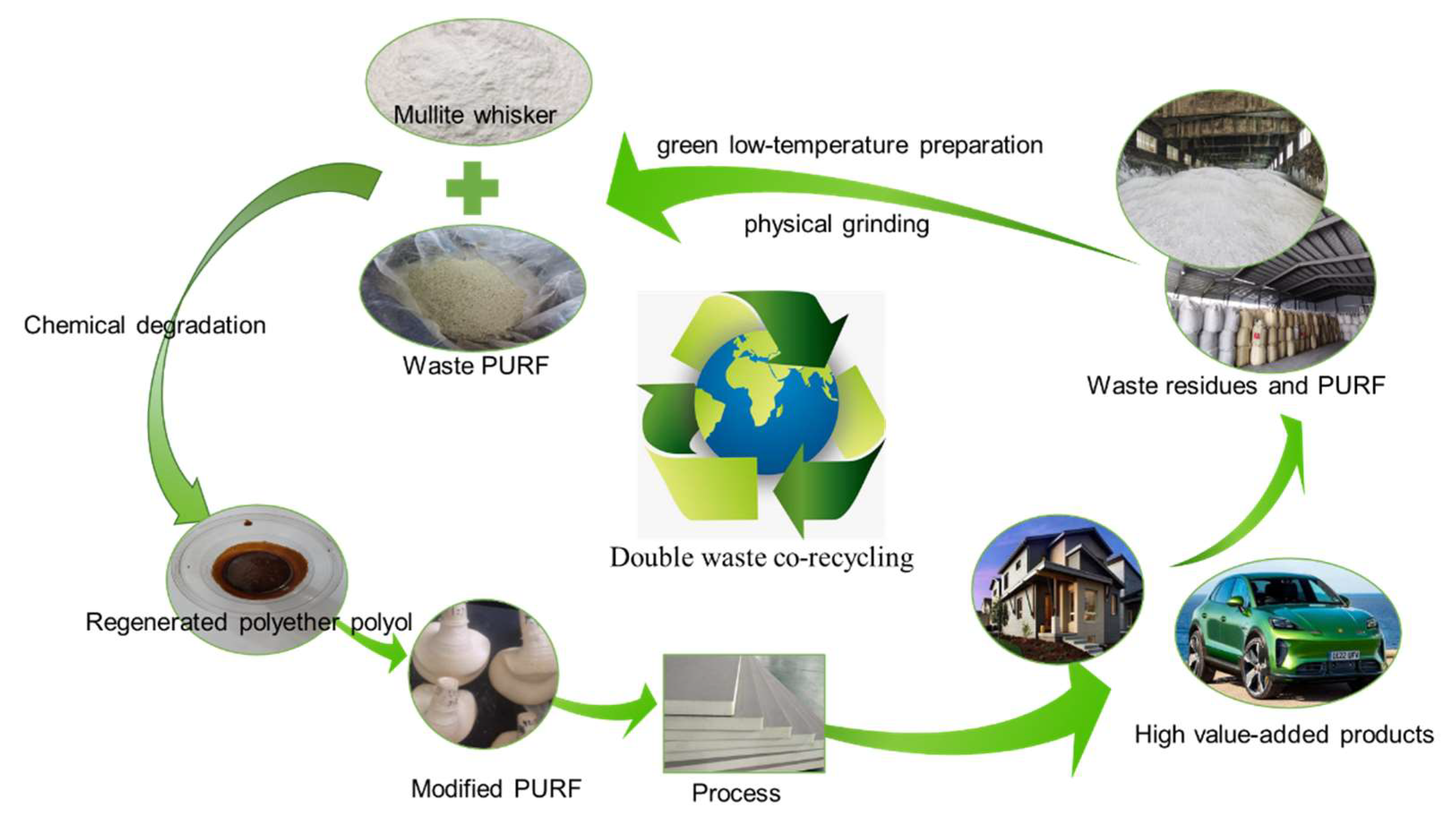
Preparation of Mullite/PU Nanocomposites by Double Waste Co-Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
3. Results and Discussion
3.1. Study on the Degradation of Waste Polyurethane
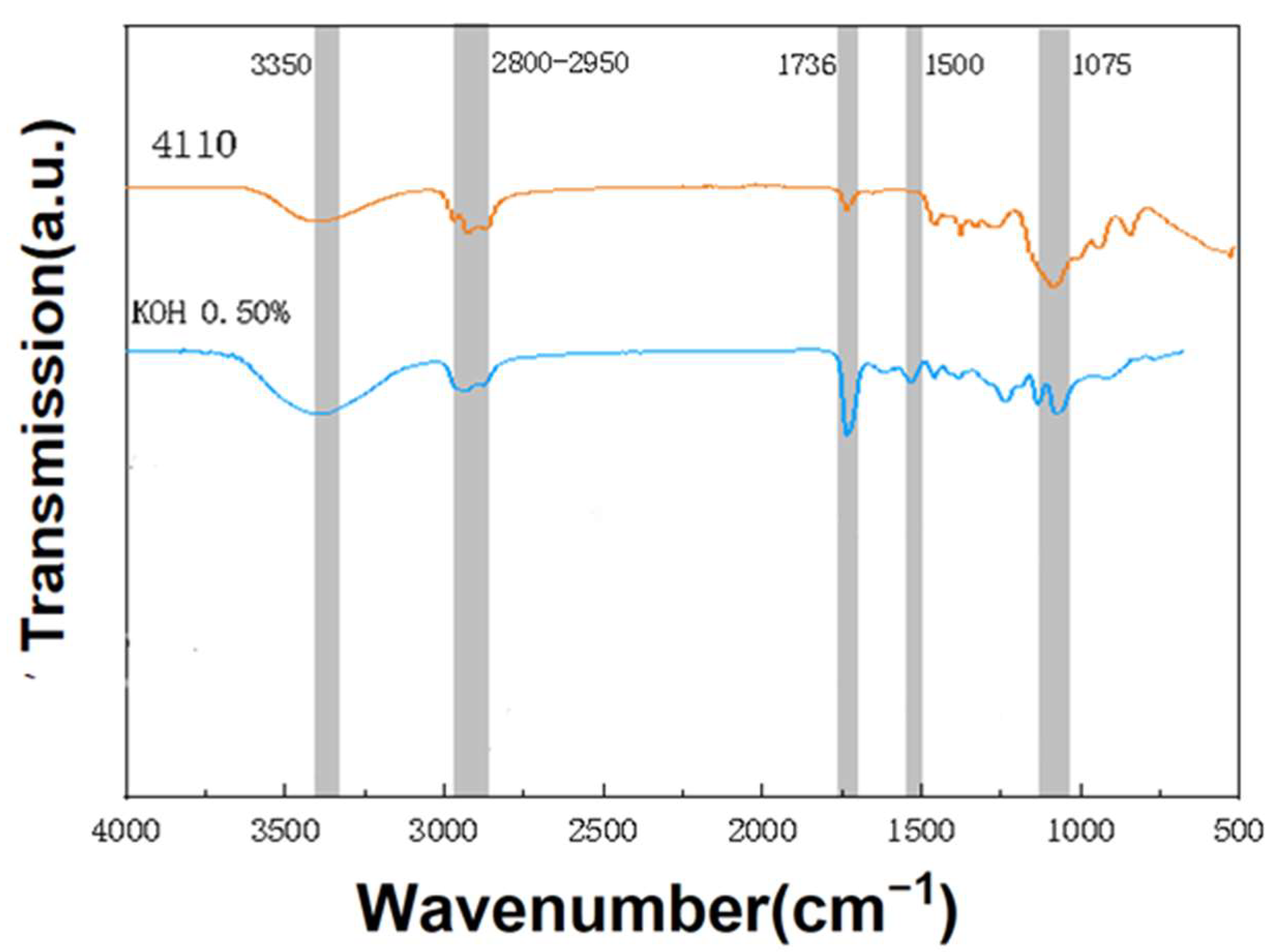
3.2. Infrared Spectroscopic Analysis of Regenerated Polyols from Waste Polyurethane
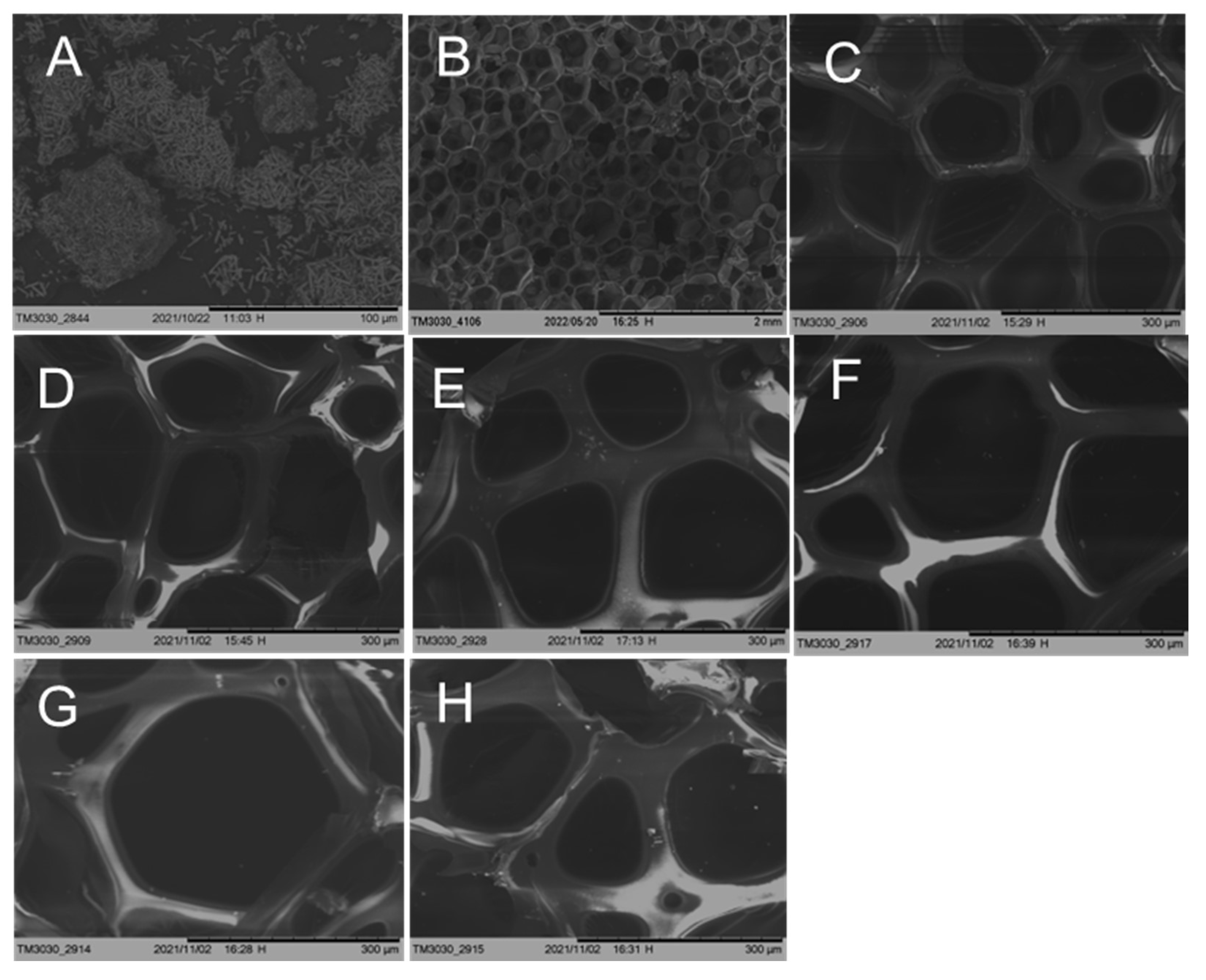
3.3. Scanning Electron Microscope (SEM) Observations
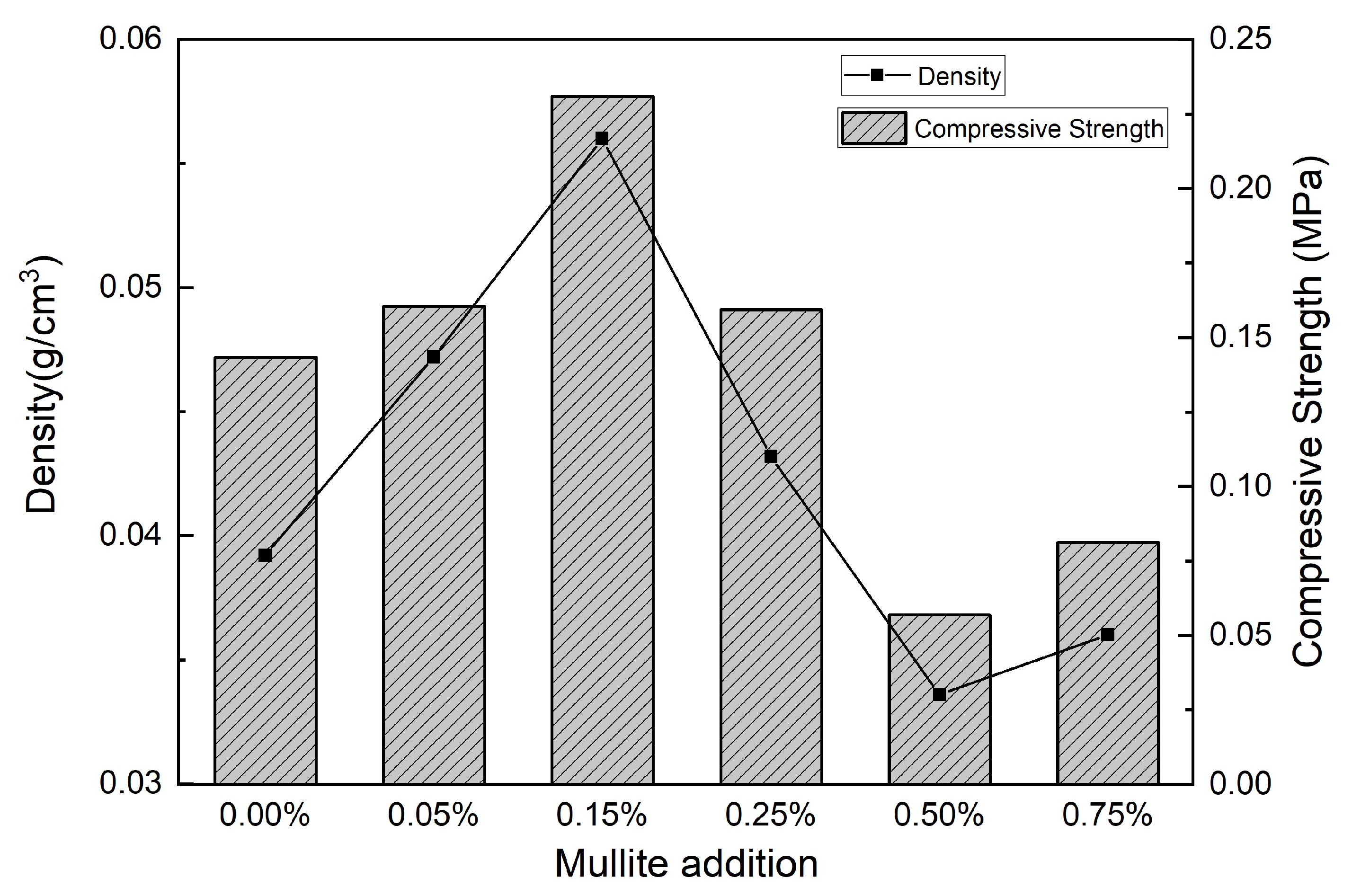
3.4. Compressive Strength and Density Test Analysis
3.5. Thermal Conductivity Test Analysis
3.6. Infrared Spectral Analysis of Mullite Modified Polyurethane
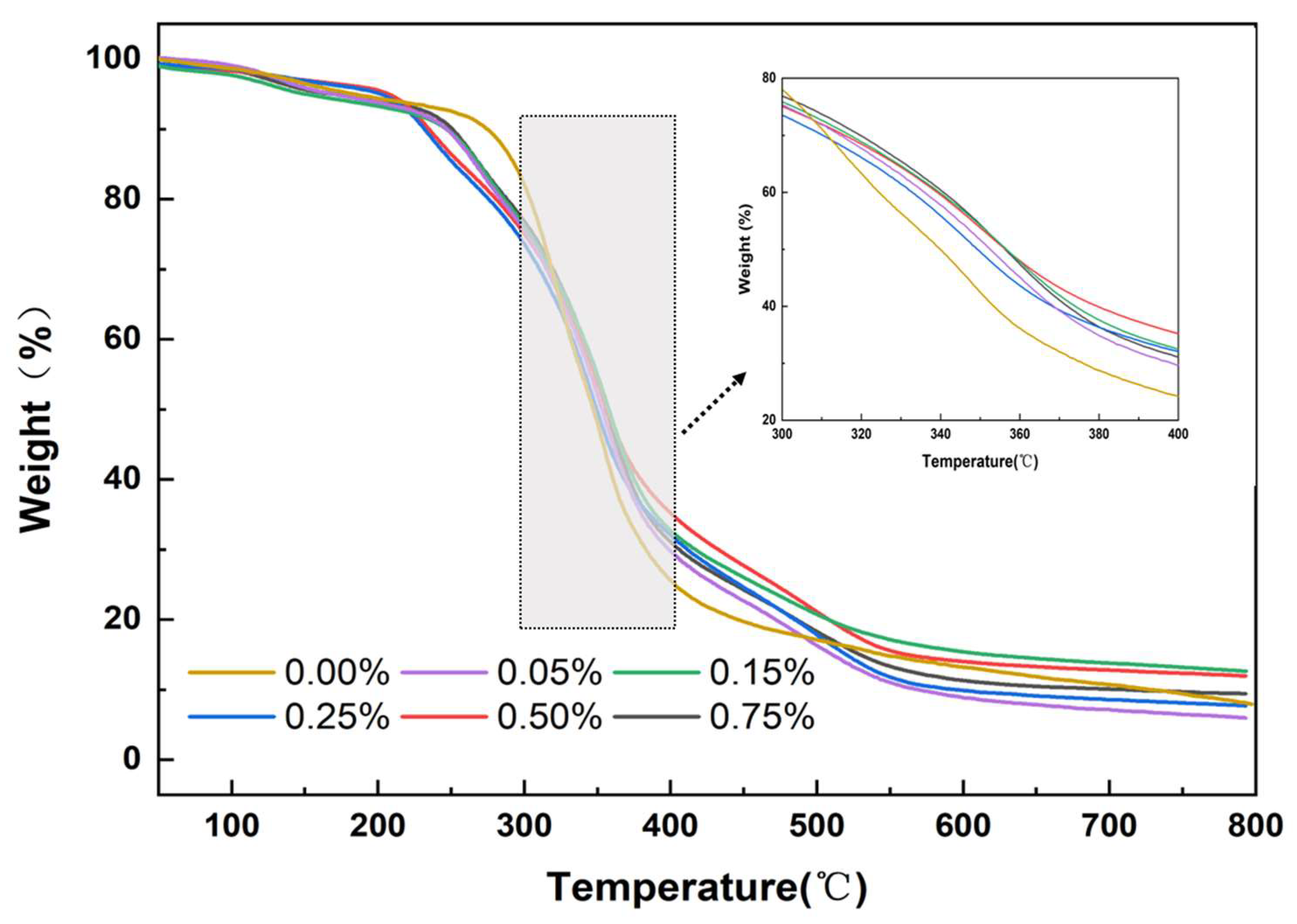
3.7. Thermogravimetric (TG) Analysis of Mullite-Modified Polyurethane Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, R.L.; Deng, G.H.; Yang, D.; Chen, H.C.; Lou, Z.D. The Utilization of Industrial Residue Incineration-power Generation and Its Application in the Engineering Project. In Proceedings of the International Conference on Civil, Architectural and Hydraulic Engineering (ICCAHE 2012), Zhangjiajie, China, 10–12 August 2012; pp. 1762–1765. [Google Scholar]
- Li, T.Z.; Zhu, X.Q.; Zhou, X.T.; Chang, J.H.; Chen, L.M.; Chen, L. Research on Porous Ceramics Synthesized by Industrial Waste Residues. In Proceedings of the 2nd International Conference on Renewable Energy and Environmental Technology (REET), Dalian, China, 19–20 August 2014; pp. 756–760. [Google Scholar]
- Schützenhofer, S.; Kovacic, I.; Rechberger, H.; Mack, S. Improvement of Environmental Sustainability and Circular Economy through Construction Waste Management for Material Reuse. Sustainability 2022, 14, 11087. [Google Scholar] [CrossRef]
- Lima, L.K.S.; Silva, K.R.; Menezes, R.R.; Santana, L.N.L.; Lira, H.L. Microstructural characteristics, properties, synthesis and applications of mullite: A review. Cerâmica 2022, 68, 126–142. [Google Scholar] [CrossRef]
- Schneider, H.; Fischer, R.X.; Schreuer, J. Mullite: Crystal Structure and Related Properties. J. Am. Ceram. Soc. 2015, 98, 2948–2967. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, X.; Li, M.; Wang, Z.; Wang, X.; Ma, Y.; Yuan, L. Fabrication and properties of mullite thermal insulation materials with in-situ synthesized mullite hollow whiskers. Ceram. Int. 2020, 46, 14474–14480. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chen, Y.C. Fabrication of mullite composites by cyclic infiltration and reaction sintering. Mater. Sci. Eng. 2001, 298, 179–186. [Google Scholar] [CrossRef]
- Zhao, G.; Rao, P.; Lu, M. Research and Application Of Mullite and Porous Mullite. China’s Ceram. 2006, 42, 13–17. [Google Scholar]
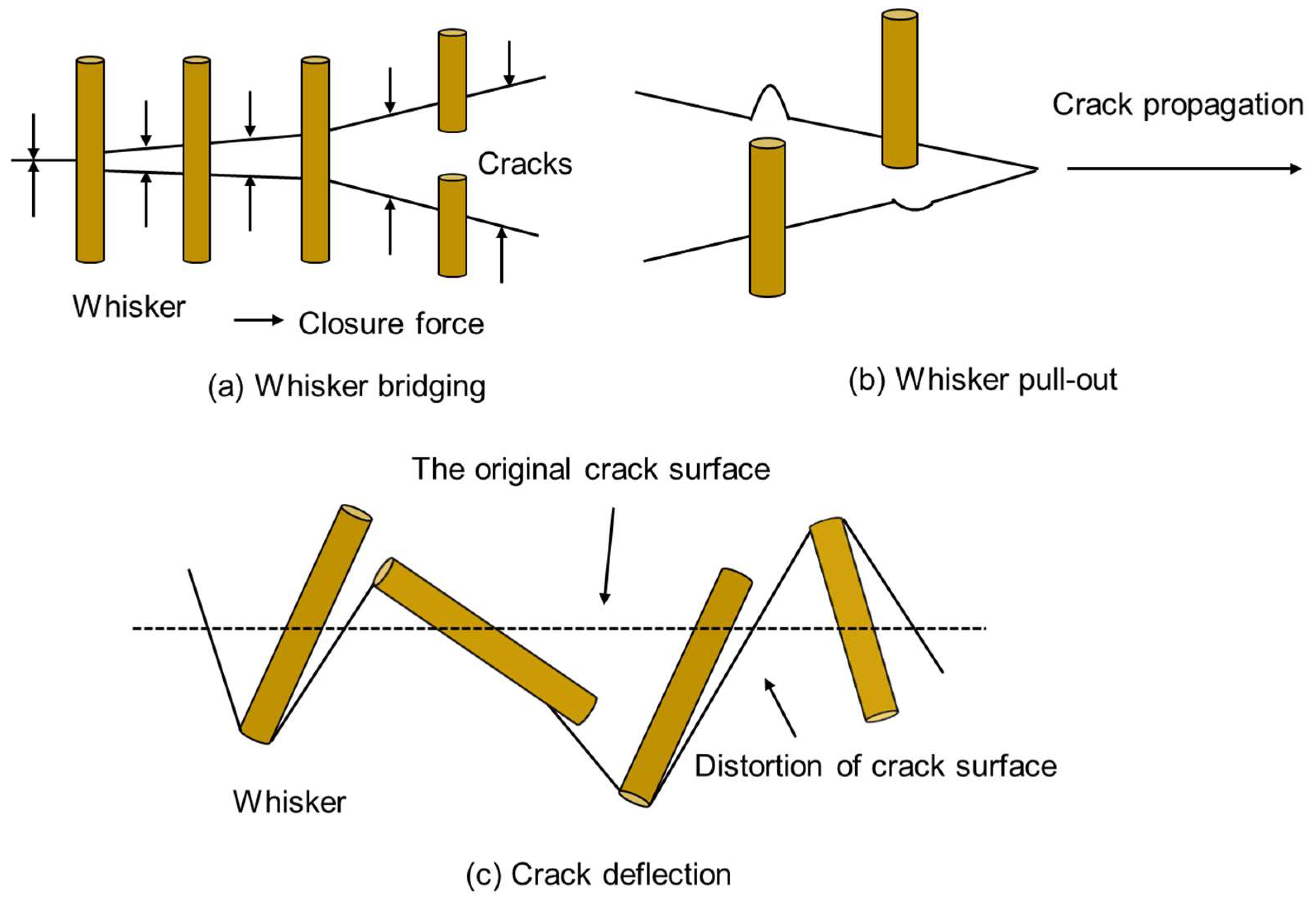
- Chen, E.; Hao, C.; Li, S.; Zhang, Y.; Ma, C. Ceramic matrix composite toughened by whiskers. New Chem. Mater. 2006, 34, 1–4. [Google Scholar]
- Liu, Y.; Wang, A.; Zhu, L.; Wang, B. Research on influencing factor of mechanical properties of SiC whisker toughened photosensitive resins. Ordnance Mater. Sci. Eng. 2009, 32, 66–70. [Google Scholar]
- Campbell, G.H.; Ruhle, M.; Dalgleish, B.J.; Evans, A.G. Whisker Toughening—A Comparison between Aluminum-Oxide and Silicon-Nitride Toughened with Silicon-Carbide. J. Am. Ceram. Soc. 1990, 73, 521–530. [Google Scholar] [CrossRef]
- Chen, E.; Chen, D. The Development of Whisker-Reinforced Polymer Composites Mechanisms. Polym. Mater. Sci. Eng. 2006, 22, 20–24. [Google Scholar]
- Li, S.; Dai, C. Status of the SiC Papticle, Whisker and Platelet Toughened Ceramic Composite. Bull. Chin. Ceram. Soc. 2004, 23, 63–65. [Google Scholar]
- Ma, F.J.; Huang, C.Z.; Niu, J.H.; Wang, L.M.; Liu, H.L.; Bai, X.L. Toughening mechanisms of Al2O3-matrix composites with SiC whiskers. Ceram. Int. 2022, 48, 17556–17563. [Google Scholar] [CrossRef]
- Sciti, D.; Pienti, L.; Fabbriche, D.D.; Guicciardi, S.; Silvestroni, L. Combined effect of SiC chopped fibers and SiC whiskers on the toughening of ZrB2. Ceram. Int. 2014, 40, 4819–4826. [Google Scholar] [CrossRef]
- Roy, J.; Das, S.; Maitra, S. Solgel-Processed Mullite Coating—A Review. Int. J. Appl. Ceram. Technol. 2015, 12, E71–E77. [Google Scholar] [CrossRef]
- Fang, J.; Hao, X.; Lin, J. Qionglai-Kaolinite Synthesis Mullite Whiskers by Using Molten Salt MetHOD. China’s Ceram. 2011, 47, 63–66. [Google Scholar]
- Zhang, B.; Cao, C.B.; Xu, Y.J.; Zhu, H.S. Synthesis of mullite whiskers using salt flux method. Chin. J. Inorg. Chem. 2005, 21, 277–280. [Google Scholar]
- Nie, J.H.; Zhang, H.; Xu, M.; Yan, Q.; Liang, Y.H. Synthesis of Mullite Whiskers Using Molten Salt Method. Rare Met. Mater. Eng. 2009, 38, 1154–1157. [Google Scholar]
- Kapilov-Buchman, K.; Bialystocki, T.; Niezni, D.; Perry, L.; Levenberg, S.; Silverstein, M.S. Porous polycaprolactone and polycarbonate poly(urethane urea)s via emulsion templating: Structures, properties, cell growth. Polym. Chem. 2021, 12, 6569–6581. [Google Scholar] [CrossRef]
- Sun, M.; Qu, G.; Geng, L.; Hou, D.; Jing, S. Fatigue Properties and Damage Characteristics of Polyurethane Mixtures under a Stress Control Mode. Sustainability 2022, 14, 10966. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane scraps via acidolysis. Chem. Eng. J. 2020, 395, 125102. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Li, P.; Shi, L. Research Process of Nano-Polyurethane Elastomer. J. Eng. Plast. Appl. 2012, 40, 91–95. [Google Scholar]
- Cao, C.L.; Cheng, J.; Di Liu, X.; Wang, R.; Zhang, J.Y.; Qu, J.; Jaeger, U. Study of Properties of One-Component Moisture-Curable Polyurethane and Silane Modified Polyurethane Adhesives. J. Adhes. Sci. Technol. 2012, 26, 1395–1405. [Google Scholar] [CrossRef]
- Ye, X. Study on Processing Technology for Polyurethane Paint. Surf. Technol. 2006, 35, 79–81. [Google Scholar]
- James, S. Polyurethane (PU) Foam Market Worth; Grand View Research, Inc.: San Francisco, CA, USA, 2022. [Google Scholar]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Phalip, V.; Averous, L. Evaluation of biological degradation of polyurethanes. Biotechnol. Adv. 2020, 39, 107457. [Google Scholar] [CrossRef]
- Sheel, A.; Pant, D. 6—Chemical Depolymerization of Polyurethane Foams via Glycolysis and Hydrolysis. In Recycling of Polyurethane Foams; Thomas, S., Rane, A.V., Kanny, K., Abitha, V.K., Thomas, M.G., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 67–75. [Google Scholar] [CrossRef]
- Nikje, M.M.A. Recycling of Polyurethane Wastes; De Gruyter: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Magnin, A.; Pollet, E.; Averous, L. Characterization of the enzymatic degradation of polyurethanes. In Enzymatic Plastic Degradation; Weber, G., Bornscheuer, U.T., Wei, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 648, pp. 317–336. [Google Scholar]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A.H. Glycolysis: An efficient route for recycling of end of life polyurethane foams. J. Polym. Res. 2021, 28, 1–19. [Google Scholar] [CrossRef]
- Amundarain, I.; Miguel-Fernandez, R.; Asueta, A.; Garcia-Fernandez, S.; Arnaiz, S. Synthesis of Rigid Polyurethane Foams Incorporating Polyols from Chemical Recycling of Post-Industrial Waste Polyurethane Foams. Polymers 2022, 14, 1157. [Google Scholar] [CrossRef]
- Simon, D.; Borreguero, A.M.; de Lucas, A.; Rodriguez, J.F. Glycolysis of viscoelastic flexible polyurethane foam wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Zhu, P.; Cao, Z.B.; Chen, Y.; Zhang, X.J.; Qian, G.R.; Chu, Y.L.; Zhou, M. Glycolysis recycling of rigid waste polyurethane foam from refrigerators. Environ. Technol. 2014, 35, 2676–2684. [Google Scholar] [CrossRef]
- Bari, S.S.; Chatterjee, A.; Mishra, S. Biodegradable polymer nanocomposites: An overview. Polym. Rev. 2016, 56, 287–328. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Baird, D.G. Preparation of polymer-clay nanocomposites and their properties. Adv. Polym. Technol. 2006, 25, 270–285. [Google Scholar] [CrossRef]
- Torres, F.G.; Saavedra, A.C. A comparison between the failure modes observed in biological and synthetic polymer nanocomposites. Polym. Plast. Technol. Mater. 2020, 59, 241–270. [Google Scholar] [CrossRef]
- Okamoto, M. Recent advances in polymer/layered silicate nanocomposites: An overview from science to technology. Mater. Sci. Technol. 2006, 22, 756–779. [Google Scholar] [CrossRef] [Green Version]
- Ismail, H.; Pasbakhsh, P.; Fauzi, M.N.A.; Abu Bakar, A. The Effect of Halloysite Nanotubes as a Novel Nanofiller on Curing Behaviour, Mechanical and Microstructural Properties of Ethylene Propylene Diene Monomer (EPDM) Nanocomposites. Polym. Plast. Technol. Eng. 2009, 48, 313–323. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Liu, X.; Zhu, Q.; Liu, Y.; Lin, X. Research Advances in Safety of Nanometer Materials and Their Risk Assessment System. Chin. J. Process Eng. 2013, 13, 893–900. [Google Scholar]
- Dai, J.; Chen, H.; Wei, L. Research progress in polyurethane/inorganic nanomaterial composites. Chem. Ind. Eng. Prog. 2014, 33, 2380–2386. [Google Scholar]
- Xuejia, D.; Yimin, W.; Dingsheng, Y.U.; XinZhong, L.I.; Bi, W. Study on Preparation and Properties of Polyamide 6/Attapulgite Nano-composite. China Plast. 2006, 20, 32–36. [Google Scholar]
- Ding, Z.; Li, J.; Xin, W.; Luo, Y. Facile and high-concentration exfoliation of montmorillonite into mono-layered nanosheets and application in multifunctional waterborne polyurethane coating. Appl. Clay Sci. 2020, 198, 105798. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Duan, J.; Wang, J. Surface morphology properties and antifouling activity of Bi2WO6/boron-grafted polyurethane composite coatings realized via multiple synergy. J. Colloid Interface Sci. 2022, 626, 815–823. [Google Scholar] [CrossRef]
- GB/TQ 12008.3-2009; Plastics—Polyether Polyols—Part 3: Determination of Hydroxyl Number. National Standard of the People’s Republic of China: Beijing, China, 2009.
- Li, X.G.; Xie, Y.B.; Huang, M.R.; Umeyama, T.; Imahori, H. Development of clean performance-tunable waterborne polyurethane using acetyl tributyl citrate for transferable holographic films. J. Clean. Prod. 2021, 279, 123496. [Google Scholar] [CrossRef]
- GB/T 6343-1986; Cellular Plastics and Rubbers—Determination of Apparent Density. National Standard of the People’s Republic of China: Beijing, China, 1986.
- Hou, M.; Li, A.; Zou, W.; Li, H.; Zhang, C. Effect of Amino-Modified Carbon Nanotubes on the Pore Structure of Polyurethane Foams. Polym. Mater. Sci. Eng. 2016, 32, 179. [Google Scholar]
- GB/T 10295-2008; Thermal Insulation-Determination of Steady-State Thermal Resistance and Related Properties-Heat Flow Meter Apparatus. National Standard of the People’s Republic of China: Beijing, China, 2008.
- Li, X.G.; Xie, Y.B.; Huang, M.R.; Umeyama, T.; Ohara, T.; Imahori, H. Effective role of eco-friendly acetyl tributyl citrate in large-scale catalyst-free synthesis of waterborne polyurethanes without volatile organic compounds. J. Clean. Prod. 2019, 237, 117543. [Google Scholar] [CrossRef]
- Wang, S.H.; Duan, H.F.; Ma, G.Z.; Hou, C.Y.; Wu, J.B.; Li, S.S.; Yang, Z.Y.; Hao, X.G. Epoxy functionalization of multiwalled carbon nanotubes for their waterborne polyurethane composite with crosslinked structure. J. Coat. Technol. Res. 2020, 17, 91–100. [Google Scholar] [CrossRef]



| Mullite Whisker Addition Amount/% | Thermal Conductivity/W/(m·K) |
|---|---|
| 0.00 | 0.0297 |
| 0.05 | 0.0189 |
| 0.15 | 0.0179 |
| 0.25 | 0.0223 |
| 0.50 | 0.0310 |
| 0.75 | 0.0301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, X.; Zhu, Y.; Liu, S.; Zhu, S.; Liu, Y. Preparation of Mullite/PU Nanocomposites by Double Waste Co-Recycling. Sustainability 2022, 14, 14310. https://doi.org/10.3390/su142114310
Gu X, Zhu Y, Liu S, Zhu S, Liu Y. Preparation of Mullite/PU Nanocomposites by Double Waste Co-Recycling. Sustainability. 2022; 14(21):14310. https://doi.org/10.3390/su142114310
Chicago/Turabian StyleGu, Xiaohua, Yanwei Zhu, Siwen Liu, Shangwen Zhu, and Yan Liu. 2022. "Preparation of Mullite/PU Nanocomposites by Double Waste Co-Recycling" Sustainability 14, no. 21: 14310. https://doi.org/10.3390/su142114310
APA StyleGu, X., Zhu, Y., Liu, S., Zhu, S., & Liu, Y. (2022). Preparation of Mullite/PU Nanocomposites by Double Waste Co-Recycling. Sustainability, 14(21), 14310. https://doi.org/10.3390/su142114310









