Abstract
The management of agricultural waste is an important issue related to environment protection, as the inappropriate disposal of this waste yields negative effects on the environment. Proper management of industrial effluents is totally aligned with sustainable development goal (SDG) number six “clean water and sanitation”, as well as partially related to other several SDG. In this work, two agricultural waste materials were used for vanillic acid recovery from wastewater. In this scenario, vanillic acid could be considered as both an organic pollutant present in several industrial effluents and a high added-value product when isolated. Therefore, its removal from wastewaters, as well as its recovery and isolation, are very interesting from environmental and economical points of view. Peanut and pistachio shells were studied as no-cost and readily accessible potential adsorbents for the removal and recovery of vanillic acid from aqueous solutions. The evolution of equilibrium isotherms of vanillic acid on both biosorbents was investigated. Three isotherm models (Langmuir, Freundlich, and Temkin) were tested to fit the experimental equilibrium data and compared. The Langmuir model provided the best correlation for vanillic acid biosorption onto both peanut and pistachio shells. Finally, the negative values of ΔG indicated that the biosorption process was spontaneous and thermodynamically favorable for both agricultural waste materials. Accordingly, peanut and pistachio shells were shown to be very efficient low-cost adsorbents, and a promising alternative for vanillic acid recovery from industrial wastewaters.
1. Introduction
Considering the high prevalence of polyphenols in industrial effluents (paper, pesticide and dye production plants, gas and coke manufacturing, tanning, textile, plastic, rubber, pharmaceutical, and petroleum factories), as well as their potential damage to living beings, makes it a significant issue to take them out before the wastewater is discharged into water resources [1]. According to the US Environmental Protection Agency (EPA), the limit of discharge for effluents containing these compounds is less than 1 mg L−1 and for the World Health Organization regulation, 0.002 mg L−1 is the permitted limit for phenol concentration in potable water [2]. The removal of these compounds from industrial effluents is necessary; however, the recovery of polyphenols with important applications is also appealing from an industrial and economic outlook [3].
Vanillic acid is a phenolic compound and one of the main micropollutants commonly present in agro-industrial wastewaters [4,5,6,7]. Nevertheless, it is also known because of its valuable use as a fragrance [8]; as an additive, flavoring, and coloring agent [8,9]; and a medical product [10,11]. In the literature, there are several techniques for vanillic acid recovery such as distillation, extractive separation, crystallization, electrodialysis, and ion exchange [12]. However, most of those techniques present different drawbacks related to secondary pollutant production, high operational costs, extreme operational conditions, and low recovery percentages. In this context, adsorption appeared as a promising alternative to those processes due to its simplicity of design, operation, and scale up; not being sensitive to toxic substances; high pollutants removal efficiencies; and low operational costs [13]. Activated carbon is a widely used as an adsorbent for removing contaminants from wastewater [14,15], but due to its expensive production and the difficulty of disposing the used carbon, it is considered not to be a viable adsorbent. Another great advantage of the adsorption technique is that several types of biological wastes, such as palm shell, peanut shell, coconut shell, castor shell, and coffee residue, can be used as low-cost adsorbent materials to recover valuable compounds from industrial effluents [16], also solving the dilemma of biomass disposal. These new adsorbents have the benefit of presenting low ash content, reasonable hardness, high surface area, and/or adequate porous structures [17]. In this line, some activated biosorbents have been employed for the treatment of olive mill wastewater phenolic compounds, with promising results [18].
The present study focuses on the adsorption of vanillic acid from aqueous mediums using two agricultural wastes—pistachio and peanut shells. Those two kinds of biowaste account for 35–45% of their 1.1 (pistachio) and 47 (peanut) million tons of annual global production [19,20,21]. According to Boumchita et al. (2017), these wastes have a major economic importance and exhibit a high affinity for phenols [22]. Experiments were performed at a constant temperature of 298 K and at atmospheric pressure. The evolution of equilibrium isotherms of vanillic acid on both biosorbents was investigated through three isotherm models (Langmuir, Freundlich, and Temkin). Adsorption process results were discussed and compared for both adsorbent materials.
2. Materials and Methods
2.1. Samples Preparation
For the experimental tests, analytical reagent grade chemicals were employed. Ultrapure water was supplied by Millipore Milli-Q Equipment. Vanillic acid was provided by Sigma Aldrich. A 4 g L−1 stock solution was prepared in a 50 mL volumetric flask by dissolving the suitable amount of the commercial product and diluting with ultrapure water. This preparation was stored at 4 °C, without being exposed to direct light. Different solutions at lower concentrations were prepared by diluting the original solution with ultrapure water.
2.2. Adsorbent Materials
Peanut and pistachio nut shells used in the batch experiments were purchased from local supermarkets in the city of Jaen, south of Spain. These natural wastes were first washed with distilled water to remove impurities. Next, these materials were dried at 110–120 °C for 24 h, burned at 700 °C for 2 h, crushed in a domestic grinder, and sieved to obtain particle sizes in the range of 60–200 mesh [23]. The powdered adsorbent was stored in an airtight container until use. No other chemical or physical treatments were used prior to the adsorption experiments.
2.3. Procedure for Adsorption Isotherms Assays
The influence of the initial concentration of vanillic acid was investigated at the following levels: 10, 20, 50, 100, 500, and 1000 mg L−1.
Vessels containing 100 mL of vanillic acid solution and 0.20 g of adsorbent materials were employed for the adsorption kinetic experiences. Vessels were kept under magnetic stirring during the whole experimental text. Several samples were extracted at defined time intervals (5, 10, 20, 40, 60, 90, and 120 min) for determination of the equilibrium vanillic acid concentration.
In their turn, adsorption isotherm studies were carried out with different initial vanillic acid concentrations ranging from 10 mg L−1 to 1000 mg L−1 at a constant adsorbent material dosage (2 g L−1).
The resulting data were fitted according to the following isotherm models: Langmuir, Freundlich, and Temkin.
2.4. Determination of Vanillic Acid by Fluorescence Spectroscopy
Fluorescence spectroscopy was employed to monitor the concentration of vanillic acid throughout the adsorption experiments. Aliquots of about 3 mL were withdrawn several times from the vessel where the biosorbents were in contact with the bulk solution. The supernatant was directly transferred to a 3 mL quartz cell and its emission spectra were recorded between 300 and 400 nm at an excitation wavelength of 278 nm. The intensity of fluorescence at 360 nm was used as an analytical signal to monitor the vanillic acid level. Fluorescence measurements were performed with a Perkin-Elmer LS50B fluorescence spectrophotometer (USA). Slits at excitation and emission monochromators were maintained at 10 nm each and the measurements were recorded at 20 °C.
2.5. Theoretical Background for Adsorption Isotherms Models
The experimental data related to the amount of equilibrium adsorbed vanillic acid by means of peanut and pistachio shells (qe), as well as the concentration of vanillic acid in the liquid phase (Ce) at room temperature, were used to describe the optimum isotherm model. The linear forms of the Langmuir, Freundlich, and Temkin equations were employed to describe the equilibrium data. The performance of each form was judged through the correlation coefficients (R2).
The equilibrium in the adsorption condition is described by equilibrium isotherms, which represent the distribution of the adsorbed solute in the adsorbent materials (adsorbed vanillic acid) and the free solute in the fluid phase (vanillic acid in solution) in equilibrium.
In this work, the adsorption capacity of the peanut and pistachio shells was evaluated as a function of vanillic acid concentration by determining its loading capacity qe (mg of pollutant/g of biosorbent) (Equation (1)).
where C0 (mg L−1) is related to the initial concentration of vanillic acid, Ce (mg L−1) refers to the equilibrium concentration of vanillic acid free in the liquid phase (mg of vanillic acid dm−3 of solution), V (m3) is the total volume of the vanillic acid solution, and ms (kg) represents the biosorbent amount.
qe = (C0 − Ce) V/ms
The Langmuir isotherm model is used to predict the adsorption of aqueous compounds onto a solid phase [24]. This mechanistic model accepts that a monolayer of adsorbed material is sorbed over a uniform adsorbent surface at a constant temperature, and that the distribution of the compound between the two phases is controlled by the equilibrium constant. The Langmuir isotherm is given by the following expression:
where Ce (mg L−1) is the equilibrium concentration in the solution (mg L−1), and qm (mg g−1) and KL (L mg−1) are the Langmuir constants, representing the maximum adsorption capacity for the solid phase (biosorbent) loading and the energy constant related to the adsorption heat, respectively.
qe = (qm KL Ce)/(1 + KL Ce)
Otherwise, the separation factor RL is a suitable characteristic of the Langmuir isotherm, which can be expressed as follows:
RL = 1/(1 + KL C0)
The RL value indicates the adsorption nature to be either unfavorable if RL > 1, linear if RL = 1, favorable if 0 < RL < 1, and irreversible if RL = 0.
On the other hand, the Freundlich isotherm standard is an empirical interaction describing solutes transmission from a liquid phase to a solid surface, which suggests that different sites with several adsorption energies are involved [25]. It is widely used to describe the adsorption characteristics for heterogeneous surfaces. The Freundlich isotherm represents the relationship between the amounts of adsorbed pollutant per mg of biosorbent, qe, and the concentration of the ionic species at equilibrium, Ce. The Freundlich exponential equation is given as follows:
where Kf and n are the Freundlich constants, and are indicators of the adsorption capacity and intensity, respectively.
qe = Kf Ce1/n
Finally, the Temkin isotherm model was tested to model the adsorption potential of peanut and pistachio shells towards vanillic acid. This model considers the effects of indirect adsorbent/adsorbate (vanillic acid) interactions on the adsorption process [26]. The model is given by Equation (5), as follows:
where A and B are Temkin isotherm constants. This isotherm assumes the adsorption heat of all molecules in the layer decreases linearly with the coverage, and that adsorption is characterized by a uniform distribution of binding energies, up to a maximum binding energy.
qe = B ln (A Ce)
3. Results and Discussion
3.1. Effect of Contact Time
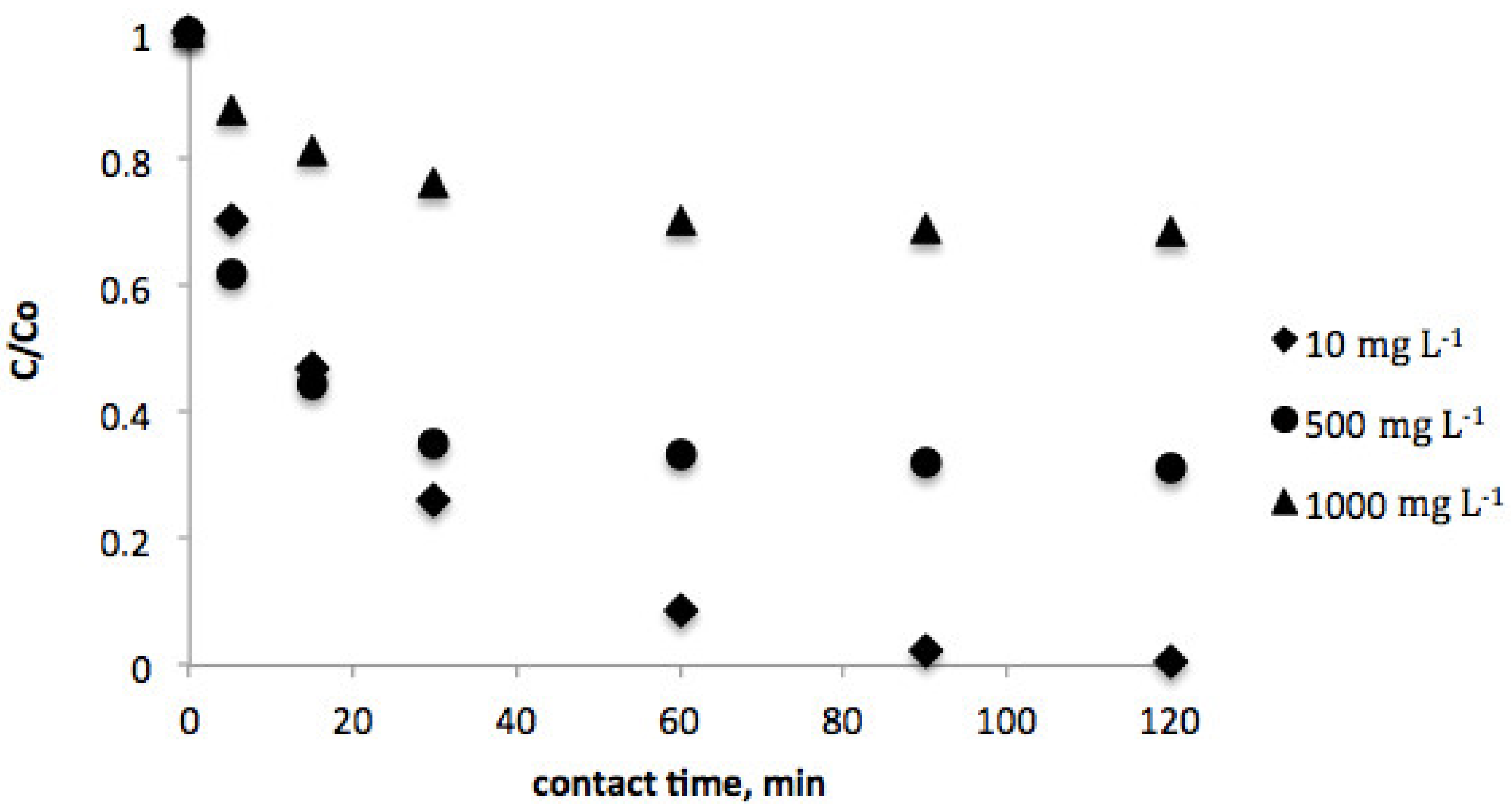
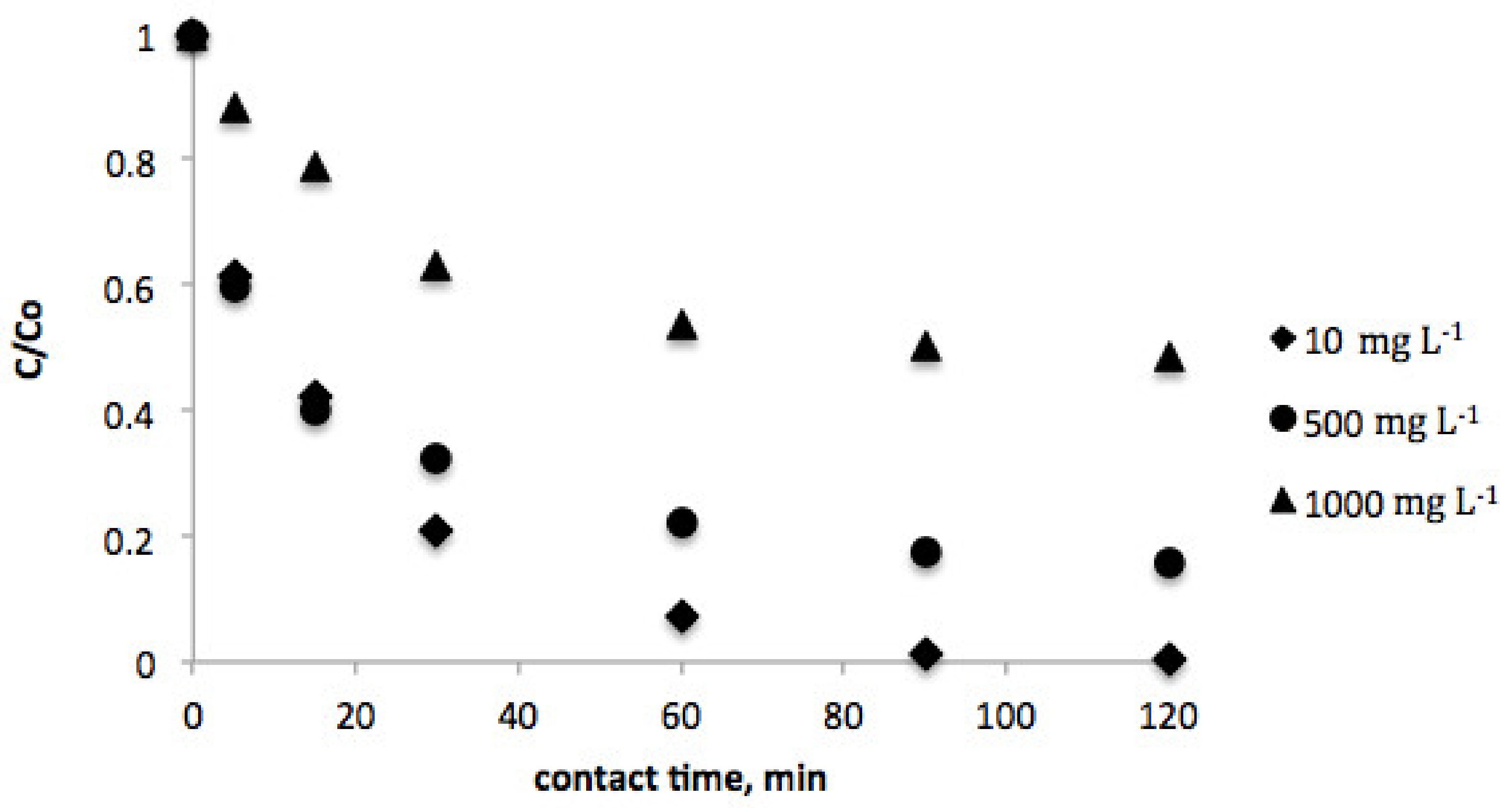
The effect of contact time was investigated in order to estimate the equilibrium concentration of vanillic acid for both biosorbents. In this sense, Figure 1 and Figure 2 show the evolution of the vanillic acid level for several contact times in the range of 0–120 min. As can be observed, equilibrium was achieved after 120 min contact time for both pistachio and peanut shells.
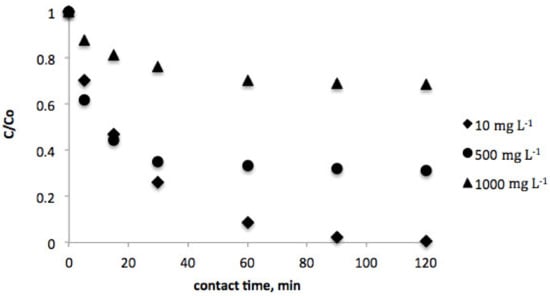
Figure 1.
Effect of contact time on the equilibrium concentration of vanillic acid (initial vanillic acid concentration = 10, 500, and 1000 mg L−1; peanut shell dosage = 2 g L−1; temperature = 298 K; volume = 100 mL).
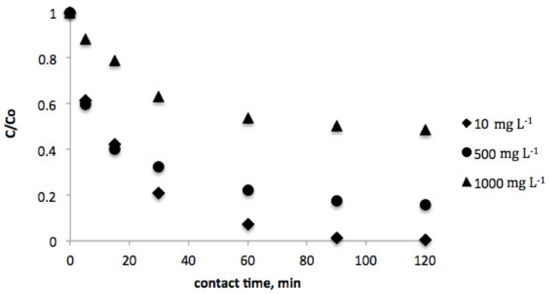
Figure 2.
Effect of contact time on equilibrium concentration of vanillic acid (initial vanillic acid concentration = 10, 500, and 1000 mg L−1; pistachio shell dosage = 2 g L−1; temperature = 298 K; volume = 100 mL).
Moreover, vanillic acid adsorption was studied at several initial concentrations (10–1000 mg L−1) for each biosorbent. The results evidenced that as the initial concentration increases, the time to reach equilibrium decreases.
Considering these empirical data, it can be assumed that the recovery efficiency was higher for peanut shell, as the equilibrum concentrations of vanillic acid were lower when this biosorbent was employed in comparison with pistachio shell.
3.2. Adsorption Isotherms Studies
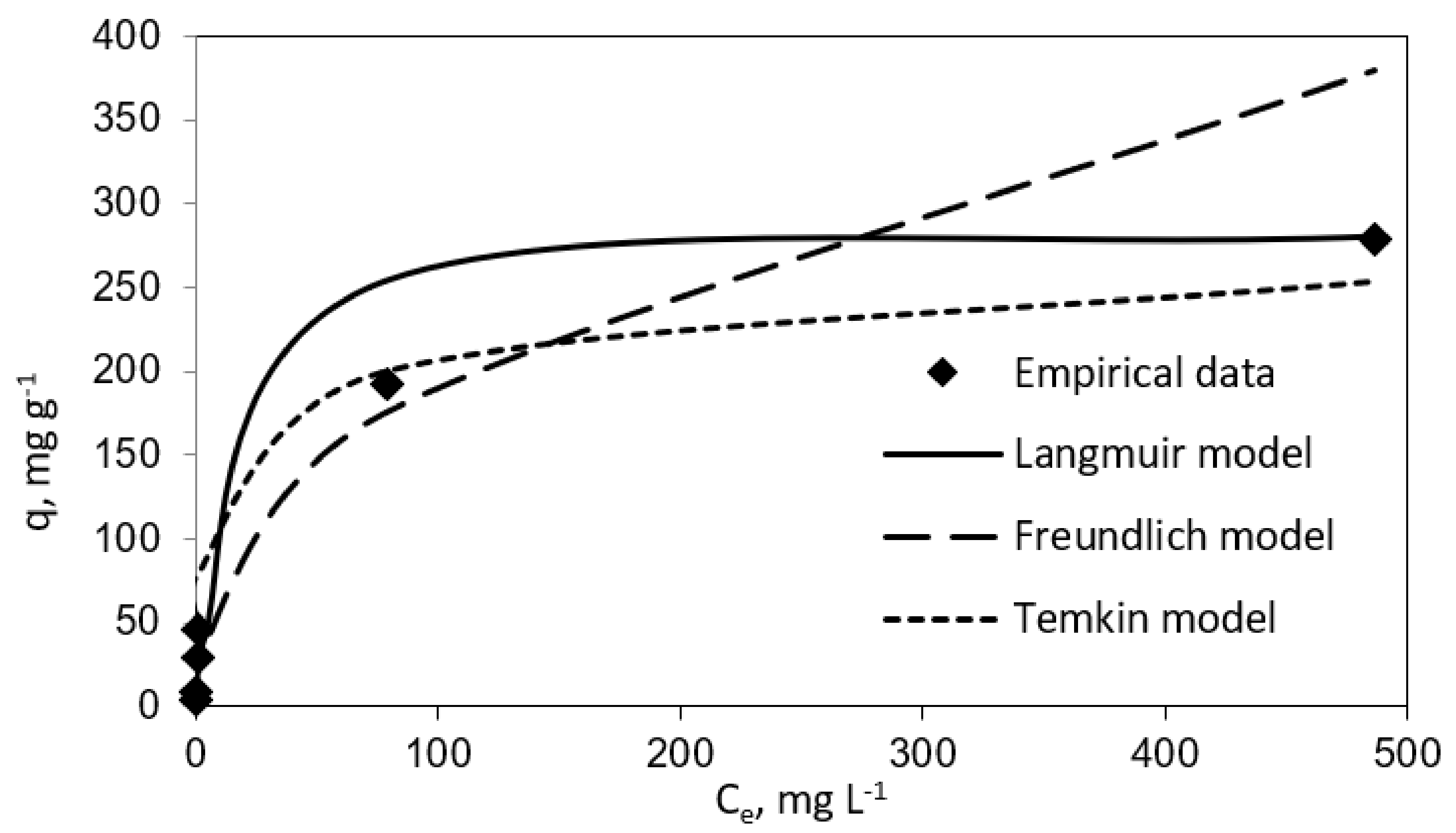
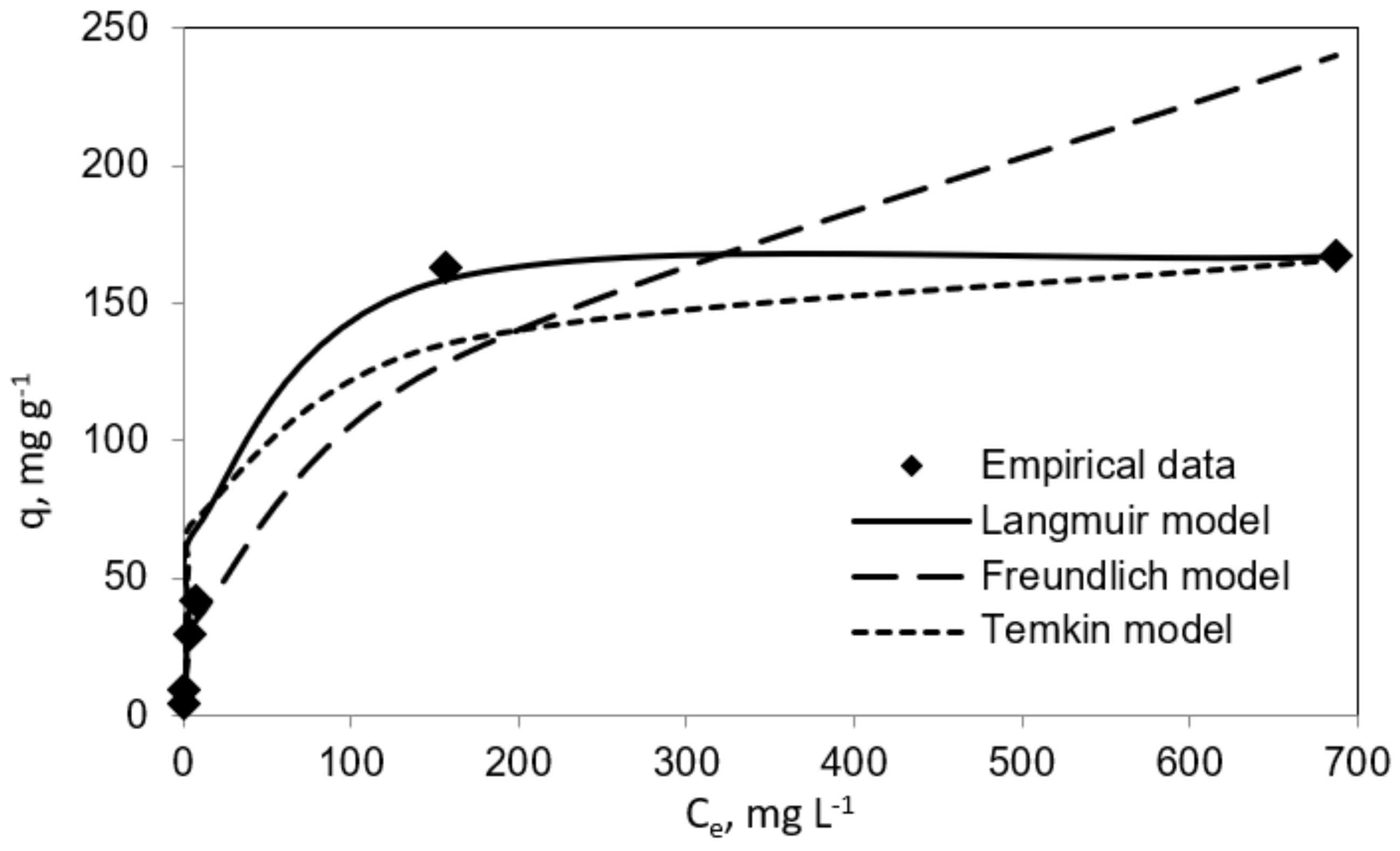
Table 1 and Table 2 report the results for the fitting to the three isotherm models. In the same line, Figure 3 and Figure 4 show experimental results graphically for further comparison and comprehension. The results plotted in these figures evidence different performances of the three isotherm models for representing the experimental equilibrium data for vanillic acid on pistachio and peanut shells. Concretely, the estimated equilibrium adsorption capacities, qe, were highly similar to the experimental values, qe,exp, for the Langmuir isotherm, as it gave the most accurate prediction of the experimental results for both adsorbent materials (R2 > 0.9 for pistachio and peanut shells). Nevertheless, the worst fitting to the experimental data was provided by the Temkin isotherm (R2 > 0.94 for peanut shell and R2 > 0.90 for pistachio shell).

Table 1.
Langmuir, Freundlich, and Temkin isotherm parameters for vanillic acid adsorption through peanut shell.

Table 2.
Langmuir, Freundlich, and Temkin isotherm parameters for vanillic acid adsorption through pistachio shell.
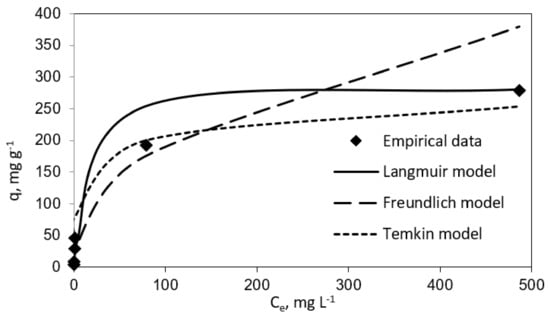
Figure 3.
Langmuir, Freundlich, and Temkin isotherms (vanillic acid concentration = 10–1000 mg L−1; peanut shell dosage = 2 g L−1; temperature = 298 K; volume = 100 mL).
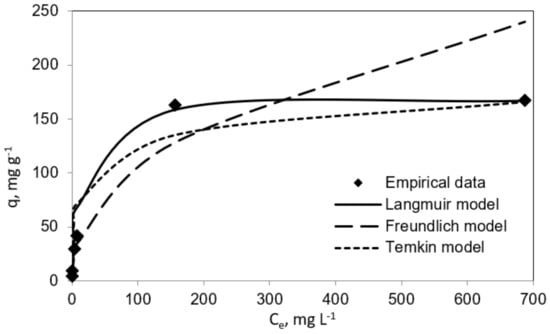
Figure 4.
Langmuir, Freundlich, and Temkin isotherms (vanillic acid concentration = 10–1000 mg L−1; pistachio shell dose = 2 mg mL−1; temperature = 298 K; volume = 100 mL).
The maximum adsorption capacity (qm) from the Langmuir isotherm model was 285.7 mg g−1 for peanut shell and 169.49 mg g−1 for pistachio shell. These results are in agreement with those obtained by Esteves et al. [18]. These authors employed agricultural waste as an adsorbent with the aim of removal phenolic compounds from olive mill wastewater. In their research work, qm values for vanillic acid adsorption onto olive tree pruning activated carbon and olive stone activated carbon were found to be 188.6 and 359.0 mg g−1, respectively. In this line, Madureira et al., 2018, investigated the adsorption of vanillic acid using a synthesized mesoporous carbon and found that the qm value was 71.90 mg g−1 [14]. It is worthy to highlight the convenience of using biosorbents versus synthetic adsorbents for the mentioned phenolic compound uptake.
On the other hand, RL values were in the range 0–1, which suggested that the sorption process was favorable when using both biosorbents.
Furthermore, as it can be observed in both Table 1 and Table 2, the n coefficient was higher than 1 for peanut and pistachio shells (n = 2.37 and n = 2.36, respectively), which indicates that the adsorption process onto these biosorbents was clearly efficient. Madureira et al., 2018, obtained similar results for vanillic acid adsorption through synthesized mesoporous carbon (n = 3.61) [14].
In the same way, from the Temkin performance shown in Figure 3 and Figure 4, the following values were calculated: A = 0.085 mL mg−1 and B = 29.26 J mol−1 for peanut shell and A = 0.2258 mL mg−1 and B = 20.72 J mol−1 for pistachio shell, which was related to the heat of sorption, indicating a physical adsorption process.
3.3. Gibbs Free Energy
Gibbs free energy was calculated from the standard thermodynamic equilibrium constant, KC (dimensionless), which is estimated as follows [27]:
where KL is the Langmuir constant expressed in L mmol−1 by means of the vanillic acid molecular mass (168.14 g mol−1).
KC= 55,500 (mmol L−1) KL
On the other hand, the standard Gibbs free energy changes (∆G°) can be calculated by means of the following equation [28]:
where T is the temperature (K) and R is the ideal gas constant (8.3145 kJ mol−1K−1).
∆G°= −RT ln KC
The estimated values are shown in Table 3 for vanillic acid adsorption onto both biosorbents. ∆G° was found to be −33.90 kJ mol−1 for pistachio shell and −34.12 kJ mol−1 for peanut shell, indicating that the adsorption processes by these natural adsorbent materials were thermodynamically favorable processes at 298 K.

Table 3.
Equilibrium parameters and Gibbs free energy of vanillic acid adsorption.
4. Conclusions
Experimental results showed that the vanillic acid level was considerably decreased with the incremental contact time. Moreover, the time required for achieving equilibrium increased with increasing the initial concentration.
On the other hand, the isotherm studies showed that the Langmuir isotherm gave the most accurate prediction of the experimental data (R2 > 0.99 for both adsorbent materials), whereas the Temkin isotherm provided the worst data fit. Moreover, the maximum adsorption capacity (qm) from the Langmuir isotherm model was 285.7 mg g−1 for peanut shell and 169.49 mg g−1 for pistachio shell. RL values were in the ranges of 0–1 (for the studied concentrations range), which implies that the sorption process became favorable. This conclusion was also supported by the n coefficient of the Freundlich isotherms (n = 2.37 for peanut shell and n = 2.36 for pistachio shell), which suggests that the adsorption process through these biosorbents was enhanced.
From the thermodynamic tests, it was found that ∆G° was −34.12 kJ mol−1 for peanut shell and −33.90 kJ mol−1 for pistachio shell, indicating that the adsorption process of vanillic acid through peanut and pistachio shell was a thermodynamic favorable process at the studied temperature (298 K).
Considering vanillic acid removal efficiencies and qm values, it can be concluded that the vanillic recovery process was more efficient for peanut shell in comparison with pistachio shell.
According to the achieved results, it was found that pistachio and peanut shells were not only inexpensive absorbents, but also had effective factors for the removal of vanillic acid from water and wastewater, making the adsorption process even more attractive for use.
Author Contributions
Conceptualization, M.D.V.-O. and D.A.-R.; methodology, M.D.V.-O.; software, M.D.V.-O. and D.A.-R.; validation, M.D.V.-O. and D.A.-R.; formal analysis, M.D.V.-O. and D.A.-R.; investigation, M.D.V.-O. and D.A.-R.; resources, M.D.V.-O. and D.A.-R.; data curation, M.D.V.-O. and D.A.-R.; writing—original draft preparation, M.D.V.-O. and A.S.F.; writing—review and editing, M.D.V.-O. and A.S.F.; visualization, M.D.V.-O.; A.S.F. and D.A.-R.; supervision, M.D.V.-O. and D.A.-R.; project administration, M.D.V.-O. and D.A.-R.; funding acquisition, D.A.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Project PID2020-115214RB-I00 funded by MCIN/AEI/10.13039/501100011033/ and ERDF, A way of making Europe, is gratefully acknowledged. Ana S. Fajardo wants to acknowledge the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant, agreement no. 843870.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jain, A.K.; Gupta, V.K.; Jain, V.K.; Suhas, S. Removal of chlorophenols using industrial wastes. Environ. Sci. Technol. 2004, 38, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, E.; Mostafapour, F.K.; Mahvi, A.H. Phenol removal from aqueous solutions using pistachio-nut shell ash as a low cost adsorbent. Fresenius Environ. Bull. 2012, 21, 2962–2968. [Google Scholar]
- Medeiros, M.C.; dos Santos, E.V.; Martínez-Huitle, C.A.; Fajardo, A.S.; Castro, S.S.L. Obtaining high-added value products from the technical cashew-nut shell liquid using electrochemical oxidation with BDD anodes. Sep. Purif. Technol. 2020, 250, 117099. [Google Scholar] [CrossRef]
- Kujawski, W.; Warszawski, A.; Ratajczak, W.; Porebski, T.; Capała, W.; Ostrowska, I. Removal of phenol from wastewater by different separation techniques. Desalination 2004, 163, 287–296. [Google Scholar] [CrossRef]
- Otero, M.; Zabkova, M.; Rodriguez, A.L. Adsorptive purification of phenol wastewaters: Experimental basis and operation of a parametric pumping unit. Chem. Eng. J. 2005, 110, 101–111. [Google Scholar] [CrossRef]
- Gupta, V.K.; Mohan, D.; Singh, K.P. Removal of 2-Aminophenol Using Novel Adsorbents. Ind. Eng. Chem. Res. 2006, 45, 1113–1122. [Google Scholar] [CrossRef]
- Cagnon, B.; Chedeville, O.; Cherrier, J.F.; Caqueret, V.; Porte, C. Evolution of adsorption kinetics and isotherms of gallic acid on an activated carbon oxidized by ozone: Comparison to the raw material. J. Taiwan Inst. Chem. Eng. 2011, 42, 996–1003. [Google Scholar] [CrossRef]
- Rosazza, J.P.N.; Huang, Z.; Dostal, L.; Volm, T.; Rosseau, B. Review: Biocatalytic transformation of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. 1995, 15, 457–471. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Narbada, A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int. J. Food Microbiol. 2003, 86, 113–122. [Google Scholar] [CrossRef]
- Delaquis, P.; Stanich, K.; Toivonen, P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J. Food Prot. 2005, 68, 1472–1476. [Google Scholar] [CrossRef]
- Vetrano, A.M.; Heck, D.E.; Mariano, T.M.; Mishin, V.; Laskin, D.L.; Laskin, J.D. Characterization of the oxidase activity in mammalian catalas. J. Biol. Chem. 2005, 280, 35372–35381. [Google Scholar] [CrossRef] [Green Version]
- Mota, M.I.F.; Pinto, P.C.R.; Loureiro, J.M.; Rodrigues, A.E. Recovery of Vanillin and Syringaldehyde from Lignin Oxidation: A Review of Separation and Purification Processes. Sep. Purif. Rev. 2016, 45, 227–259. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Madureira, J.; Melo, R.; Cabo Verde, S.; Matos, I.; Bernardo, M.; Noronha, J.P.; Margaça, F.M.A.; Fonseca, I.M. Recovery of phenolic compounds from multi-component solution by a synthesized activated carbon using resorcinol and formaldehyde. Water Sci. Technol. 2018, 77, 456–466. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 14830. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Chen, R.; Du, H.; Zhang, J.; Shi, L.; Qin, Y.; Yue, L.; Wang, J. Synthesis of activated carbon from peanut shell as dye adsorbents for wastewater treatment. Adsorpt. Sci. Technol. 2019, 37, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Sumboja, A.; Prakoso, B.; Ma, Y.; Irwan, F.R.; Hutani, J.J.; Mulyadewi, A.; Mahbub, M.A.A.; Zong, Y.; Liu, Z. FeCo Nanoparticle-Loaded Nutshell-Derived Porous Carbon as Sustainable Catalyst in Al-Air Batteries. Energy Mater. Adv. 2021, 2021, 7386210. [Google Scholar] [CrossRef]
- Esteves, B.M.; Morales-Torres, S.; Madeira, L.M.; Maldonado-H’odar, F.J. Specific adsorbents for the treatment of OMW phenolic compounds by activation of bio-residues from the olive oil industry. J. Environ. Manag. 2022, 306, 114490. [Google Scholar] [CrossRef]
- Yang, T.; Lua, A.C. Characteristics of activated carbons prepared from pistachio-nut shells by physical activation. J. Colloid Interface Sci. 2003, 267, 408–417. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy–power rivals lithium ion capacitors. Energy Environ.Sci. 2015, 8I, 941–955. [Google Scholar] [CrossRef]
- Xu, S.-D.; Zhao, Y.; Liu, S.; Ren, X.; Chen, L.; Shi, W.; Wang, X.; Zhang, D. Curly hard carbon derived from pistachio shells as high-performance anode materials for sodium-ion batteries. J. Mater. Sci. 2018, 53, 12334–12351. [Google Scholar] [CrossRef]
- Boumchita, S.; Lahrichi, A.; Benjelloun, Y.; Lairini, S.; Nenov, V.; Zerrouq, F. Application of Peanut shell as a low-cost adsorbent for the removal of anionic dye from aqueous solutions. J. Mater. Environ. Sci. 2017, 8, 2353–2364. [Google Scholar]
- Igwegbe, C.A.; Ighalo, J.O.; Ghosh, S.; Ahmadi, S.; Ugonabo, V.I. Pistachio (Pistacia vera) waste as adsorbent for wastewater treatment: A review. Biomass Convers. Biorefnery 2021, 1–19. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physicochim. URSS 1940, 12, 217–222. [Google Scholar]
- Kopinke, F.-D.; Georgi, A.; Goss, K.-U. Comment on “Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solution: A critical review”. Water Res. 2018, 129, 520–521. [Google Scholar] [CrossRef]
- Alzaydien, A.S. Adsorption of methylene blue from aqueous solution onto a low-cost natural Jordanian Tripoli. Am. J. Appl. Sci. 2009, 5, 197–208. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).