Simple Summary
This study was based on the determination of podocyst excystment, start time of strobilation, duration of strobilation, and cumulative ephyra numbers of the edible jellyfish Rhopilema esculentum (Kishinouye, 1891) podocysts which were preserved at 2 ± 1 °C for more than 12 months. To this end, the podocysts were tested under nine combinations of three variable temperatures (simulated temperatures increasing from different starting dates of 14.5 °C on 1 April, 18 °C on 1 May, and 23.2 °C on 1 July respectively to natural levels) and three salinities (20, 25, and 30). Podocyst excystment and ephyrae production occurred in all treatments. Higher temperature and lower salinity significantly facilitated the podocyst excystment and accelerated the start time of strobilation (p < 0.05). Significantly greater ephyra numbers were produced with lower salinity (20 and 25) and temperatures increasing from 18 °C on 1 May to natural levels (p < 0.05).
Abstract
As one of the edible jellyfish species, Rhopilema esculentum (Kishinouye, 1891) is a traditional fishery resource and an important economic aquaculture species in China. However, facing the current situation of natural resources exhaustion and problems of breeding population frequent disease, quantity, and quality of seedlings in artificial breeding cannot satisfy the market demand. Temperature and salinity have been considered to play crucial roles in regulating R. esculentum asexual reproduction. This study examined the combined effects by exposing post-preserved R. esculentum podocysts (preserved at 2 ± 1 °C for more than 12 months) to three variable temperatures (simulated temperatures increasing from different starting dates of 14.5 °C on 1 April, 18 °C on 1 May, and 23.2 °C on 1 July, respective to natural levels) and three salinities (20, 25, and 30). Podocyst excystment, the start time of strobilation, duration of strobilation, and cumulative ephyra numbers were tested for 45 days and transfer rates from podocysts to ephyrae were analyzed to assess the most optimal combination of temperature and salinity. The results showed that podocyst excystment and ephyrae production occurred in all treatments. Higher temperature and lower salinity significantly facilitated the podocyst excystment and accelerated the start time of strobilation (p < 0.05). Significantly greater ephyra numbers were produced with lower salinity (20 and 25) and temperatures increasing from 18 °C on 1 May to natural levels (p < 0.05). There were significant interactions between temperature and salinity on the cumulative ephyra numbers and transfer rates from podocysts to ephyrae (p < 0.05). These results suggested that R. esculentum podocysts for long-term preservation at low temperature could be recycled. Temperature and salinity regulation can affect the number and time of R. esculentum seedlings to achieve high production and satisfy the market demand for real-time seedling supply. This conclusion would provide a scientific basis for the innovative methods of sustainable utilization of the edible jellyfish (R. esculentum) resources.
1. Introduction
Rhopilema esculentum (Kishinouye, 1891) is one of the important traditional fishing, artificial enhancement release, and pond-cultured species in China, with an annual output value of more than CNY ten billion [1,2]. R. esculentum medusae are rich in nutrients, low in fat, delicious, and have a healthy role in disease prevention [2,3]. In recent years, it has also been deeply developed into cosmetics and health care products, which increased the economic value and broad market prospects [4,5]. Compared to other aquaculture species, such as fish, shrimp, and crab, the outstanding advantages of the culture of R. esculentum medusae are faster growth, shorter breeding cycles, lower costs, quicker results, and higher incomes [2]. In some provinces of China (such as Liaoning, Shandong, and Jiangsu), artificial enhancement release and pond-cultured R. esculentum medusae have become a characteristic industry [2,6]. There are significant social, economic, and ecological benefits to related industries including fishing, aquaculture, processing, trade, and especially seedling breeding which is the key basis for the entire R. esculentum industry.
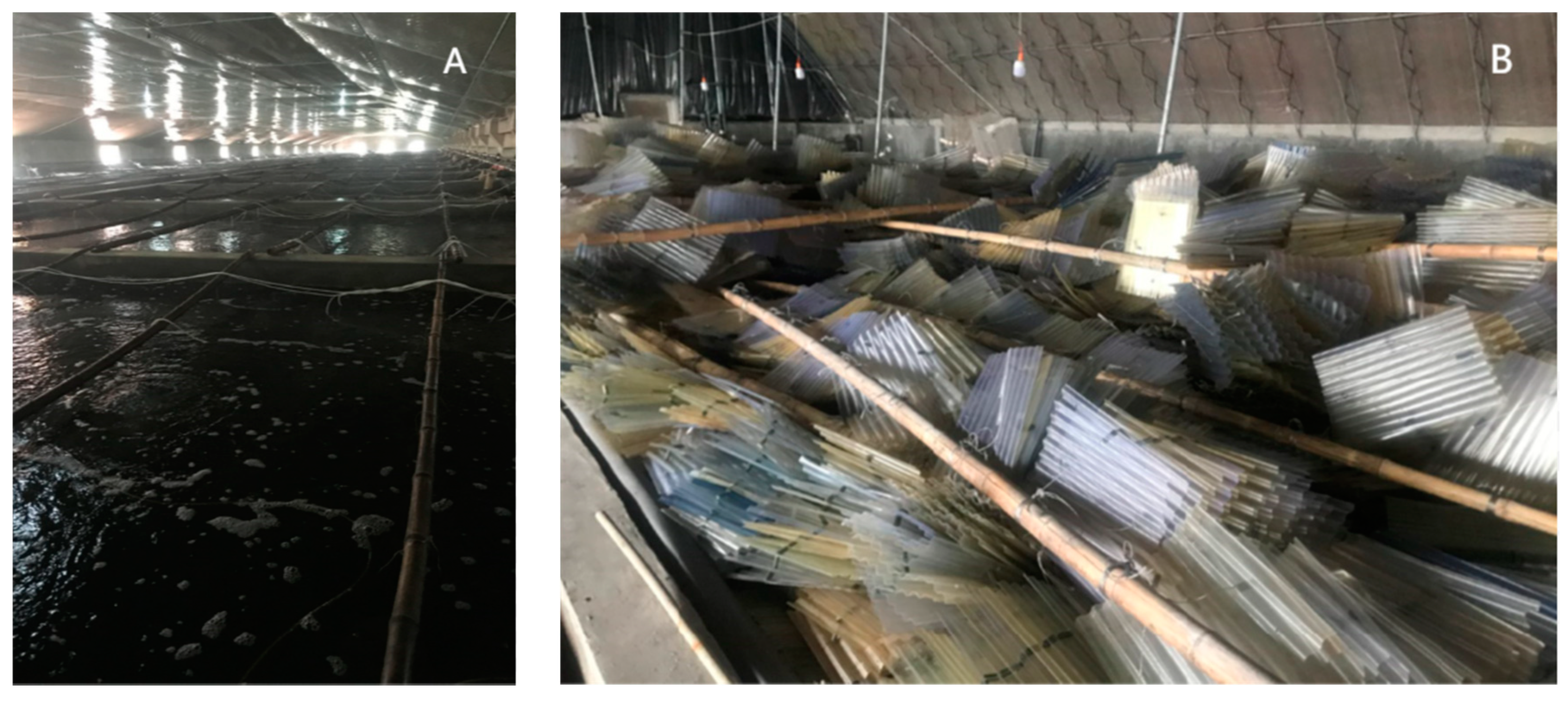
In the current traditional seedling breeding process, a large number of polyps have been acquired by catching some mature medusae together in the pool of the breeding plant in autumn. Then these polyps were fed to grow up by overwintering, and they began to release ephyrae when the temperature increased in spring (Figure 1A). When the strobilation finished, these polyps and their podocysts would be discarded (Figure 1B). Therefore, podocyst reproduction has not been utilized in the breeding process. However, The podocysts of the giant jellyfish play an important role in the reproduction of the polyps and even the ephyrae population [7,8]. Nemopilema nomurai podocysts are capable of dormancy for at least 6 years [9]. Aurelia aurita s.l. podocysts are capable of surviving for up to 3.2 years [10]. R. esculentum polyps are able to replenish the population through podocyst reproduction in the natural life cycle [11]. If R. esculentum podocysts can be further developed and used, the breeding process will save biological resources and reduce the negative impacts on the environment. Therefore, we thought about whether R. esculentum podocysts can be recycled after long-term preservation at low temperatures. If the podocysts can be recycled, how can we get more polyps and ephyrae by replaying them to natural water temperatures and suitable salinity in different months?
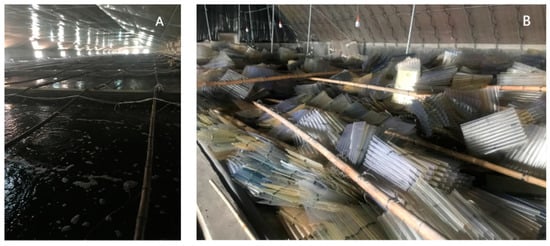
Figure 1.
Yingkou Jellyfish (R. esculentum) seedling breeding farm (A). Plenty of discarded polythene sheeting attached with podocysts and post-strobilated polyps (B). Source: Ming Sun.
At present, R. esculentum has been systematically studied through basic theories and applied biology, such as life history [11], morphological structure [12,13], experimental ecology [14,15,16,17,18,19], population genetics [20,21,22], population distribution and natural feeding habitat [23,24], enhancement release [25,26,27], artificial culture technology [28,29,30], and pond polyculture [31,32,33,34]. Laboratory studies have shown that abundant food supply [18,35], aeration [35], and darkness [36] are beneficial to podocyst reproduction. Temperature and salinity are implicated as key environmental factors playing a critical role in controlling podocyst reproduction and strobilation [15,17,19,36,37,38,39,40,41]. Compared to higher salinity, the range from 20 to 22 was the optimum salinity for podocyst reproduction [36], with a similar result of the salinity between 18 and 22 being the optimum range for podocyst excystment [37]. In contrast, podocyst reproduction increased with salinity increasing from 18 to 26 [39]. Therefore, conclusions about the effect of salinity on podocyst reproduction are inconsistent and need to be further studied. In addition, previous studies only focused on the effects of constant temperature [36,37,38,39]. Because of seasonal characteristics in local climate change, R. esculentum podocysts after overwintering may experience seasonal seawater temperature warming. The combined effects of variational temperature and salinity on podocyst excystment and strobilation may significantly regulate the abundance of following ephyrae, which was thus worthy of study. This study was conducted to test the effects of variable temperatures (T1 was simulated water temperature of local breeding plant from 14.5 °C on 1 April to 19 °C on 15 May, T2 was simulated water temperature of local breeding plant from 18 °C on 1 May to 22 °C on 14 June, and T3 was a control group of natural temperature in the laboratory from 23.2 °C on 1 July to 25.1 °C on 14 August) and salinities (20, 25, and 30) on podocyst recycling of R. esculentum, which has not been reported in the literature. Unlike most previous studies conducted at the individual level, testing the materials that produced podocysts by polyps in a short period or overwintering through short-term low temperatures for about 2–3 months, our experiment was carried out at the whole colony level using triplicates of at least 400 podocysts in each of 9 combinations of temperature and salinity and testing on the materials that podocysts were preserved on long-term at low temperatures for more than 12 months. The study’s aim is to determine the recycling likelihood of R. esculentum podocysts that have undergone long-term preservation at low temperatures and to clarify the specific regulating effects of temperatures and salinities, simulated with seasonal variation. The research results have important significance in improving the quality and efficiency of jellyfish (R. esculentum) seedling breeding, further developing and reusing biological resources, and promoting and upgrading industrial transformation.
2. Materials and Methods
2.1. Origin of Podocysts
The podocysts were produced from asexual reproduction by a polyp population for seedling breeding production, provided by Yingkou Jellyfish (R. esculentum) seedling breeding farm when they finished production in spring 2020. These polyps originated from sexual reproduction by medusae collected from Yingkou Jellyfish (R. esculentum) aquaculture ponds in August 2019. During the previous seedling breeding period, these polyps were attached to a polythene sheeting of 30 × 40 cm. Ten strings, each with twenty polyethylene sheets connected at 10 cm intervals by rope, were hung in a horizontal position by one bamboo. Ten pieces of bamboo were placed in one tank (4 m × 4 m × 1.5 m) with fresh filtered seawater (Figure 1A). Polyps were fed with newly hatched Artemia salina nauplii (body length: ca. 200 μm) every day, resulting in cumulatively producing a number of podocysts (average 10–20 podocysts each polyp) before strobilating in spring. Plenty of polyethylene sheeting attached with podocysts and post-strobilated polyps are usually discarded when production is finished (Figure 1B). Two strings with forty polyethylene sheets were transported by a truck from Yingkou Jellyfish (R. esculentum) seedling breeding farm to our institute on 15 June 2020. They were cultured in a plastic container (1.2 m × 1.2 m × 0.8 m) in filtered seawater of natural temperatures gradually decreasing from 20 °C to 2 °C for 10 days and salinities ranging from 29 to 33 in dark conditions, then preserved by controllable temperature cold storage with low water temperatures 2 ± 1 °C for more than 12 months. During the low temperature preserved period, we replenished the fresh filtered seawater at the same low temperature every week and did no feeding. Seawater for the culture and experiments was collected from the coast of Dalian in the Northern Yellow Sea, filtered through sand, and stored in dark tanks (Salinity: 31 ± 1; PH: 8.1 ± 1; DO > 5 mg/L).
2.2. Laboratory Testing
A microcosm experiment was performed in the jellyfish ecological laboratory of LNOFSRI (Liaoning Ocean and Fisheries Science Research Institute), located in Dalian, China, from July to August 2021. Three polythene sheets inhabited by massive podocysts and a few post-strobilated polyps were taken out from cold storage and cut into pieces of about 10 × 10 cm. Pieces of sheeting with at least 400 podocysts of about 20 to 30 families (10–20 podocysts produced by a mother polyp is a family) were arbitrarily selected; then, extra polyps and podocysts were removed with a dissecting needle. One piece of sheeting was placed into a sealed plastic container (15 × 15 × 3 cm) and kept in the dark. Each testing group had three replicates with three plastic containers filled with seawater of each testing temperature and salinity.
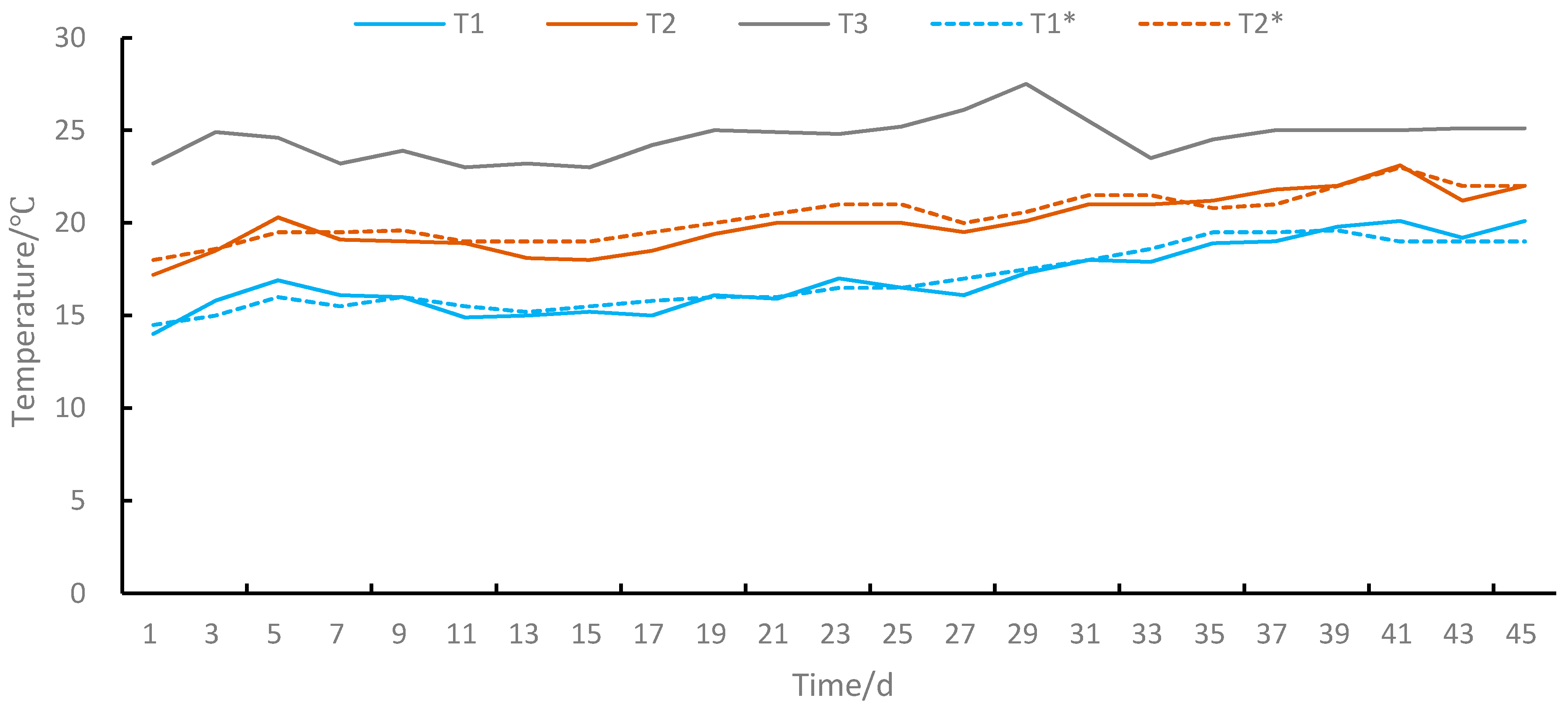
In the laboratory testing, podocyst excystment and strobilation of polyps produced by testing podocysts were investigated under 9 combinations of 3 variable temperatures (T1, T2, and T3) and 3 salinities (S1, S2, and S3 were 20, 25, and 30, respectively). We preset three variable temperature groups (T1, T2, and T3) according to the monitoring results of water temperature in the local breeding plant by CTD (Star-Oddi, DST-CTD). T1 was the preset temperature according to the previous year’s monitoring data of water temperature in the breeding plant from 14.5 °C on 1 April to 19 °C on 15 May, T2 was the preset temperature according to the previous year’s monitoring data of water temperature in the breeding plant from 18 °C on 1 May to 22 °C on 14 June, and T3 was a control group of natural temperature in the laboratory from 23.2 °C on 1 July to 25.1 °C on 14 August (Figure 2).
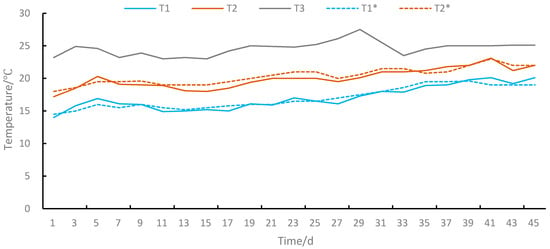
Figure 2.
Temperature curve. T1 was a simulation group of actual measured temperatures in the #1 incubator from 14 °C to 20.1 °C, T1* was the preset temperature according to the previous year’s monitoring data of water temperature in the breeding plant from 14.5 °C on 1 April to 19 °C on 15 May, T2 was the simulation group of actual measured temperatures in the #2 incubator from 17.2 ℃ to 22 ℃, T2* was the preset temperature according to the previous year’s monitoring data of water temperature in the breeding plant from 18 °C on 1 May to 22 °C on 14 June, T3 was a control group of the natural temperature in the laboratory from 23.2 °C on 1 July to 25.1 °C on 14 August.
During all testing, intelligent incubators (GXZ—280B; GXZ—280C, produced by Ningbo Jiangnan Instrument Factory) were used to maintain preset temperatures and were carried out in total darkness. We calibrated and recorded the actual water temperature daily with a thermometer (HS-001) by placing a separate beaker containing 500 mL seawater in the placing incubators. Seawater in each testing container was gradually diluted with deionized water stepwisely at a rate of 2–3 salinity changes per 2 h to achieve the desired salinities. A salinometer (PAL-06) was used to measure salinity. They were observed, recorded, and fed at 2-day intervals. Polyps produced by testing podocysts were fed with Artemia salina nauplii (body length: ca. 200 μm) with 1 ind. 4 tentacles polyps−1, 2 ind. 8 tentacles polyps−1, and 4 ind. 16 tentacles polyps−1 and the seawater was renewed with the same temperature and salinity after feeding for 2 h. The duration of the testing was 45 days, from 1 July 2011 to 14 August 2011, until strobilation finished in all treatments.
2.3. Index Measurements and Statistical Analysis
The number of podocyst excystments and ephyra numbers was observed and recorded when feeding every two days. The transfer rates from podocysts to ephyrae was calculated by the ratio of the final cumulative ephyra numbers to the initial podocyst numbers. The statistical analyses were determined using SPSS 20. The experimental data were expressed as mean ± SD (n = 3). Duncan’s multiple comparison was used to test the differences among groups. To assess the effects of temperature and salinity on podocyst excystment and strobilation (i.e., cumulative podocyst excystment, cumulative ephyra numbers, the start time of strobilation, duration of strobilation, and transfer rates from podocysts to ephyrae), one-way ANOVA was used after testing for normality and equality in the variance of the data. The combined effects of temperature and salinity on podocyst excystment and strobilation (i.e., cumulative podocyst excystment, cumulative ephyra numbers, the start time of strobilation, duration of strobilation, and transfer rates from podocysts to ephyrae) were tested by two-way ANOVA.
3. Results
3.1. Podocyst Excystment
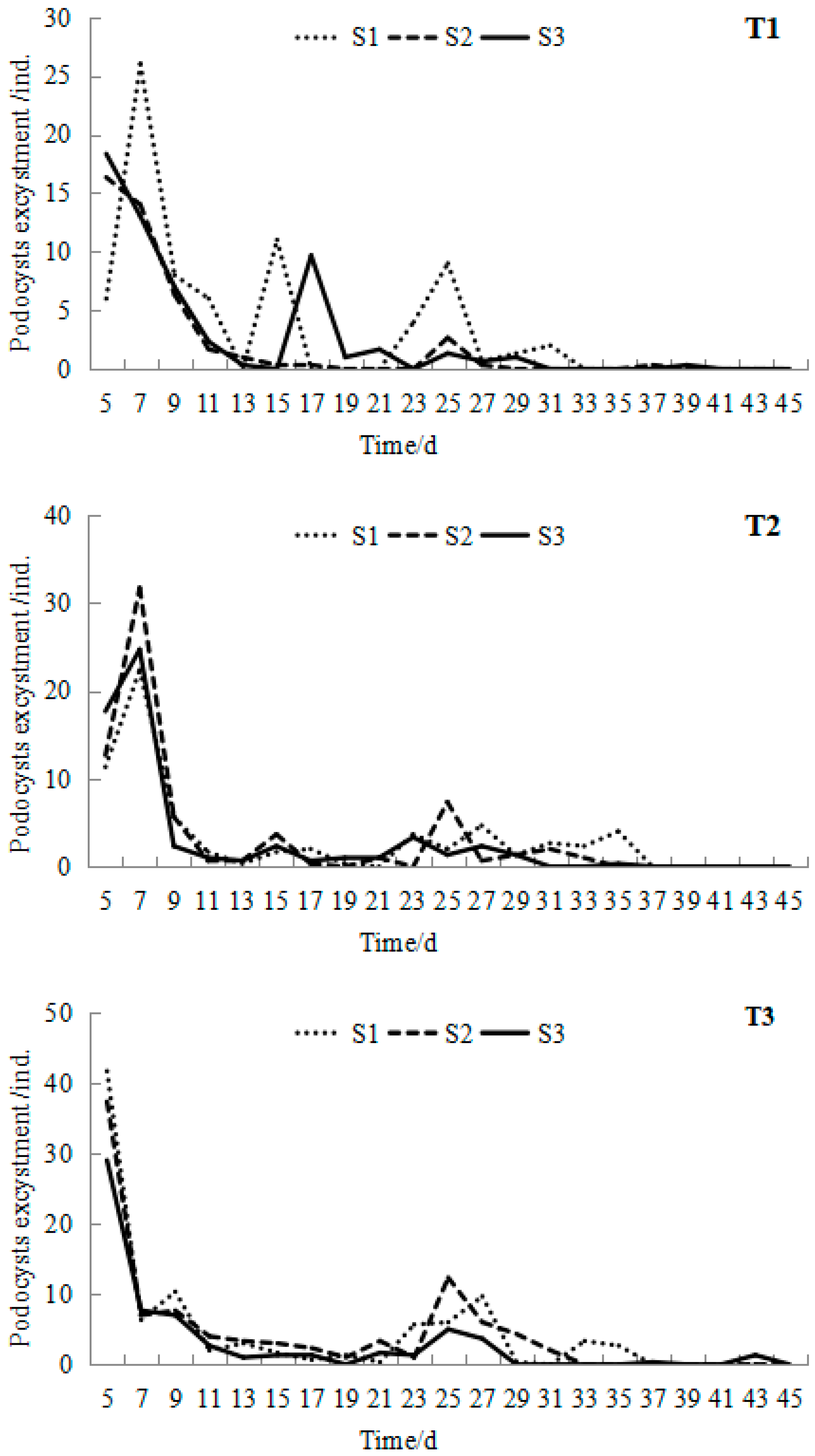
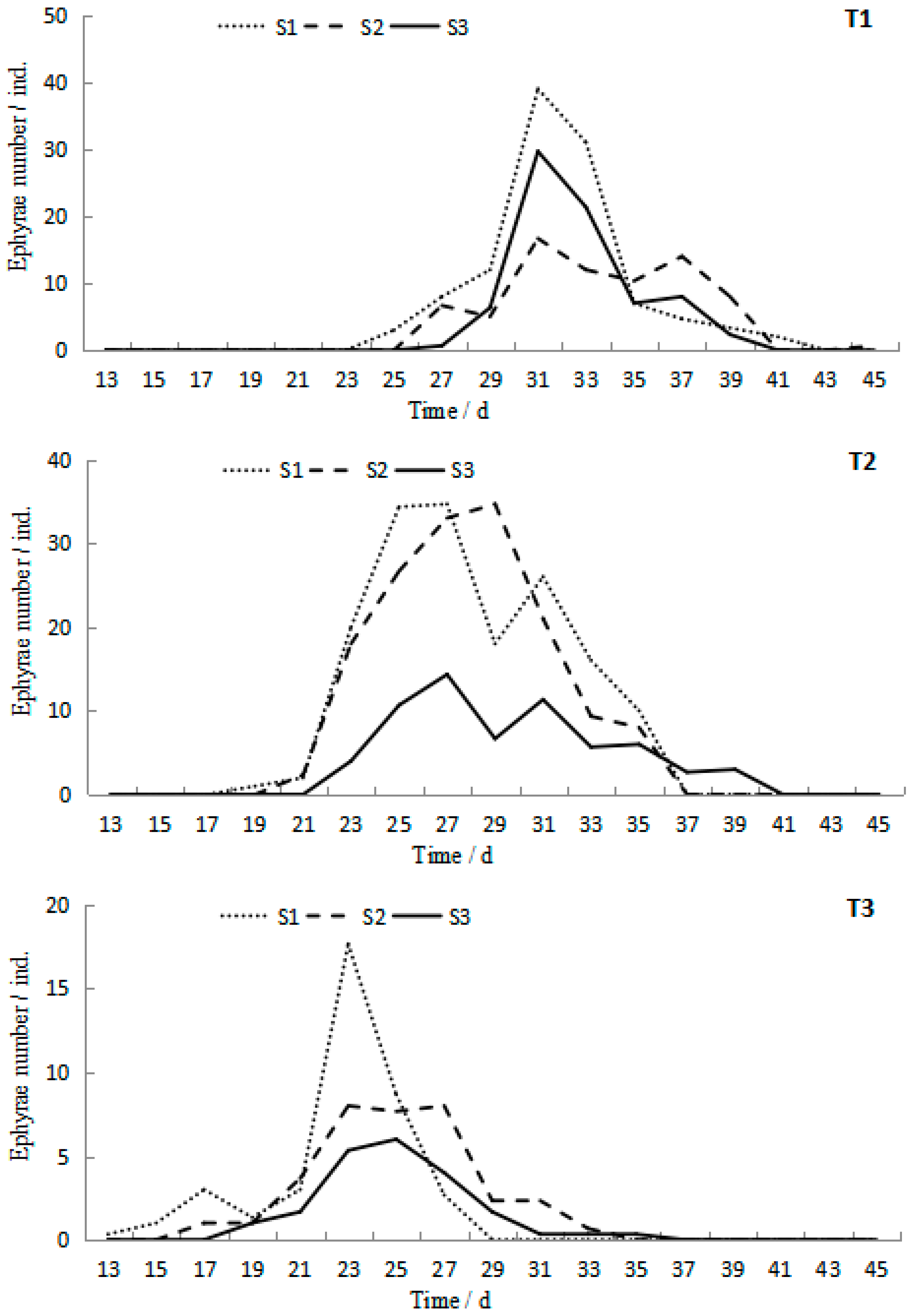
After preservation at a low temperature (2 ± 1 °C) for more than 12 months, R. esculentum podocysts began to excyst with the stimulus of temperature warming. Podocyst excystment occurred in all treatments (Figure 3). All the peak values of podocyst excystment appeared in the first week, and the number showed a trend of fluctuation and decline with time increase. The mean peak value of each group ranged from 16 to 42, with the minimum mean peak value occurring on the fifth day at 16.9 °C in the T1S2 group and the maximum mean peak value occurring on the fifth day at 24.6 °C in the T3S1 group.
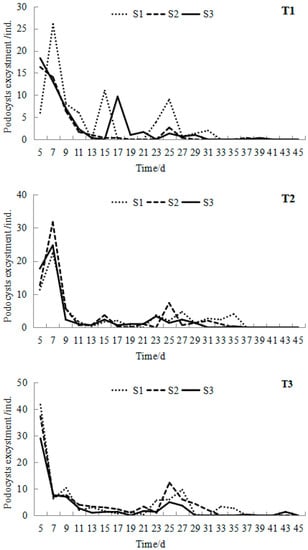
Figure 3.
Mean podocyst excystment at different temperatures and salinities during the 45-day experiment of Rhopilema esculentum.
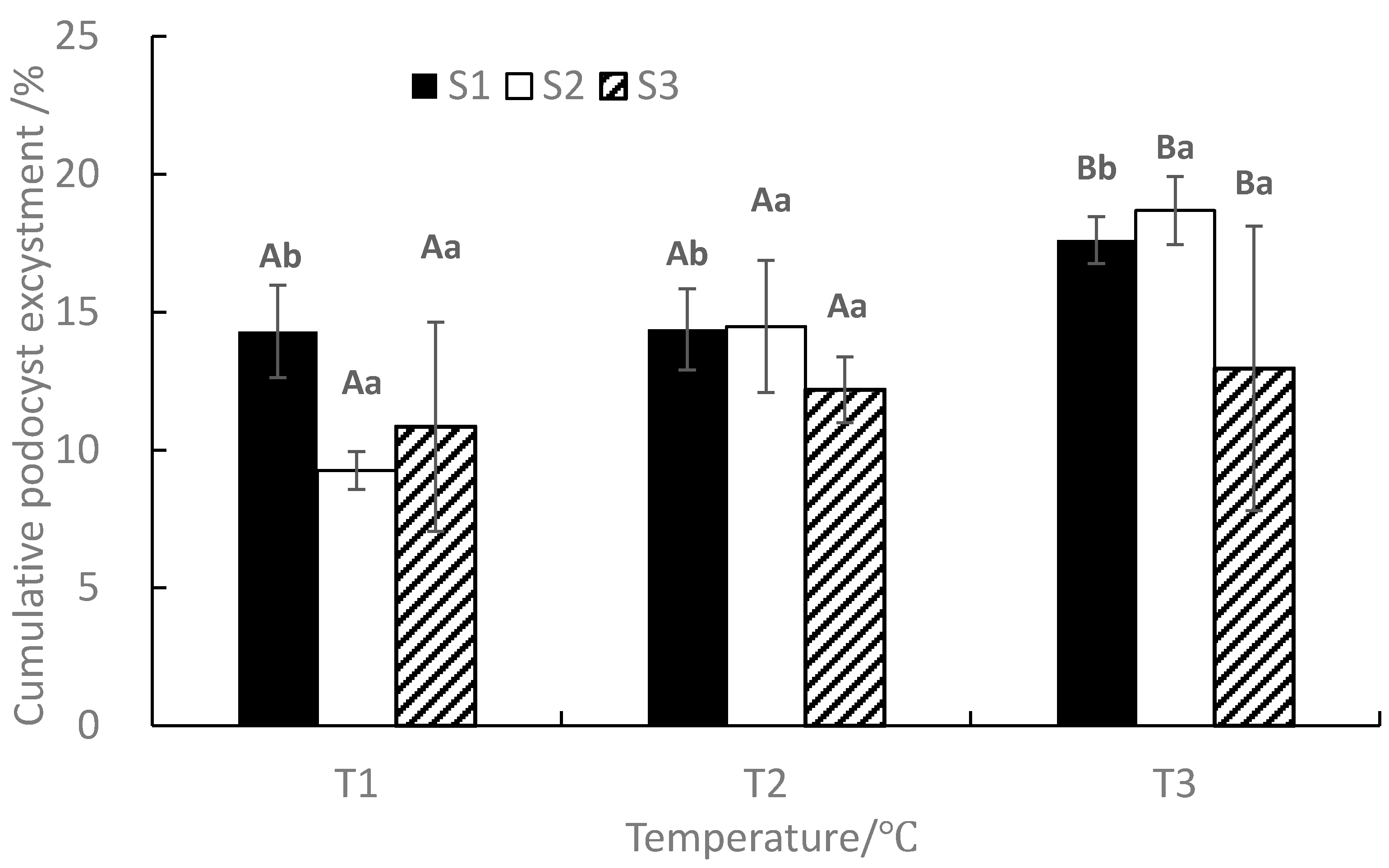
At the end of the experiment, the mean cumulative podocyst excystment rate ranged from 9.26 to 18.68%, with the maximum value occurring in T3S2 and the minimum value occurring in T1S2 (Figure 4). Under the same salinity, The mean cumulative podocyst excystment rate increased with the increase of temperature. ANOVA results showed that temperature and salinity significantly affected the cumulative podocyst excystment rate (p < 0.05), whereas the interaction between temperature and salinity was not significant (p > 0.05, Table 1).
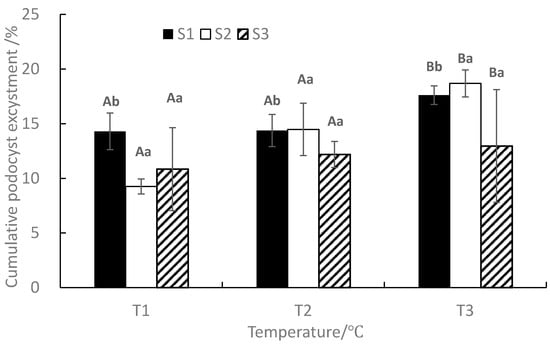
Figure 4.
Effects of different temperatures and salinities on cumulative podocyst excystment of Rhopilema esculentum. Vertical lines: SD. Means with different capital letters at different temperatures and different lowercase letters at different salinities are significantly different (p < 0.05) according to Duncan’s multiple comparison.

Table 1.
Summary of two-way ANOVA results testing the effects of temperature and salinity on the cumulative podocyst excystment, start time of strobilation, duration of strobilation, cumulative ephyra numbers, and transfer rates from podocysts to ephyrae in various cases. p < 0.05 was considered to be statistically significant and is shown in bold.
3.2. Strobilation
Polyps produced by the testing podocysts began to strobilate on the 13th day at 23.2 °C in the T3S1 group. Then strobilation successively occurred in all treatments (Figure 5). However, all the polyps strobilated only one time during the whole experiment. The start time of strobilation and the peak duration presented a tendency to delay with temperature decreasing and salinity increasing. ANOVA results showed that temperature and salinity significantly affected the start time of strobilation (p < 0.05), whereas their interaction was not significant (p > 0.05, Table 1). The duration of strobilation ranged from 11 to 18 days, with the maximum value occurring in T2S1 and the minimum value occurring in T3S3.
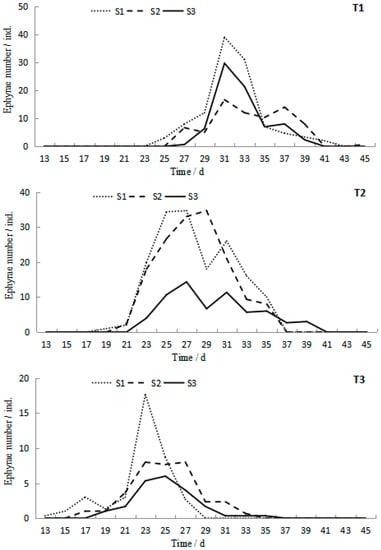
Figure 5.
Ephyra numbers at different temperatures and salinities during the 45-day experiment of Rhopilema esculentum.
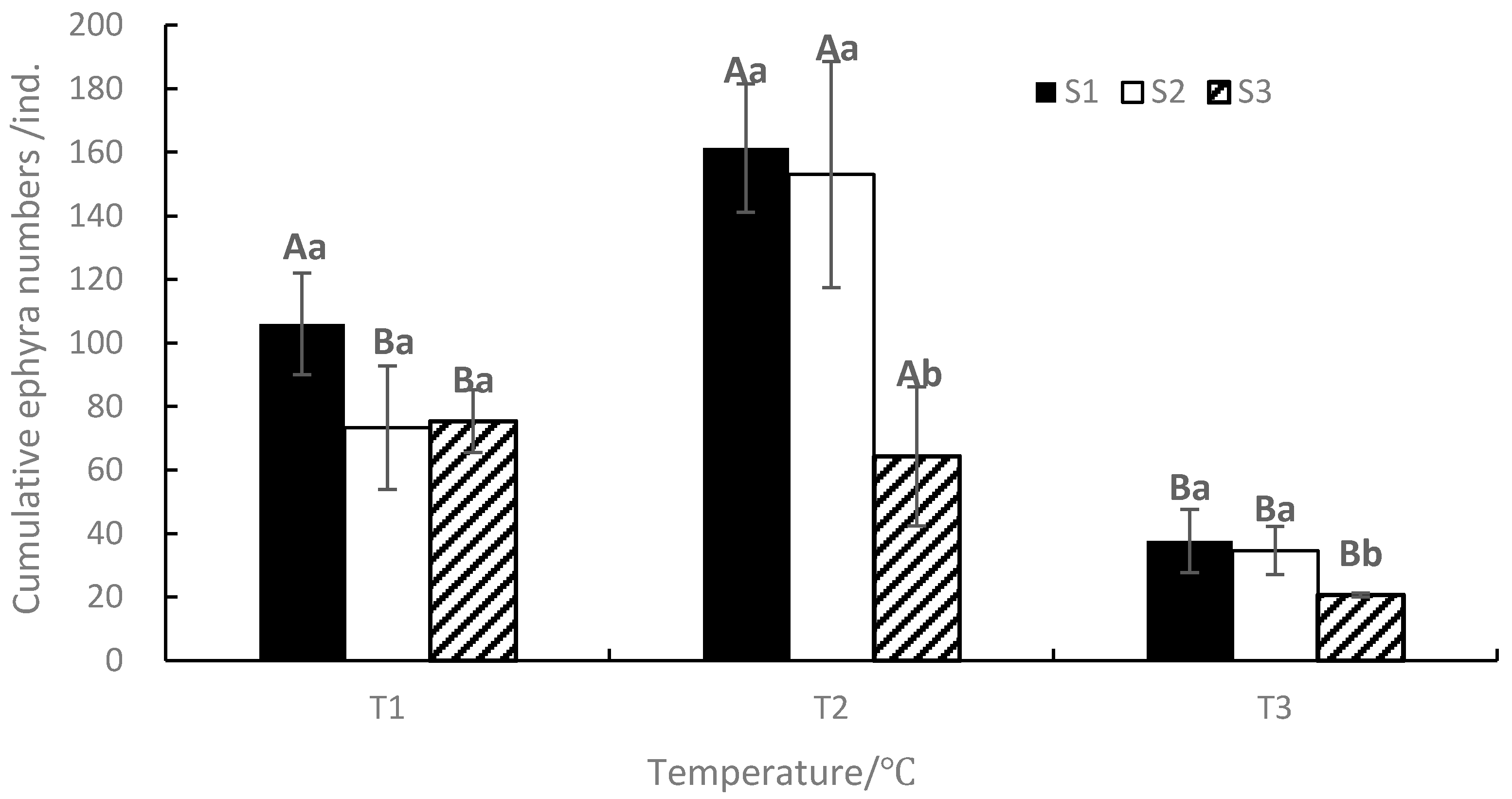
At the end of the experiment, the mean cumulative ephyra numbers ranged from 21 to 161, with the maximum value occurring in T2S1 and the minimum value occurring in T3S3 (Figure 6). The mean cumulative ephyra numbers of S1 are the most under the same temperature, and the mean cumulative ephyra numbers of T2 are the most under the same salinity. ANOVA results showed that temperature, salinity, and their interaction significantly affected the duration of strobilation and the cumulative ephyra numbers (p < 0.05, Table 1).
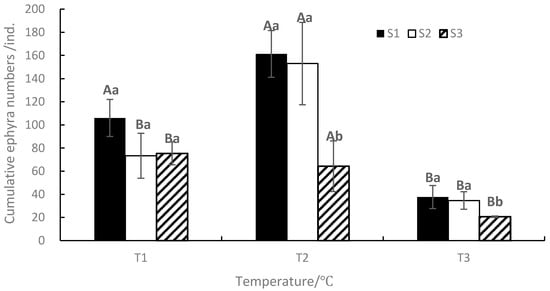
Figure 6.
Effects of different temperatures and salinities on the cumulative ephyra numbers. Vertical lines: SD. Means with different capital letters at different temperatures and different lowercase letters at different salinities are significantly different (p < 0.05) according to Duncan’s multiple comparison.
3.3. Transfer Rates from Podocysts to Ephyrae
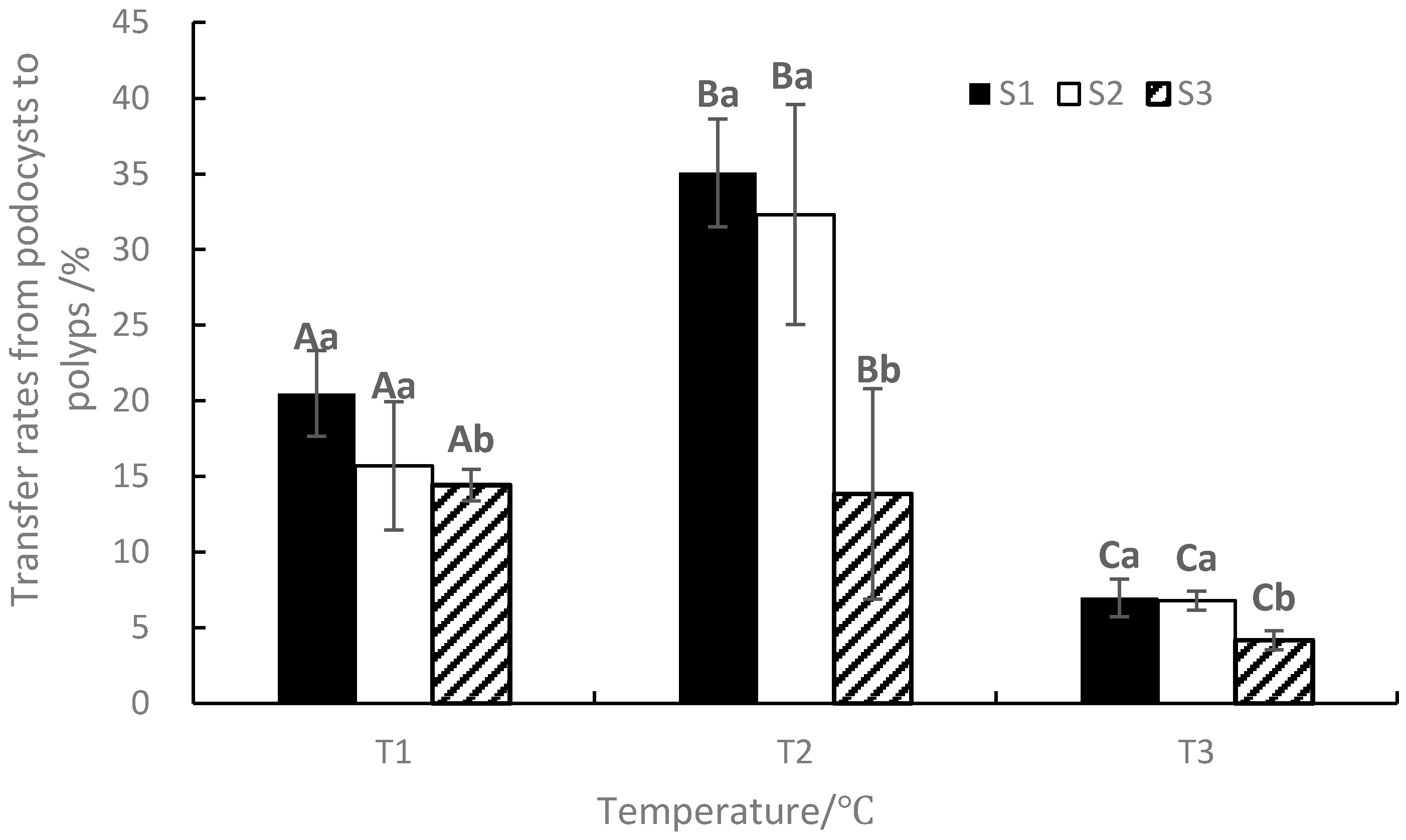
At the end of the experiment, the transfer rates from podocysts to ephyrae ranged from 4.16 to 35.08%, with the maximum value occurring in T2S1 and the minimum value occurring in T3S3 (Figure 7). The changing trend was similar to the mean cumulative ephyra numbers. ANOVA results showed that temperature, salinity, and their interaction significantly affected the transfer rates from podocysts to ephyrae (p < 0.05, Table 1).
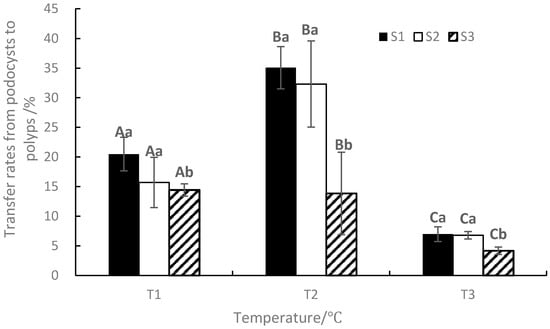
Figure 7.
Effects of different temperatures and salinities on the transfer rates from podocysts to ephyrae. Vertical lines: SD. Means with different capital letters at different temperatures and different lowercase letters at different salinities are significantly different (p < 0.05) according to Duncan’s multiple comparison.
4. Discussion
The life cycle of R. esculentum consists of alternating generations of planktonic jellyfish reproducing sexually and benthic polyps reproducing asexually [11]. The life cycle stage used for releasing in natural sea areas or inshore ponds is the planktonic ephyra stage. It only takes about two months to grow to a mature medusa stage that is ready for processing and selling. Correspondingly, a large number of ephyrae are required, and the seedling breeding process has spawned another industry. Ephyrae only derive from polyps by strobilating, while polyps derive from two ways. One is from the sexual reproduction of mature medusae and the other is from the asexual reproduction of polyps by producing podocysts. Therefore, podocyst reproduction and polyp strobilation are the key processes affecting seedling yield.
Polyps and podocysts are too small to be found in natural waters. Almost all reports on R. esculentum asexual reproduction have been carried out in the laboratory. Few studies on R. esculentum podocysts showed that the suitable range of temperature and salinity is wide. They can overwinter at low temperatures (−1.6–3.2 °C) for about three months and the survival rate is 100% [37]. However, how long can R. esculentum podocysts survive at low temperatures? Our experiments showed that podocyst excystment and subsequent strobilation occurred in all treatments, the cumulative podocyst excystment ranged from 7.84% to 22.01%, and the transfer rates from podocysts to ephyrae ranged from 4.16% to 35.08%. The results fully proved that R. esculentum podocysts, preserved at 1–3 °C for more than 12 months, still had the ability to excyst, and could be recycled and bred under appropriate environmental conditions.
The influences of environmental factors on the asexual reproduction of many cnidarian jellyfish species have been extensively studied over the last several decades [42,43,44]. Temperature and salinity were implicated as key environmental factors playing an important role in determining the annual presence and production of scyphomedusa [45], including N. nomurai [46,47], Aurelia aurita, Rhizostoma pulmo, Cotylorhiza tuberculata [48], and R. esculentum [15,37]. R. esculentum podocysts did not excyst below 10 °C and would die when salinity was below 8. Podocyst excystment increased with the increase of temperature in the range of 15–30 °C. The optimum salinity range for R. esculentum podocyst excystment was 18–22 [37]. Temperatures (16, 20, and 24 °C) and salinities (18, 22, and 26) in the combined experiments showed that both temperature and salinity had significant effects on the number of podocysts formed, but the interaction was not significant [39]. Our results showed that rapid high temperature and lower salinity were beneficial to podocyst excystment (p < 0.05). This conclusion was consistent with previous research, which could indicate that the control effects of temperature and salinity on podocyst excystment did not change because of long-term preservation at low temperatures. In our research, the start time of strobilation and the peak duration presented a tendency to delay with temperature decreasing and salinity increasing, which was in accord with previous conclusions that as the temperature increased, ephyrae would be released earlier [39]. In general, strobilation of overwintering polyps of R. esculentum occurred in spring when the temperature rose to 13–19 °C [15,17,19,38]. However, the water temperature has been increased above 20 °C rapidly to speed up the strobilation in artificial breeding [15,28,40]. In this study, strobilation of R. esculentum polyps occurred when temperatures rose from 3 °C to 19–25 °C, indicating warming of seawater temperature was also a trigger for these polyps produced by podocysts of post-preservation at low temperature for a long time.
In northern China, the earliest time to release seedlings of R. esculentum ephyrae is usually in April or May in spring when the pond water temperature reaches 15 °C, then continuously the seedlings are released into the pond in spring and summer, and continuously the adult medusae are caught after two months. In the breeding process, there has always been a shortage of seedlings to satisfy the market demand. In order to expand the duration of the seedling supply, R. esculentum polyps have been preserved at low temperatures for a long time [41]. Although this method can expand the duration of the seedlings’ supply, the increasing occurrence of many polyps detaching from the attachment plate proposed a new problem. In this study, the testing object targeted R. esculentum podocysts, a key stage of long-term preservation, which is more resistant to adverse environments than the polyps. We try to solve the bottleneck problem of breeding industrial development by innovating the method of long-term preserved podocysts at low temperatures.
Our testing results proved that the polyps produced by podocysts could strobilate in a short time and release ephyrae with warming temperature, even finishing the whole process within 45 days. Therefore, it is feasible to obtain ephyrae by R. esculentum podocysts for long-term preservation at low temperatures. In addition, You et al. proposed that the optimization of low-temperature duration and temperature change level might improve the reproductive efficiency of strobilation, which needs to be further studied [41]. In our research, the transfer rates from podocysts to ephyrae in T2S1 and T2S2 are significantly higher than other treatments, which indicated that compared to warming the podocysts to natural water temperature in early 1 April, delaying warming on 1 May would obtain significantly greater ephyra numbers. Different temperatures and salinity resulted in significantly different ephyra numbers and occurring times. These research data could provide a scientific basis for precise control methods in the future.
5. Conclusions
Podocyst excystment and ephyrae production occurred in all test treatments. Higher temperature and lower salinity significantly facilitated the podocyst excystment and accelerated the start time of strobilation (p < 0.05). Significantly greater ephyra numbers were produced with lower salinity and temperatures increasing from 18 °C on 1 May to natural levels (p < 0.05). There were significant interactions between temperature and salinity on the cumulative ephyra numbers and the transfer rates from podocysts to ephyrae (p < 0.05). These results suggested that R. esculentum podocysts preserved for a long time at low temperatures could be recycled. Temperature and salinity regulation can affect the numbers of R. esculentum seedlings and the time to achieve high production and satisfy the market demand for real-time seedling supply. This conclusion would provide a scientific basis for the innovative methods of sustainable utilization of the edible jellyfish (R. esculentum) resources.
Author Contributions
M.S. conducted the experiments, analyzed the data, and wrote the paper. F.C. took part in writing the paper. Y.D. took part in feeding the polyps. J.S. designed the experiments and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Liaoning Academy of Agricultural Sciences Dean Fund Program, grant numbers 2021MS0505; the National Natural Science Foundation of China, grant numbers 41906138 and 31770458; and the National Key R&D Program of China, grant numbers 2017YFC1404401.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dong, J.; Jiang, L.X.; Tan, K.F.; Liu, H.Y.; Purcell, J.E.; Li, P.J.; Ye, C.C. Stock enhancement of the edible jellyfish (Rhopilema esculentum Kishinouye) in Liaodong Bay, China: A review. Hydrobiologia 2009, 616, 113–118. [Google Scholar] [CrossRef]
- Li, Y.F.; Li, Y.L.; Zhou, Z.C.; Tian, M.L.; Bao, X.B.; He, C.B. Pond culture of edible jellyfish Rhopilema esculentum in North China coast: Research progress. Fish. Sci. 2020, 39, 286–294. [Google Scholar]
- You, K.; Chi, X.P.; Ma, C.H.; Liu, X.T.; Mu, Y.C.; Han, Z.Q.; Gao, Z.X.; Ding, M.L.; Luo, C.Y. Analysis on the development of Rhopilema Esculentum industry in China. Chin. Fish. Econ. 2012, 30, 108–112. [Google Scholar]
- Li, Y.F.; Zheng, M.X.; Zhu, F.; Lin, J. Preparation and physicochemical properties of jellyfish collagen. J. Fuzhou Univ. 2018, 46, 286–294. [Google Scholar]
- You, K.; Ma, C.H.; Gao, H.; Li, F.; Zhang, M.; Wang, Q.B. Research on the jellyfish (Rhopilema esculentum Kishinouye) and associated aquaculture techniques in China:current status. Aquac. Int. 2007, 15, 479–488. [Google Scholar] [CrossRef]
- Jiao, J.J.; Zhang, Y.Q.; Zhang, J.H.; Li, L.Z.; Qiu, S.Y. Jellyfish Rhopilema esculentum stock enhancement in coastal Shandong province: Review and thinking. Fish. Sci. 2021, 40, 141–150. [Google Scholar]
- Arai, M.N. The potential importance of podocysts to the formation of scyphozoan blooms: A review. Hydrobiologia 2009, 616, 241–246. [Google Scholar] [CrossRef]
- Thein, H.; Ikeda, H.; Uye, S.I. Ecophysiological characteristics of podocysts in Chrysaora pacifica (Goette) and Cyanea nozakii Kishinouye (Cnidaria: Scyphozoa: Semaeostomeae): Effects of environmental factors on their production, dormancy and excystment. J. Exp. Mar. Biol. Ecol. 2013, 446, 151–158. [Google Scholar] [CrossRef]
- Kawahara, M.; Ohtsu, K.; Uye, S.I. Bloom or non-bloom in the giant jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae): Roles of dormant podocysts. J. Plankton Res. 2013, 35, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Thein, H.; Ikeda, H.; Uye, S.I. The potential role of podocysts in perpetuation of the common jellyfish Aurelia aurita s.l. (Cnidaria: Scyphozoa) in anthropogenically perturbed coastal waters. Hydrobiologia 2012, 690, 157–167. [Google Scholar] [CrossRef]
- Ding, G.W.; Chen, J.K. The life history of Rhopilema esculent a kishinouye. J. Fish. China 1981, 5, 93–104. [Google Scholar]
- Sun, M.; Dong, J.; Zhao, Y.; Fu, Z.L. Morphological studies on advanced metephyrae of Nemopilema nomurai and Rhopilema esculentum. Prog. Fish. Sci. 2010, 31, 48–53. [Google Scholar]
- Liu, C.S.; Wan, Y.; Gao, F.; Chen, S.Q.; Wang, A.M.; Gu, Z.F. Ultrastructure of the embryonic development and metamorphosis of Rhopilema esculentum. Acta Hydrobiol. Sin. 2018, 42, 1019–1026. [Google Scholar]
- Hao, Z.L.; Zheng, B.; Li, Q.; Li, X.Y.; Chen, T.J. Research Progress on the effects of environmental conditions on the growth and development of jellyfish. J. Dalian Fish. Univ. 2015, 30, 444–448. [Google Scholar]
- Chen, J.K.; Ding, G.W. The effect of temperature on the strobilation of Rhopilema esculenta Kishinouye. Acta Zool. Sin. 1983, 29, 195–206. [Google Scholar]
- Chen, J.K.; Ding, G.W.; Liu, C.Y. The effect of light on the strobilation of Rhopilema esculenta Kishinouye. Oceanol. Et Limnol. Sin. 1984, 15, 310–316. [Google Scholar]
- Chen, J.K.; Ding, G.W. On the seasonal regularity of strobilation of edible medusa. J. Fish. China 1984, 8, 55–68. [Google Scholar]
- Guo, P. Effect of nutritional condition on the formation and germination of the podocyst of scyphistomae of Rhopilema esculenta Kishinouye. J. Fish. China 1990, 14, 206–211. [Google Scholar]
- You, K.; Ma, C.H.; Gao, H.W.; Li, F.; Wei, R. The Effects of temperature decrease on the scyphistomae strobilation of Jellyfish, Rhopilema esculentum Kishinouye. J. World Aquac. Soc. 2008, 39, 706–711. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, B.; Wang, W.B.; Dong, J. Genetic diversity analysis of Rhopilema esculentum population in Liaodong Bay based on COI wave gene. Fish. Sci. 2016, 35, 404–409. [Google Scholar]
- Li, Y.L.; Dong, J.; Wang, B.; Li, Y.P.; Yu, X.G.; Fu, J.; Wang, W.B. Genetic characterization of different populations of Rhopilema esculentum based on the mito-chondrial COI sequence. Chin. J. Appl. Ecol. 2016, 27, 2340–2347. [Google Scholar]
- Di, M.Y.; Zhou, Z.C.; Li, Y.F.; Tian, M.L.; Hou, H.M.; Li, Y.L.; Bao, X.B.; He, C.B. Analysis of genetic diversity in four wild populations of jellyfish Rhopilema esculenta by microsatellite markers. Fish. Sci. 2018, 37, 762–768. [Google Scholar]
- Zhang, J.H.; Qiu, S.R.; Qiao, F.Q.; Li, Z.; Geng, B.L. Relationship between Distribution of Jellyfish Resources and Zooplankton in Coastal Southern Shandong. Fish. Sci. 2013, 32, 98–101. [Google Scholar]
- Sun, M.; Wang, B.; Li, Y.L.; Wang, A.Y.; Dong, J.; Ma, T.Y.; Ban, Y.L. Feeding habitats and trophic levels of Rhopilema esculentum Kishinouye in Liaodong Bay based on analyzing carbon and nitrogen stable isotopes. Chin. J. Appl. Ecol. 2016, 27, 1103–1108. [Google Scholar]
- Liang, W.B.; Jiang, L.X.; Yu, S.L. The Review and developmental Strategies on the Enhancement and Releasement of Jellyfish in Offshore Fishing Areas in Liaoning Province. Fish. Sci. 2007, 26, 423–424. [Google Scholar]
- Zhou, Y.D. The retrospection and prospect of releasing and enhancement of fishery resources in Zhejiang coastal waters. Mar. Fish. 2004, 26, 131–139. [Google Scholar]
- Liu, C.Y.; Bi, Y.P. A Method of Recapture Rate in Jellyfish Ranching. Fish. Sci. 2006, 25, 150–151. [Google Scholar]
- Chen, S.Q.; Zhang, Y.; Wang, Y.G.; Yu, D.X. Breeding of jellyfish (Rhopilema esculenta Kishinouye). Mar. Sci. 2004, 28, 4–7. [Google Scholar]
- Zhang, M.; Lu, D.X. Rhopilema esculentum culture techniques in Nantong area. Hebei Fish. 2015, 12, 44–47. [Google Scholar]
- Jia, Q.H.; Yang, P.W.; Chen, X.X.; Bai, H.F.; Li, X.C.; Liu, G.; Shen, H.B.; Wen, S.E. Rhopilema esculentum artificial cultivation and managemen. Mod. Agric. Technol. 2015, 15, 272–273. [Google Scholar]
- Guo, K.; Zhao, W.; Dong, S.L.; Jiang, Z.Q. Structure of suspended particles andorganic carbon storage in jellyfish-shellfish-fish-prawn polyculture ponds. Acta Ecol. Sin. 2016, 36, 1872–1880. [Google Scholar]
- Sun, X.W.; Zheng, W.J.; Huang, Y.D. Polyculture of Jellyfish, Tiger Shrimp, and Razor Clam (Sinonovacula constricta) in a Pond in Nanton. Chin. J. Fish. 2017, 30, 38–41. [Google Scholar]
- Wang, B.; Tian, J.S.; Zhou, Z.C. Food web in jellyfish-shrimp-shellfish polyculture pond. Chin. J. Appl. Ecol. 2021, 32, 2028–2034. [Google Scholar]
- Zhao, Z.L.; Guan, X.Y.; Wang, B.; Dong, Y.; Zhou, Z.C. Bacterial community composition in a polyculture system of Rhopilema esculenta, Penaeus monodon and Ruditapes Philipp. Aquac. Res. 2019, 50, 973–978. [Google Scholar] [CrossRef]
- You, K.; Ma, C.H.; Wang, S.J.; Gao, T.X.; Li, J.L.; Wang, X.W. Effects of aeration and feeding on podocyst germination of jellyfish, Rhopolema esculentum Kishinouye. J. Fish. Sci. China 2010, 17, 1353–1357. [Google Scholar]
- Lu, N.; Jiang, S.; Chen, J.K. Effects of temperature, salinity and light on the podoctst generation of Rhopilema esculenta kishinouye. Fish. Sci. 1997, 16, 3–8. [Google Scholar]
- Jiang, S.; Lu, N.; Chen, J.K. Effect of temperature, salinity and light on the germination of the podocyst of Rhopilema esculenta kishinouye. Fish. Sci. 1993, 12, 1–4. [Google Scholar]
- Zhang, X.L.; Cheng, Y.X.; Chen, S.Q.; Chen, C.Y.; Zhang, Y. The effect of temperature on strobilation and early stage growth of jellyfish, Rhopilema esculenta. J. Shanghai Fish. Univ. 2006, 15, 182–185. [Google Scholar]
- Wu, Y.; Li, S.F.; Yan, L.P.; Jiang, Y.Z.; Cheng, J.H. Effects of temperature and salinity on asexual reproduction of Rhopilema Esculenta. J. Anhui Agric. Sci. 2009, 37, 11414–11418. [Google Scholar]
- You, K.; Ma, C.H.; Wang, S.J.; Gao, T.X.; Yao, Z.H.; Wang, X.W. The effect of short-term low temperature on strobilation of jellyfish, Rhopilema esculenta kishinouve. Acta Hydrobiol. Sin. 2010, 34, 1223–1227. [Google Scholar] [CrossRef]
- You, K.; Wang, S.J.; Ma, C.H.; Gao, T.X.; Wang, Y.Z.; Yang, C.H. Long-term low temperature effect on jellyfish strobilation. Adv. Mar. Sci. 2011, 29, 215–220. [Google Scholar]
- Purcell, J.E. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Mar. Ecol. Prog. Ser. 2007, 348, 183–196. [Google Scholar] [CrossRef]
- Liu, W.C.; Lo, W.T.; Purcell, J.E.; Chang, H.H. Effect of temperature and light intensity on asexual reproduction of the scyphozoan, Aurelia aurita (L.) in Taiwan. Hydrobiology 2009, 616, 247–258. [Google Scholar] [CrossRef]
- Sun, M.; Chai, Y.; Dong, J.; Fu, Z.L.; Liu, Z.G.; Lin, J.Q.; Wang, J.H. Effects of environmental factors on polyp survival and reproduction of Aurelia ap.1. Acta Ecol. Sin. 2017, 37, 1309–1317. [Google Scholar]
- Willcox, S.; Moltschaniwskyj, N.A.; Crawford, C. Asexual reproduction in scyphistoma of Aurelia sp.: Effects of temperature and salinity in an experimental study. J. Exp. Mar. Biol. Ecol. 2007, 353, 107–114. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, S.; Jin, X.; Li, C.L. Associations of large jellyfish distributions with temperature and salinity in the Yellow Sea and East China Sea. Hydrobiology 2012, 690, 81–96. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Purcell, J.E.; Li, Y.L.; Duan, Y.; Wang, A.Y.; Wang, B. Testing the influence of previous-year temperature and food supply on development of Nemopilema nomurai blooms. Hydrobiology 2015, 754, 85–96. [Google Scholar] [CrossRef]
- Purcell, J.E.; Atienza, D.; Fuentes, V.; Olariaga, A.; Tilves, U.; Colahan, C.; Gili, J.M. Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. Hydrobiology 2012, 690, 169–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).