First Evidence of Microplastic Presence in Bed Load Sediments of a Small Urban Stream in Warsaw
Abstract
:1. Introduction
2. Materials and Methods
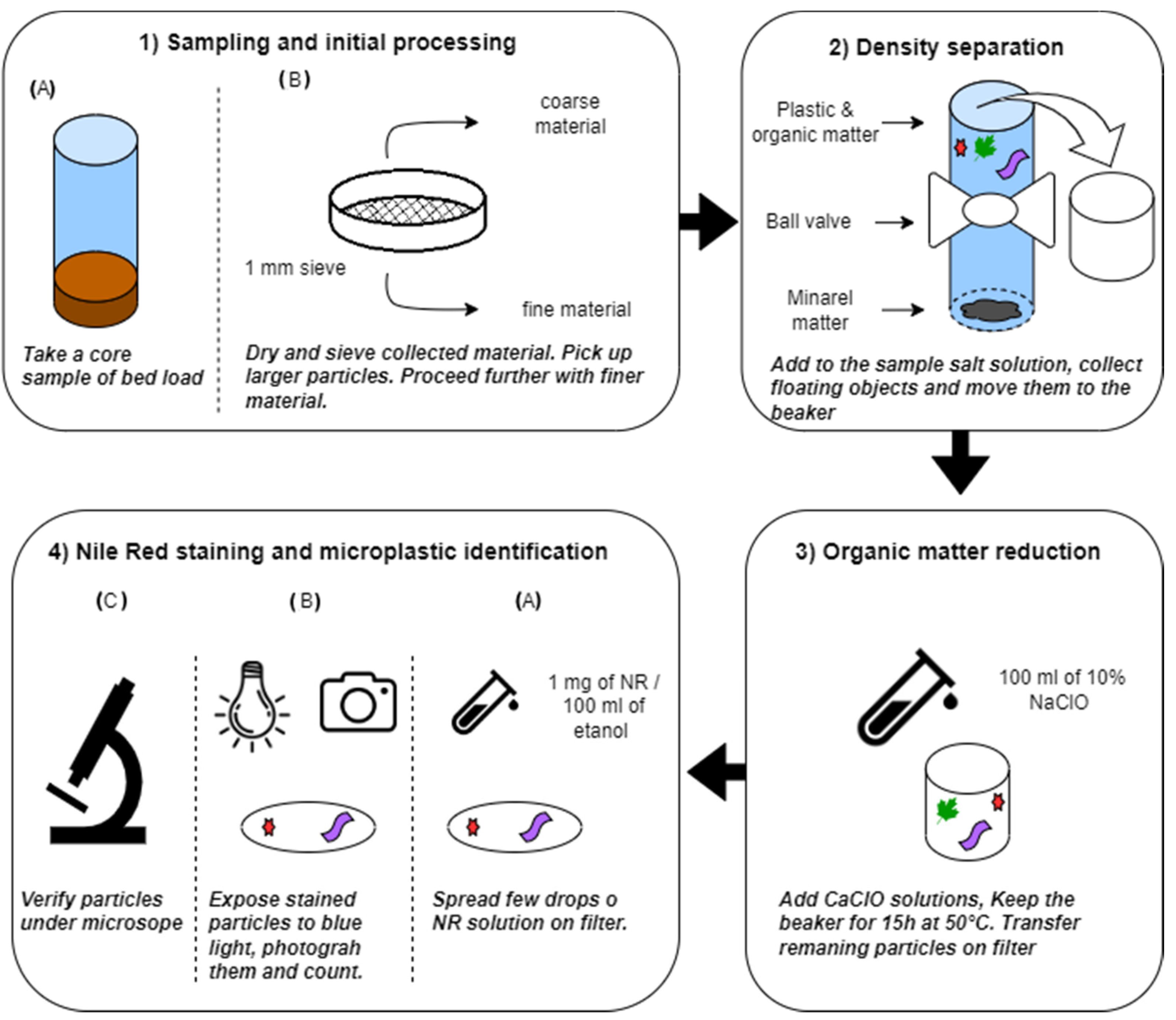
2.1. Overview of the Applied Procedure
2.2. Study Catchment and Sampling Cross Sections
2.3. Initial Processing
2.4. Density Separation
2.5. Organic Matter Reduction
2.6. Nile Red Staining and Microplastic Identification
3. Results and Discussion
4. Conclusions
- Microplastics were present in the channel of Służew Creek in Warsaw, where the estimated number of particles ranged from 191 to 279 pieces per 30 g of bed load sediment for the selected sampling sites;
- The presence of the largest particles (more than 1 mm in size) most likely depends on the management of the catchment area and the number of tributaries, while the abundance of the finest particles (less than 1 mm in size) could also be determined by the meteorological conditions;
- Small reservoirs may reduce the load of particles and thus enhance the quality of urban runoff;
- The number of particles seems to increase in the catchment area and factors influencing microplastic accumulation in the study catchment should be investigated in detail in further works;
- There is a need for further broad research focusing among others on: (i) the standardization of methods and laboratory procedures (leading to microplastic detection) in relation to the type of sample, and (ii) thee identification of specific polymers and the verification of obtained results with the use of the NR staining method.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Sun, X.; Liang, J.; Zhu, M.; Zhao, Y.; Zhang, B. Microplastics in Seawater and Zooplankton from the Yellow Sea. Environ. Pollut. 2018, 242, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in Marine Mammals Stranded around the British Coast: Ubiquitous but Transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in Wild Fish from North East Atlantic Ocean and Its Potential for Causing Neurotoxic Effects, Lipid Oxidative Damage, and Human Health Risks Associated with Ingestion Exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef] [PubMed]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in Marine Organism: Environmental and Toxicological Effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef]
- Alimba, C.G.; Faggio, C. Microplastics in the Marine Environment: Current Trends in Environmental Pollution and Mechanisms of Toxicological Profile. Environ. Toxicol. Pharmacol. 2019, 68, 61–74. [Google Scholar] [CrossRef]
- Chatterjee, S.; Sharma, S. Microplastics in Our Oceans and Marine Health. Field Actions Sci. Rep. J. Field Actions 2019, 19, 54–61. [Google Scholar]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and Synthetic Particles Ingested by Deep-Sea Amphipods in Six of the Deepest Marine Ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef] [Green Version]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A Theoretical Assessment of Microplastic Transport in River Catchments and Their Retention by Soils and River Sediments. Environ. Sci Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Käppler, A.; Biniasch, O.; Feldens, P.; Stollberg, N.; Lange, X.; Fischer, D.; Eichhorn, K.-J.; Pollehne, F.; Oberbeckmann, S.; et al. Tracing Microplastics in Aquatic Environments Based on Sediment Analogies. Sci. Rep. 2019, 9, 15207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.; Dimzon, I.K.; Eubeler, J.; Knepper, T.P. Analysis, Occurrence, and Degradation of Microplastics in the Aqueous Environment. In Freshwater Microplastics: Emerging Environmental Contaminants? Wagner, M., Lambert, S., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2018; pp. 51–67. ISBN 978-3-319-61615-5. [Google Scholar]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A Review of Microplastic Distribution in Sediment Profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Frey, P.; Church, M. Bedload: A Granular Phenomenon. Earth Surf. Process. Landf. 2011, 36, 58–69. [Google Scholar] [CrossRef]
- Liro, M.; Emmerik, T.V.; Wyżga, B.; Liro, J.; Mikuś, P. Macroplastic Storage and Remobilization in Rivers. Water 2020, 12, 2055. [Google Scholar] [CrossRef]
- Kataoka, T.; Nihei, Y.; Kudou, K.; Hinata, H. Assessment of the Sources and Inflow Processes of Microplastics in the River Environments of Japan. Environ. Pollut. 2019, 244, 958–965. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Simpson, M.D.; Nicholson, K.N.; Neumann, K. Exploratory Study of Microplastics in the Eastern Himalayas of Nepal. AGU Fall Meet. Abstr. 2019, 2019, 52. [Google Scholar]
- Pegado, T.d.S.S.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First Evidence of Microplastic Ingestion by Fishes from the Amazon River Estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- Gerolin, C.R.; Pupim, F.N.; Sawakuchi, A.O.; Grohmann, C.H.; Labuto, G.; Semensatto, D. Microplastics in Sediments from Amazon Rivers, Brazil. Sci. Total Environ. 2020, 749, 141604. [Google Scholar] [CrossRef]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic Polar Waters: The First Reported Values of Particles in Surface and Sub-Surface Samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosker, T.; Guaita, L.; Behrens, P. Microplastic Pollution on Caribbean Beaches in the Lesser Antilles. Mar. Pollut. Bull. 2018, 133, 442–447. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in Surface Waters and Sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yin, L.; Wen, X.; Du, C.; Wu, L.; Long, Y.; Liu, Y.; Ma, Y.; Yin, Q.; Zhou, Z.; et al. Microplastics in Sediment and Surface Water of West Dongting Lake and South Dongting Lake: Abundance, Source and Composition. Int. J. Environ. Res. Public Health 2018, 15, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banasik, K.; Krajewski, A.; Sikorska, A.; Hejduk, L. Curve Number Estimation for a Small Urban Catchment from Recorded Rainfall-Runoff Events. Arch. Environ. Prot. 2014, 40, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Krajewski, A.; Sikorska-Senoner, A.E.; Hejduk, A.; Hejduk, L. Variability of the Initial Abstraction Ratio in an Urban and an Agroforested Catchment. Water 2020, 12, 415. [Google Scholar] [CrossRef] [Green Version]
- Krajewski, A.; Sikorska-Senoner, A.E. Suspended Sediment Routing through a Small On-Stream Reservoir Based on Particle Properties. J. Soils Sediments 2021, 21, 1523–1538. [Google Scholar] [CrossRef]
- Starzyński, J. Wilanów: Dzieje Budowy Pałacu Za Jana III; PWN: Warszawa, Poland, 1976. [Google Scholar]
- Krajewski, A.; Wasilewicz, M.; Sikorska, A.; Banasik, K. Operation of Detention Pond in Urban Area—Example of Wyścigi Pond in Warsaw. In Environmental Engineering V; Pawłowska, M., Pawłowski, L., Eds.; CRC Press: Leiden, The Netherlands, 2017; ISBN 978-1-315-28197-1. [Google Scholar]
- Wojtkowska, M.; Karwowska, E.; Chmielewska, I.; Bekenova, K.; Wanot, E. Copper and Cadmium in Bottom Sediments Dredged from Wyścigi Pond, Warsaw, Poland--Contamination and Bioaccumulation Study. Environ. Monit. Assess. 2015, 187, 737. [Google Scholar] [CrossRef] [Green Version]
- Khuyen, V.T.K.; Le, D.V.; Fischer, A.R.; Dornack, C. Comparison of Microplastic Pollution in Beach Sediment and Seawater at UNESCO Can Gio Mangrove Biosphere Reserve. Glob. Chall. 2021, 5, 2100044. [Google Scholar] [CrossRef]
- Schröder, K.; Kossel, E.; Lenz, M. Microplastic Abundance in Beach Sediments of the Kiel Fjord, Western Baltic Sea. Environ. Sci. Pollut. Res. 2021, 28, 26515–26528. [Google Scholar] [CrossRef]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of Density Separation for the Rapid Recovery of Microplastics from Sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef] [Green Version]
- Masura, J.; Baker, J.; Foster, G.; Courtney, A. Laboratory Methods for the Analysis of Microplastics in the Marine Environment; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015.
- Hitchcock, J.N. Storm Events as Key Moments of Microplastic Contamination in Aquatic Ecosystems. Sci. Total Environ. 2020, 734, 139436. [Google Scholar] [CrossRef]
- Narmadha, V.; Jose, J.; Patil, S.; Farooqui, M.O.; Srimuruganandam, B.; Saravanadevi, S.; Krishnamurthi, K. Assessment of Microplastics in Roadside Suspended Dust from Urban and Rural Environment of Nagpur, India. Int. J. Environ. Res. 2020, 14, 629–640. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A Rapid-Screening Approach to Detect and Quantify Microplastics Based on Fluorescent Tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeiren, P.; Muñoz, C.; Ikejima, K. Microplastic Identification and Quantification from Organic Rich Sediments: A Validated Laboratory Protocol. Environ. Pollut. 2020, 262, 114298. [Google Scholar] [CrossRef]
- Coppock, R.L.; Cole, M.; Lindeque, P.K.; Queirós, A.M.; Galloway, T.S. A Small-Scale, Portable Method for Extracting Microplastics from Marine Sediments. Environ. Pollut. 2017, 230, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, J.C.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Selection of Microplastics by Nile Red Staining Increases Environmental Sample Throughput by Micro-Raman Spectroscopy. Sci. Total Environ. 2021, 783, 146979. [Google Scholar] [CrossRef]
- Liu, F.; Vianello, A.; Vollertsen, J. Retention of Microplastics in Sediments of Urban and Highway Stormwater Retention Ponds. Environ. Pollut. 2019, 255, 113335. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a Quick and Efficient Method of Removing Organic Matter without Damaging Microplastic Samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Rocha-Santos, T.; Prata, J.C.; Duarte, A.C.; Girão, A.V.; Lopes, P.; Cristovão, T.; da Costa, J.P. A Straightforward Method for Microplastic Extraction from Organic-Rich Freshwater Samples. Sci. Total Environ. 2022, 815, 152941. [Google Scholar] [CrossRef] [PubMed]
- Lavoy, M.; Crossman, J. A Novel Method for Organic Matter Removal from Samples Containing Microplastics. Environ. Pollut. 2021, 286, 117357. [Google Scholar] [CrossRef] [PubMed]
- Mbachu, O.; Jenkins, G.; Pratt, C.; Kaparaju, P. Enzymatic Purification of Microplastics in Soil. MethodsX 2021, 8, 101254. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell. Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 Mm to 20 Μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prata, J.C.; Alves, J.R.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Major Factors Influencing the Quantification of Nile Red Stained Microplastics and Improved Automatic Quantification (MP-VAT 2.0). Sci. Total Environ. 2020, 719, 137498. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Roy, P.D.; Kutralam-Muniasamy, G. Analyzing Microplastics with Nile Red: Emerging Trends, Challenges, and Prospects. J. Hazard Mater. 2022, 423, 127171. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Reis, V.; Matos, J.T.V.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. A New Approach for Routine Quantification of Microplastics Using Nile Red and Automated Software (MP-VAT). Sci. Total Environ. 2019, 690, 1277–1283. [Google Scholar] [CrossRef]
- Scircle, A.; Cizdziel, J.V. Detecting and Quantifying Microplastics in Bottled Water Using Fluorescence Microscopy: A New Experiment for Instrumental Analysis and Environmental Chemistry Courses. J. Chem. Educ. 2020, 97, 234–238. [Google Scholar] [CrossRef]
- Wiggin, K.J.; Holland, E.B. Validation and Application of Cost and Time Effective Methods for the Detection of 3–500 μm Sized Microplastics in the Urban Marine and Estuarine Environments Surrounding Long Beach, California. Mar. Pollut. Bull. 2019, 143, 152–162. [Google Scholar] [CrossRef]
- Godoy, V.; Prata, J.C.; Blázquez, G.; Almendros, A.I.; Duarte, A.C.; Rocha-Santos, T.; Calero, M.; Martín-Lara, M.Á. Effects of Distance to the Sea and Geomorphological Characteristics on the Quantity and Distribution of Microplastics in Beach Sediments of Granada (Spain). Sci. Total Environ. 2020, 746, 142023. [Google Scholar] [CrossRef] [PubMed]
- NIH—National Institutes of Health. Available online: https://imagej.nih.gov/ij/ (accessed on 22 June 2022).
- Patchaiyappan, A.; Dowarah, K.; Zaki Ahmed, S.; Prabakaran, M.; Jayakumar, S.; Thirunavukkarasu, C.; Devipriya, S.P. Prevalence and Characteristics of Microplastics Present in the Street Dust Collected from Chennai Metropolitan City, India. Chemosphere 2021, 269, 128757. [Google Scholar] [CrossRef]
- Sekudewicz, I.; Dąbrowska, A.M.; Syczewski, M.D. Microplastic Pollution in Surface Water and Sediments in the Urban Section of the Vistula River (Poland). Sci. Total Environ. 2021, 762, 143111. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman Imaging for Microplastics Analysis: State of the Art, Challenges and Prospects. TRAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Hengstmann, E.; Fischer, E.K. Nile Red Staining in Microplastic Analysis-Proposal for a Reliable and Fast Identification Approach for Large Microplastics. Environ. Monit. Assess. 2019, 191, 612. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and Quantification of Microplastics Using Nile Red Staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Patchaiyappan, A.; Ahmed, S.Z.; Dowarah, K.; Jayakumar, S.; Devipriya, S.P. Occurrence, Distribution and Composition of Microplastics in the Sediments of South Andaman Beaches. Mar. Pollut. Bull. 2020, 156, 111227. [Google Scholar] [CrossRef]
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; De Witte, B.; Janssen, C.; Everaert, G. Microplastic Detection and Identification by Nile Red Staining: Towards a Semi-Automated, Cost- and Time-Effective Technique. Sci. Total Environ. 2022, 823, 153441. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Habibi, N.; Behbehani, M. Micro-Nano Plastic in the Aquatic Environment: Methodological Problems and Challenges. Animals 2022, 12, 297. [Google Scholar] [CrossRef]

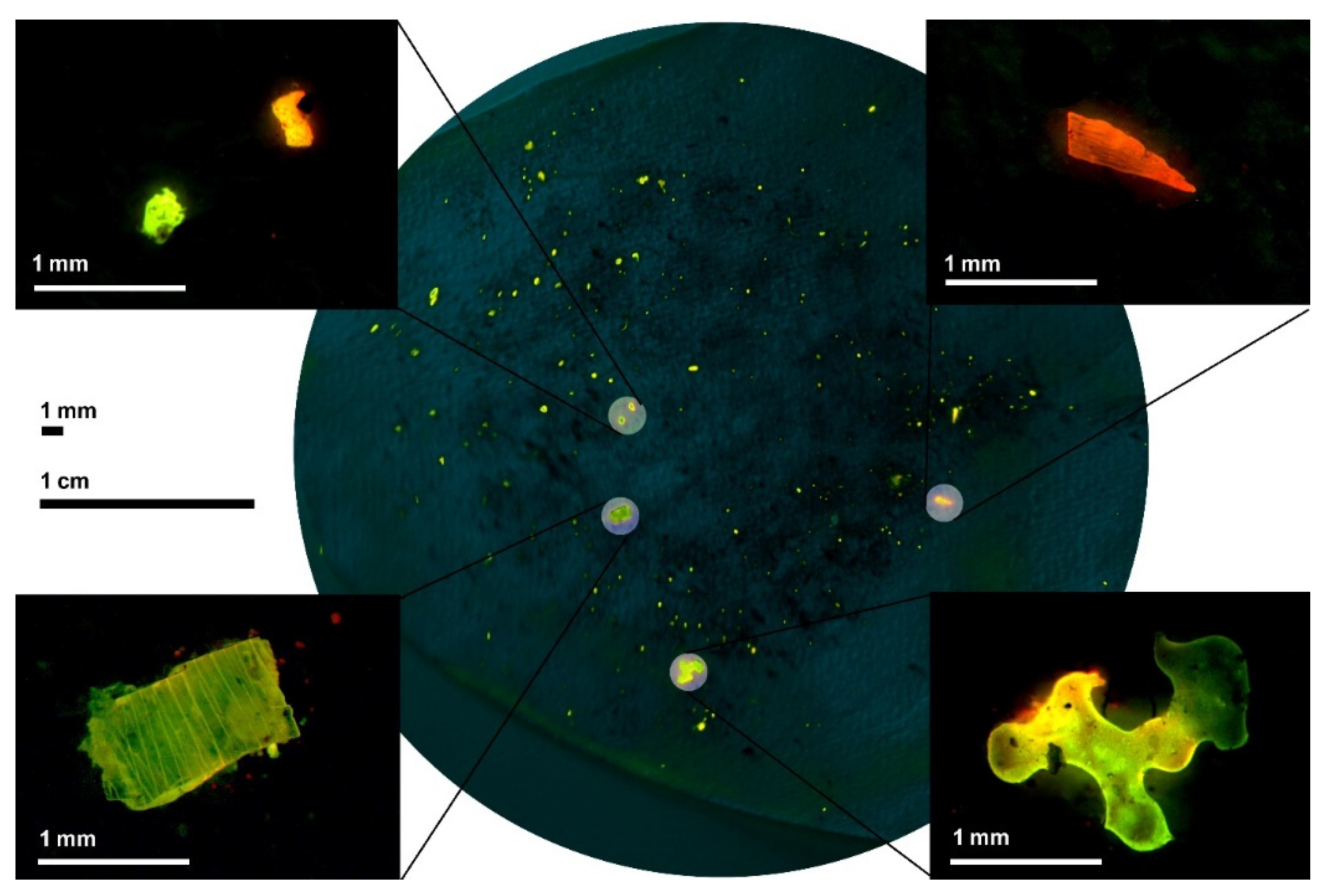
| No. | Name/Location of the Sampling Site | Number of Microplastic Particles Captured on 1 mm Sieve | Number of Microplastic Particles Captured on Filter (Pieces/30 g of Bed Load Sediment) | Total Number of Microplastic Particles (Pieces/30 g of Bed Load Sediment) | Median Particle Surface in the Sample (mm2) |
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |
| 1 | S1, Służew Creek | 0 | 213 | 213 | 0.0057 |
| 2 | S2, Grabów Channel | 6 | 185 | 191 | 0.0076 |
| 3 | S3, Służew Creek | 3 | 276 | 279 | 0.0062 |
| 4 | S4, Służew Creek | 1 | 249 | 250 | 0.0123 |
| Average | 3 | 231 | 233 | 0.00795 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewski, A.; Hejduk, A.; Hejduk, L. First Evidence of Microplastic Presence in Bed Load Sediments of a Small Urban Stream in Warsaw. Sustainability 2022, 14, 16017. https://doi.org/10.3390/su142316017
Krajewski A, Hejduk A, Hejduk L. First Evidence of Microplastic Presence in Bed Load Sediments of a Small Urban Stream in Warsaw. Sustainability. 2022; 14(23):16017. https://doi.org/10.3390/su142316017
Chicago/Turabian StyleKrajewski, Adam, Agnieszka Hejduk, and Leszek Hejduk. 2022. "First Evidence of Microplastic Presence in Bed Load Sediments of a Small Urban Stream in Warsaw" Sustainability 14, no. 23: 16017. https://doi.org/10.3390/su142316017
APA StyleKrajewski, A., Hejduk, A., & Hejduk, L. (2022). First Evidence of Microplastic Presence in Bed Load Sediments of a Small Urban Stream in Warsaw. Sustainability, 14(23), 16017. https://doi.org/10.3390/su142316017







