Biogas Slurry as an Alternative to Chemical Fertilizer: Changes in Soil Properties and Microbial Communities of Fluvo-Aquic Soil in the North China Plain
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Area Description
2.2. Experimental Design and Agricultural Managements
2.3. Soil Sampling and Physicochemical Analysis
2.4. Soil DNA Extraction and Microbial High-Throughput Sequencing
2.5. Statistical Analysis
3. Results
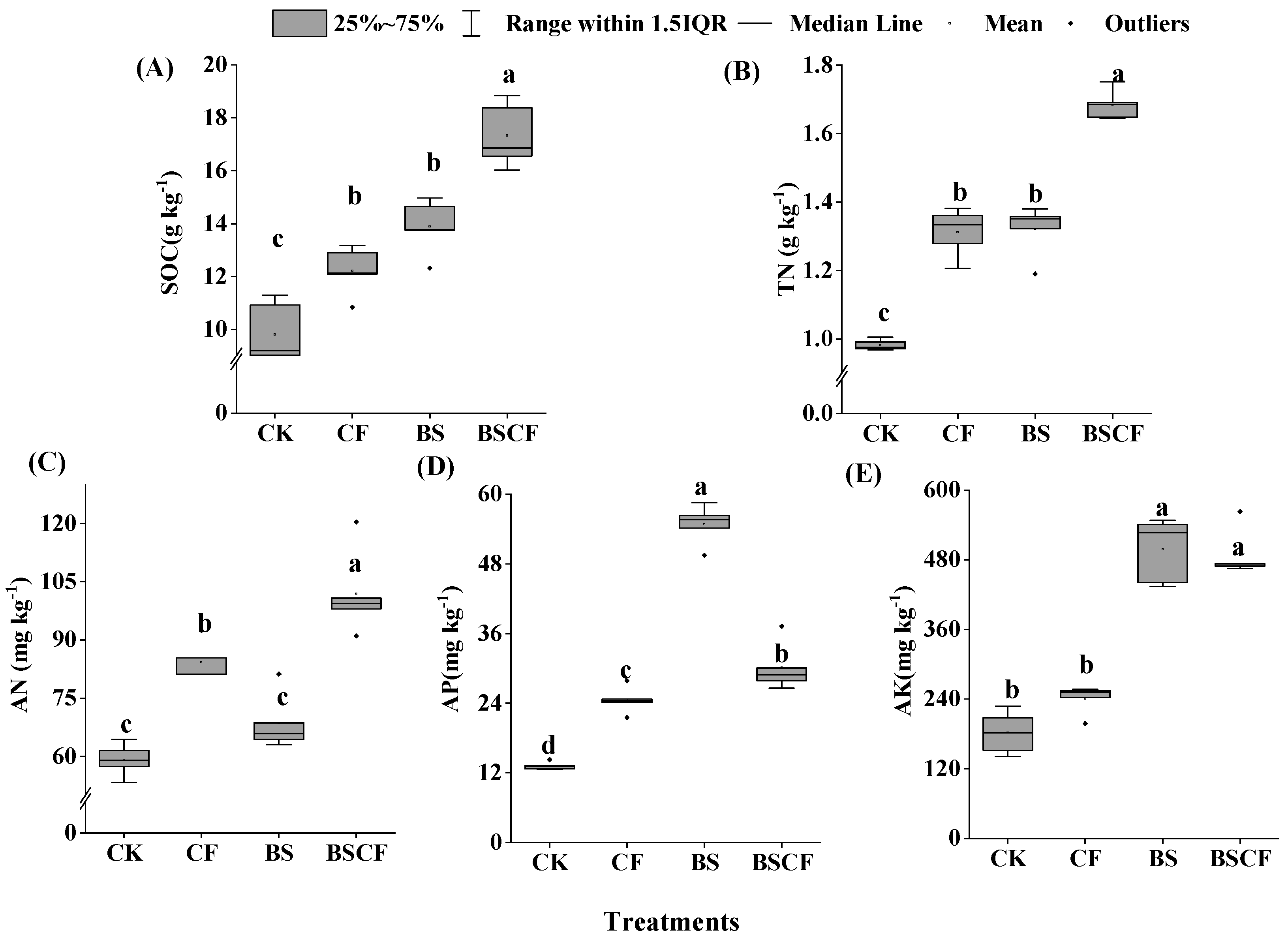
3.1. Soil Physicochemical Properties
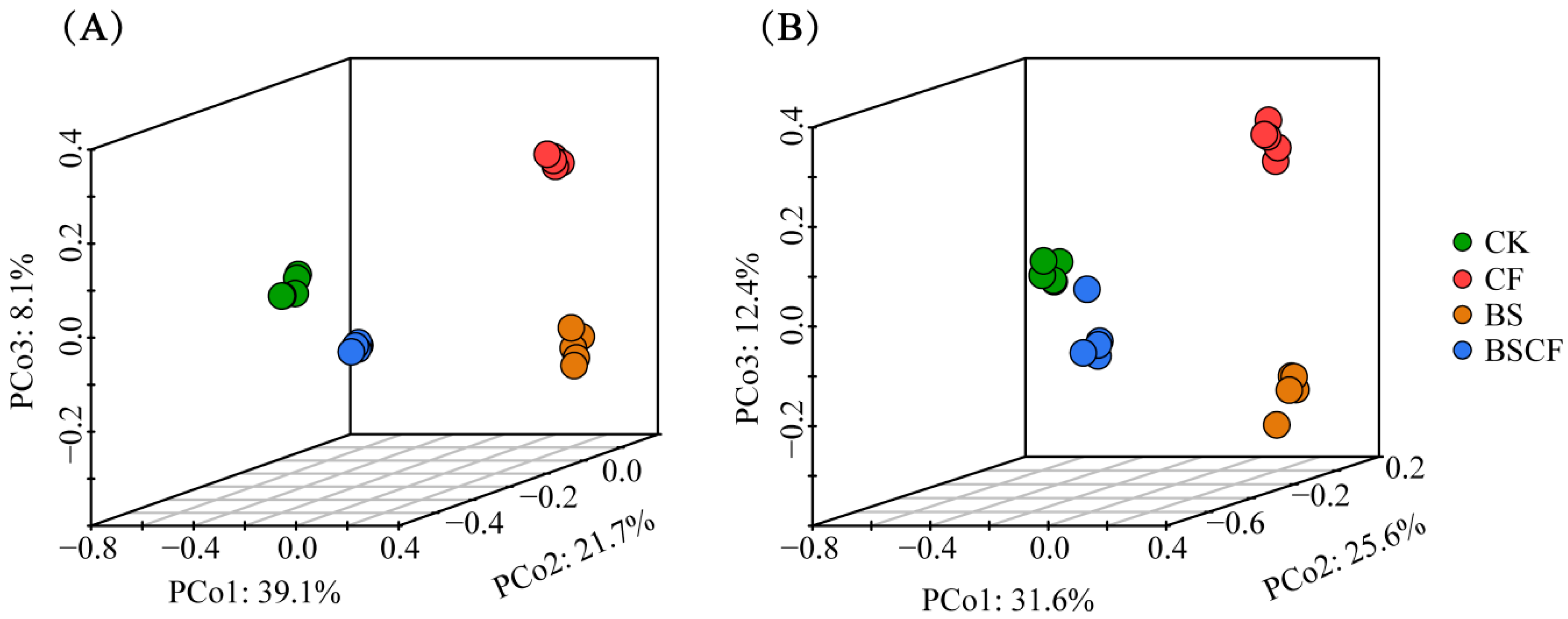
3.2. Changes in Composition and Diversity of Microbial Communities
3.3. Relationships between Microbial Characteristics and Soil Properties
4. Discussion
4.1. Impacts on Soil Physicochemical Properties
4.2. Impacts on Soil Microbial Community Composition and Diversity
4.3. Implications for Development of Sustainable Crop Production and Animal Husbandry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavalcante, J.S.; Favaretto, N.; Dieckow, J.; Cherobim, V.F.; Barth, G. Long-term surface application of dairy liquid manure to soil under no-till improves carbon and nitrogen stocks. Eur. J. Soil Sci. 2019, 71, 1132–1143. [Google Scholar]
- Terhoeven-Urselmans, T.; Scheller, E.; Raubuch, M.; Ludwig, B.; Joergensen, R.G. CO2 evolution and N mineralization after biogas slurry application in the field and its yield effects on spring barley. Appl. Soil Ecol. 2009, 42, 297–302. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, L.M.; Sharma, S.; Singh, N. Overview on agricultural potentials of biogas slurry (BGS): Applications, challenges, and solutions. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Juárez, M.F.D.; Zangerle, M.; Insam, H. Effects of digestate on soil chemical and microbiological properties: A comparative study with compost and vermicompost. J. Hazard Mater. 2015, 302, 267. [Google Scholar] [CrossRef]
- Bosch-Serra, à.D.; Yagüe, M.R.; Poch, R.M.; Molner, M.; Junyent, B.; Boixadera, J. Aggregate strength in calcareous soil fertilized with pig slurries. Eur. J. Soil Sci. 2017, 68, 449–461. [Google Scholar] [CrossRef]
- Niyungeko, C.; Liang, X.; Liu, C.; Zhou, J.; Chen, L.; Lu, Y.; Tiimub, B.M.; Li, F. Effect of biogas slurry application on soil nutrients, phosphomonoesterase activities, and phosphorus species distribution. J. Soils Sediments 2020, 20, 900–910. [Google Scholar] [CrossRef]
- Tang, J.; Davy, A.J.; Wang, W.; Zhang, X.H.; Wu, D.F.; Hu, L.; Yin, J.Z. Effects of biogas slurry on crop yield, physicochemical properties and aggregation characteristics of lime concretion soil in wheat–maize rotation in the North China Plain. J. Soil Sci. Plant Nutr. 2022, 22, 2406–2417. [Google Scholar] [CrossRef]
- Wang, W.G.; Zhang, Y.H.; Liu, Y.; Jiang, N.; Deng, L.W. Managing liquid digestate to support the sustainable biogas industry in China: Maximizing biogas linked agroecosystem balance. GCB Bioenergy 2021, 13, 880–892. [Google Scholar] [CrossRef]
- Abubaker, J.; Cederlund, H.; Arthurson, V.; Pell, M. Bacterial community structure and microbial activity in different soils amended with biogas residues and cattle slurry. Appl. Soil Ecol. 2013, 72, 171–180. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Wu, H.; Yan, S.; Guo, D.; Wang, G.; Ma, Y. Soil chemical and microbial responses to biogas slurry amendment and its effect on Fusarium wilt suppression. Appl. Soil Ecol. 2016, 107, 116–123. [Google Scholar] [CrossRef]
- Tang, Y.F.; Wen, G.L.; Li, P.P.; Dai, C.; Han, J.G. Effects of biogas slurry application on crop production and soil properties in a rice–wheat rotation on coastal reclaimed farmland. Water Air Soil Pollut. 2019, 230, 51. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Q.K.; Liu, D.H.; Hu, C.; Sun, J.W.; Wang, X.B.; Liang, G.Q.; Zhou, W. Composition, predicted functions, and co-occurrence networks of fungal and bacterial communities- Links to soil organic carbon under long-term fertilization in a rice-wheat cropping system. Eur. J. Soil Biol. 2020, 100, 103226. [Google Scholar] [CrossRef]
- Xu, M.; Xian, Y.; Wu, J.; Gu, Y.F.; Yang, G.; Zhang, X.H.; Peng, H.; Yu, X.Y.; Xiao, Y.L.; Li, L. Effect of biogas slurry addition on soil properties, yields, and bacterial composition in the rice-rape rotation ecosystem over 3 years. J. Soil Sediments 2019, 19, 2534–2542. [Google Scholar] [CrossRef]
- Li, J.S.; Duan, N.; Guo, S.; Shao, L.; Lin, C.; Wang, J.H.; Hou, J.; Meng, J.; Han, M.Y. Renewable resource for agricultural ecosystem in China: Ecological benefit for biogas by-product for planting. Ecol. Inform. 2012, 12, 101–110. [Google Scholar] [CrossRef]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Zheng, X.B.; Fan, J.B.; Cui, J.; Wang, Y.; Zhou, J.; Ye, M.; Sun, M.M. Effects of biogas slurry application on peanut yield, soil nutrients, carbon storage, and microbial activity in an Ultisol soil in southern China. J. Soil Sediments 2016, 16, 449–460. [Google Scholar] [CrossRef]
- Du, Z.J.; Xiao, Y.T.; Qi, X.B.; Liu, Y.; Fan, X.Y.; Li, Z.Y. Peanut-shell biochar and biogas slurry improve soil properties in the North China Plain: A four-year field study. Sci. Rep. 2018, 8, 13724. [Google Scholar] [CrossRef]
- Lu, R.K. Soil and Agro-Chemical Analysis Methods; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- Fei, C.; Zhang, S.; Li, J.; Liang, B.; Ding, X. Partial substitution of rice husk for manure in greenhouse vegetable fields: Insight from soil carbon stock and aggregate stability. Land Degrad. Dev. 2021, 32, 3962–3972. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Banitalebi, G.; Mosaddeghi, M.R.; Shariatmadari, H. Feasibility of agricultural residues and their biochars for plant growing media: Physical and hydraulic properties. Waste Manage. 2019, 87, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, F.; Li, W.; Ning, Q.; Lia, J.W.; Zhang, J.B.; Ma, D.H.; Zhang, C.Z. Organic amendment mitigates the negative impacts of mineral fertilization on bacterial communities in Shajiang black soil. Appl. Soil Ecol. 2019, 150, 103457. [Google Scholar] [CrossRef]
- Ye, G.P.; Lin, Y.X.; Liu, D.Y.; Chen, Z.M.; Luo, J.F.; Boland, N.; Fan, J.B.; Ding, W.X. Long-term application of manure over plant residues mitigates acidification, builds soil organic carbon and shifts prokaryotic diversity in acidic Ultisols. Appl. Soil Ecol. 2019, 133, 24–33. [Google Scholar] [CrossRef]
- Badagliacca, G.; Petrovicova, B.; Pathan, S.I.; Roccotelli, A.; Romeo, M.; Monti, M.; Gelsomino, A. Use of solid anaerobic digestate and no-tillage practice for restoring the fertility status of two Mediterranean orchard soils with contrasting properties. Agric. Ecosyst. Environ. 2020, 300, 107010. [Google Scholar]
- de Sosal, L.L.; Glanville, H.C.; Marshall, M.R.; Schnepf, A.; Cooper, D.M.; Hill, P.W.; Binley, A.; Jones, D.L. Stoichiometric constraints on the microbial processing of carbon with soil depth along a riparian hillslope. Biol. Fertil. Soils 2018, 54, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Insam, H.; Gomez-Brandon, M.; Ascher, J. Manure-based biogas fermentation residues: Friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Wentzel, S.; Schmidt, R.; Piepho, H.P.; Semmler-Busch, U.; Joergensen, R.G. Response of soil fertility indices to long-term application of biogas and raw slurry under organic farming. Appl. Soil Ecol. 2015, 96, 99–107. [Google Scholar] [CrossRef]
- Sommer, S.G.; Zhang, G.Q.; Bannink, A.; Chadwick, D.; Webb, J. Algorithms determining ammonia emission from buildings housing cattle and pigs and from manure stores. Adv. Agron. 2006, 89, 261–335. [Google Scholar]
- Rahaman, M.A.; Zhan, X.Y.; Zhang, Q.W.; Li, S.Q.; Lv, S.M.; Long, Y.T.; Zeng, H.L. Ammonia volatilization reduced by combined application of biogas slurry and chemical fertilizer in maize–wheat rotation system in North China Plain. Sustainability 2020, 12, 4400. [Google Scholar]
- Christensen, M.L.; Hjorth, M.; Keiding, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar]
- Hernández, D.; Polo, A.; Plaza, C. Long-term effects of pig slurry on barley yield and N use efficiency under semiarid Mediterranean conditions. Eur. J. Agron. 2013, 44, 78–86. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, C.G.; Kang, H. Temporal dynamics of bacterial and fungal communities in a genetically modified (GM) Rice Ecosystem. Microb. Ecol. 2010, 61, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. Isme J. 2012, 6, 1007–1017. [Google Scholar] [PubMed]
- Ren, C.J.; Chen, J.; Deng, J.; Zhao, F.Z.; Han, X.H.; Yang, G.H.; Tong, X.G.; Feng, Y.Z.; Shelton, S.; Ren, G.X. Response of microbial diversity to C:N:P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fertil. Soils. 2017, 53, 1–12. [Google Scholar]
- Francioli, D.; Elke, S.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 289. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.Q.; Wang, Y.; Li, Y.; Li, P.P.; Chen, L.; Jie, X.L.; Hu, D.S.; Feng, B.; Yue, K.; et al. Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 2020, 151, 108017. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, S.X.; Zheng, X.Q.; Zhang, J.Q.; Bai, N.L.; Zhang, H.Y.; Lv, W.G. Effects of biogas slurry combined with chemical fertilizer on soil bacterial and fungal community composition in a paddy field. Front. Microbiol. 2021, 12, 2338. [Google Scholar] [CrossRef]
- Stursová, M.; Žifčáková, L.; Leigh, M.B.; Burgess, R.; Baldrian, P. Cellulose utilization in forest litter and soil: Identification of bacterial and fungal decomposers. FEMS Microbiol. Ecol. 2012, 80, 735–746. [Google Scholar]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.; Huber, J.A.; O’Connor, M.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Ye, G.P.; Lin, Y.X.; Luo, J.F.; Di, H.J.; Lindsey, S.; Liu, D.Y.; Fan, J.B.; Ding, W.X. Responses of soil fungal diversity and community composition to long-term fertilization: Field experiment in an acidic Ultisol and literature synthesis. Appl. Soil Ecol. 2020, 145, 103305. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, E.P.; Neal, A.L.; Zhang, X.X.; Li, Z.Y.; Xiao, Y.T.; Du, Z.J.; Gao, F.; Fan, X.Y.; Hu, C. Reducing water use by alternate-furrow irrigation with livestock wastewater reduces antibiotic resistance gene abundance in the rhizosphere but not in the non-rhizosphere. Sci. Total Environ. 2019, 648, 12–24. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Biogas Slurry | Chemical Fertilizer | |||||
|---|---|---|---|---|---|---|---|
| Application Amount | N | P | K | N | P | K | |
| m3 hm−2 | kg hm−2 | kg hm−2 | kg hm−2 | kg hm−2 | kg hm−2 | kg hm−2 | |
| CK | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CF | 0.00 | 0.00 | 0.00 | 0.00 | 180.00 | 39.30 | 74.70 |
| BS | 120.00 | 180.00 | 36.00 | 58.80 | 0.00 | 3.30 | 15.90 |
| BSCF | 60.00 | 90.00 | 18.00 | 29.40 | 90.00 | 21.30 | 45.30 |
| Treatments | BD | WHC | wMWD | pH | EC |
|---|---|---|---|---|---|
| g cm−3 | % | mm | μs cm−1 | ||
| CK | 1.37 ± 0.04 a | 17.18 ± 0.79 c | 0.42 ± 0.04 d | 7.90 ± 0.06 a | 247.80 ± 32.32 d |
| CF | 1.35 ± 0.02 ab | 18.98 ± 0.87 bc | 0.84 ± 0.16 c | 7.36 ± 0.17 c | 401.60 ± 25.13 c |
| BS | 1.30 ± 0.02 b | 19.93 ± 0.86 b | 1.08 ± 0.15 b | 7.38 ± 0.11 c | 696.00 ± 16.70 a |
| BSCF | 1.31 ± 0.03 b | 22.21 ± 1.50 a | 1.53 ± 0.04 a | 7.67 ± 0.04 b | 574.40 ± 19.84 b |
| Treatments | Chao1 | Observed-Species | Shannon Index | Simpson Index |
|---|---|---|---|---|
| Bacteria | ||||
| CK | 5211.55 ± 108.81 d | 4670.46 ± 92.13 d | 10.66 ± 0.05 d | 0.998 ± 0.002 b |
| CF | 6718.14 ± 134.41 c | 5868.18 ± 114.77 c | 11.00 ± 0.10 c | 0.997 ± 0.007 b |
| BS | 7162.74 ± 118.52 b | 6274.82 ± 90.13 b | 11.37 ± 0.05 b | 0.999 ± 0.002 ab |
| BSCF | 8080.80 ± 36.00 a | 7628.96 ± 68.62 a | 11.74 ± 0.02 a | 0.999 ± 0.002 a |
| Fungi | ||||
| CK | 403.23 ± 31.07 b | 399.06 ± 30.79 b | 6.30 ± 0.14 a | 0.966 ± 0.004 a |
| CF | 413.30 ± 14.42 b | 409.60 ± 14.49 b | 6.33 ± 0.10 a | 0.969 ± 0.003 a |
| BS | 628.41 ± 40.99 a | 622.96. ± 40.87 a | 6.83 ± 0.07 a | 0.975 ± 0.003 a |
| BSCF | 590.05 ± 32.85 a | 584.88 ± 33.24 a | 6.51 ± 0.21 a | 0.967 ± 0.007 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Yin, J.; Davy, A.J.; Pan, F.; Han, X.; Huang, S.; Wu, D. Biogas Slurry as an Alternative to Chemical Fertilizer: Changes in Soil Properties and Microbial Communities of Fluvo-Aquic Soil in the North China Plain. Sustainability 2022, 14, 15099. https://doi.org/10.3390/su142215099
Tang J, Yin J, Davy AJ, Pan F, Han X, Huang S, Wu D. Biogas Slurry as an Alternative to Chemical Fertilizer: Changes in Soil Properties and Microbial Communities of Fluvo-Aquic Soil in the North China Plain. Sustainability. 2022; 14(22):15099. https://doi.org/10.3390/su142215099
Chicago/Turabian StyleTang, Jiao, Jinzhong Yin, Anthony J. Davy, Feifei Pan, Xu Han, Shaonan Huang, and Dafu Wu. 2022. "Biogas Slurry as an Alternative to Chemical Fertilizer: Changes in Soil Properties and Microbial Communities of Fluvo-Aquic Soil in the North China Plain" Sustainability 14, no. 22: 15099. https://doi.org/10.3390/su142215099
APA StyleTang, J., Yin, J., Davy, A. J., Pan, F., Han, X., Huang, S., & Wu, D. (2022). Biogas Slurry as an Alternative to Chemical Fertilizer: Changes in Soil Properties and Microbial Communities of Fluvo-Aquic Soil in the North China Plain. Sustainability, 14(22), 15099. https://doi.org/10.3390/su142215099






