Abstract
The large discharges of wastewater in different natural receiving environments, particularly the marine environment, have massively contributed to pollution. This study examined the physicochemical and microbiological quality of wastewater discharged along the Mediterranean Sea. This investigation is one of the few works carried out by scientists along with the Mediterranean countries. Wastewater sampling was carried out in Egypt, Morocco, Algeria, Tunisia, and Italy. Analyses confirmed that the quality of treated wastewater was always better than the ones registered as raw wastewater. In addition, the quality of the treated wastewater and seawater appeared to be adequate and satisfied the standard and recommended limits in vigor in all these Mediterranean countries. These results showed that the wastewater treatment process used in Italy allowed a higher reduction in most tested parameters (COD: 80.44%, BOD5: 58.9%, OM: 70.74%, TOC: 78.4%). The bacteriological quality of these waters and their diversification was assessed by the denaturing gradient gel electrophoresis (DGGE) technique. Firmicutes, Gamma-Proteobacteria, and Beta-Proteobacteria in 42% of the DGGE DNA bands, and predominately some lineages affiliated to cultivable and non-cultivable clones of Bacteroides (58%), were found. The examination of antibiotic susceptibility at the level of 18 strains isolated from various arbitrary water samples showed that most of these isolates exhibited resistance to at least one antibiotic family, and 11 isolates exhibited 100% resistance against aztreonam. This result confirms the large antibiotic-resistance spreading and circulation registered these last decades in the natural environment, conditioned by the extensive and non-controlled use of antibiotics for human therapy and animal feeding.
1. Introduction
The development of Mediterranean countries has required implementing a policy of environmental protection associated with an improvement in scientific and technical knowledge of treatments and recycling, and the recovery of solid and liquid residues in agriculture and/or their discharge into the receiving environments. This development is focused on prioritizing actions of applied research-oriented policies by the countries of the Mediterranean rim in this field. Most of these countries, such as Tunisia, are characterized by the aridity of their climate and the irregularity of the regime of precipitations, which makes water resources rare and poorly distributed in time and space.
As is well known, water as a support for life is one of the essential substances on earth, representing a key link in the food chain. Unfortunately, however, many industrial, domestic, and agricultural activities can be a source of pollution to this resource [1,2]. Wastewater treatment in rural and urban areas is a vital requirement, but often this treatment is problematic. This wastewater needs to be treated as it is regularly discharged into the natural environment, especially the sea, rivers, and lakes. Wastewater contains a wide variety of microorganisms that cause serious diseases to humans, animals, and vegetation; although most are harmless, and can be useful and used as an essential tool for the bioremediation of recalcitrant toxic compounds and inactivation of water pathogens [3,4,5]. According to the World Health Organization, waterborne diseases such as cholera, typhoid, and hepatitis cause approximately two million deaths per year in some rural areas of third world countries, most of which are in children under five [6]. Thus, adequate biological (secondary treatment processes) and physicochemical treatments should be considered to improve the sanitary quality of wastewater [7,8].
Thus, given the increasing water needs in various countries’ economic sectors, mainly agriculture-treated wastewater, it is an important potential water resource to be valorized. Some major problems and constraints hinder the reuse of this water in agriculture, such as the mastery of adequate management and protection techniques for this non-conventional resource, and the rationalization of its use according to its quality. The evaluation of the short-, medium-, and long-term impacts on the natural environment, in particular the marine environment, the socio-economic environment, and health, is essentially an important component to be studied with great attention. The eutrophication of aquatic environments (coastal areas, rivers, lakes, groundwater) resulting from an increase in load of organic matter and phosphate and nitrate contents is the most striking example [9]. Thus, the input of treated or untreated wastewater into the main receiving environment, i.e., the ocean, is still confronted with a lack of fundamental and applied knowledge about its evolution and its impact on the coastal and general marine system.
In Mediterranean countries, the percentage of the population connected to municipal sewerage systems is high. While many villages are still not equipped with sewage treatment plants, the cost of installation increases significantly as population density decreases [10]. From there, much of the pre-treated or treated wastewater is discharged into the marine and coastal environments. However, these coastal marine environments are subject to perpetual physical, chemical, and bacteriological changes [11,12,13,14].
The study of whole microbial communities has so far been studied using culture-dependent approaches, limited by cultivability and contamination by heterotrophic bacteria. The development of advanced molecular techniques such as DGGE is a requirement for the detection of bacteria in the VBNC state that cannot grow on standard culture media [15]. These bacteria, in response to various stresses, may decrease in cell size [16]. The solution to these problems is the use of metagenomic approaches based on DNA and rRNA gene sequence analysis. Thus, wastewater samples have been screened to recover particular pathogenic bacterial strains, mainly characterized via their genealogic affiliation, denaturing gradient gel electrophoresis (DGGE), a typically used molecular method for rapid fingerprint analysis of microbial community structure, phenotype/genotype of antimicrobial resistance, integrons, plasmid types, and molecular typing (multi-locus sequence typing; MLST). [17]. Recent microbiological studies focus on applying molecular methods for the characterization of the diversity of different microbial communities in natural receiving environments, specifically the marine environment.
Similarly, in recent decades, the widespread overuse and misuse of antibiotics in human medicine and other sectors have had unintended consequences. Antibiotics of pharmaceutical origin are now found in large quantities in wastewater and fresh and marine water bodies [18]. With the widespread and massive distribution of large quantities of antibiotics, the likelihood of horizontal transfer of resistance genes between pathogenic and non-pathogenic bacteria is a major problem. A growing awareness of the dangers posed by a post-antibiotic era has led to an increase in work documenting antibiotic resistance in the environment [17,19,20,21].
It is within this broad and specific framework of monitoring characteristics that this collaborative work between research teams, from five countries around the Mediterranean, is based. The main objectives of this study are: (i) the assessment of physicochemical pollution parameters and some mineral element contents in raw and treated wastewater, and in coastal seawater collected from five Mediterranean countries; Tunisia, Algeria, Morocco, Egypt, and Italy; and (ii) an evaluation of wastewater treatment procedures concerning the prevailing total bacterial communities and their density and diversity in the wastewater treatment systems of these different countries; and lastly (iii) the determination of some antibacterial susceptibility profiles at some isolates, and the frequency of some antibiotic resistance characteristics.
2. Materials and Methods
2.1. Sampling Sites
A sampling of raw and treated wastewater and seawater was carried out in five Mediterranean countries, namely Tunisia, Algeria, Morocco, Egypt, and Italy, during February 2017 (Figure 1). One liter of water was collected aseptically from each site at a depth of one meter and in a sterilized glass bottle. All collected samples were immediately transported to the laboratory in a cooler at 4–6 °C and kept in the dark at 4 °C until analysis.
Figure 1.
Location of water sampling areas. Tunisia (IT: 35°28′28″ N; 11°02′27″ E/OT: 35°28′49″ N; 11°03′32″ E/SWT: 35°28′20″ N; 11°03′14″ E); Algeria (IA: 31°11′59″ N; 29°57′53″ E/OA: 31°09′05″ N; 29°50′31″ E); Morocco (IM: 33°34′40″ N; 5°08′36.7″ W/OM: 33°32′39″ N; 5°10′37″ W); Egypt (IE: 31°12′ 08.4″ N; 29°55.9′57″ E/OE: 31°11′59.4″ N; 29°57′53.8″ E/SWE: 31°09′05.9″ N; 29°50′31.2″ E); Italy (II: 38°06′50″ N; 14°51′46″ E/OI: 38°06′52″ N; 14°51′42″ E/SWI: 38°06′56″ N; 14°51′38″ E).
2.2. Physicochemical Analysis
Physicochemical analyses were carried out on raw, treated wastewater, and seawater samples. These analyses included pH, electrical conductivity (EC), and turbidity (Tub), and were conducted according to the USA standard methods by using a conductometer WTW 315i, a pH-meter WTW, and an AQUALITIC turbidity-meter. Chemical oxygen demand (COD), biological oxygen demand (BOD5), organic matter (OM), total organic carbon (TOC), suspended solid (SS), nitrate (NO3−), and absorbable organic halide (AOX) were analyzed using a portable UV analyzer (Pastel UV, Secomam, Alès, France). Zinc, copper, and iron concentrations were also examined by an atomic absorption spectrophotometer (Varian Atomic Absorption AA Spectrometer 200 series (AA240Z) Varian spectrometers, Markham, ON, Canada).
2.3. UPLC-MS/MS Analysis
Chemical analysis regarding the detection of some dominant antibiotics in the wastewater was achieved by using a validated UPLC-MS/MS. This analysis was carried out as described by Tahrani et al. [22] using a Waters Acquity UPLC® system interfaced with a Xevo TQ-S tandem quadrupole mass spectrometer.
2.4. Total DNA Extraction
The genomic DNA of each wastewater sample was extracted using a bead-beating process, as described by Lemarchand et al. [23]. For seawater samples, one liter of each sample was consecutively filtered through 0.45 and 0.22 µm sterile cellulose nitrate membrane filters. Then, the filters were cut into pieces and transferred to 15 mL sterile falcon tubes. Cell lysis was performed in the presence of 1.8 mL of lysis buffer and 90 μL of lysozyme by incubating the mix at 37 °C for 30 min. Next, 210 µL of 10% SDS and 50 μL proteinase K were added, mixed, and incubated for 2 h at 55 °C in a shaking incubator. The lysis product was then mixed with phenol/chloroform/isoamyl alcohol (25:24:1). After centrifugation, the aqueous phase was transferred to a new tube, mixed with chloroform/isoamyl alcohol (24:1) and centrifuged (13,000 g/5 min). The supernatant containing the DNA was precipitated with isopropanol at −20 °C overnight. Finally, the DNA was isolated by centrifugation, washed with ethanol and dissolved in TAE buffer. Obtained DNA was analyzed by electrophoresis in agarose gels to check its purity and molecular size.
2.5. PCR Amplification and DGGE Analysis
A denaturing gradient gel electrophoresis analysis of the bacterial community in water samples (treated, untreated wastewater, and seawater) was conducted. PCR amplification targeting the 16S rDNA gene was performed by using universal primers unique to the bacterium domain: 907R (3′-CCGTCAATTCCTTTGATGTTT-5′) and 357F (3′-TACGGGAGGCAGCAG-5′ with a 5′-end GC-clamp) targeting a portion of the 16S rRNA gene that includes the hypervariable V3–V5 regions [24,25]. PCR reactions were performed in a 25 µL final volume containing 1x PCR buffer, 2.5 mM MgCl2, 0.12 mM dNTPs, and 0.3 mM of each primer and 1 U Taq DNA polymerase, applying the following thermic protocol: 94 °C for 4 min, followed by 10 cycles of 94 °C for 30 s, 61 °C for 1 min, and 72 °C for 1 min; followed by a further 20 cycles of 94 °C for 30 s, 56 °C for 1 min, and 72 °C for 1 min; and a final extension at 72 °C for 10 min. The presence and length of PCR products were confirmed by electrophoresis in 1% w/v agarose gel before DGGE analysis.
The PCR products were run on a 7% (w/v) polyacrylamide gel in 1x TAE pH 7.4 with a denaturing gradient of 40 to 60% [24,25]. Electrophoresis was carried out at 60 °C for 18 h at a constant voltage of 90 V. After electrophoresis, the polyacrylamide gel was stained for 30 min in ethidium bromide solution, then washed with distilled water and photographed on a UV trans-illumination table. Dominant DGGE bands were excised and eluted overnight at 37 °C with weak shaking in 80 µL of sterilized distilled water. The eluted DNA fragments were reamplified by PCR using the same universal primer without the GC-clamp as described in the previous paragraph, and then sequenced and deposited in GenBank. The DGGE band profiles were examined using Image J software (version 1.46, NIH, Bethesda, MD, USA) and XLSTAT (version 2021.2, ADDINSOFT INC, New York, NY, USA), which allows the density and migration of the bands to be converted into numerical values known as principal components. DGGE profiles were also utilized to generate matrices indicating the presence or absence (scored as 1 or 0, respectively) of specific bands, and a dendrogram was created using MVSP software (Multi Variate Statistical Package, version 3.21, KCS, Wales, UK) by applying the UPGMA algorithm (unweighted pair group method with arithmetic mean). In order to express the diversity of the bacterial community, the Shannon diversity index (H) was calculated, as described by Sun et al. [26], using the DGGE profiles.
2.6. Bacteria Isolation and Antimicrobial Susceptibility Test
One hundred milliliters of each water sample were filtered through cellulose nitrate membranes (0.22 µm pore size), which were placed onto different culture medium plates (SS-agar for Salmonella strains; MacConkey-agar for E. coli strains; TCBS-agar for Vibrio strains; Slanetz and Bartley-agar for Enterococcus bacteria; TTC Tergitol-agar for Coliform bacteria) and incubated for 48 h at 37 °C. All analyses were made in duplicate. Different bacterial strains were selected and purified by repeated streaking on the same medium based on morphological characteristics of the colony. Antimicrobial susceptibility of all isolates was performed using the disc diffusion method, as recommended by the Clinical and Laboratory Standards Institute [27]. Twelve antibacterial agents were tested: Amikacin (AK/30 µg), Cefotaxime (CTX/30 µg), Aztreonam (ATM/30 µg), Imipenem (IMI/10 µg), Tetracycline (T/30 µg), Azithromycin (ATH/15 µg), Ciprofloxacin (CIP/5 µg), Trimethoprim (TM/5 µg), Rifampicin (RP/5 µg), Amoxicillin (A/25 µg), Chloramphenicol (C/10 µg), and Ceftazidime (CAZ/30 µg).
2.7. Detection of Antimicrobial Resistance Genes
Six antimicrobial genes conferring resistance to tetracycline (tetA, tetB), sulfonamide (sul1), and β-lactamase (blaTEM, blaSHV, blaCTX-M) were investigated by specific PCR using the primers and conditions described by Dhaouadi et al. [28].
2.8. Statistical Analysis
All results are the average of three determinations. An analysis of variance was carried out using the SPSS 10.05 statistical program (SPSS for Windows; SPSS, Inc., Chicago, IL, USA), and means were separated by the least significant difference according to the Student–Newman–Keuls test (at p ≤ 0.05).
3. Results and Discussion
3.1. Water Quality Monitoring
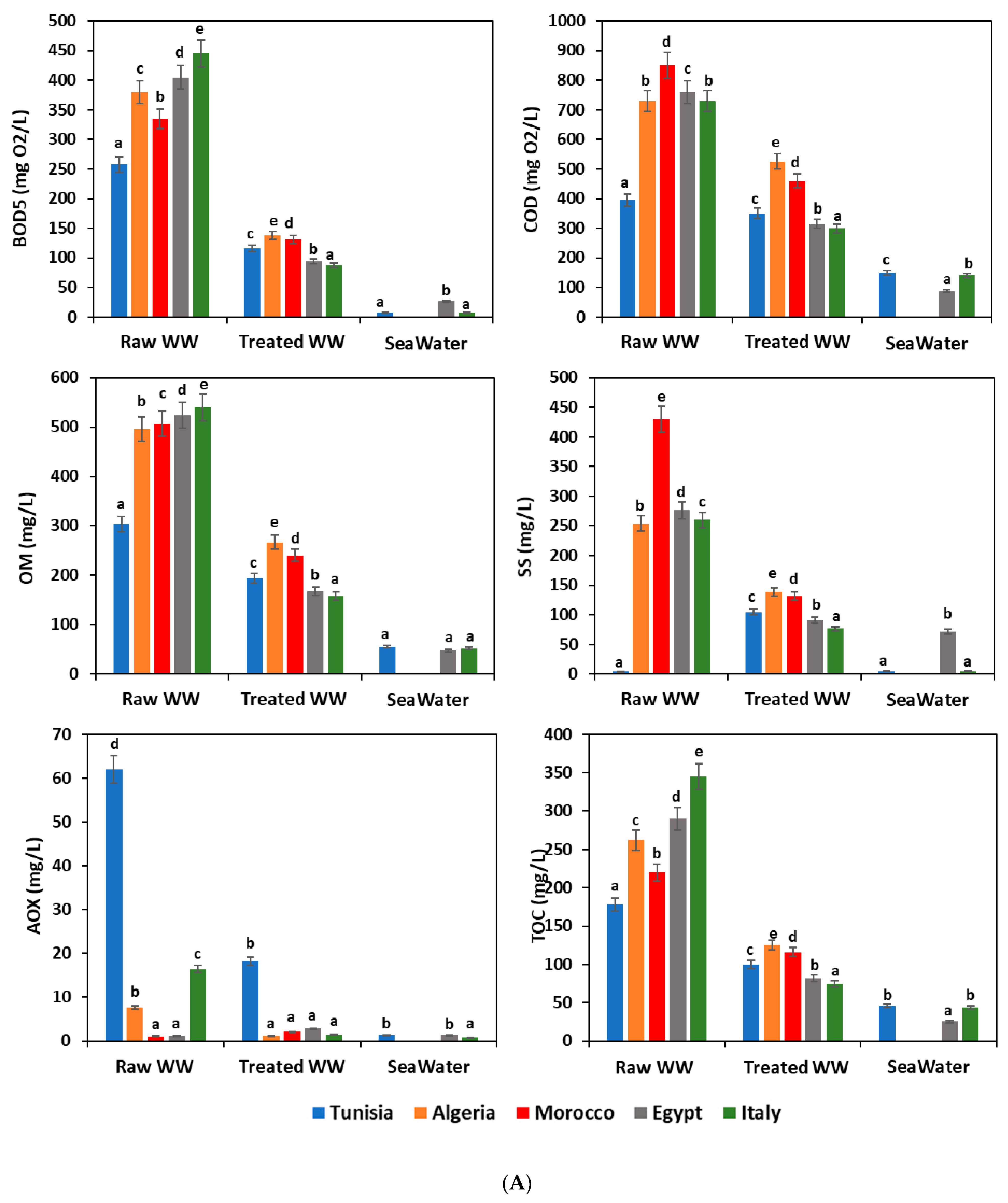
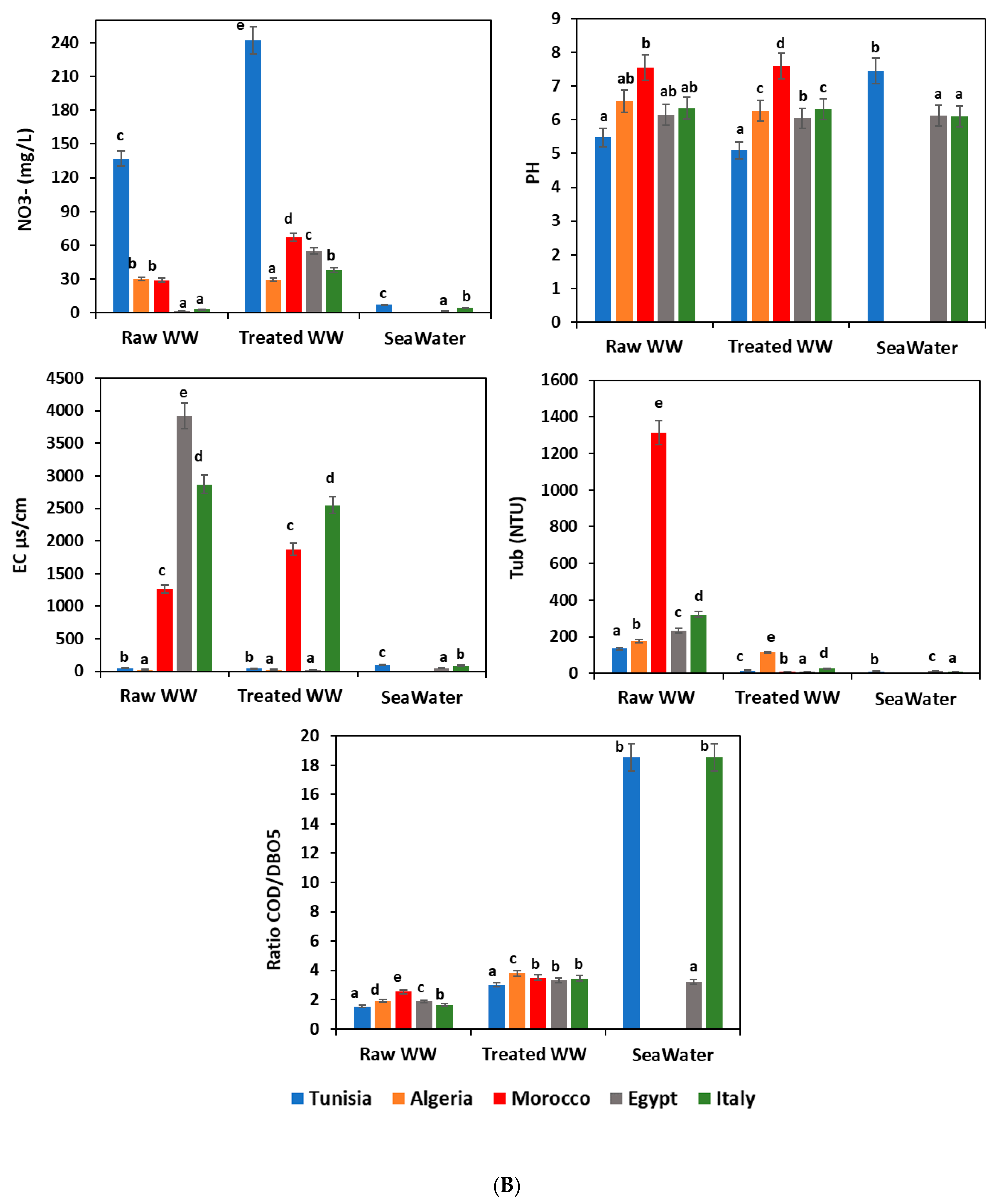
Figure 2A,B show a complete overview of the analyzed waste- and seawater quality parameters at each sampling site. According to the water recommendation limits in the considered Mediterranean countries [29,30,31,32,33], the highest values were noticed for the most tested parameters for the untreated wastewater; however, these values were below the permissible levels for BOD5, COD, OM, SS, AOX, TOC, and Tub in the WWTP effluents. Comparison with other sampling sites, as well wastewater from the raw WWTP, showed the highest values for the most quality parameters because of the high organic loads initially registered and usually received in the treatment plants. Seawater usually showed lower pollutant content than those registered for raw and treated wastewater. This finding is explained by the phenomenon of dilution of the pollutants carried by wastewater in the sea.
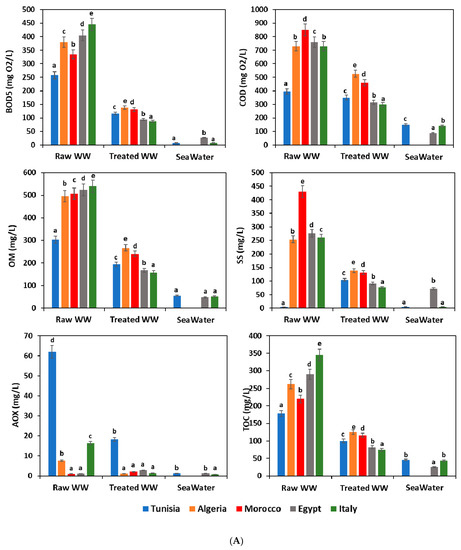
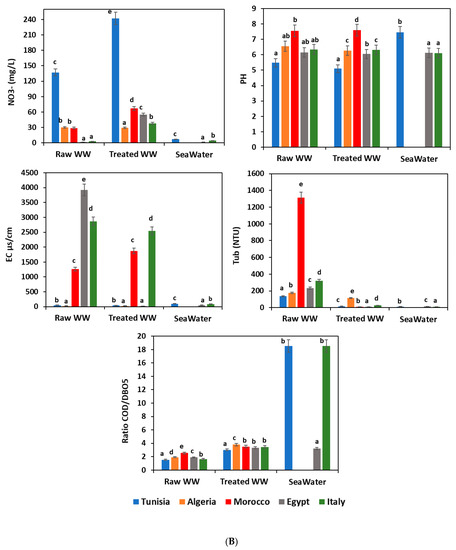
Figure 2.
(A). Physicochemical characteristics of different water samples. WW: wastewater; BOD5: biochemical oxygen demand; COD: chemical oxygen demand; OM: organic matter; SS: suspended solid; AOX: absorbable organic halide; TOC: total organic carbon; means (n = 3) followed by the same lowercase letter within columns are not significantly different according to the Student–Newman–Keuls test (p ≤ 0.05). (B). Physicochemical characteristics of different water samples. WW: wastewater; NO3−: nitrate; PH: potential hydrogen; EC: electrical conductivity; Tub: turbidity; BOD5: biochemical oxygen demand; COD: chemical oxygen demand; means (n = 3) followed by the same lowercase letter within columns are not significantly different according to the Student–Newman–Keuls test (p ≤ 0.05).
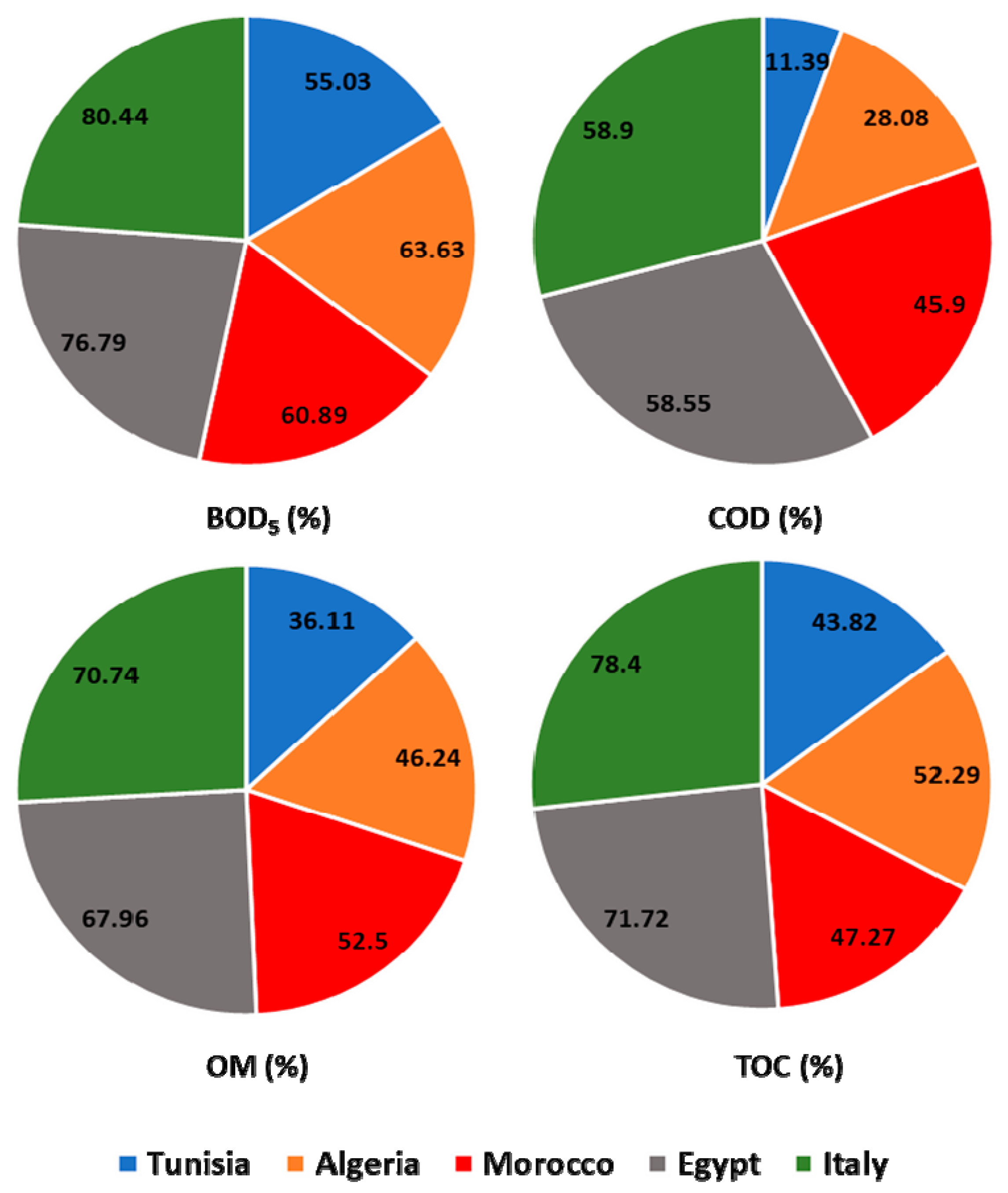
Similar studies reported high rates of these quality parameters in raw wastewater influent at WW plants from Tunisia and Algeria, with values up to 1720, 1450, and 747 mg/L for COD, TSS, and BOD5, respectively [34,35,36]. In the other hand, other research showed that the average effluent content of several quality parameters like COD, BOD5, TSS, and NO3− showed an increase as compared to treated wastewater samples [37,38]. This result was linked to the performance and outcome of the wastewater treatment process used. WWTP of Italy presented a higher reduction (p ≤ 0.05) in most tested parameters between the raw and treated wastewater (Figure 2A,B). Therefore, the process of wastewater treatment used in Italy allowed satisfactory removal of COD, BOD5, OM, and TOC; with average efficiencies of 80.44%, 58.9%, 70.74%, and 78.4%, respectively (Figure 3). The treatment processes used in Egyptian plants came afterwards with efficiency in the order of 76.79%, 58.55%, 67.96%, and 71.72% for COD, BOD5, OM, and TOC, respectively. On the other hand, the Tunisian wastewater plants were revealed to be the least efficient treatment system (Figure 2A,B and Figure 3). The effectiveness of pollutant removal can be related to several biological and physical processes that might occur between wastewater and other components governed and ruled by pollutants, plants, and microbial communities [39].
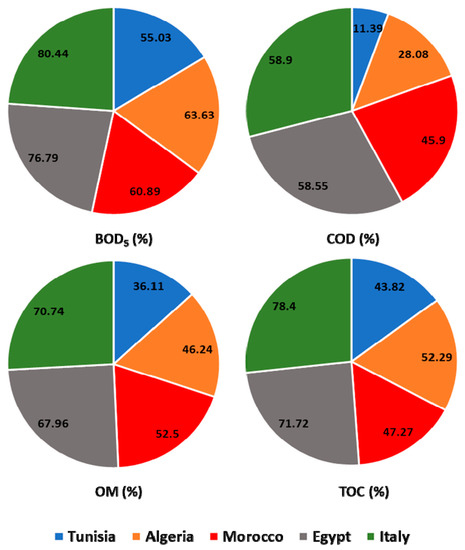
Figure 3.
Performance of the different wastewater treatment systems.
pH is an important factor that determines the suitability of treated wastewater for various purposes, including toxicity to animals and plants. In the present study, the pH remained near neutrality and the average values in all water samples ranged from 5.1 ± 0.05 to 8.06 ± 0.2 (Figure 2B).
Concerning the seawater samples, our results showed that treated wastewater discharged directly into the sea did not affect water quality. The average values for examined parameters were within the acceptable limits. Results showed that wastewater in all Mediterranean countries was properly treated (Figure 2A,B). Other similar studies reported that wastewater discharges in the sea degrade the water quality [40,41,42]. High-level loads of physicochemical pollutants could cause multiple harmful effects on humans and other living organisms, and lead to an important change in the main structural and functional characteristics of marine ecosystems [41].
Organic matter removal at the wastewater treatment plants has fluctuated between 36.11% and 70.74%. To estimate the potential biodegradability of organic matter, the COD/BOD5 ratio was calculated for all water samples (influent and effluent wastewater plants, and seawater), and the results showed a significant difference (p ≤ 0.05) between the different countries (Figure 2B). Furthermore, Afsa et al. [37] observed similar results in the COD/BOD5 ratio in wastewater ranging between 1.7 ± 0.5, 3.0 ± 0.3, and 2.2 ± 0.2 for WWTP influents, WWTP effluents, and seawater, respectively. Several studies suggest that a biodegradation process could be expected in this case [35,37,43].
During the various treatment steps, wastewater becomes depleted by reducing organic materials and enriching in oxygen, providing oxygen for other elements such as carbon, nitrogen, and sulfur. Thus, there would be an indirect enrichment of the water in oxidized materials such as CO2, SO2, and SO4 and NO, NO2, and NO3. These phenomena of oxidation explain the increase of NO3- content in all treated wastewater samples (29.2 ± 2, 38 ± 5, 55 ± 3, 67 ± 2, and 242 ± 3 mg/L in Algeria, Italy, Egypt, Morocco, and Tunisia, respectively). However, the Tunisian treated wastewater, exceeding the limits in vigor, was revealed to be below the standards recommended in Mediterranean countries (Figure 2A,B). Numerous studies found nitrates are very toxic to human and aquatic organisms [44,45,46,47]. However, the average values were 7 ± 0.5, 5 ± 1, 4 ± 1, 0.25 ± 0.01, and 4.6 ± 0.3 mg/L for seawater in Tunisia, Algeria, Morocco, Egypt and Italy, respectively. All of these values were within the recommended standard.
Regarding absorbable organic halide (AOX), their contents have been revealed to be higher in all influent wastewater samples as compared to those registered in effluents and seawater samples (Figure 2A). There was a significant difference (p ≤ 0.05) between the raw wastewater samples of different countries studied, with average AOX contents of 62 ± 3, 7.6 ± 3.606, 0.08 ± 0.021, 0.23 ± 0.006, and 16.40 ± 5 mg/L in Algeria, Morocco, Egypt, and Italy, respectively. However, there were no significant differences (p > 0.05) in the treated wastewater discharged in the seawater of Algeria, Morocco, Egypt, and Italy with values of 0.21 ± 0.01, 2.1 ± 2, 2.8 ± 1.5, and 0.43 ± 0.006 mg/L, respectively. Only the Tunisian treated wastewater showed a higher average AOX content of 18.20 ± 3 mg/L. The high AOX content in effluents has been highlighted, and this high AOX content was linked to raw wastewater sources as the paper mill industry, the oil refining and petrochemical industries, leather manufacturing, textile factories, plastics factories, pesticides factories, and pharmaceutical industries [48].
3.2. Metal Analysis
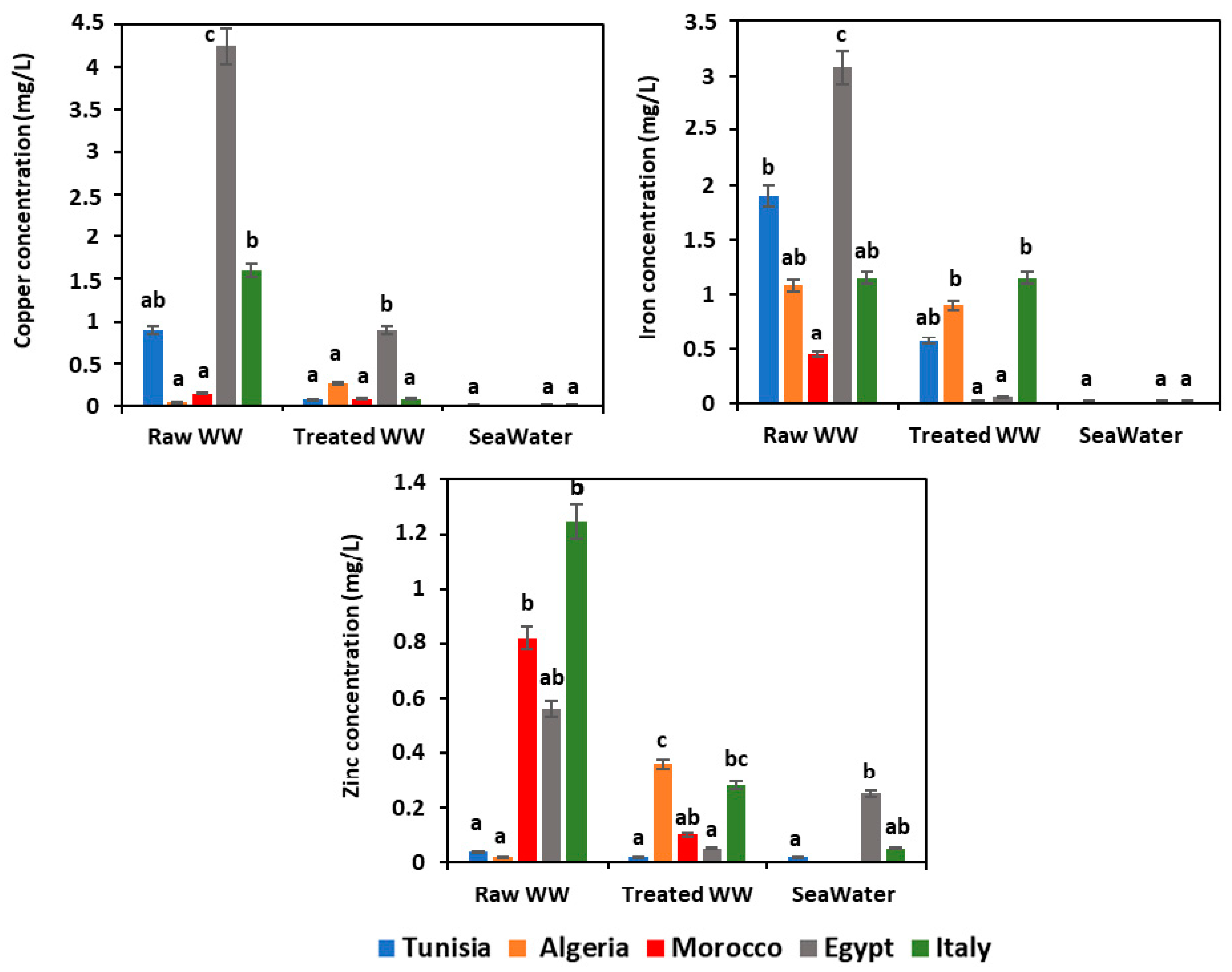
Some mineral elements are considered to be potentially toxic to humans if present in high concentrations and exceed the regulations in vigor [49,50,51]. Fortunately, low metal contents were recorded for the following metals that did not exceed the norms in vigor of each Mediterranean country [29,30,31,32,33] (Figure 4). Nevertheless, Egyptian raw wastewater has presented a slightly higher level of copper of 4.24 ± 0.50 mg/L and iron of 3.08 ± 0.5 mg/L. Furthermore, the metal contents recorded in seawater samples of the different Mediterranean countries showed no significant difference (p > 0.05) and almost appeared to be at the same level.
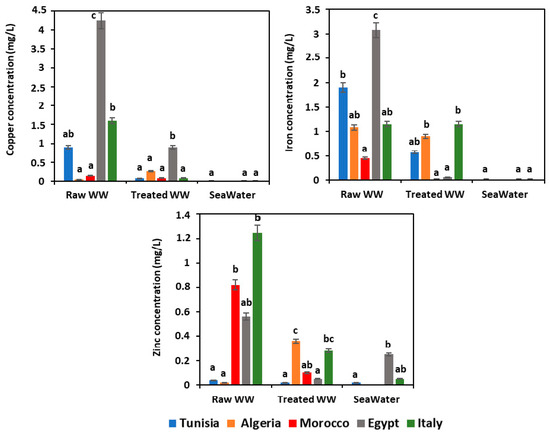
Figure 4.
Heavy metal analysis. WW: wastewater; means (n = 3) followed by the same lowercase letter within columns are not significantly different according to the Student–Newman–Keuls test (p ≤ 0.05).
On the other hand, chromatographic analyses revealed the entire absence of antibiotics, and the microbiological community via molecular investigation showed various bacterial resistances to a wide range of antibiotics. This result indicated that antibiotics are unstable and labile compounds in water and will be transformed by pursuing arbitrary bio-physicochemical interactions in waters. Moreover, the permanent and massive release of antibiotics in wastewater during this last decade has caused and led to the dissemination of multiple microbial antibiotic resistances in the natural environment. This microbial antibiotic resistance gained by bacteria could be exploited as a potential and efficient agent for antimicrobial bioremediation of a polluted environment, because these bacteria could use the antibiotics as carbon and energy sources [52].
3.3. Bacterial Community Structure
To have an overall view of the bacterial community prevailing in the different water samples examined in raw and treated wastewater and seawater sampled from various Mediterranean countries, DGGE fingerprinting analysis targeting the V3–V5 hypervariable region of the 16S rRNA gene was investigated. DGGE profile analysis revealed a large variability in bacterial community structure (Figure 5).
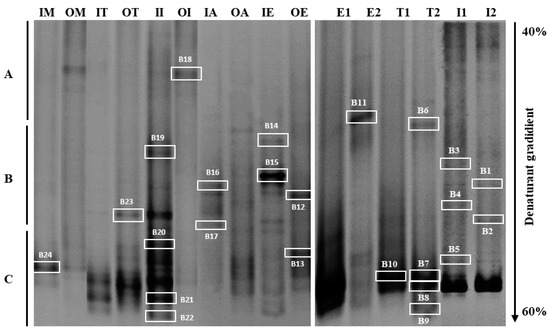
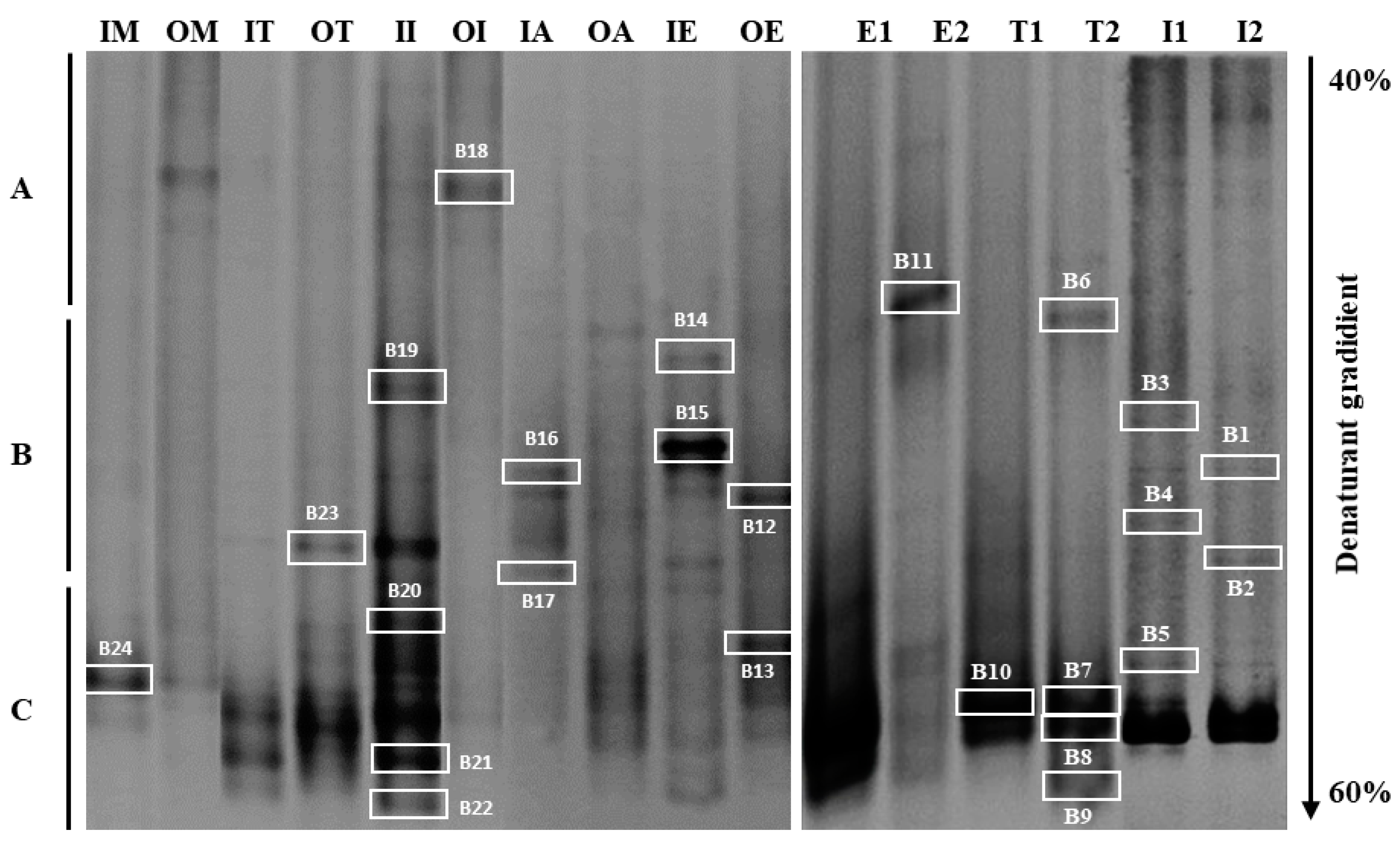
Figure 5.
DGGE patterns show the bacterial community structure of wastewater samples (raw and treated WW) and seawater samples based on variability of the V3–V5 region of 16S rRNA. The gradient of the urea and formamide ranged from 40% to 60%. Three types of DNA bands were defined with correlation to the running level, as short (A), medium (B) and long migration bands (C). Marked bands were excised and sequenced. IM (raw WW Morocco); OM (treated WW Morocco); IT (raw WW Tunisia); OT (treated WW Tunisia); II (raw WW Italy); OI (treated WW Italy); IA (raw WW Algeria); OA (treated WW Algeria); IE (raw WW Egypt); OE (treated WW Egypt); E1 (Egypt filtered 0.45 µm); E2 (Egypt filtered 0.22 µm); T1 (Tunisia filtered 0.45 µm); T2 (Tunisia filtered 0.22 µm); I1 (Italy filtered 0.45 µm); I2 (Italy filtered 0.22 µm).
Electrophoresis gel examination showed several DNA bands with variable intensities and migration distances. Some bands were specific to a site of sampling, whereas other bands were common over one sample. Visual analysis of DGGE profiles revealed a variation in migration, allowing them to be classified into three levels of migration: short migration bands, medium migration bands, and long migration bands (Figure 5). DNA bands characterized by a medium migration and corresponding to high GC content bacteria seemed predominant in all wastewaters.
The profiles of the treated wastewater in Italy (OI) and of Morocco (OM) characterized by DNA bands of low migration (low GC content) differed from the other water samples examined. However, the profiles showed similar intense DNA bands of medium migration for the different samples. Likewise, Khouja et al. [35], during a DGGE study in wastewater treatment plants, showed clear variations in bacterial abundance and microbial diversity species between treatment steps. This last study underlined the importance of this technique in a specific microbiological investigation. However, this technique allows the revelation only of 1 to 2% of the microbial communities existing in the natural environment, which presents the dominant species [15].
Results showed considerable differences in DGGE communities between different water samples, particularly before and after treatment. More DNA bands were detected in raw wastewater than those observed in treated wastewater (Figure 5). A relatively complex bacterial community in the raw wastewater of Italy (II) and Egypt (IE) was noted, reflecting a higher biodiversity (II with 8 bands; IE with 9 bands). Many DNA bands disappeared from non-treated to treated wastewater, revealing and affirming the performance of Italy and Egypt’s treatment plants.
For seawater samples, the DGGE profiles showed a relative difference between seawater samples filtered with 0.45 µm and those filtered with 0.22 µm (Figure 5). Two common bands with long migration were observed in all water samples of the Mediterranean Sea. This result agrees with a previous studies, which have shown that microbial community composition in the pre-filter fraction (0.45 µm) differs from the one of smaller size fraction (0.22 µm) [53,54]. Some bands of the 0.45 and 0.22 µm filterable seawater fractions (S1, S5, and S8) appeared to be common in the DGGE profiles of samples. However, bands S11, S9, S6, and S2 detected in seawater fraction patterns filtered with 0.22 µm were absent in 0.45 µm filtered samples. This shows that corresponding bacteria (S11, S9, S7, and S2) have a small size. These cells could be starvation forms, inducing cell reduction or simply small cells [16].
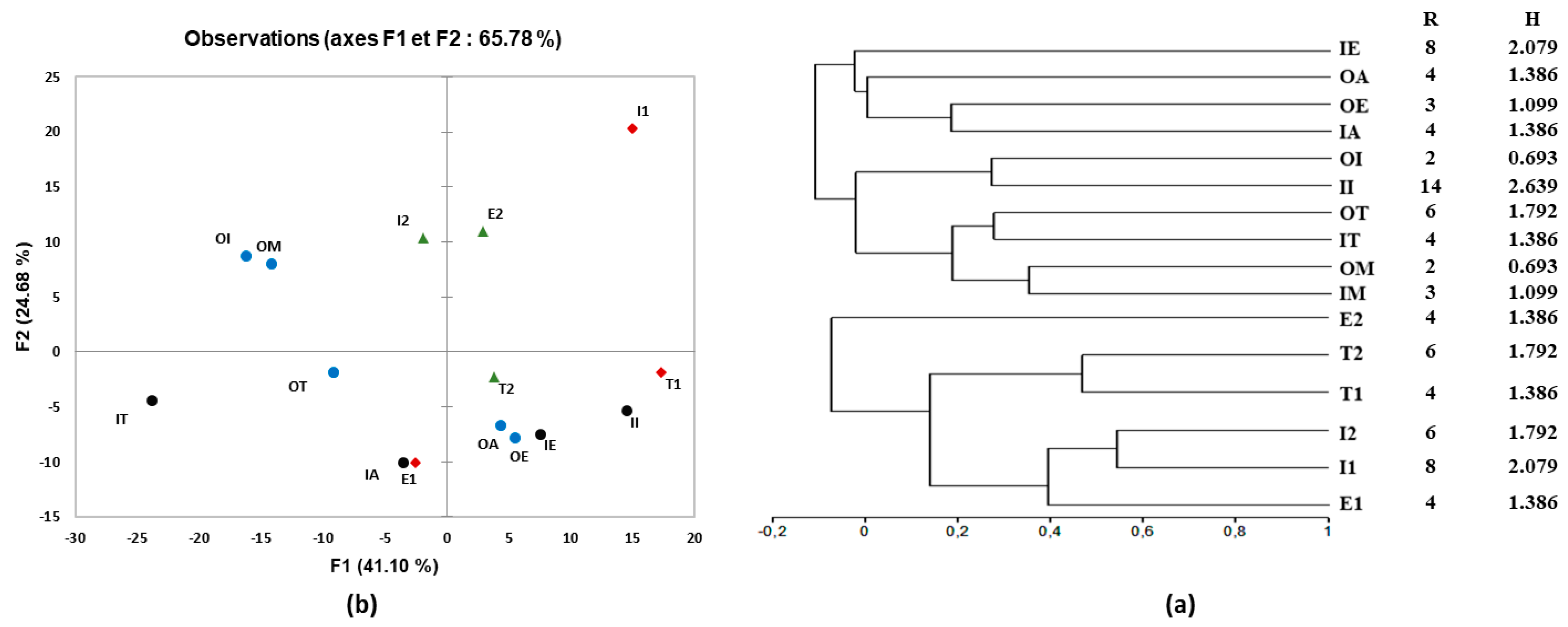
In addition, the Shannon index of diversity (H) was used to estimate microbial diversity in water samples in Mediterranean countries (Tunisia, Algeria, Morocco, Egypt, and Italy). The result showed that the raw wastewater in Italy had the most diversified bacterial group based on the diversity index (Figure 6a), but decreased in treated wastewater to 0.693, suggesting that bacterial diversity was affected during the treatment process. Thus, the variation in wastewater treatment technologies and plant processes significantly affected the effluent bacterial community (Figure 5 and Figure 6). Several studies mentioned that quantitative changes between bacteria in WWTPs were affected by wastewater characteristics conditioned and influenced by the operational and technological system, the climate, and the geographic location [55,56]. For seawater sampled from Morocco, microbial diversity was found to be more complex than that obtained in other countries (Figure 6a).
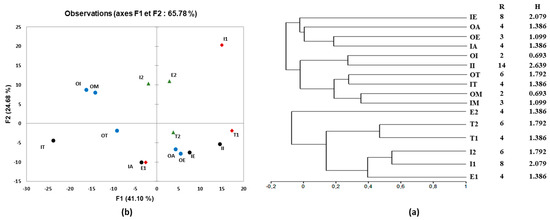
Figure 6.
(a) Clustering analysis of species richness (R) and Shannon diversity (H) and (b) principal component analysis (PCA) of DGGE patterns of bacterial communities in wastewater and seawater samples.
Furthermore, Figure 6a illustrates the similarities between DGGE profiles. The distribution of bacterial communities in different water samples collected from the five Mediterranean countries and the DGGE DNA-band profiles were used to construct UPGMA dendrograms according to Pearson’s correlation (Figure 6a). The bacterial community diversity registered in Morocco, Tunisia, and Italy’s raw and treated wastewater showed a similarity of 35%, 28%, and 27%, respectively (Figure 6a). In contrast, a low similarity was observed for the raw and treated wastewater of Algeria and Egypt.
In agreement with other work, our study confirmed the changes in bacterial communities from influent to effluent, since many DNA bands present in the influent appeared to be absent from the DGGE pattern in the effluent, with new DNA bands appearing [15,35,57].
Concerning seawater samples, the dendrogram showed that microbial communities presented around 53 to 46% of similarity between E1 (seawater pre-filtered with 0.45 µm) and I2 (seawater filtered with 0.22 µm), and between seawater T1 and T2 collected from Tunisia, respectively (Figure 6a). Principal component analysis (PCA) was also used to examine the relationships between the different water samples and the intensities of DGGE bands. Figure 6b confirmed the differences observed visually by displaying a variation of 41.10% and 24.68% according to the first and second principal axes. In general, we observed a significant difference between the microbial communities of different waters, as confirmed by the previous analysis in Figure 6.
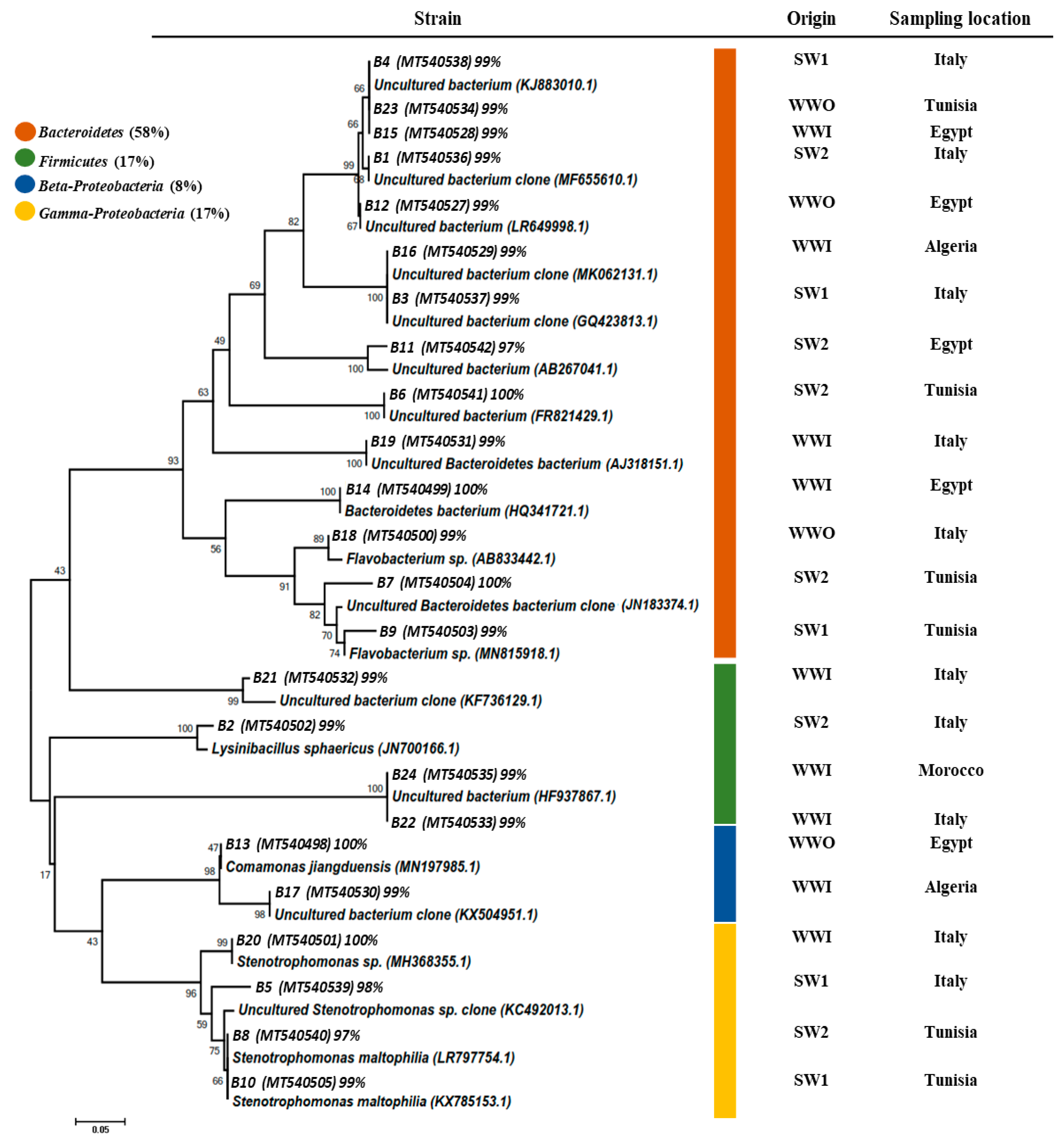
To assess this diversity, 24 DGGE DNA bands were excised from gels, sequenced, and analyzed. Figure 7 summarizes the affiliation of the retrieved sequences. DGGE results showed a high proportion of Bacteroidetes represented by 14 sequences and 58% of the bacterial 16S rRNA gene sequences found and recovered from all water samples. A significant part of the eluted DNA bands (67%) was found to be most similar to those of yet uncultured microorganisms, with the majority affiliated with the phylum Bactroidetes (11/14 sequences; 78%). DNA bands assigned to Proteobacteria and represented by the beta and gamma subclasses showed 25% (n = 6). Gamma-Proteobacteria (n = 4, 17%) were represented by Stenotrophomonas sp. (B20), Stenotrophomonas maltophilia (B7 and B10), and a single sequence affiliated to an Uncultured Stenotrophomonas sp. (B5). Beta-proteobacteria (n = 2, 5%) were represented by Comamonas jianduensis (B13) and an Uncultured bacterium (B17). The remaining four sequences were assigned to Firmicutes (n = 4, 17%) and related to Uncultured bacterium (B21, B22, and B24) and Lysinibacillus sphaericus (B2).
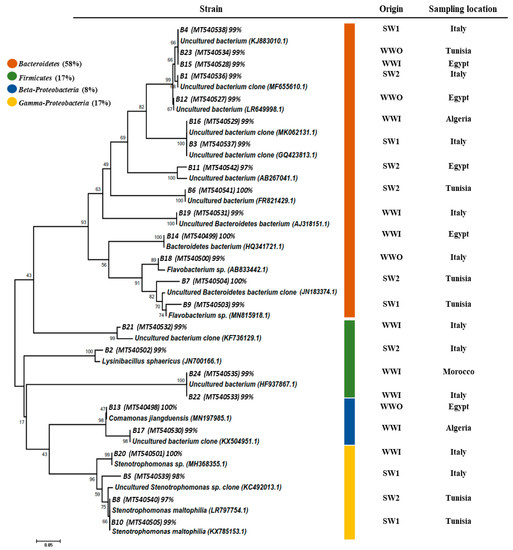
Figure 7.
Neighbor joining tree, which represents the phylogenetic relationship of the most abundant 16S rRNA sequences retrieved from wastewater (raw and treated WW) and seawater samples. The phylogenetic dendrogram was evaluated by performing a bootstrap analysis of 1000 data sets using the MEGA 6.0 program. 16S rRNA sequence accession numbers are in the brackets.
These results, confirmed by several other studies, underline the dominance of species belonging to the Bacteroides and Proteobacteria phylum in wastewater [55,57]. In marine waters, gram-positive and gram-negative bacteria may decrease their cell size when undergoing nutrient starvation. This result can be supported by the change and abrupt passing of the bacteria from a very rich environment like wastewater to a more or less depleted environment like seawater. According to several studies, Flavobacterium was detected in seawater that flowed through 0.45 µm filters [16,58]. DNA band B9 affiliated with Flavobacterium sp. was detected in our study in the seawater that flowed through the 0.45 µm filter. The results highlighted that Flavobacterium cells might have changed their fundamental metabolism and reduced their size to adapt to the stressful marine environment. Thus, under unfavorable conditions, the Bacteroides group may change their form and become morphologically smaller [59].
3.4. Antibiotic Resistance Profiles
To get multi-antibiotic-resistant bacteria in different sources of water, five growth mediums were used to isolate different genera of cultivable bacteria from wastewater collected in the five Mediterranean countries. According to their morphology, 18 strains were obtained from wastewater, including 9 strains from treated wastewater, 6 strains from raw wastewater, and 3 strains from seawater (Table 1). The susceptibility of the 18 isolates to 12 antimicrobial agents is shown in Table 1. All isolates were resistant to at least two antibiotic classes. They revealed an important frequency of resistance to the 12 antibiotics tested: Amikacin (4/18; 22%), Cefotaxime (8/18; 44%), Aztreonam (11/18; 61%), Imipenem (4/18; 22%), Tetracycline (5/18; 28%), Azithromycin (7/18; 39%), Ciprofloxacin (4/18; 22%), Trimethoprim (9/18; 50%), Rifampicin (6/18; 33%), Amoxicillin (4/18; 22%), Chloramphenicol (8/18; 44%), and Ceftazidime (10/18; 56%). A total of 50% of isolates (9/18) harbored one or more resistance genes (tetA, tetB, sul1, blaTEM, blaSHV, and blaCTX-M), which demonstrates their role as reservoirs of tetracycline-, sulfonamide-, and β-lactam-encoding genes. The phenotype of each isolate might be due to the acquisition of several genetic elements such as plasmids, transposons, and integrons, which are known as important vehicles of resistance genes [36,60].

Table 1.
Phenotypic and genotypic characteristics of bacterial strains isolated from different water samples.
Among the five tetracycline-resistant isolates, tetA and tetB genes were found in three (60%) and one (20%) isolate, respectively. The sul1 gene encoding sulfonamide resistance was detected in five isolates (55.6%) (Table 1). Five isolates carried β-lactamase-encoding genes. The blaCTX-M gene was found in three isolates and the blaTEM and blaSHV genes were separately detected in only one isolate. However, nine isolates were negative for all tested genes. Several studies have shown antibiotic-resistant bacteria in polluted or non-polluted environments. This genetic flexibility allowed them to survive in altered environments, because of their ability to reach and transfer resistance genes [52,61,62,63,64,65].
4. Conclusions
Abatement of pollutants in the wastewater to permissible concentrations required for natural environment protection is needed. Major results obtained in this study showed that the wastewater treatment plants of Italy and Egypt were highly efficient in pollutant load reduction, and overall, achieved a satisfactory performance especially for COD, BOD5, OM, and TOC removal. Furthermore, results showed a general low content of all tested metals below the standards in vigor in the different Mediterranean countries considered. Moreover, a high bacterial diversity assessed by a DGGE approach was registered in water collected from the five Mediterranean countries. The diversity of bacterial communities was ultimately influenced by changes in environmental conditions. Microbial diversity may be an important indicator of the overall performance of the treatment system. However, multi-antibiotic bacterial resistance is a current major public health problem highlighting the need for a permanent surveillance system, and concern about the spread of antibiotic resistance in natural ecosystems, especially in water.
Author Contributions
W.H., I.M. and H.B.M. conceived and designed the experiments; W.H. carried out the functional experiments, analyzed the data and wrote the manuscript; I.M. carried out the functional experiments and revised the manuscript; J.V.L. carried out the analytical experiments and analyzed the data; W.H., A.G.P., A.B., G.D.B., N.K., R.A., A.H., N.H.K. and H.B.M. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by Research Unit of Analysis and Process Applied to the Environmental (APAE), Higher Institute of Applied Sciences and Technology of Mahdia and by the Tunisian Ministry of Higher Education and Scientific Research. We would like to thank all the contributors from different Mediterranean countries who supported the fieldwork and the samples collection.
Conflicts of Interest
The authors declare that they have no conflict of interest regarding the publication of this article.
References
- Jebara, A.; Lo Turco, V.; Potortì, A.G.; Bartolomeo, G.; Ben Mansour, H.; Di Bella, G. Organic pollutants in marine samples from Tunisian coast: Occurrence and associated human health risks. Environ. Pollut. 2021, 271, 116266. [Google Scholar] [CrossRef] [PubMed]
- Jebara, A.; Albergamo, A.; Rando, R.; Potortì, A.G.; Lo Turco, V.; Ben Mansour, H.; Di Bella, G. Phthalates and non-phthalate plasticizers in Tunisian marine samples: Occurrence, spatial distribution and seasonal variation. Mar. Pollut. Bull. 2021, 163, 111967. [Google Scholar] [CrossRef] [PubMed]
- Cyprowski, M.; Szarapińska-Kwaszewska, J.; Dudkiewicz, B.; Krajewski, J.A.; Szadkowska-Stańczyk, I. Exposure assessment to harmful agents in workplaces in sewage plant workers. Med. Pract. 2005, 56, 213–222. [Google Scholar]
- Cyprowski, M.; Stobnicka-Kupiec, A.; Ławniczek-Wałczyk, A.; Bakal-Kijek, A.; Gołofit-Szymczak, M.; Górny, R.L. Anaerobic bacteria in wastewater treatment plant. Int. Arch. Occup. Environ. Health 2018, 91, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerardi, M.H.; Zimmerman, M.C. Wastewater Pathogens; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- WHO (World Health Organization). Water Supply and Sanitation Collaborative Council and Operation/Maintenance Network. Tools for Assessing the Operation and Maintenance Status of Water Supply and Sanitation in Developing Countries; Document WHO/SDE/WSH/00.3; WHO: Geneva, Switzerland, 2000.
- Cotruvo, J.; Dufour, A.; Rees, G.; Bartram, J.; Carr, R.; Cliver, D.O.; Craun, G.F.; Fayer, R.; Gannon, V.P.J. Waterborne Zoonoses: Identification, Causes, and Control; World Health Organization: Geneva, Switzerland, 2004.
- Koivunen, J.; Siitonen, A.; Heinonen-Tanski, H. Elimination of enteric bacteria in biological–chemical wastewater treatment and tertiary filtration units. Water Res. 2003, 37, 690–698. [Google Scholar] [CrossRef]
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Biostability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef] [Green Version]
- WHO (World Health Organization). A Regional Overview of Wasterwater Management and Reuse in the Eastern Mediterranean Region (No. WHO-EM/CEH/139/E); WHO: Geneva, Switzerland, 2005.
- Hacioglu, N.; Dulger, B. Monthly variation of some physico-chemical and microbiological parameters in Biga Stream (Biga, Canakkale, Turkey). Afr. J. Biotechnol. 2009, 8, 1929–1937. [Google Scholar]
- Jebara, A.; Lo Turco, V.; Faggio, C.; Licata, P.; Nava, V.; Potortì, A.G.; Crupi, R.; Ben Mansour, H.; Di Bella, G. Monitoring of Environmental Hg Occurrence in Tunisian Coastal Areas. Int. J. Environ. Res. Public Health 2021, 18, 5202. [Google Scholar] [CrossRef]
- Lamine, I.; Alla, A.A.; Bourouache, M.; Moukrim, A. Monitoring of Physicochemical and Microbiological Quality of Taghazout Seawater (Southwest of Morocco): Impact of the New Tourist Resort “Taghazout Bay”. J. Ecol. Eng. 2019, 20, 79–89. [Google Scholar] [CrossRef]
- Sreenivasulu, G.; Jayaraju, N.; Sundara Raja Reddy, B.C.; Lakshmi Prasad, T. Physico-chemical parameters of coastal water from Tupilipalem coast southeast coast of India. J. Coast. Sci. 2015, 2, 34–39. [Google Scholar]
- Mehri, I.; Turki, Y.; Chérif, H.; Khessairi, A.; Hassen, A.; Gtari, M. Influence of biological treatment and ultraviolet disinfection system on Pseudomonas spp. diversity in wastewater as assessed by denaturing gradient gel electrophoresis. CLEAN–Soil Air Water 2014, 42, 578–585. [Google Scholar] [CrossRef]
- Haller, C.M.; Rölleke, S.; Vybiral, D.; Witte, A.; Velimirov, B. Investigation of 0.2 μm filterable bacteria from the Western Mediterranean Sea using a molecular approach: Dominance of potential starvation forms. FEMS Microbiol. Ecol. 2000, 31, 153–161. [Google Scholar] [CrossRef]
- Hassen, B.; ABBASSI, M.S.; Benlabidi, S.; Ruiz-Ripa, L.; Mama, O.M.; Ibrahim, C.; Hassen, A.; Hammami, S.; Torres, T. Genetic characterization of ESBL-producing Escherichia coli and Klebsiella pneumoniae isolated from wastewater and river water in Tunisia: Predominance of CTX-M-15 and high genetic diversity. Environ. Sci. Pollut. Res. Int. 2020, 27, 44368–44377. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sghaier, S.; Abbassi, M.S.; Pascual, A.; Serrano, L.; Díaz-De-Alba, P.; Ben Said, M.; Hassen, B.; Ibrahim, C.; Hassen, A.; Lopez-Cerero, L. ESBL-producing Enterobacteriaceae from animal origins and wastewater in Tunisia: The first detection of O25b-B23-CTX-M-27-ST131 Escherichia coli and CTX-M-15-OXA-204-producing Citrobacter freundii from wastewater. J. Glob. Antimicrob. Resist. 2019, 17, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Hassen, B.; Abbassi, M.S.; Ruiz-Ripa, L.; Mama, O.M.; Ibrahim, C.; Benlabidi, S.; Hassen, A.; Torres, C.; Hammami, S. Detection and characterization of extended-spectrum β-lactamase-producing and colistin-resistant Enterobacteriaceae from the biological industrial wastewater treatment plant in Tunisia with detection of the colistin-resistance mcr-1 gene. FEMS Microbiol. Ecol. 2021, 97, fiaa231. [Google Scholar] [CrossRef]
- Tahrani, L.; Mehri, I.; Reyns, T.; Anthonissen, R.; Verschaeve, L.; Khalifa, A.B.H.; Joris, V.L.; Hassen, A.; Ben Mansour, H. UPLC-MS/MS analysis of antibiotics in pharmaceutical effluent in Tunisia: Ecotoxicological impact and multi-resistant bacteria dissemination. Arch. Microbiol. 2018, 200, 553–565. [Google Scholar] [CrossRef]
- Lemarchand, K.; Berthiaume, F.; Maynard, C.; Harel, J.; Payment, P.; Bayardelle, P.; Masson, L.; Brousseau, R. Optimization of microbial DNA extraction and purification from raw wastewater samples for downstream pathogen detection by microarrays. J. Microbiol. Methods 2005, 63, 115–126. [Google Scholar] [CrossRef]
- Hassen, W. Biodegradation of Pesticides Used in Agricultural Soils; European University Edition: Sarrebruck, Allemagne, 2020; ISBN 978-613-9-53921-5. (In French) [Google Scholar]
- Ammeri, R.W.; Hassen, W.; Hidri, Y.; Di Rauso Simeone, G.; Hassen, A. Macrophyte and indigenous bacterial co-remediation process for pentachlorophenol removal from wastewater. Int. J. Phytoremediat. 2021, 24, 271–282. [Google Scholar] [CrossRef]
- Sun, S.; Guo, Z.; Yang, R.; Sheng, Z.; Cao, P. Analysis of microbial diversity in tomato paste wastewater through PCR-DGGE. Biotechnol. Bioprocess Eng. 2013, 18, 111–118. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Information Supplement CLSI Document M100-S23; CLSI: Philadelphia, PA, USA, 2013. [Google Scholar]
- Dhaouadi, S.; Soufi, L.; Hamza, A.; Fedida, D.; Zied, C.; Awadhi, E.; Mtibaa, M.; Hassen, B.; Cherif, A.; Torres, C.; et al. Co-occurrence of mcr-1 mediated colistin resistance and β-lactamases encoding genes in Multidrug-resistant Escherichia coli from broiler chickens with colibacillosis in Tunisia. J. Glob. Antimicrob. Resist. 2020, 22, 538–545. [Google Scholar] [CrossRef] [PubMed]
- TUA. Legislative Decree. n.152 03/04/2001. Unified Environmental Code. Decreto Legislativo. Norme in Materia Ambientale—Testo Unico dell’Ambiente. 2016. Available online: www.gazzettaufficiale.it/eli/gu/2006/04/14/88/so/96/sg/pdf (accessed on 17 May 2018).
- Ministère del’Environnement du Maroc. Normes Marocaines, Bulletin Officiel du Maroc; N° 5062 du 30 ramadan, 1423; Ministère del’Environnement du Maroc: Rabat, Morocco, 2002.
- JORA (Journal Officiel de la République Algérienne). Décret Exécutif n° 06-141 du 20 Rabie El Aouel 1427 Correspondant au 19 Avril 2006, Section 1, Article 3; JORA: Algérie, 2006. [Google Scholar]
- NTPA-001; Normative Act Regarding the Determination of Limits of Loading with Pollutants of Industrial and Town Wastewaters at Evacuation in Natural Receptors. Egyptian Official Monitor: Alexandria, Egypt, 2005.
- NT106 002; Tunisian Standard NT106 002 Defines the Maximum Authorized Concentrations of the Various Pollutants in Water before Discharge into the Receiving Environment. Environmental Ministry: Tunis, Tunisia, 2018.
- Hamaidi-Chergui, F.; Errahmani, M.B.; Demiai, A.; Hamaidi, M.S. Monitoring of physico-chemical characteristics and performance evaluation of a wastewater treatment plant in Algeria. In Proceedings of the 3rd International Conference—Water Resources and Wetlands, Tulcea, Romania, 8–10 September 2014. [Google Scholar]
- Khouja, I.; Sullivansealey, K.; M’hiri, F.; Ouzari, H.I.; Saidi, N. Spatial–temporal variation of treatment performance and bacterial community diversity in a hybrid constructed wetland. Int. J. Environ. Sci. Technol. 2020, 17, 3217–3230. [Google Scholar] [CrossRef]
- Nasri, E.; Subirats, J.; Sànchez-Melsió, A.; Ben Mansour, H.; Borrego, C.M.; Balcázar, J.L. Abundance of carbapenemase genes (blaKPC, blaNDM and blaOXA-48) in wastewater effluents from Tunisian hospitals. Environ. Pollut. 2017, 229, 371–374. [Google Scholar] [CrossRef]
- Afsa, S.; Hamden, K.; Martin, P.A.L.; Ben Mansour, H. Occurrence of 40 pharmaceutically active compounds in hospital and urban wastewaters and their contribution to Mahdia coastal seawater contamination. Environ. Sci. Pollut. Res. 2020, 27, 1941–1955. [Google Scholar] [CrossRef]
- Hamaidi-Chergui, F.; Errahmani, M.B. Water quality and physicochemical parameters of outgoing waters in a pharmaceutical plant. Appl. Water Sci. 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A.; Antonopoulou, M.; Konstantinou, I.K. Removal of endocrine disrupting chemicals in HSF and VF pilot-scale constructed wetlands. Chem. Eng. J. 2016, 294, 146–156. [Google Scholar] [CrossRef]
- Houda, B.; Dorra, G.; Chafai, A.; Emna, A.; Khaled, M. Impact of a mixed “industrial and domestic” wastewater effluent on the southern coastal sediments of Sfax (Tunisia) in the Mediterranean Sea. Int. J. Environ. Res. 2011, 5, 691–704. [Google Scholar]
- Reopanichkul, P.; Carter, R.W.; Worachananant, S.; Crossland, C.J. Wastewater discharge degrades coastal waters and reef communities in southern Thailand. Mar. Environ. Res. 2010, 69, 287–296. [Google Scholar] [CrossRef]
- Roberts, R.L. Apparatus for Filtering Water or Wastewater. U.S. Patent No. 9517947, 13 December 2016. [Google Scholar]
- Idrissi, Y.A.; Alemad, A.; Aboubaker, S.; Daifi, H.; Elkharrim, K.; Belghyti, D. Caractérisation physico-chimique des eaux usées de la ville d’Azilal-Maroc-/[Physico-chemical characterization of wastewater from Azilal city-Morocco-]. Int. J. Innov. Appl. Stud. 2015, 11, 556–566. [Google Scholar]
- Kocour Kroupová, H.; Valentová, O.; Svobodová, Z.; Šauer, P.; Máchová, J. Toxic effects of nitrite on freshwater organisms: A review. Rev. Aquac. 2018, 10, 525–542. [Google Scholar] [CrossRef]
- Mazzitelli, J.Y.; Budzinski, H.; Cachot, J.; Geffard, O.; Marty, P.; Chiffre, A.; François, A.; Bonnafe, E.; Geret, F. Evaluation of psychiatric hospital wastewater toxicity: What is its impact on aquatic organisms? Environ. Sci. Pollut. Res. 2018, 25, 26090–26102. [Google Scholar] [CrossRef] [PubMed]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- WHO (World Health Organization). Nitrate and Nitrite in Drinking-Water; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Osman, W.H.W.; Abdullah, S.R.S.; Mohamad, A.B.; Kadhum, A.A.H.; Rahman, R.A. Simultaneous removal of AOX and COD from real recycled paper wastewater using GAC-SBBR. J. Environ. Manag. 2013, 121, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Bua, G.D.; Licata, P.; Cicero, N.; Dugo, G. Trace elements in Thunnus thynnus from Mediterranean Sea and benefit–risk assessment for consumers. Food Addit. Contam. Part B 2015, 8, 175–181. [Google Scholar] [CrossRef]
- Di Bella, G.; Lo Turco, V.; Potortì, A.G.; Bua, G.D.; Fede, M.R.; Dugo, G. Geographical discrimination of Italian honey by multi-element analysis with a chemometric approach. J. Food Compos. Anal. 2015, 44, 25–35. [Google Scholar] [CrossRef]
- Potortì, A.G.; Di Bella, G.; Lo Turco, V.; Rando, R.; Dugo, G. Non-toxic and potentially toxic elements in Italian donkey milk by ICP-MS and multivariate analysis. J. Food Compos. Anal. 2013, 31, 161–172. [Google Scholar] [CrossRef]
- Tahrani, L.; Soufi, L.; Mehri, I.; Najjari, A.; Hassen, A.; Van Loco, J.; Reyns, T.; Cherif, A.; Ben Mansour, H. Isolation and characterization of antibiotic-resistant bacteria from pharmaceutical industrial wastewaters. Microb. Pathog. 2015, 89, 54–61. [Google Scholar] [CrossRef]
- Simon, M.; Grossart, H.P.; Schweitzer, B.; Ploug, H. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat. Microb. Ecol. 2002, 28, 175–211. [Google Scholar] [CrossRef] [Green Version]
- Padilla, C.C.; Ganesh, S.; Gantt, S.; Huhman, A.; Parris, D.J.; Sarode, N.; Stewart, F.J. Standard filtration practices may significantly distort planktonic microbial diversity estimates. Front. Microbiol. 2015, 6, 547. [Google Scholar] [CrossRef] [Green Version]
- Cydzik-Kwiatkowska, A.; Zielińska, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, Z.; Yang, Y.; Mei, X.; Wu, Z. Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res. 2013, 47, 859–869. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, X.; Zhu, L. Activated sludge bacterial communities of typical wastewater treatment plants: Distinct genera identification and metabolic potential differential analysis. AMB Express 2018, 8, 184. [Google Scholar] [CrossRef]
- Miteva, V.I.; Brenchley, J.E. Detection and isolation of ultrasmall microorganisms from a 120,000-year-old Greenland glacier ice core. Appl. Environ. Microbiol. 2005, 71, 7806–7818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obayashi, Y.; Suzuki, S. High growth potential and activity of 0.2 µm filterable bacteria habitually present in coastal seawater. Biogeosci. Discuss. 2017, 1–19. [Google Scholar] [CrossRef]
- Akrami, F.; Rajabnia, M.; Pournajaf, A. Resistance integrons: A Mini review. Casp. J. Intern. Med. 2019, 10, 370–376. [Google Scholar]
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Jara, D.; Bello-Toledo, H.; Domínguez, M.; Cigarroa, C.; Fernández, P.; Vergara, L.; Quezada-Aguiluz, M.; Opazo-Capurro, A.; Lima, C.A.; González-Rocha, G. Antibiotic resistance in bacterial isolates from freshwater samples in Fildes Peninsula, King George Island, Antarctica. Sci. Rep. 2020, 10, 3145. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef]
- Smaldone, G.; Marrone, R.; Cappiello, S.; Martin, G.A.; Oliva, G.; Cortesi, M.L.; Anastasio, A. Occurrence of antibiotic resistance in bacteria isolated from seawater organisms caught in Campania Region: Preliminary study. BMC Vet. Res. 2014, 10, 161. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).