Comprehensive Utilization and Sustainable Development of Bauxite in Northern Guizhou on a Background of Carbon Neutralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Literature Method
- (1)
- Comprehensive study of the geological characteristics, development horizon and distribution law of associated mineral resources in Guizhou.
- (2)
- Evaluation of associated mineral resources and optimization of prospecting targets in Guizhou.
- (3)
- Establishing a pilot project of comprehensive investigation, evaluation and re-use technology of associated mineral tailings resources in Guizhou.
2.2. Supplementary Test
3. Results
3.1. Data Collection from the Literature
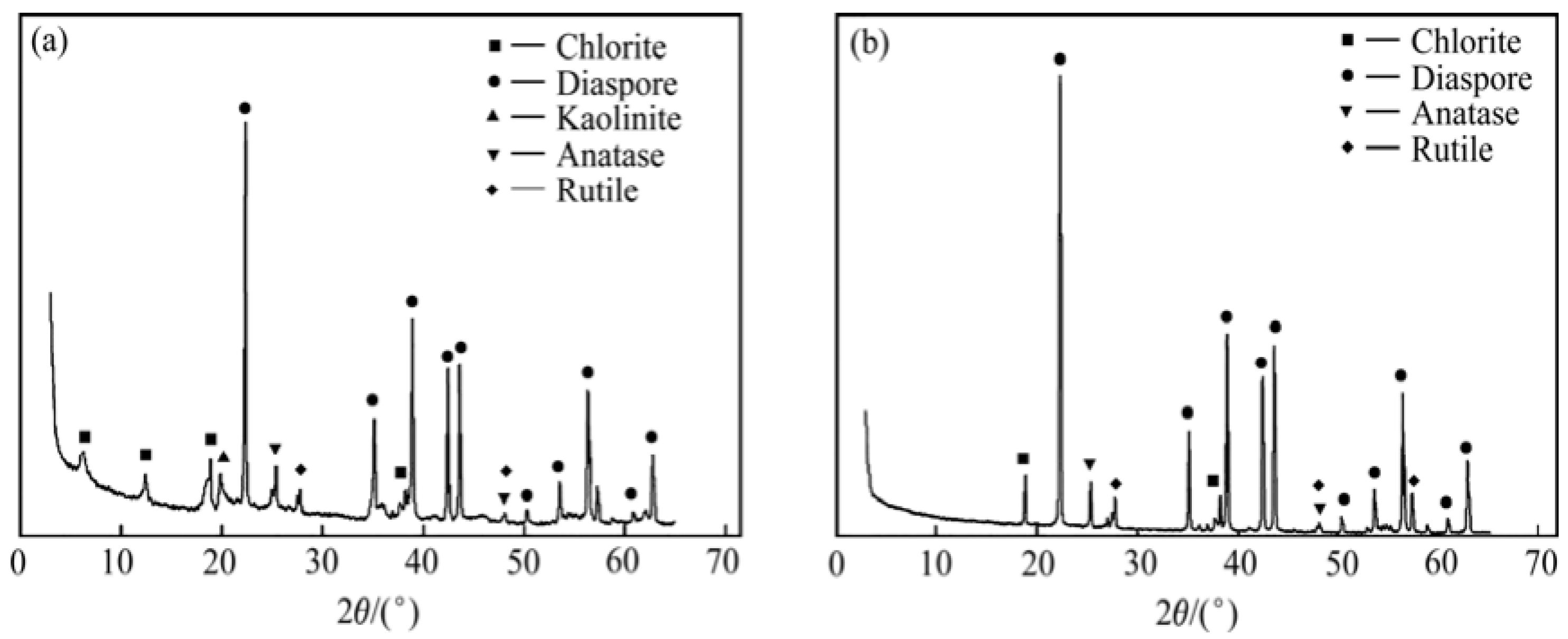
3.2. Mineral Characterization
3.3. Chemical Characterization
3.3.1. Major Element Characterization
3.3.2. Trace Element Characterization
4. Discussion
4.1. Data Analysis from the Literature
4.2. Enrichment and Migration of Associated Ga, Li and Rare Earth Elements
4.3. Metallogenic Process Analysis
4.3.1. Mineralization
4.3.2. The Ore-Forming Process
4.4. Comprehensive Utilization Analysis of Associated Ga, Li and Rare Earth Elements
4.4.1. Analysis of the Comprehensive Utilization of Ga and Li
4.4.2. Analysis of the Comprehensive Utilization of Rare Earth Elements
5. Conclusions
5.1. Urgent Demand for Science and Technology
5.2. Accelerating Breakthrough Strategic Actions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, C.; Min, Z. Characteristics of Heavy Mineral Assemblages in Bauxite Deposits in Northern Guizhou and Their Geological Significance. Chin. J. Nonferrous Met. 2021, 31, 1106–1119. [Google Scholar]
- Lapparent, J.D.E. Les Bauxites de la France Méridionale; Imprimerie Nationale: Paris, France, 1930; pp. 1–187. [Google Scholar]
- Du, Y.; Zhou, Q.; Jin, Z.; Ling, W.; Zhang, X.; Yu, J.; Wang, X.; Yu, W.; Huang, X. Research progress on basic geology and mineralization of bauxite in Wuzhengdao area, northern Guizhou. Geol. Sci. Technol. Inf. 2013, 32, 1–6. [Google Scholar]
- Liu, Y.; Zhou, W.; Cheng, G.; Cui, T.; Long, H. Study on characteristics of ore-bearing rock series and metallogenic regularity of bauxite in Guizhou. Acta Mineral. Sinica 2016, 36, 289–294. [Google Scholar]
- Lei, Z.; Weng, S.; Chen, Q.; Xiong, X.; Pan, Z.; He, X.; Chen, H. Lithofacies palaeogeography of the Dazhuyuan Age of the Early Permian in the Wuzhengdao area, northern Guizhou, and its ore-controlling significance for bauxite. Geol. Sci. Technol. Inf. 2013, 32, 8–12. [Google Scholar]
- Cui, T.; Jiao, Y.; Du, Y.; Yu, W.; Ji, B.; Lei, Z.; Weng, S.; Jin, Z.; He, X. Palaeosalinity identification of bauxite formation environment in Wuzhengdao area, northern Guizhou. Geol. Sci. Technol. Inf. 2013, 32, 46–51. [Google Scholar]
- Liu, C.; Jin, Z.; Guo, J. Sedimentary facies of freshwater sedimentary bauxite deposit in Wuzhengdao area, northern Guizhou. J. Cent. South Univ. 2015, 46, 962–969. [Google Scholar]
- Wang, X.; Jiao, Y.; Du, Y.; Ling, W.; Wu, L. Rare earth element geochemical characteristics of bauxite in Wuzhengdao area, northern Guizhou. Geol. Sci. Technol. Inf. 2013, 32, 27–33. [Google Scholar]
- Emmalou Van Tilburg, N. Why adults participate? J. Ext. 1992, 30, 12–13. [Google Scholar]
- Thongmak, M. Inquiring into lifelong learning intention: Comparisons of gender, employment status, and media exposure. Int. J. Lifelong Educ. 2021, 40, 72–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wu, H.; Ding, X.; Ling, W.; Lei, Z.; Weng, S.; Ma, Q.; Du, Y. Micro-area element geochemical characteristics and metallogenic significance of oolitic bauxite in Wuzhengdao area, northern Guizhou. Geol. Sci. Technol. Inf. 2013, 32, 62–70. [Google Scholar]
- Zhang, Y.; Ling, W.; Wu, H. Geochemical characteristics of different types of bauxite ores in northern Guizhou and their implications for mineralization. Geol. Sci. Technol. Inf. 2013, 32, 71–79. [Google Scholar]
- Yin, K. Mineralization and metallogenic model of Wuzhengdao bauxite deposit in northern Guizhou. Acta Sedimentol. Sin. 2009, 27, 452–457. [Google Scholar]
- Zarasvandi, A.; Carranza, E.J.M.; Ellahi, S.S. Geological, geochemical, and mineralogical characteristics of the Mandan and Deh-now bauxite deposits, Zagros Fold Belt, Iran. Ore Geol. Rev. 2012, 48, 125–138. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Deng, J.; Zhang, Q.; Sun, S.; Meng, J. Mineralogical and geochemical investigations of the Dajia Salento-type bauxite deposits, western Guangxi, China. J. Geochem. Explor. 2010, 105, 137–152. [Google Scholar] [CrossRef]
- Wang, D.; Li, P.; Qu, W.; Yin, L.; Zhao, Z.; Lei, Z.; Wen, S. Discovery and comprehensive evaluation of tungsten and lithium in Dazhuyuan bauxite deposit, Guizhou Province. Sci. China Earth Sci. 2013, 43, 44–51. [Google Scholar]
- Yu, W.; Du, Y.; Gu, S. Multi-stage leaching of Early Permian bauxite in Wuzhengdao area, northern Guizhou, and its ore-controlling significance. Geol. Sci. Technol. Inf. 2013, 32, 34–39. [Google Scholar]
- Jin, Z.; Zou, L.; Zhang, L.; Zheng, M.; Han, Y. Metallogenic model and prospecting model of bauxite in Wu-Zheng-Dao area, Guizhou. Acta Sedimentol. Sin. 2018, 36, 914–926. [Google Scholar]
- Calagari, A.A.; Abedini, A. Geochemical investigations on Permo-Triassic bauxite horizon at Kanisheeteh, east of Bukan, West-Azarbaidjan, Iran. J. Geochem. Explor. 2007, 94, 1–18. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Z.; Guo, J. Effect of sea level change on the enrichment and mineralization of sedimentary bauxite: A case study of bauxite in northern Guizhou. Chin. J. Nonferrous Met. 2018, 28, 985–993. [Google Scholar]
- Anawati, J.; Azimi, G. Recovery of scandium from Canadian bauxite residue utilizing acid baking followed by water leaching. Waste Manag. 2019, 95, 549–559. [Google Scholar] [CrossRef]
- Gu, J.; Huang, Z.; Fan, H.; Jin, Z.; Yan, Z.; Zhang, J. Mineralogy, geochemistry, and genesis of lateritic bauxite deposits in the Wuchuan–Zheng’an–Daozhen area, Northern Guizhou Province, China. J. Geochem. Explor. 2013, 130, 44–59. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Kazantzis, N.K.; Emmert, M.H. Process for Scandium Recovery from Jamaican Bauxite Residue: A Probabilistic Economic Assessment. Mater. Today Proc. 2019, 9, 578–586. [Google Scholar] [CrossRef]
- Zhou, K.; Teng, C.; Zhang, X.; Peng, C.; Chen, W. Enhanced selective leaching of scandium from red mud. Hydrometallurgy 2018, 182, 57–63. [Google Scholar] [CrossRef]
- Li, G.; Ye, Q.; Deng, B.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Extraction of scandium from scandium-rich material derived from bauxite ore residues. Hydrometallurgy 2018, 176, 62–68. [Google Scholar] [CrossRef]
- Zhu, X.; Niu, Z.; Li, W.; Zhao, H.; Tang, Q. A novel process for recovery of aluminum, iron, vanadium, scandium, titanium and silicon from red mud. J. Environ. Chem. Eng. 2020, 8, 103528. [Google Scholar] [CrossRef]
- Mameli, P.; Mongelli, G.; Oggiano, G.; Dinelli, E. Geological, geochemical and mineralogical features of some bauxite deposits from Nurra (Western Sardinia, Italy): Insights on conditions of formation and parental affinity. Int. J. Earth Sci. 2007, 96, 887–902. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Yang, S.; Ma, X.; Liu, L.; Sun, X. Regional multi-sources of Carboniferous karstic bauxite deposits in North China Craton: Insights from multi-proxy provenance systems. Sediment. Geol. 2021, 421, 105958. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Zhao, F.; Liu, D.; Zou, Y.; Zhang, W.; Liu, X.; Li, L.; Zhao, L. Geological and geochemical characteristics of karst bauxite-bearing sequences in Xiabu area, Central Shanxi Province, North China. J. Geochem. Explor. 2021, 230, 106849. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, M.; Yan, J.; Chen, X. Zircon U-Pb Ages and Geochemistry of the Granite in the Xintianling Tungsten Deposit, SE China: Implications for Geodynamic Settings of the Regional Tungsten Mineralization. Minerals 2022, 12, 952. [Google Scholar] [CrossRef]
- Onghena, B.; Borra, C.R.; Van Gerven, T.; Binnemans, K. Recovery of scandium from sulfation-roasted leachates of bauxite residue by solvent extraction with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Sep. Purif. Technol. 2017, 176, 208–219. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Wu, Y.; Lei, Q.; Peng, C.; Chen, W. Separation and recovery of iron and scandium from acid leaching solution of red mud using D201 resin. J. Rare Earths 2019, 38, 1322–1329. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, W.; Yang, M.; Yan, J. Guizhou Karst Carbon Sink and Sustainability—An Overview. Sustainability 2022, 14, 11518. [Google Scholar] [CrossRef]
- Wang, W.W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q.; et al. Recovery of gallium from Bayer red mud through acidic-leaching-ionexchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Nayak, S.; Devi, N. Studies on extraction of gallium (III) from chloride solution using Cyphos IL 104 and its removal from photodiodes and red mud. Hydrometallurgy 2017, 171, 191–197. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Selective leaching ofrare earth elements frombauxite residue (redmud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 2016, 164, 125–135. [Google Scholar] [CrossRef]
- Davris, P.; Balomenos, E.; Panias, D.; Paspaliaris, I. Chapter 12—Leaching Rare Earth Elements from Bauxite Residue Using Brønsted Acidic Ionic Liquids. In Rare Earths Industry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 183–197. [Google Scholar]
- Deng, B.; Li, G.; Luo, J.; Ye, Q.; Liu, M.; Rao, M.; Jiang, T.; Bauman, L.; Zhao, B. Selectively leaching the iron-removed bauxite residues with phosphoric acid for enrichment of rare earth elements. Sep. Purif. Technol. 2019, 227, 115714. [Google Scholar] [CrossRef]
- Zhang, X.K.; Zhou, K.G.; Chen, W.; Lei, Q.Y.; Huang, Y.; Peng, C.H. Recovery of iron and rare earth elements from red mud through an acid leaching-stepwise extraction approach. J. Cent. South Univ. 2019, 26, 458–466. [Google Scholar] [CrossRef]
- Zhao, F.P.; Yang, Z.Q.; Wei, Z.S. Polyethylenimine-modified chitosan materials for the recovery of La(III) from leachates of bauxiteresidue. Chem. Eng. J. 2020, 388, 124307. [Google Scholar] [CrossRef]
- Yang, W.; Min, Z.; Yang, M.; Yan, J. Exploration of the Implementation of Carbon Neutralization in the Field of Natural Resources under the Background of Sustainable Development—An Overview. Int. J. Environ. Res. Public Health 2022, 19, 14109. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Jin, Z.; Zheng, M.; Liu, L.; Huang, Z.; Ye, L.; Wu, S.; Zeng, D.; Gu, J. Guizhou bauxite Distribution characteristics and enrichment mechanism of lithium in ore-bearing rocks [J/OL]. Acta Geol. Sinica 2022, 1, 225. [Google Scholar] [CrossRef]

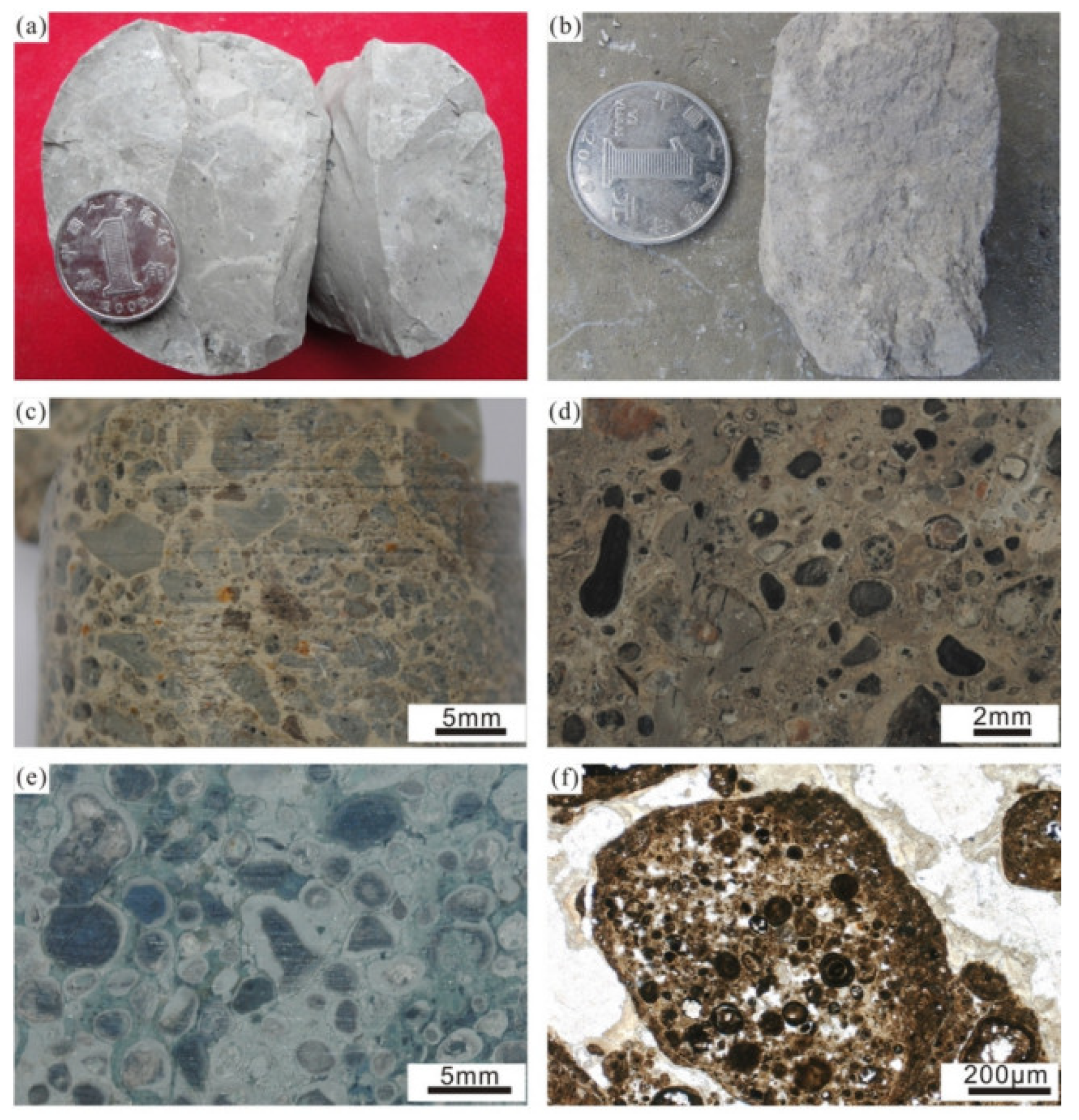

| Ore Field Division | Serial Number | County/City | Name | Companion | Grade of Associated Elements | Al2O3 (%) | Associated Resources (t) | Bauxite Resources (10,000 t) |
|---|---|---|---|---|---|---|---|---|
| Minerals | ||||||||
| Guiyang-Qingzhen bauxite ore field | 1 | Xiuwen County | Xiaoshan Dam | Ga,Sc, Ge | 0.0031~0.005; | 68.47 | Ga:666 | 1343 |
| 0.00287~0.00752; | ||||||||
| 0.000243~0.000547 | ||||||||
| 2 | Xiuwen County | Arrow shaft punch | Ga | 0.0022~0.0136 | 65.36 | 220.59 | 407 | |
| 3 | Xiuwen County | Dry Dam | Ga,Sc | Ga:0.006 | 63.52 | Ga:777 | 646 | |
| 4 | Xiuwen County | Changchong | Ga,Sc | Ga:0.005 | 63.4 | Ga:388 | 786 | |
| 5 | Qingzhen city | Cat Farm | Ga,Ge,Sc | 0.0058; 0.001~0.1; | 67.6 | Ga:9317 | 178508 | |
| 0.065 | ||||||||
| 6 | Qingzhen city | Lin Dao | Ga,Sc,Zr | 0.0067; | 66.32 | Ga:543 | 689 | |
| 0.0023~0.0072; | ||||||||
| 0.01 | ||||||||
| 7 | Qingzhen city | Yan Long | Ga | 0.0073 | 70.85 | 558.59 | 680 | |
| 8 | Qingzhen city | Mai Ba | Ga | 0.0087 | 65.68 | 1006 | 1255 | |
| 9 | Qingzhen city | Meg | Ga, Sc | 0.0061; | 67.49 | Ga:276 | 600 | |
| 0.00287~0.00752 | ||||||||
| 10 | Guiyang City | Cape Hill | Ga | 0.0094 | 66.05 | 1081.54 | 817 | |
| Zunyi-Xifeng bauxite ore field | 11 | Zunyi County | Rear Slot | Ga.V | 0.0132; | 67.04 | Ga:1594 | 993 |
| 0.0139~0.0674 | ||||||||
| 12 | Zunyi County | Chuanzhu Temple | Ga | 0.0134 | 65.65 | 818 | 544 | |
| 13 | Zunyi County | New Station | Ga | 0.012 | 60.22 | - | 331 | |
| 14 | Zunyi County | Gou Jiang | Ga | 0.014 | 65.22 | 905 | 678 | |
| 15 | Zunyi County | Song Jia Dalin | Ga | 0.0109 | 63.5 | 743.95 | 513 | |
| 16 | Zunyi County | Fairy Rock | Ga | 0.0098 | 57.45 | 1499 | - | |
| 17 | Kaiyang County | Zhao Jiawan | Ga | 0.0157 | 61.43 | - | 428.5 | |
| 18 | Kaiyang County | Xinzhai | Ga | 0.0155 | 70.47 | - | - | |
| 19 | Xifeng County | Shuitou village | Ga | 0.0135 | 62.26 | - | - | |
| Wuchuan-Zhengan-Daozhen bauxite ore field | 20 | Daozhen, Wuchuan, Xinmin, Zhengan County | Dazhuyuan | Ga,W,Li,Zr | 0.0086; 0.1~0.22; | 68.85 | Ga:5448.25 | 3564 |
| Li2O:0.0984; 0.0818 | Li2O: | |||||||
| 62337.97 | ||||||||
| 21 | Wuchuan County | Wachangping | Ga.Li | Ga:0.01~0.03; | 73 | Ga:7372; | 4397 | |
| Li2O5:0.1-0.5 | Li2O5:69188 |
| Sample | Deep (m) | Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | TiO2 | MnO | Fe2O3 | LOI | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZK14904-4 | 386.7 | 0.54 | 1.96 | 30.05 | 39.08 | 0.04 | 3.79 | 0.27 | 2.12 | 0.01 | 8.11 | 13.89 | 99.86 |
| ZK14904-5 | 387.0 | 0.43 | 2.40 | 31.02 | 36.59 | 0.07 | 2.06 | 0.15 | 1.77 | 0.02 | 10.69 | 14.73 | 99.93 |
| ZK14904-7 | 387.9 | 0.03 | 1.93 | 54.65 | 7.89 | 0.05 | 0.01 | 0.26 | 4.22 | 0.05 | 15.42 | 15.38 | 99.89 |
| ZK14904-8 | 388.1 | 0.04 | 1.81 | 58.57 | 8.22 | 0.09 | 0.05 | 0.35 | 5.24 | 0.05 | 11.74 | 13.73 | 99.89 |
| ZK14904-9 | 388.6 | 0.02 | 1.61 | 61.13 | 7.96 | 0.10 | 0.01 | 0.01 | 3.20 | 0.03 | 12.44 | 13.38 | 99.89 |
| ZK14904-10 | 389.0 | 0.06 | 2.00 | 56.75 | 9.95 | 0.09 | 0.01 | 0.01 | 2.71 | 0.04 | 15.43 | 12.86 | 99.91 |
| ZK14904-13 | 390.5 | 0.05 | 0.66 | 67.31 | 3.55 | 0.10 | 0.01 | 0.09 | 3.34 | 0.02 | 6.52 | 18.31 | 99.96 |
| ZK14904-14 | 390.9 | 0.04 | 0.92 | 60.59 | 5.58 | 0.07 | 0.01 | 0.01 | 2.75 | 0.02 | 10.61 | 19.35 | 99.95 |
| ZK14904-15 | 391.6 | 0.04 | 1.24 | 54.81 | 7.7 | 0.12 | 0.01 | 0.01 | 3.99 | 0.03 | 14.57 | 17.41 | 99.93 |
| ZK14904-16 | 392.1 | 0.04 | 1.20 | 53.65 | 7.93 | 0.07 | 0.01 | 0.01 | 3.41 | 0.02 | 15.11 | 18.47 | 99.92 |
| ZK14904-17 | 393.2 | 0.04 | 1.38 | 57.40 | 7.77 | 0.07 | 0.01 | 0.01 | 3.05 | 0.02 | 13.67 | 16.50 | 99.92 |
| ZK14904-19 | 393.4 | 0.06 | 2.54 | 45.80 | 13.53 | 0.10 | 0.01 | 0.01 | 2.51 | 0.04 | 21.45 | 13.89 | 99.94 |
| ZK14904-20 | 393.5 | 0.04 | 3.55 | 36.82 | 18.38 | 0.09 | 0.01 | 0.01 | 2.01 | 0.04 | 27.67 | 11.29 | 99.91 |
| ZK14904-21 | 394.1 | 0.13 | 3.94 | 32.07 | 34.35 | 0.08 | 0.44 | 0.05 | 1.55 | 0.03 | 14.94 | 12.35 | 99.93 |
| ZK14904-22 | 395.3 | 0.22 | 3.47 | 30.47 | 34.13 | 0.10 | 1.14 | 0.07 | 1.52 | 0.03 | 17.95 | 10.77 | 99.87 |
| ZK14904-23 | 396.5 | 0.22 | 3.10 | 30.36 | 33.58 | 0.08 | 1.23 | 0.08 | 1.63 | 0.03 | 19.27 | 10.35 | 99.93 |
| ZK14904-24 | 397.6 | 0.30 | 2.30 | 32.03 | 36.95 | 0.08 | 1.78 | 0.07 | 1.76 | 0.02 | 14.15 | 10.55 | 99.99 |
| ZK14904-25 | 398.3 | 0.31 | 2.53 | 30.60 | 35.13 | 0.06 | 1.69 | 0.11 | 1.71 | 0.03 | 17.63 | 10.11 | 99.91 |
| ZK14904-26 | 398.8 | 0.03 | 1.32 | 62.33 | 6.83 | 0.12 | 0.01 | 0.05 | 3.38 | 0.03 | 11.33 | 14.47 | 99.90 |
| ZK14904-27 | 399.6 | 0.13 | 3.63 | 33.83 | 37.99 | 0.05 | 0.18 | 0.08 | 2.39 | 0.01 | 8.24 | 13.42 | 99.95 |
| ZK702-2 | 199.6 | 0.04 | 0.87 | 75.16 | 4.04 | 0.05 | 0.02 | 0.01 | 4.44 | 0.01 | 0.77 | 14.53 | 99.94 |
| ZK702-3 | 201.7 | 0.07 | 0.69 | 38.69 | 42.07 | 0.04 | 0.16 | 0.10 | 1.70 | 0.01 | 1.83 | 14.55 | 99.91 |
| ZK702-4 | 202.3 | 0.19 | 0.51 | 36.56 | 42.8 | 0.04 | 0.38 | 0.12 | 1.63 | 0.01 | 3.16 | 14.56 | 99.96 |
| ZK702-5 | 203.5 | 0.08 | 3.47 | 32.25 | 34.05 | 0.04 | 0.07 | 0.07 | 1.79 | 0.14 | 16.00 | 11.99 | 99.95 |
| ZK702-6 | 204.8 | 0.59 | 1.38 | 35.17 | 41.85 | 0.08 | 1.73 | 0.12 | 1.46 | 0.04 | 5.78 | 11.76 | 99.96 |
| ZK3402-3 | 297.7 | 0.19 | 4.22 | 40.33 | 31.88 | 0.05 | 1.62 | 0.08 | 3.00 | 0.01 | 4.52 | 14.00 | 99.90 |
| ZK3402-4 | 298.3 | 0.08 | 4.95 | 40.27 | 23.75 | 0.06 | 0.38 | 0.06 | 2.07 | 0.01 | 12.42 | 15.90 | 99.95 |
| ZK3402-6 | 299.6 | 0.03 | 1.12 | 72.27 | 4.49 | 0.12 | 0.04 | 0.01 | 3.05 | 0.01 | 4.22 | 14.55 | 99.91 |
| ZK3402-7 | 299.8 | 0.03 | 1.13 | 71.50 | 4.52 | 0.06 | 0.03 | 0.01 | 3.73 | 0.01 | 3.31 | 15.58 | 99.91 |
| ZK3402-8 | 300.0 | 0.03 | 1.41 | 69.01 | 5.53 | 0.08 | 0.03 | 0.01 | 3.01 | 0.01 | 6.40 | 14.40 | 99.92 |
| ZK3402-9 | 300.3 | 0.02 | 1.38 | 69.35 | 5.87 | 0.09 | 0.03 | 0.01 | 2.91 | 0.01 | 5.73 | 14.51 | 99.91 |
| ZK3402-10 | 300.6 | 0.03 | 4.17 | 51.55 | 14.74 | 0.11 | 0.03 | 0.01 | 2.42 | 0.04 | 14.51 | 12.28 | 99.89 |
| ZK3402-11 | 302.6 | 0.07 | 4.77 | 35.75 | 31.09 | 0.06 | 0.29 | 0.05 | 1.93 | 0.03 | 13.81 | 12.06 | 99.91 |
| ZK3402-12 | 303.5 | 0.11 | 3.80 | 35.99 | 35.35 | 0.07 | 0.66 | 0.08 | 1.90 | 0.02 | 9.49 | 12.46 | 99.93 |
| ZK3402-13 | 303.9 | 0.20 | 3.58 | 34.92 | 36.40 | 0.08 | 1.71 | 0.10 | 1.65 | 0.02 | 9.90 | 11.38 | 99.94 |
| ZK3402-14 | 305.0 | 0.28 | 3.34 | 34.29 | 36.64 | 0.09 | 2.57 | 0.10 | 1.68 | 0.02 | 10.72 | 10.19 | 99.92 |
| ZK3402-15 | 305.5 | 0.35 | 2.74 | 32.97 | 37.88 | 0.10 | 4.13 | 0.13 | 1.36 | 0.02 | 11.5 | 8.77 | 99.95 |
| ZK3402-16 | 307.3 | 0.63 | 1.48 | 33.97 | 43.01 | 0.09 | 6.08 | 0.17 | 1.60 | 0.01 | 4.56 | 8.33 | 99.93 |
| ZK5604-2 | 521.3 | 0.10 | 0.79 | 50.80 | 29.15 | 0.05 | 0.17 | 0.01 | 1.88 | 0.01 | 2.52 | 14.48 | 99.96 |
| ZK5604-3 | 523.5 | 0.11 | 0.30 | 37.58 | 43.87 | 0.06 | 0.11 | 0.04 | 2.17 | 0.01 | 1.25 | 14.40 | 99.90 |
| ZK5604-5 | 525.3 | 0.09 | 0.25 | 35.33 | 42.21 | 0.06 | 0.12 | 0.07 | 1.40 | 0.01 | 4.75 | 15.70 | 99.99 |
| ZK5604-6 | 526.0 | 0.35 | 0.28 | 36.55 | 44.46 | 0.06 | 0.63 | 0.08 | 1.42 | 0.01 | 2.01 | 14.12 | 99.97 |
| ZK5604-7 | 528.4 | 0.05 | 2.64 | 30.34 | 30.80 | 0.06 | 0.01 | 0.08 | 1.47 | 0.07 | 23.54 | 10.85 | 99.91 |
| ZK5604-8 | 529.9 | 0.07 | 0.85 | 35.39 | 41.35 | 0.05 | 0.09 | 0.10 | 1.41 | 0.01 | 6.13 | 14.48 | 99.93 |
| ZK5604-9 | 531.3 | 0.27 | 2.84 | 28.54 | 34.89 | 0.15 | 3.92 | 0.15 | 1.24 | 0.04 | 19.6 | 8.32 | 99.96 |
| Sample | Deep (m) | Li | V | Zr | Hf | Nb | Ta | Ni | Cr | Ga | Sr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ZK14904-4 | Bauxite | 386.7 | 524.1 | 328.2 | 526.5 | 13.6 | 51.6 | 3.4 | 3.2 | 335.4 | 55.7 | 230.0 |
| ZK14904-5 | 387.0 | 731.2 | 325.7 | 467.1 | 13.3 | 39.5 | 2.8 | 4.8 | 351.5 | 64.9 | 256.4 | |
| ZK14904-6 | 387.1 | 232.0 | 502.5 | 741.4 | 25.4 | 114.6 | 7.3 | 16.1 | 383.9 | 111.6 | 90.6 | |
| ZK14904-7 | 387.9 | 265.5 | 516.9 | 714.5 | 22.8 | 89.8 | 6.5 | 23.8 | 465.5 | 112.3 | 81.2 | |
| ZK14904-8 | 388.1 | 194.5 | 456.9 | 471.4 | 13.1 | 60.9 | 4.0 | 16.2 | 289.9 | 86.7 | 100.5 | |
| ZK14904-9 | 388.6 | 185.8 | 546.5 | 525.2 | 16.1 | 64.4 | 4.7 | 14.6 | 620.5 | 96.0 | 130.4 | |
| ZK14904-10 | 389.0 | 231.7 | 494.9 | 467.3 | 14.1 | 57.5 | 4.0 | 21.8 | 407.1 | 81.6 | 102.4 | |
| ZK14904-12 | 390.3 | 87.8 | 263.2 | 458.1 | 14.4 | 67.9 | 4.5 | 5.4 | 241.7 | 78.6 | 117.2 | |
| ZK14904-13 | 390.5 | 84.7 | 270.0 | 468.9 | 14.9 | 69.0 | 5.0 | 5.4 | 239.1 | 80.8 | 109.5 | |
| ZK14904-14 | 390.9 | 167.7 | 303.9 | 456.5 | 13.3 | 58.1 | 4.3 | 8.0 | 200.8 | 74.4 | 57.9 | |
| ZK14904-15 | 391.6 | 172.8 | 329.0 | 1835.0 | 20.9 | 98.0 | 7.0 | 7.7 | 394.4 | 72.0 | 171.2 | |
| ZK14904-16 | 392.1 | 240.8 | 612.2 | 624.5 | 19.1 | 73.5 | 5.4 | 13.0 | 387.1 | 88.8 | 63.7 | |
| ZK14904-17 | 393.2 | 190.2 | 319.7 | 493.2 | 14.3 | 64.8 | 4.8 | 20.4 | 209.9 | 83.2 | 75.7 | |
| ZK14904-19 | 393.4 | 217.5 | 252.5 | 471.3 | 12.4 | 53.2 | 3.8 | 51.6 | 157.5 | 73.3 | 140.0 | |
| ZK14904-20 | 393.5 | 299.4 | 304.6 | 330.1 | 8.9 | 42.4 | 2.9 | 97.8 | 364.2 | 79.5 | 155.6 | |
| ZK14904-21 | 394.1 | 525.1 | 179.6 | 294.2 | 8.1 | 33.3 | 2.3 | 312.9 | 84.1 | 34.0 | 235.3 | |
| ZK14904-22 | 395.3 | 413.0 | 178.2 | 289.8 | 8.3 | 32.0 | 2.3 | 238.1 | 108.8 | 34.5 | 261.2 | |
| ZK14904-23 | 396.5 | 517.1 | 230.5 | 323.8 | 8.9 | 35.3 | 2.5 | 250.5 | 148.4 | 40.4 | 224.5 | |
| ZK14904-24 | 397.6 | 540.4 | 193.2 | 358.4 | 9.7 | 37.9 | 2.6 | 297.0 | 133.9 | 42.7 | 205.7 | |
| ZK14904-25 | 398.3 | 690.5 | 287.4 | 366.9 | 10.1 | 38.6 | 2.7 | 225.3 | 229.8 | 40.9 | 194.4 | |
| ZK14904-26 | 398.8 | 150.4 | 375.2 | 519.2 | 15.8 | 75.1 | 5.3 | 10.7 | 258.3 | 84.9 | 173.0 | |
| ZK14904-27 | 399.6 | 694.7 | 176.7 | 419.1 | 13.0 | 55.3 | 3.8 | 332.2 | 212.6 | 65.5 | 161.9 | |
| ZK14904-28 | S1hj | 401.9 | 70.7 | 221.7 | 173.1 | 5.0 | 21.0 | 1.5 | 90.2 | 155.1 | 40.3 | 226.9 |
| ZK202-2 | Bauxite | 199.6 | 54.7 | 238.2 | 813.6 | 25.6 | 109.2 | 6.7 | 3.7 | 372.4 | 100.3 | 23.4 |
| ZK202-3 | 201.7 | 550.9 | 431.3 | 462.2 | 9.7 | 44.1 | 2.6 | 75.7 | 171.5 | 43.5 | 80.9 | |
| ZK202-4 | 202.3 | 368.1 | 252.3 | 442.4 | 9.8 | 41.4 | 2.5 | 137.1 | 187.9 | 25.4 | 91.7 | |
| ZK202-5 | 203.5 | 461.2 | 118.4 | 375.6 | 8.8 | 38.1 | 2.7 | 202.7 | 148.0 | 43.5 | 54.1 | |
| ZK202-6 | 204.8 | 468.0 | 221.4 | 387.8 | 8.9 | 33.0 | 2.2 | 165.9 | 189.1 | 35.7 | 154.5 | |
| ZK202-7 | C2h | 206.8 | 2.3 | 20.7 | 19.2 | 0.8 | 0.9 | 0.1 | 24.0 | 5.2 | 1.3 | 467.8 |
| ZK3402-3 | Bauxite | 297.7 | 647.0 | 173.2 | 576.8 | 17.1 | 68.4 | 4.5 | 4.9 | 619.7 | 76.2 | 147.8 |
| ZK3402-4 | 298.3 | 534.5 | 144.1 | 485.2 | 13.0 | 44.4 | 3.0 | 13.0 | 511.9 | 65.6 | 95.4 | |
| ZK3402-5 | 298.4 | 654.3 | 193.5 | 450.3 | 12.0 | 48.1 | 3.1 | 11.9 | 504.9 | 67.3 | 143.5 | |
| ZK3402-6 | 299.6 | 42.1 | 353.4 | 576.8 | 17.4 | 63.3 | 4.6 | 5.7 | 799.4 | 116.1 | 142.0 | |
| ZK3402-7 | 299.8 | 51.6 | 420.6 | 900.3 | 23.2 | 91.8 | 5.8 | 12.1 | 821.4 | 131.1 | 48.0 | |
| ZK3402-8 | 300.0 | 77.6 | 408.8 | 884.6 | 17.4 | 63.2 | 4.3 | 8.4 | 786.2 | 110.3 | 53.1 | |
| K3402-9 | 300.3 | 86.4 | 358.8 | 532.9 | 17.2 | 63.0 | 4.5 | 11.3 | 754.1 | 115.3 | 72.8 | |
| ZK3402-10 | 300.6 | 603.9 | 338.6 | 498.6 | 14.4 | 55.4 | 3.8 | 48.1 | 553.8 | 83.3 | 173.9 | |
| ZK3402-11 | 302.6 | 1239.9 | 309.9 | 412.5 | 12.2 | 45.6 | 2.9 | 114.0 | 489.2 | 54.1 | 113.0 | |
| ZK3402-12 | 303.5 | 1139.5 | 297.5 | 342.8 | 11.9 | 42.9 | 3.0 | 185.4 | 297.5 | 55.0 | 166.1 | |
| ZK3402-13 | 303.9 | 965.0 | 335.8 | 346.1 | 10.0 | 37.9 | 2.6 | 196.1 | 335.8 | 52.9 | 200.6 | |
| ZK3402-14 | 305.0 | 953.8 | 443.4 | 444.7 | 10.9 | 40.1 | 2.7 | 161.2 | 443.4 | 48.0 | 210.5 | |
| ZK3402-15 | 305.5 | 876.6 | 338.8 | 310.8 | 8.8 | 29.8 | 2.1 | 120.4 | 338.8 | 51.0 | 238.2 | |
| ZK3402-16 | 307.3 | 500.0 | 319.5 | 344.5 | 10.0 | 36.1 | 2.4 | 90.4 | 319.5 | 34.6 | 277.6 | |
| ZK3402-17 | S1hj | 307.8 | 134.2 | 311.1 | 344.8 | 9.0 | 34.2 | 2.2 | 104.8 | 311.1 | 41.2 | 314.4 |
| ZK3402-18 | 308.6 | 53.2 | 150.7 | 178.9 | 4.9 | 23.4 | 1.6 | 74.3 | 150.7 | 37.9 | 179.4 | |
| ZK5604-2 | Bauxite | 521.3 | 5261.7 | 487.2 | 484.1 | 12.8 | 43.4 | 2.8 | 32.4 | 350.0 | 50.6 | 85.7 |
| ZK5604-3 | 523.5 | 968.1 | 189.7 | 491.8 | 13.7 | 50.7 | 3.4 | 93.1 | 265.2 | 27.9 | 91.2 | |
| ZK5604-5 | 525.3 | 383.9 | 128.6 | 277.0 | 8.0 | 31.4 | 2.0 | 99.5 | 98.0 | 26.8 | 66.4 | |
| ZK5604-6 | 526.0 | 349.2 | 125.9 | 279.3 | 7.8 | 32.7 | 2.1 | 114.0 | 113.3 | 34.1 | 87.4 | |
| ZK5604-7 | 528.4 | 800.1 | 193.3 | 292.8 | 8.4 | 32.8 | 2.2 | 159.4 | 180.6 | 67.1 | 82.8 | |
| ZK5604-8 | 529.9 | 576.0 | 167.5 | 300.9 | 8.3 | 32.9 | 2.2 | 77.9 | 163.3 | 27.2 | 80.2 | |
| ZK5604-9 | 531.3 | 147.8 | 164.2 | 297.9 | 8.1 | 27.8 | 1.9 | 95.5 | 152.9 | 36.4 | 337.4 | |
| ZK5604-10 | C2h | 532.8 | 41.0 | 28.7 | 47.5 | 1.2 | 4.9 | 0.3 | 60.1 | 28.8 | 7.5 | 282.3 |
| ZK3228-3 | Bauxite | 621.2 | 1664.6 | 310.7 | 587.9 | 17.5 | 57.8 | 3.3 | 14.8 | 404.7 | 85.7 | 101.0 |
| ZK3228-4 | 621.5 | 1318.7 | 334.8 | 653.6 | 22.2 | 84.6 | 4.9 | 11.6 | 777.2 | 140.4 | 103.2 | |
| ZK3228-5 | 621.6 | 6.7 | 303.9 | 876.4 | 30.8 | 116.8 | 6.8 | 6.1 | 667.4 | 135.7 | 55.2 | |
| ZK3228-6 | 622.6 | 4.8 | 297.3 | 866.8 | 32.0 | 114.9 | 7.0 | 6.1 | 648.5 | 135.1 | 53.8 | |
| ZK3228-7 | 623.4 | 97.5 | 325.3 | 558.4 | 21.7 | 82.8 | 5.3 | 38.4 | 369.3 | 98.3 | 79.4 | |
| ZK3228-9 | 624.5 | 488.9 | 170.0 | 391.1 | 9.8 | 36.1 | 2.5 | 102.6 | 193.0 | 40.1 | 320.6 | |
| ZK3228-12 | C2h | 626.9 | 1.0 | 24.4 | 1.0 | 0.0 | 0.2 | 0.0 | 27.6 | 2.1 | 0.2 | 127.8 |
| ZK3228-13 | 627.3 | 1.0 | 27.3 | 4.8 | 0.1 | 0.7 | 0.1 | 26.5 | 3.6 | 1.0 | 108.1 | |
| ZK3228-14 | 627.9 | 6.4 | 36.6 | 57.5 | 2.1 | 3.4 | 0.4 | 29.5 | 19.6 | 4.4 | 203.1 | |
| ZK3228-15 | S1hj | 628.5 | 30.0 | 128.8 | 181.2 | 5.1 | 17.5 | 1.3 | 80.8 | 96.1 | 21.9 | 121.9 |
| Sample | Ba | Dy | Er | Eu | Ga | Sm | Sn | Sr | Ta | Tb | Th | Tm | U | V | W | Y | Li | Sc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | |
| C2h-1 | 129.0 | 5.81 | 3.03 | 1.21 | 2.4 | 4.72 | 1 | 578 | 0.1 | 1.07 | 1.9 | 0.35 | 10.10 | 28 | <1 | 48.3 | 6.0 | 3.1 |
| S1hj-1 | 680.0 | 13.40 | 7.08 | 3.34 | 31.2 | 17.10 | 5 | 350 | 1.7 | 2.50 | 39.0 | 0.93 | 8.14 | 148 | 3 | 81.8 | 36.8 | 19.5 |
| S1hj-2 | 512.0 | 4.81 | 2.79 | 1.16 | 25.0 | 6.39 | 4 | 209 | 1.1 | 0.83 | 19.6 | 0.44 | 4.22 | 135 | 2 | 27.9 | 26.6 | 14.8 |
| Zky82909-1 | 11.0 | 0.17 | 0.15 | 0.04 | 0.6 | 0.24 | <1 | 1770 | 0.1 | 0.03 | 0.7 | 0.03 | 5.12 | 27 | <1 | 1.6 | 5.3 | 0.8 |
| Zky82909-2 | 576.0 | 5.79 | 3.47 | 1.71 | 44.3 | 8.41 | 6 | 419 | 1.9 | 1.04 | 30.6 | 0.55 | 16.40 | 458 | 3 | 31.8 | 80.2 | 23.5 |
| Zky82909-3 | 751.0 | 8.52 | 4.46 | 5.55 | 53.2 | 33.60 | 9 | 278 | 3.0 | 1.73 | 36.6 | 0.69 | 14.15 | 291 | 5 | 39.7 | 650.0 | 29.2 |
| Zky82909-4 | 356.0 | 6.32 | 4.57 | 1.46 | 55.8 | 5.54 | 12 | 461 | 4.0 | 1.02 | 51.8 | 0.83 | 16.20 | 326 | 7 | 36.5 | 2160.0 | 33.8 |
| Zky82909-5 | 226.0 | 11.05 | 7.27 | 3.75 | 159.5 | 19.90 | 17 | 643 | 4.8 | 1.90 | 93.7 | 1.26 | 18.60 | 691 | 22 | 61.7 | 281.0 | 39.7 |
| Zky82909-6 | 22.2 | 12.90 | 8.87 | 1.74 | 120.5 | 7.79 | 22 | 246 | 6.4 | 1.91 | 92.8 | 1.47 | 21.90 | 641 | 9 | 77.1 | 8.5 | 38.9 |
| Zky82909-7 | 21.1 | 8.96 | 5.61 | 3.19 | 106.5 | 17.30 | 16 | 373 | 4.0 | 1.64 | 59.2 | 0.94 | 16.15 | 397 | 7 | 50.4 | 39.8 | 32.4 |
| Zky82909-8 | 89.8 | 6.44 | 3.61 | 3.24 | 55.2 | 14.70 | 10 | 381 | 2.8 | 1.19 | 39.7 | 0.61 | 9.09 | 195 | 5 | 31.5 | 660.0 | 32.6 |
| Zky82909-9 | 306.0 | 5.27 | 2.96 | 1.77 | 28.5 | 4.86 | 7 | 165 | 1.9 | 0.86 | 26.2 | 0.48 | 7.28 | 219 | 3 | 28.6 | 690.0 | 37.7 |
| Zky82909-10 | 242 | 6.27 | 4.08 | 2.62 | 67 6 | 12.40 | 12 | 226 | 3.3 | 1.12 | 48.1 | 0.65 | 11.60 | 299 | 6 | 35.0 | 2160.0 | 31.1 |
| Zky82909-11 | 20.2 | 8.96 | 5.91 | 1.29 | 125.0 | 5.43 | 15 | 79 | 4.4 | 1.40 | 65.5 | 0.92 | 13.10 | 656 | 8 | 54.7 | 57.4 | 35.3 |
| Zk15-2-1 | 200.0 | 211.00 | 98.40 | 46.50 | 44.8 | 241.00 | 3 | 388 | 1.4 | 36.90 | 19.2 | 12.50 | 59.60 | 218 | 2 | 1200.0 | 1210.0 | 90.6 |
| Zk15-2-2 | 87.9 | 9.64 | 6.12 | 3.86 | 53.0 | 19.20 | 9 | 406 | 2.3 | 1.76 | 33.8 | 1.03 | 30.00 | 503 | 8 | 44.7 | 1120.0 | 40.7 |
| Zk15-2-3 | 124.0 | 14.55 | 9.60 | 1.67 | 70.8 | 5.51 | 11 | 119 | 3.9 | 2.11 | 56.9 | 1.50 | 19.35 | 510 | 17 | 86.5 | 1370.0 | 42.8 |
| Zk15-2-4 | 142.5 | 31.40 | 18.50 | 4.05 | 117.0 | 13.90 | 15 | 164 | 6.9 | 4.66 | 94.3 | 2.62 | 42.90 | 762 | 8 | 198.5 | 284.0 | 50.6 |
| Zk15-2-5 | 16.2 | 23.70 | 13.75 | 3.51 | 168.5 | 12.95 | 13 | 135 | 5.0 | 3.82 | 70.0 | 1.95 | 45.20 | 723 | 6 | 135.0 | 5.3 | 42.5 |
| Zk15-2-6 | 15.0 | 21.50 | 13.20 | 2.41 | 158.5 | 8.16 | 16 | 78 | 4.5 | 3.28 | 89.8 | 1.91 | 54.90 | 723 | 5 | 120.0 | 3.1 | 46.8 |
| Zk15-2-7 | 806.0 | 5.69 | 4.39 | 1.44 | 27.9 | 8.14 | 9 | 355 | 2.8 | 0.81 | 37.3 | 0.78 | 9.47 | 336 | 4 | 36.0 | 1500.0 | 32.3 |
| Zk15-2-8 | 540.0 | 13.00 | 5.38 | 7.44 | 28.5 | 51.70 | 7 | 447 | 2.0 | 3.33 | 30.9 | 0.82 | 10.50 | 429 | 3 | 60.8 | 910.0 | 27.1 |
| Zk15-2-9 | 457.0 | 19.20 | 6.24 | 13.15 | 29.6 | 82.80 | 7 | 412 | 1.7 | 5.38 | 27.3 | 0.82 | 11.25 | 546 | 3 | 79.7 | 850.0 | 26.3 |
| Zk15-2-10 | 35.1 | 21.30 | 10.55 | 3.45 | 2.9 | 11.35 | 1 | 274 | 0.1 | 3.64 | 1.1 | 1.22 | 3.03 | 37 | <1 | 181.5 | 9.0 | 7.1 |
| Zk15-2-11 | 39.3 | 1.12 | 0.63 | 0.30 | 2.1 | 1.25 | 1 | 318 | 0.1 | 0.19 | 1.5 | 0.09 | 7.09 | 24 | <1 | 9.0 | 7.0 | 1.9 |
| Zk403-1 | 29.6 | 7.20 | 4.48 | 0.67 | 48.3 | 1.98 | 16 | 65 | 4.4 | 0.98 | 43 2 | 0.72 | 10.85 | 137 | 9 | 43.9 | 690.0 | 19.0 |
| Zk403-2 | 60.6 | 6.20 | 3.81 | 0.90 | 40.1 | 2.26 | 13 | 101 | 3.5 | 0.88 | 48.3 | 0.62 | 9.20 | 99 | 5 | 39.7 | 530.0 | 14.1 |
| Zk403-3 | 11.2 | 9.77 | 6.54 | 0.86 | 76.4 | 3.33 | 17 | 63 | 5.3 | 1.34 | 54.4 | 1.02 | 13.45 | 216 | 8 | 58.8 | 101.0 | 26.1 |
| Zk403-4 | 53.7 | 8.74 | 6.46 | 1.01 | 74.3 | 5.70 | 16 | 175 | 6.0 | 1.20 | 67.1 | 1.07 | 8.87 | 304 | 8 | 48.2 | 152.5 | 48.9 |
| Zk403-5 | 8.9 | 8.28 | 6.34 | 0.97 | 75.2 | 5.88 | 17 | 224 | 5.1 | 1.23 | 63.5 | 1.08 | 7.88 | 313 | 7 | 45.2 | 198.0 | 45.7 |
| Zk6A03-1 | 150.5 | 5.05 | 3.42 | 0.41 | 49.9 | 1.07 | 10 | 138 | 3.7 | 0.63 | 40.3 | 0.52 | 7.86 | 263 | 6 | 36.9 | 830.0 | 14.4 |
| Zk6A03-2 | 14.6 | 9.97 | 7.42 | 0.44 | 104.5 | 1.42 | 14 | 42 | 5.1 | 1.13 | 92.6 | 1.12 | 11.35 | 270 | 29 | 71.1 | 130.5 | 23.6 |
| Zk6A03-3 | 10.9 | 16.90 | 13.55 | 0.72 | 103.5 | 2.44 | 21 | 82 | 8.6 | 1.87 | 76.8 | 1.96 | 14.30 | 235 | 15 | 118.5 | 26.7 | 35.7 |
| Zk6A03-4 | 10.1 | 11.20 | 9.47 | 0.51 | 96.8 | 2.01 | 17 | 61 | 5.8 | 1.17 | 64.7 | 1.36 | 11.10 | 205 | 9 | 77.5 | 16.7 | 32.0 |
| Zk6A03-5 | 8.3 | 11.20 | 10.15 | 0.47 | 72.7 | 1.56 | 15 | 98 | 5.4 | 1.17 | 55.1 | 1.55 | 10.05 | 310 | 8 | 76.8 | 87.7 | 38.5 |
| Zk6A03-6 | 7.9 | 12.50 | 11.90 | 1.31 | 72.1 | 5.55 | 14 | 62 | 4.8 | 1.61 | 57.8 | 1.97 | 7.84 | 243 | 7 | 80.0 | 116.5 | 34.4 |
| Zk6A03-7 | 75.2 | 13.85 | 10.60 | 1.92 | 31.6 | 8.75 | 9 | 238 | 2.6 | 1.95 | 34.4 | 1.86 | 4.81 | 191 | 5 | 64.0 | 690.0 | 25.0 |
| Zk1204-1 | 139.5 | 2.82 | 1.97 | 0.36 | 26.6 | 0.99 | 4 | 501 | 2.3 | 0.41 | 35.6 | 0.29 | 6.27 | 230 | 4 | 17.2 | 1290.0 | 21.5 |
| Zk1204-5 | 84.3 | 6.44 | 5.02 | 0.50 | 23.7 | 2.01 | 9 | 103 | 3.1 | 0.78 | 35.9 | 0.76 | 8.07 | 93 | 3 | 31 3 | 420.0 | 33.6 |
| Name of the Deposit | Lithology | Number of Samples (Piece) | Al2O3 | SiO2 | Fe2O3 | TiO2 | K2O | Na2O | Li (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| Xinmin | Bauxite | 11 | 67.10 | 10.69 | 3.04 | 2.79 | 0.20 | 0.53 | 360 |
| Bauxite | 3 | 48.65 | 31.90 | 2.65 | 1.50 | 0.49 | 0.78 | 417 | |
| Yanping | Bauxite | 4 | 54.40 | 20.28 | 8.32 | 2.21 | 1.05 | 0.63 | 1004 |
| Peach Garden | Bauxite | 3 | 52.71 | 20.62 | 6.73 | 1.25 | 0.34 | 0.72 | 539 |
| Wachangping | Bauxite | 8 | 46.72 | 27.51 | 5.53 | 1.31 | 0.49 | 0.71 | 656 |
| Bauxite | 5 | 60.16 | 15.79 | 4.57 | 2.02 | 0.15 | 0.44 | 805 | |
| Rear Slot | Bauxite | 30 | 65.63 | 11.26 | 3.88 | 2.91 | 2.08 | 0.03 | 73 |
| Xianrenyan in Zunyi | Bauxite | 30 | 63.83 | 7.02 | 11.91 | 2.80 | 0.48 | 0. 03 | 73 |
| Correlation coefficient with Li | −0.240 | 0.227 | 0.001 | 0.261 | 0.093 | 0.441 | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Zhang, M.; Tao, C.; Yan, J. Comprehensive Utilization and Sustainable Development of Bauxite in Northern Guizhou on a Background of Carbon Neutralization. Sustainability 2022, 14, 14301. https://doi.org/10.3390/su142114301
Yang W, Zhang M, Tao C, Yan J. Comprehensive Utilization and Sustainable Development of Bauxite in Northern Guizhou on a Background of Carbon Neutralization. Sustainability. 2022; 14(21):14301. https://doi.org/10.3390/su142114301
Chicago/Turabian StyleYang, Wu, Min Zhang, Cui Tao, and Jun Yan. 2022. "Comprehensive Utilization and Sustainable Development of Bauxite in Northern Guizhou on a Background of Carbon Neutralization" Sustainability 14, no. 21: 14301. https://doi.org/10.3390/su142114301





