF Anode Electrode
2.3. MFC System Construction and Operation
- V: Voltage (V)
- R: External resistance in Ω
- I: Current in amps
2.4. Investigation of Cost Estimation of MFC with MnCo2O4@CF
2.5. Chromatographic Analysis
- C0 = Initial concentration of DCF.
- Ct = the residual concentration of the DCF after a reaction time.
- η= the DCF removal efficiency%.
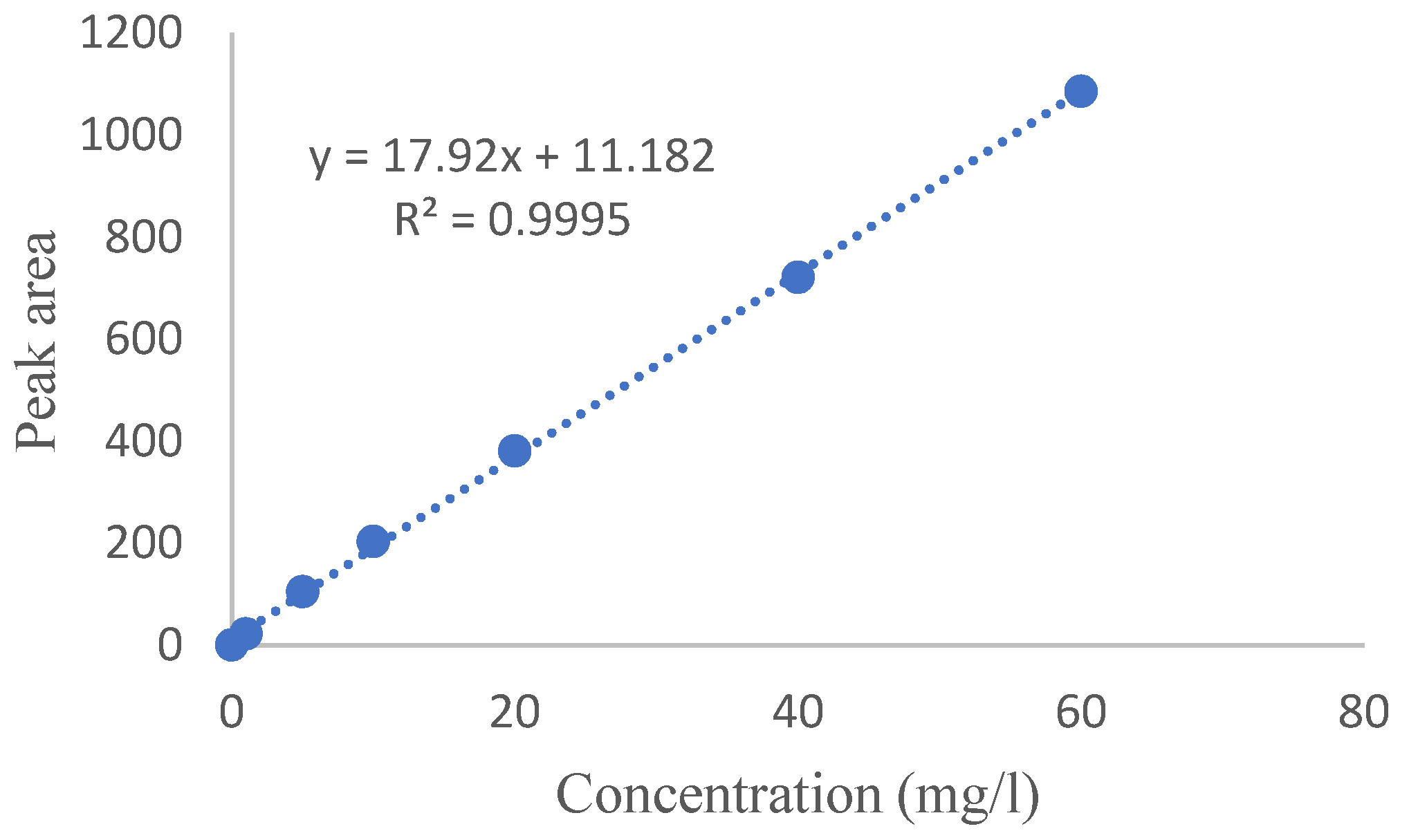
Calibration Curve Drawing
2.6. Electrochemical Measurements
3. Results and Discussion
3.1. Characteristics of the Modified Electrode
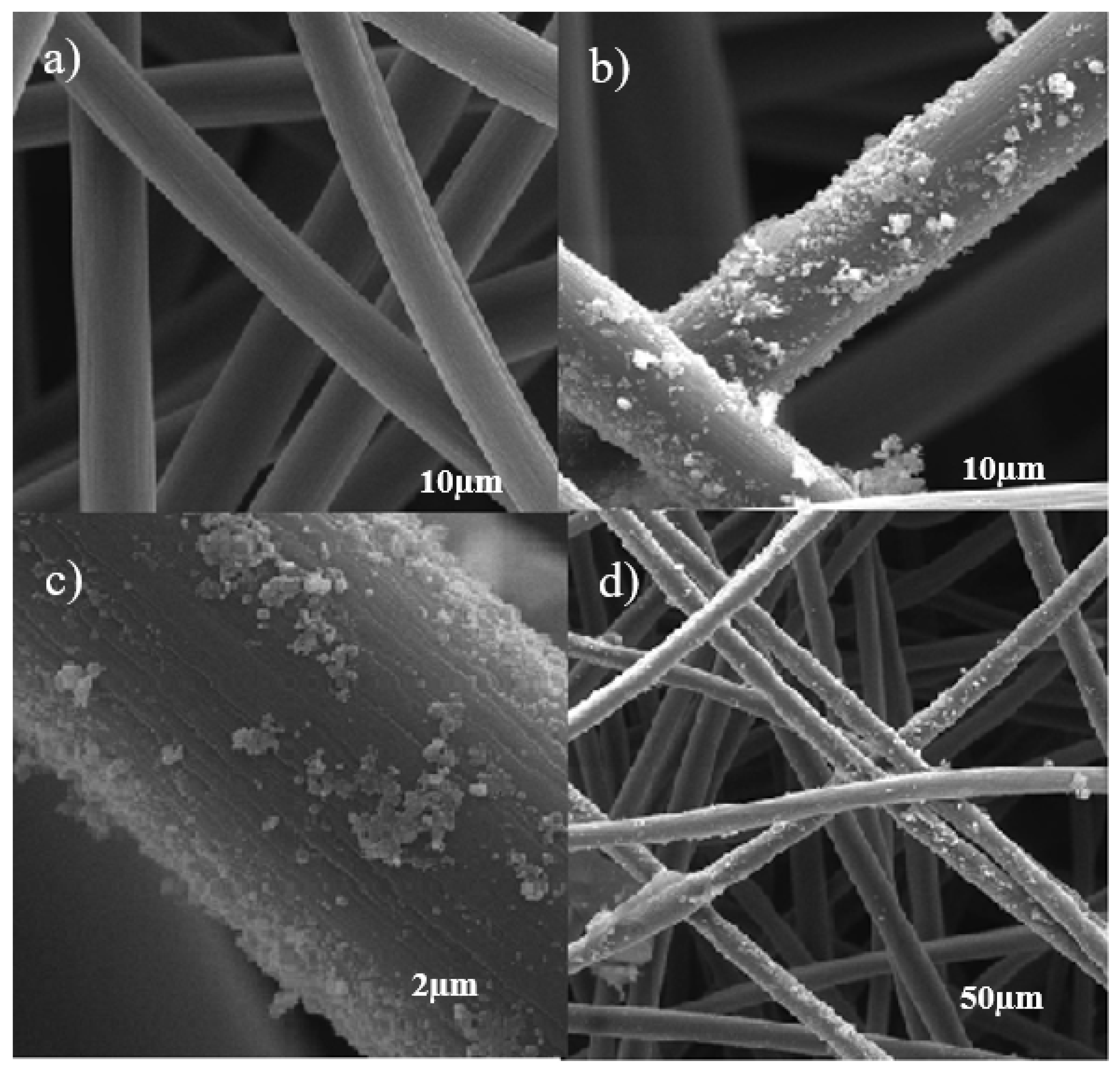
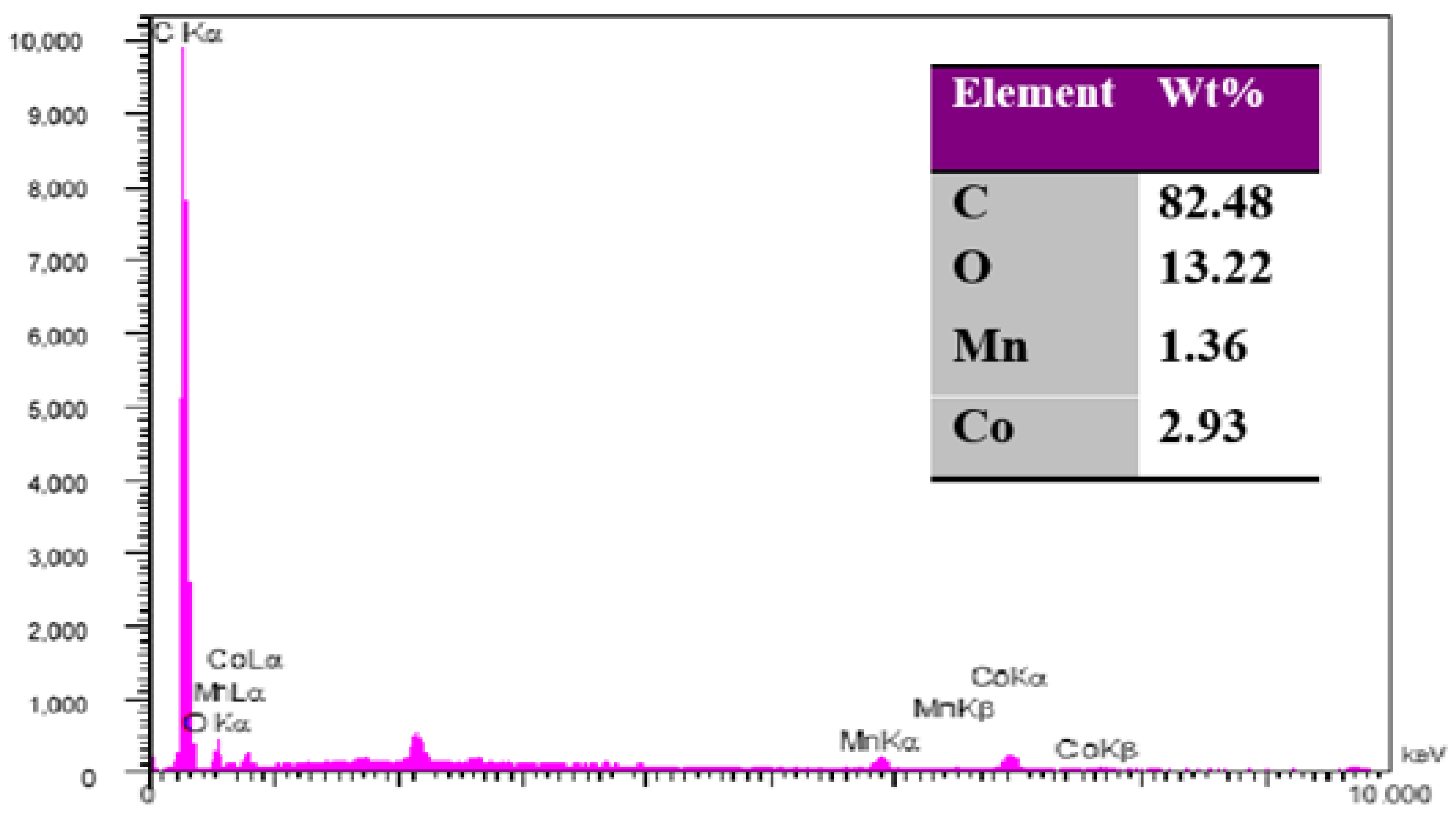
3.1.1. Electrode Characterization Using SEM and EDS
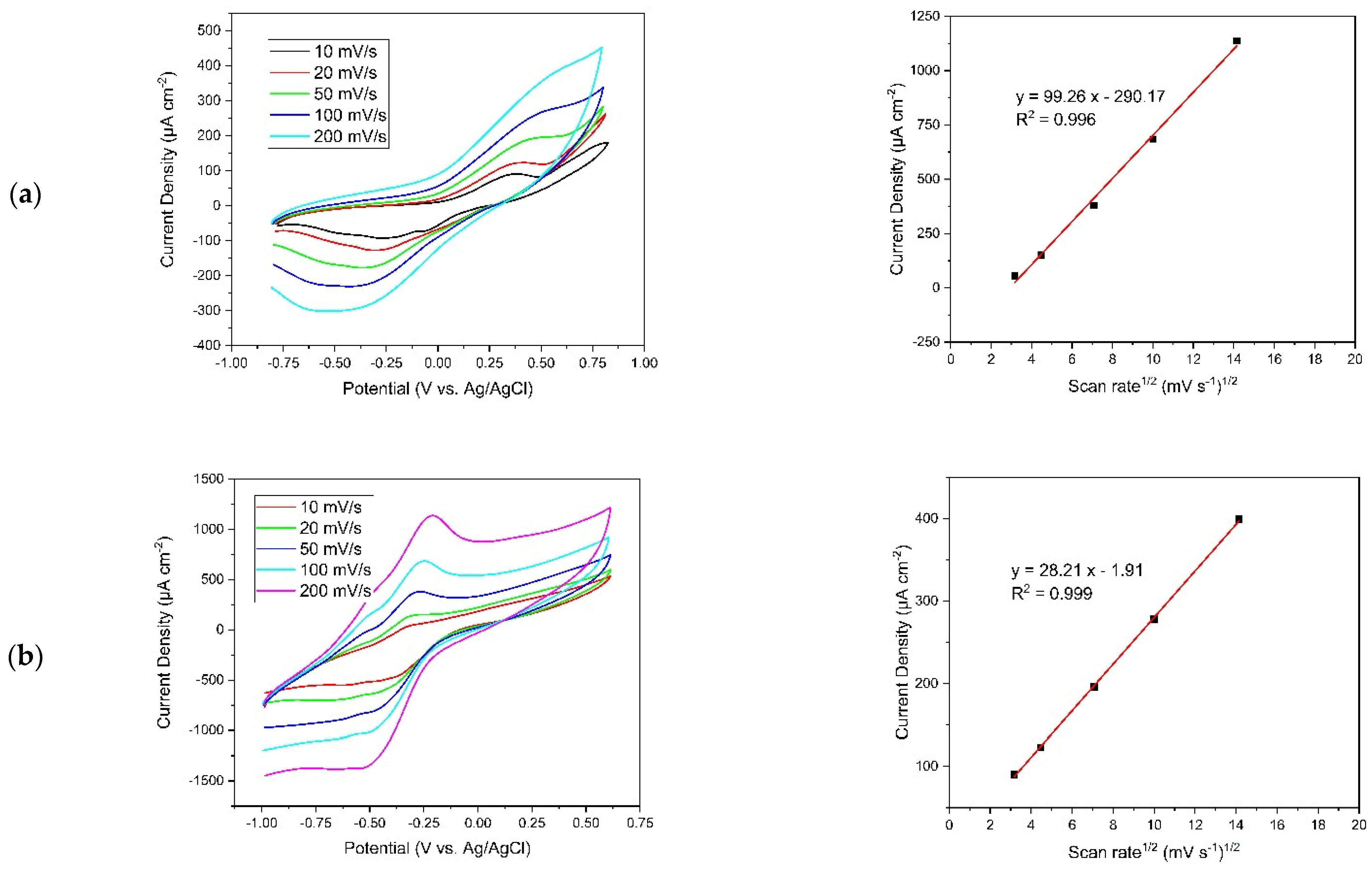
3.1.2. Cyclic Voltammetry
3.1.3. Electrochemical Impedance Spectroscopy (EIS)
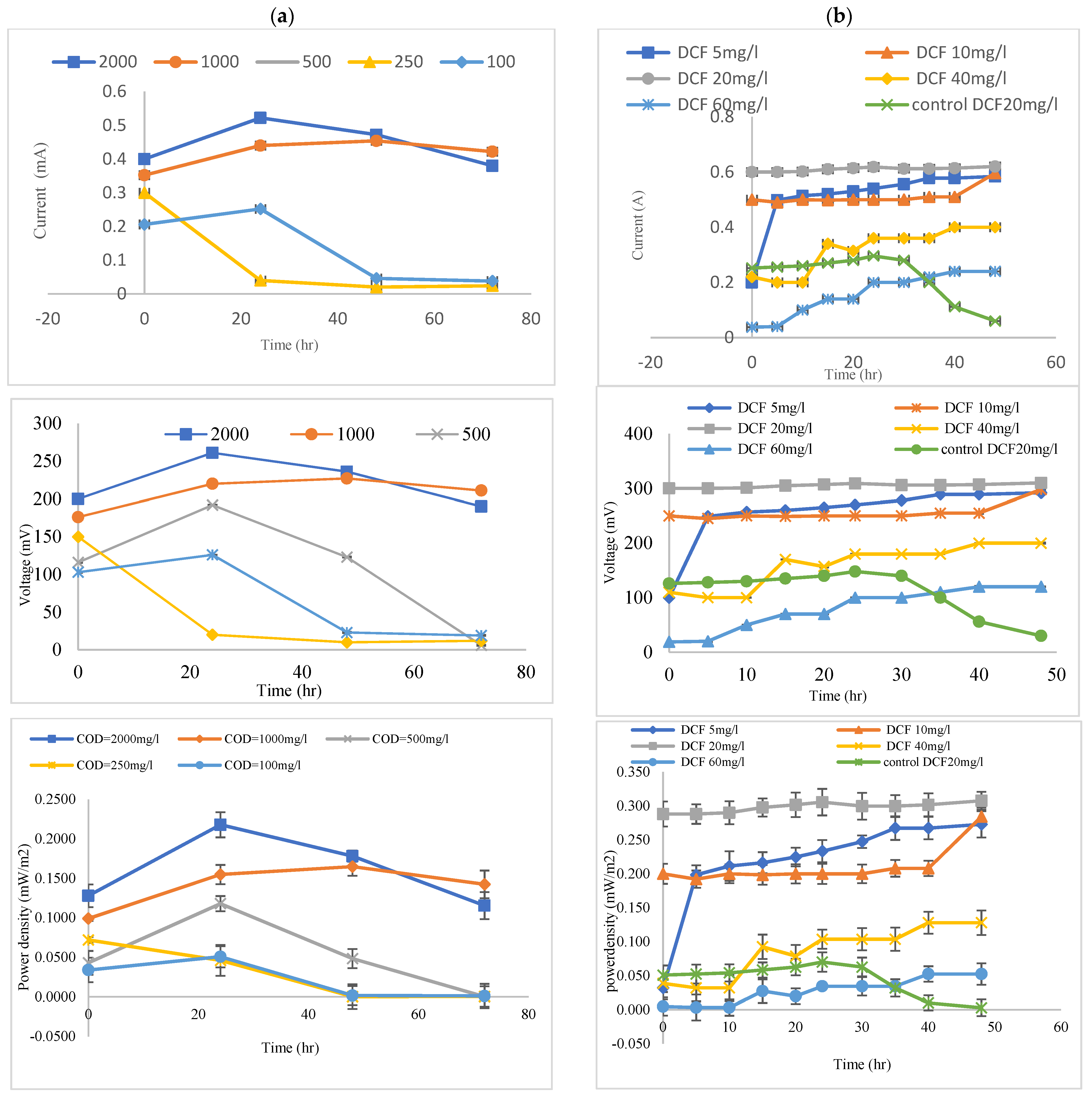
3.2. Effects of the Coated Anode with MnCo2O4 @ CF and Uncoated on Voltage Generation
3.3. Efficiency Coated Anode with MnCo2O4 @ CF, and Uncoated (CF) in the Removal of COD from Wastewater
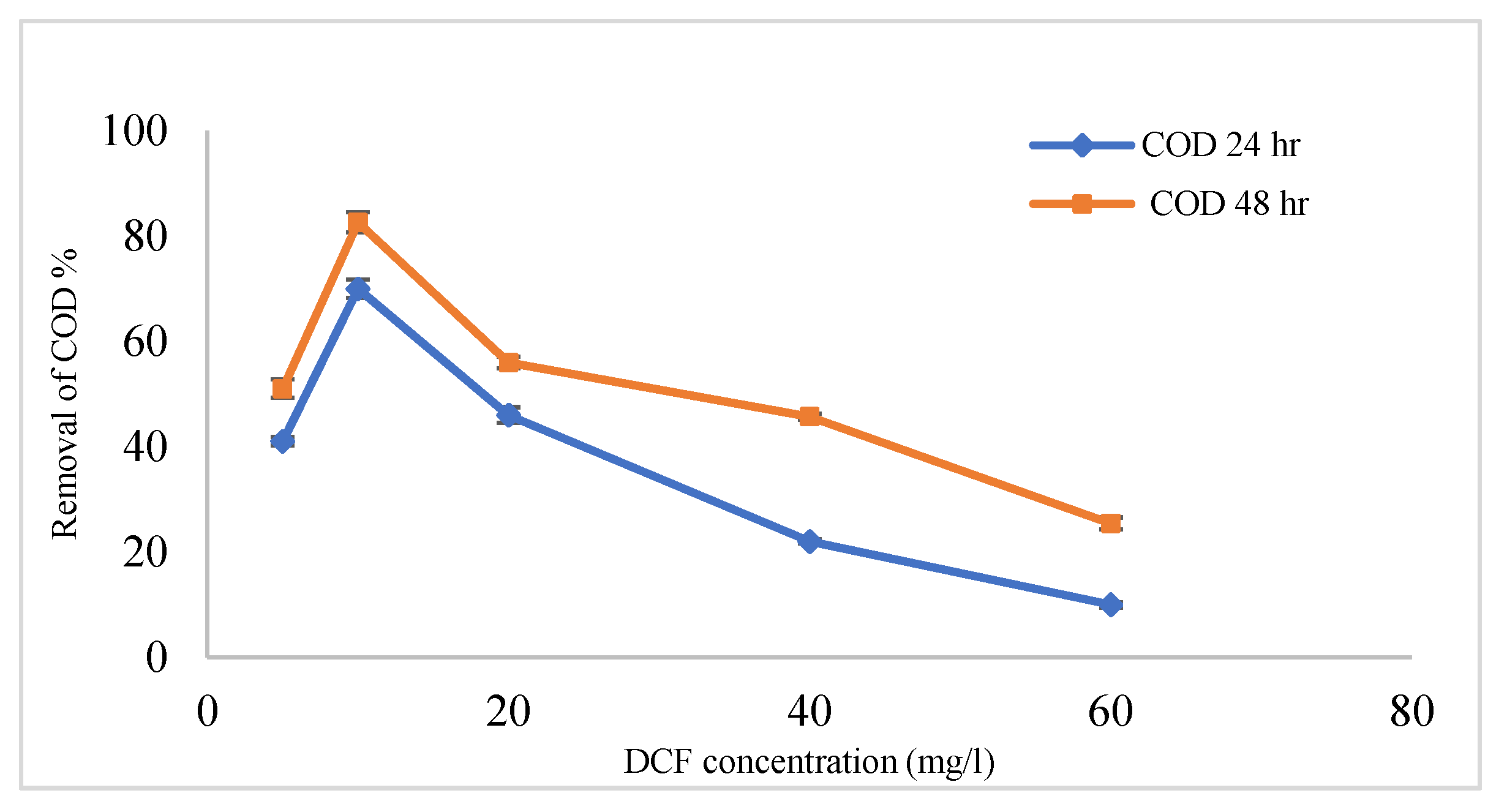
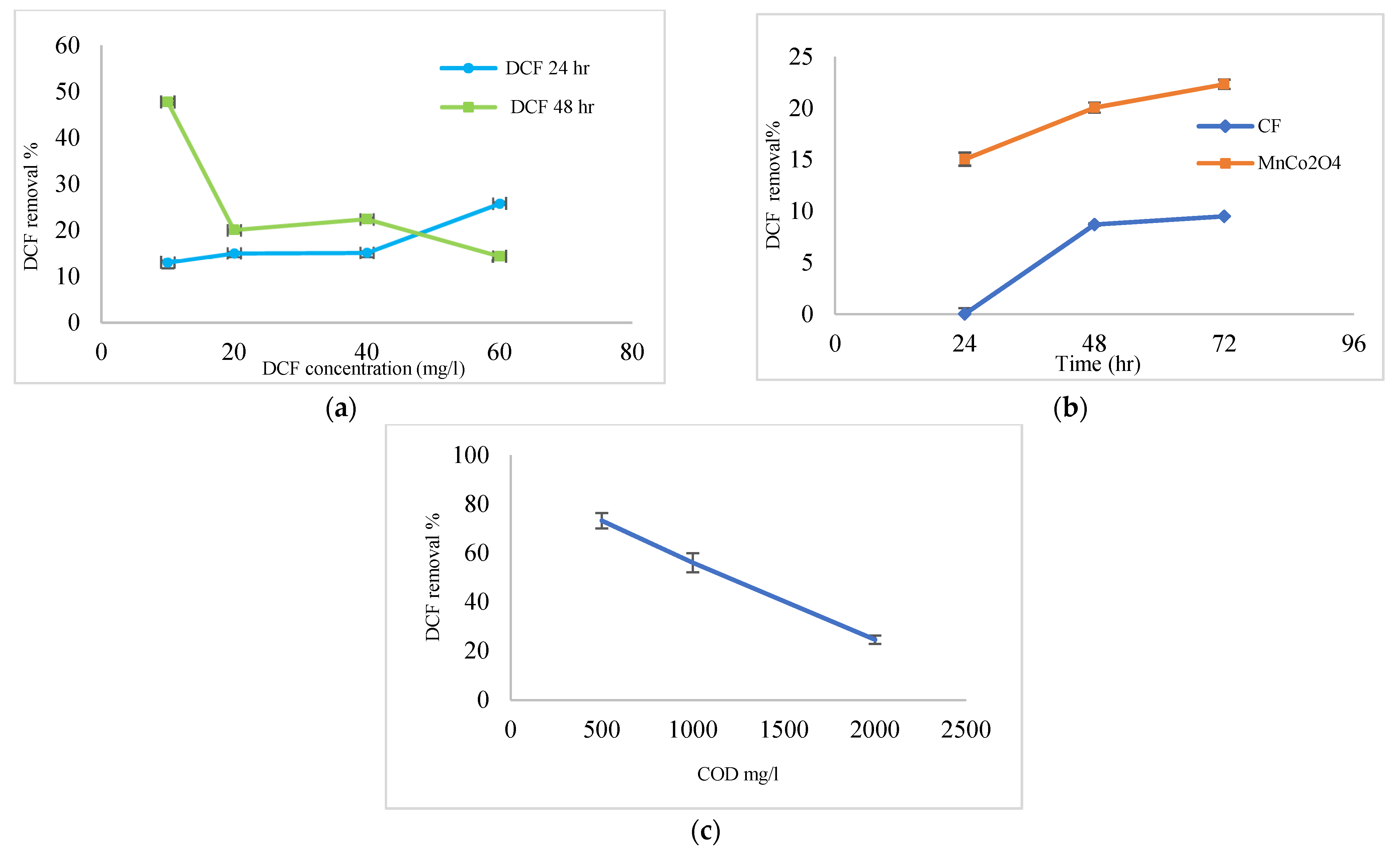
3.4. Efficiency Coated Anode with MnCo2O4 @ CF and Uncoated (CF) in the Removal of DCF Sodium from Wastewater
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; GEIßEN, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Pocostales, P.; Alvarez, P.; Oropesa, A. Diclofenac removal from water with ozone and activated carbon. J. Hazard. Mater. 2009, 163, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Mazouzi, Y.; Miche, A.; Loiseau, A.; Beito, B.; Méthivier, C.; Knopp, D.; Salmain, M.; Boujday, S. Design and Analytical Performances of a Diclofenac Biosensor for Water Resources Monitoring. ACS Sens. 2021, 6, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef] [Green Version]
- Hossain, A.; Nakamichi, S.; Habibullah-AL-Mamun, M.; Tani, K.; Masunaga, S.; Matsuda, H. Occurrence and ecological risk of pharmaceuticals in river surface water of Bangladesh. Environ. Res. 2018, 165, 258–266. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): Occurrence and potential risk assessments. Sci. Total Environ. 2018, 642, 230–240. [Google Scholar] [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical sensors and biosensors for the determination of diclofenac in pharmaceutical, biological and water samples. Talanta Open 2021, 3, 100026. [Google Scholar] [CrossRef]
- Qiu, B.; Hu, Y.; Liang, C.; Wang, L.; Shu, Y.; Chen, Y.; Cheng, J. Enhanced degradation of diclofenac with Ru/Fe modified anode microbial fuel cell: Kinetics, pathways and mechanisms. Bioresour. Technol. 2020, 300, 122703. [Google Scholar] [CrossRef]
- Smaali, A.; Berkani, M.; Merouane, F.; Vasseghian, Y.; Rahim, N.; Kouachi, M. Photocatalytic-persulfate-oxidation for diclofenac removal from aqueous solutions: Modeling, optimization and biotoxicity test assessment. Chemosphere 2021, 266, 129158. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, N.; Gao, L.; Yuan, R.; Chen, H.; Hou, X.; Hou, J.; Wang, F.; Zhou, B. Removal of diclofenac in effluent of sewage treatment plant by photocatalytic oxidation. Water 2020, 12, 2902. [Google Scholar] [CrossRef]
- Saravanan, M.; Karthika, S.; Malarvizhi, A.; Ramesh, M. Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: Hematological, biochemical, ionoregulatory and enzymological responses. J. Hazard. Mater. 2011, 195, 188–194. [Google Scholar] [CrossRef]
- Pfluger, P.; Dietrich, D.R. Effects on pharmaceuticals in the environment—An overview and principle considerations. In Pharmaceuticals in the Environment; Springer: Berlin/Heidelberg, Germany, 2001; pp. 11–17. [Google Scholar]
- Ouada, S.B.; Ali, R.B.; Cimetiere, N.; Leboulanger, C.; Ouada, H.B.; Sayadi, S. Biodegradation of diclofenac by two green microalgae: Picocystis sp. and Graesiella sp. Ecotoxicol. Environ. Saf. 2019, 186, 109769. [Google Scholar] [CrossRef] [PubMed]
- Urase, T.; Kikuta, T. Separate estimation of adsorption and degradation of pharmaceutical substances and estrogens in the activated sludge process. Water Res. 2005, 39, 1289–1300. [Google Scholar] [CrossRef]
- Rigobello, E.S.; Dantas, A.D.B.; Di Bernardo, L.; Vieira, E.M. Removal of diclofenac by conventional drinking water treatment processes and granular activated carbon filtration. Chemosphere 2013, 92, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.-Y.; Chen, Z.-W.; Li, M.-M.; Xiang, Q.-H.; Wang, X.-X.; Miao, H.-F.; Ruan, W.-Q. Degradation of diclofenac sodium using Fenton-like technology based on nano-calcium peroxide. Sci. Total Environ. 2021, 773, 144801. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.A.; Zouari, N.; Al-Ghouti, M.A. Removal of pesticides from water and wastewater: Chemical, physical and biological treatment approaches. Environ. Technol. Innov. 2020, 19, 101026. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Effects of carbon source, nitrogen source, and natural algal powder-derived carbon source on biodegradation of tetracycline (TEC). Bioresour. Technol. 2019, 288, 121567. [Google Scholar] [CrossRef]
- Aguilera Flores, M.M.; Ávila Vázquez, V.; Medellín Castillo, N.A.; Carranza Álvarez, C.; Cardona Benavides, A.; Ocampo Pérez, R.; Labrada Delgado, G.J.; Durón Torres, S.M. Ibuprofen degradation and energy generation in a microbial fuel cell using a bioanode fabricated from devil fish bone char. J. Environ. Sci. Health Part A 2021, 56, 874–885. [Google Scholar] [CrossRef]
- Xie, B.; Liang, H.; You, H.; Deng, S.; Yan, Z.; Tang, X. Microbial community dynamic shifts associated with sulfamethoxazole degradation in microbial fuel cells. Chemosphere 2021, 274, 129744. [Google Scholar] [CrossRef]
- Maddalwar, S.; Nayak, K.K.; Kumar, M.; Singh, L. Plant microbial fuel cell: Opportunities, challenges, and prospects. Bioresour. Technol. 2021, 341, 125772. [Google Scholar] [CrossRef]
- Peng, X.; Cao, J.; Xie, B.; Duan, M.; Zhao, J. Evaluation of degradation behavior over tetracycline hydrochloride by microbial electrochemical technology: Performance, kinetics, and microbial communities. Ecotoxicol. Environ. Saf. 2020, 188, 109869. [Google Scholar] [CrossRef] [PubMed]
- Dessie, Y.; Tadesse, S. Nanocomposites as Efficient Anode Modifier Catalyst for Microbial Fuel Cell Performance Improvement. J. Chem. Rev. 2021, 3, 320–344. [Google Scholar]
- Dessie, Y.; Tadesse, S.; Adimasu, Y. Improving the performance of graphite anode in a Microbial Fuel Cell via PANI encapsulated α-MnO2 composite modification for efficient power generation and methyl red removal. Chem. Eng. J. Adv. 2022, 10, 100283. [Google Scholar] [CrossRef]
- Nandy, A.; Sharma, M.; Venkatesan, S.V.; Taylor, N.; Gieg, L.; Thangadurai, V. Comparative evaluation of coated and non-coated carbon electrodes in a microbial fuel cell for treatment of municipal sludge. Energies 2019, 12, 1034. [Google Scholar] [CrossRef] [Green Version]
- Sallam, E.R.; Fetouh, H.A. Comparative Study for the Production of Sustainable Electricity from Marine Sediment Using Recyclable Low-Cost Solid Wastes Aluminum Foil and Graphite Anodes. ChemistrySelect 2022, 7, e202103972. [Google Scholar] [CrossRef]
- Sallam, E.; Khairy, H.; Elnouby, M.; Fetouh, H. Sustainable electricity production from seawater using Spirulina platensis microbial fuel cell catalyzed by silver nanoparticles-activated carbon composite prepared by a new modified photolysis method. Biomass Bioenergy 2021, 148, 106038. [Google Scholar] [CrossRef]
- Schmidt, S.; Hoffmann, H.; Garbe, L.-A.; Schneider, R.J. Liquid chromatography–tandem mass spectrometry detection of diclofenac and related compounds in water samples. J. Chromatogr. A 2018, 1538, 112–116. [Google Scholar] [CrossRef]
- Sallam, E.R.; Khairy, H.M.; Elshobary, M.; Fetouh, H.A. Application of Algae for Hydrogen Generation and Utilization. In Handbook of Research on Algae as a Sustainable Solution for Food 2022, Energy, and the Environment; IGI Global: Hershey, PA, USA, 2022. [Google Scholar]
- Wang, Y.-Q.; Huang, H.-X.; Li, B.; Li, W.-S. Novelly developed three-dimensional carbon scaffold anodes from polyacrylonitrile for microbial fuel cells. J. Mater. Chem. A 2015, 3, 5110–5118. [Google Scholar] [CrossRef]
- Wu, X.Y.; Tong, F.; Song, T.S.; Gao, X.Y.; Xie, J.J.; Zhou, C.C.; Zhang, L.X.; Wei, P. Effect of zeolite-coated anode on the performance of microbial fuel cells. J. Chem. Technol. Biotechnol. 2015, 90, 87–92. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, D.; Yue, X.; Feng, C.; Wei, C. Ruthenium oxide-coated carbon felt electrode: A highly active anode for microbial fuel cell applications. J. Power Sources 2012, 210, 26–31. [Google Scholar] [CrossRef]
- Peng, X.; Yu, H.; Wang, X.; Zhou, Q.; Zhang, S.; Geng, L.; Sun, J.; Cai, Z. Enhanced performance and capacitance behavior of anode by rolling Fe3O4 into activated carbon in microbial fuel cells. Bioresour. Technol. 2012, 121, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, P.; Yang, X.; Jiang, Y.; Bian, Y.; Chen, C.; Zhang, X.; Huang, X. Binder-free graphene and manganese oxide coated carbon felt anode for high-performance microbial fuel cell. Biosens. Bioelectron. 2016, 81, 32–38. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Abdelkareem, M.A.; Obaid, M.; Chae, S.-H.; Park, M.; Kim, H.Y.; Barakat, N.A. Cobalt oxides-sheathed cobalt nano flakes to improve surface properties of carbonaceous electrodes utilized in microbial fuel cells. Chem. Eng. J. 2017, 326, 497–506. [Google Scholar] [CrossRef]
- Tahir, K.; Miran, W.; Jang, J.; Maile, N.; Shahzad, A.; Moztahida, M.; Ghani, A.A.; Kim, B.; Lee, D.S. MnCo2O4 coated carbon felt anode for enhanced microbial fuel cell performance. Chemosphere 2021, 265, 129098. [Google Scholar] [CrossRef]
- Ye, Z.; Li, T.; Ma, G.; Dong, Y.; Zhou, X. Metal-Ion (Fe, V, Co, and Ni)-doped MnO2 ultrathin nanosheets supported on Carbon fiber paper for the oxygen evolution reaction. Adv. Funct. Mater. 2017, 27, 1704083. [Google Scholar] [CrossRef]
- Cakici, M.; Kakarla, R.R.; Alonso-Marroquin, F. Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem. Eng. J. 2017, 309, 151–158. [Google Scholar] [CrossRef]
- Zarshad, N.; Wu, J.; Rahman, A.U.; Ni, H. Fe-MnO2 core-shell heterostructure for high-performance aqueous asymmetrical supercapacitor. J. Electroanal. Chem. 2020, 871, 114266. [Google Scholar] [CrossRef]
- Kumar, Y.; Chopra, S.; Gupta, A.; Kumar, Y.; Uke, S.; Mardikar, S. Low temperature synthesis of MnO2 nanostructures for supercapacitor application. Mater. Sci. Energy Technol. 2020, 3, 566–574. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gad-Allah, T.A.; El-Khatib, K.; El-Gohary, F. Power generation using spinel manganese–cobalt oxide as a cathode catalyst for microbial fuel cell applications. Bioresour. Technol. 2011, 102, 10459–10464. [Google Scholar] [CrossRef]
- Dessie, Y.; Tadesse, S.; Eswaramoorthy, R.; Adimasu, Y. Biosynthesized α-MnO2-based polyaniline binary composite as efficient bioanode catalyst for high-performance microbial fuel cell. All Life 2021, 14, 541–568. [Google Scholar] [CrossRef]
- Yu, L.; Yuan, Y.; Tang, J.; Zhou, S. Thermophilic Moorella thermoautotrophica-immobilized cathode enhanced microbial electrosynthesis of acetate and formate from CO2. Bioelectrochemistry 2017, 117, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Davari, E.; Johnson, A.D.; Mittal, A.; Xiong, M.; Ivey, D.G. Manganese-cobalt mixed oxide film as a bifunctional catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2016, 211, 735–743. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, B.; Li, B.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. Study on the characteristics of a high capacity nickel manganese cobalt oxide (NMC) lithium-ion battery—An experimental investigation. Energies 2018, 11, 2275. [Google Scholar] [CrossRef] [Green Version]
- Khilari, S.; Pandit, S.; Varanasi, J.L.; Das, D.; Pradhan, D. Bifunctional manganese ferrite/polyaniline hybrid as electrode material for enhanced energy recovery in microbial fuel cell. ACS Appl. Mater. Interfaces 2015, 7, 20657–20666. [Google Scholar] [CrossRef]
- Hu, M.; Li, X.; Xiong, J.; Zeng, L.; Huang, Y.; Wu, Y.; Cao, G.; Li, W. Nano-Fe3C@ PGC as a novel low-cost anode electrocatalyst for superior performance microbial fuel cells. Biosens. Bioelectron. 2019, 142, 111594. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, H.; Li, P.; Sun, L.; Hou, F.; Li, B. Treatment of pharmaceutical wastewater for reuse by coupled membrane-aerated biofilm reactor (MABR) system. RSC Adv. 2015, 5, 69829–69838. [Google Scholar] [CrossRef]
- Amari, S.; Boshrouyeh Ghandashtani, M. Non-steroidal anti-inflammatory pharmaceutical wastewater treatment using a two-chambered microbial fuel cell. Water Environ. J. 2020, 34, 413–419. [Google Scholar] [CrossRef]
- Zhao, J.; Xin, M.; Zhang, J.; Sun, Y.; Luo, S.; Wang, H.; Wang, Y.; Bi, X. Diclofenac inhibited the biological phosphorus removal: Performance and mechanism. Chemosphere 2020, 243, 125380. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Ganguly, K.; Chaudhuri, K.; Chakrabarty, A. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int. J. Antimicrob. Agents 2000, 14, 249–251. [Google Scholar] [CrossRef]
- Wu, Y.; Fu, H.; Wen, H.; Chen, F.; Dai, Z.; Yang, A. Degradation of Diclofenac Sodium in Microbial fuel cells. IOP Conf. Ser. Earth Environ. Sci. 2019, 369, 012011. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Freguia, S.; Rabaey, K.; Yuan, Z.; Keller, J. Electron and carbon balances in microbial fuel cells reveal temporary bacterial storage behavior during electricity generation. Environ. Sci. Technol. 2007, 41, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ. Sci. Technol. 2007, 41, 3708–3714. [Google Scholar] [CrossRef]
- Xu, H.; Quan, X.; Xiao, Z.; Chen, L. Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem. Eng. J. 2018, 335, 539–547. [Google Scholar] [CrossRef]
- Zwiener, C.; Frimmel, F. Short-term tests with a pilot sewage plant and biofilm reactors for the biological degradation of the pharmaceutical compounds clofibric acid, ibuprofen, and diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Kuramitz, H.; Nakata, Y.; Kawasaki, M.; Tanaka, S. Electrochemical oxidation of bisphenol A. Application to the removal of bisphenol A using a carbon fiber electrode. Chemosphere 2001, 45, 37–43. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Yanina, S.; Mclean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef]


| COD (mg/L) | ||||||
|---|---|---|---|---|---|---|
| Time (h) | 2000 (mg/L) | 1000 (mg/L) | 500 (mg/L) | 250 (mg/L) | 100 (mg/L) | COD (1000 mg/L) DCF (20 mg/L) |
| 24 | 53.3 ± 0.19 | 76.92 ± 1.19 | 74.4 ± 1.57 | 58.8 ± 0.35 | 57.5 ± 1.15 | 46 ± 1.49 |
| 48 | 85.33 ± 0.23 | 80.77 ± 0.18 | 76.75 ± 1.06 | 70.4 ± 0.27 | 74.5 ± 1.09 | 56 ± 1.11 |
| 72 | 94.67 ± 0.02 | 89.74 ± 0.13 | 76.75 ± 1.12 | 80 ± 0.30 | 75.7 ± 0.55 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morovati, R.; Hoseini, M.; Azhdarpoor, A.; Dehghani, M.; Baghapour, M.A.; Yousefinejad, S. Removal of Diclofenac Sodium from Wastewater in Microbial Fuel Cell by Anode Modified with MnCo2O4. Sustainability 2022, 14, 13907. https://doi.org/10.3390/su142113907
Morovati R, Hoseini M, Azhdarpoor A, Dehghani M, Baghapour MA, Yousefinejad S. Removal of Diclofenac Sodium from Wastewater in Microbial Fuel Cell by Anode Modified with MnCo2O4. Sustainability. 2022; 14(21):13907. https://doi.org/10.3390/su142113907
Chicago/Turabian StyleMorovati, Roya, Mohammad Hoseini, Abooalfazl Azhdarpoor, Mansooreh Dehghani, Mohammad Ali Baghapour, and Saeed Yousefinejad. 2022. "Removal of Diclofenac Sodium from Wastewater in Microbial Fuel Cell by Anode Modified with MnCo2O4" Sustainability 14, no. 21: 13907. https://doi.org/10.3390/su142113907






