Effect of Chemical Pre-Treatment on the Catalytic Performance of Oil Palm EFB Fibre Supported Magnetic Acid Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
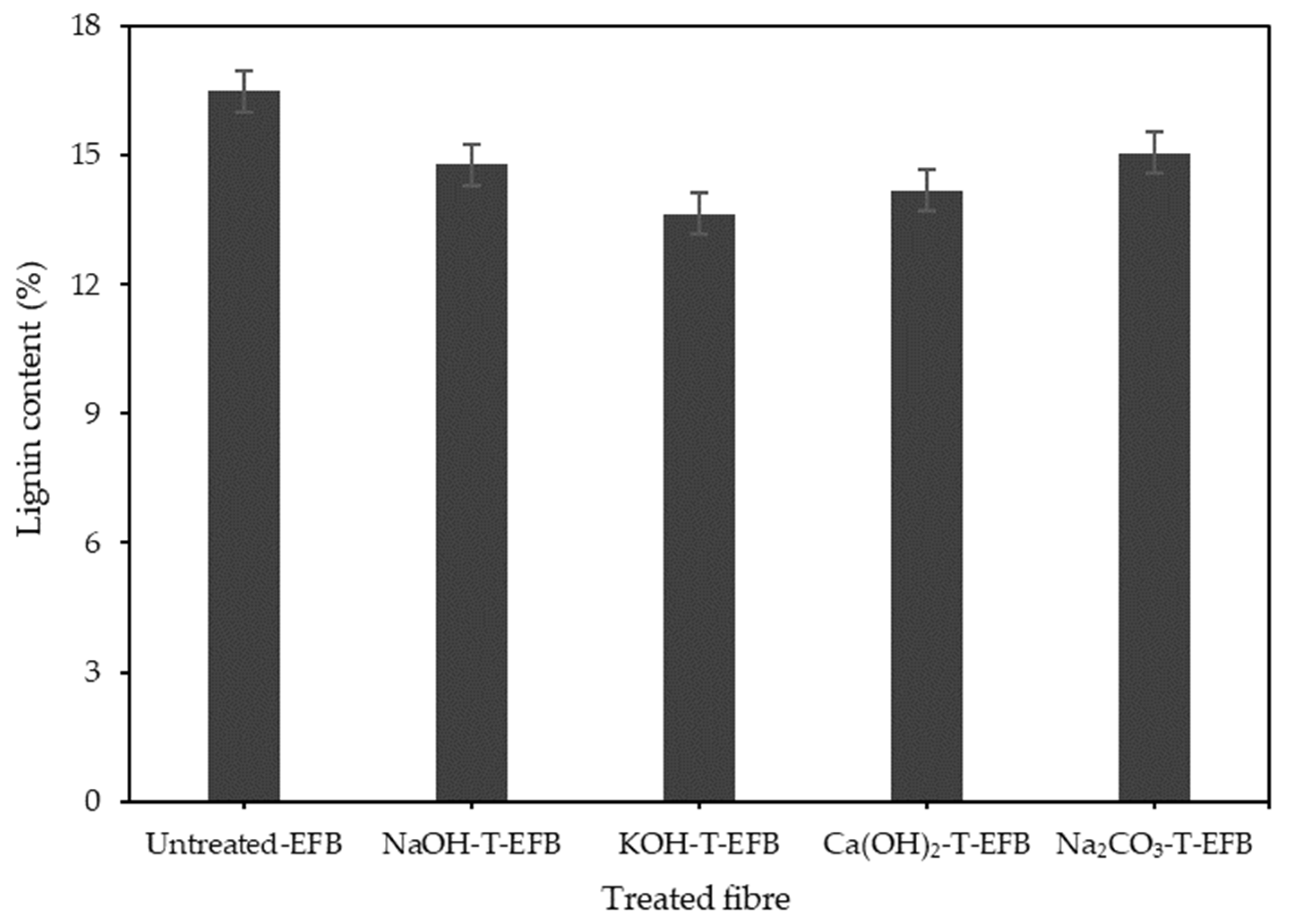
2.2. EFB Fibre Chemical Pre-Treatment
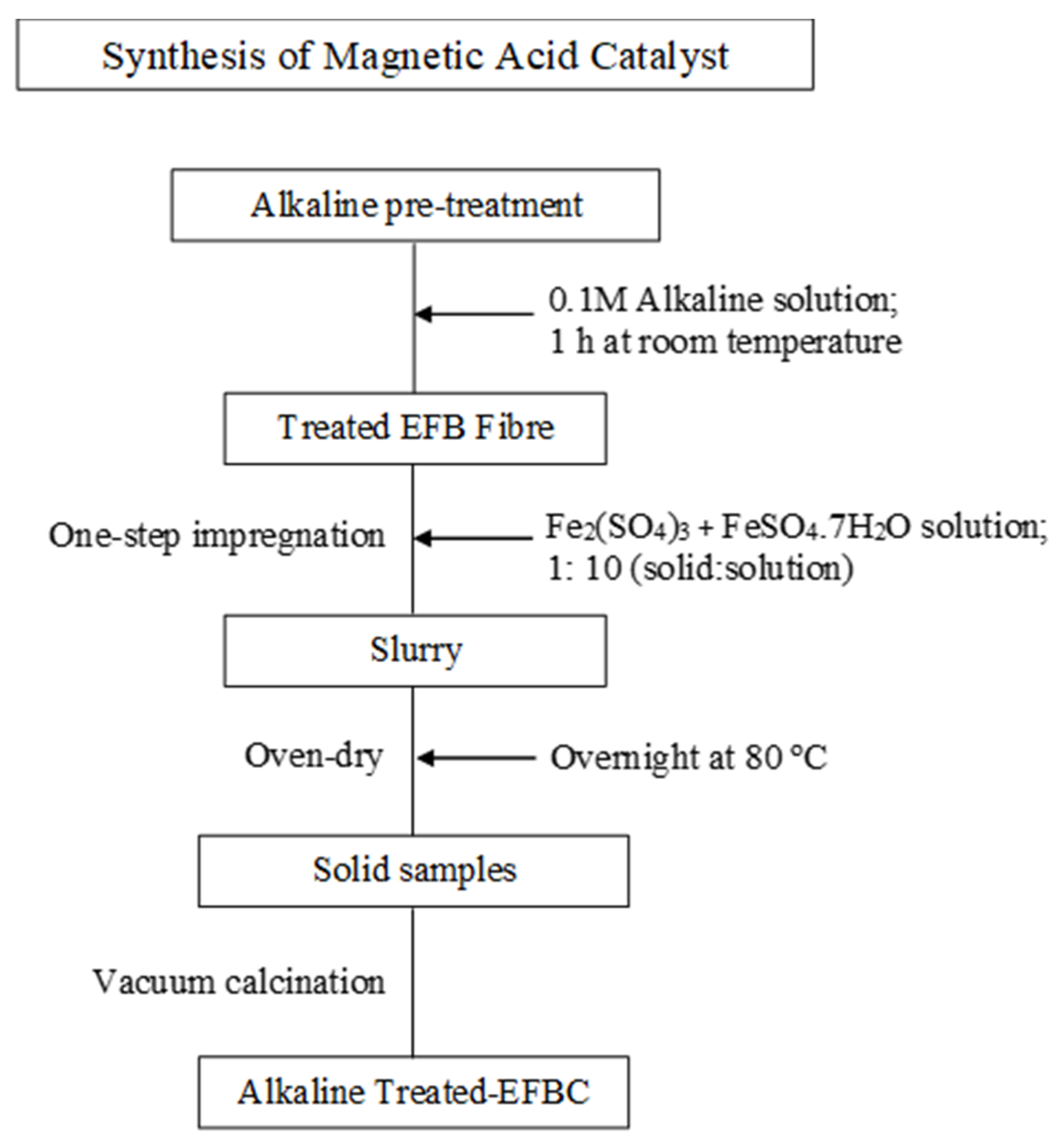
2.3. Synthesis of the Magnetic Solid Catalyst
2.4. Characterisation of the Fibre and Catalyst
2.5. Catalytic Performance
3. Results and Discussion
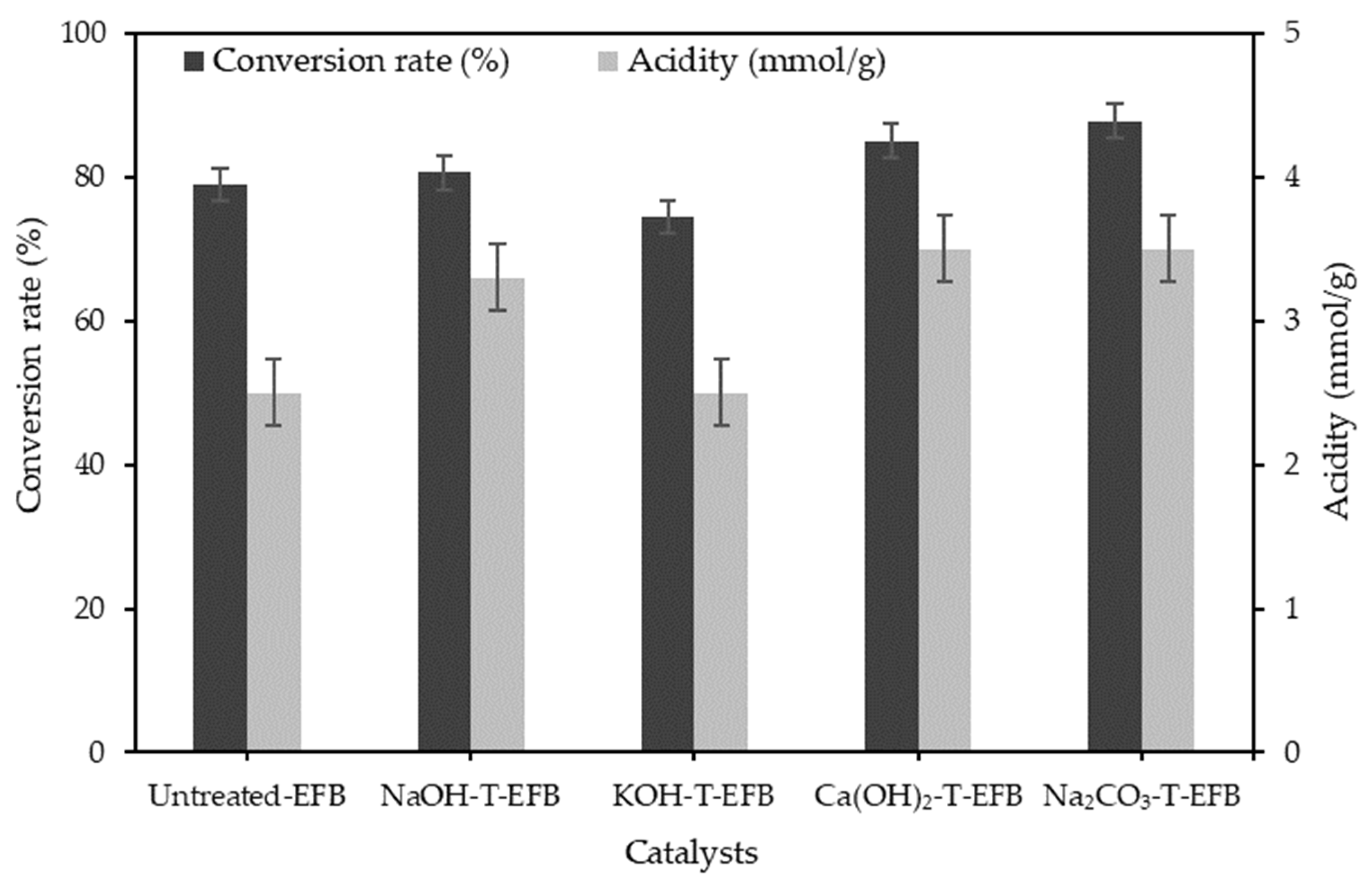
3.1. Effect of Various Chemical Pre-Treatments on the Esterification Reaction
3.2. Single Factor Optimisation
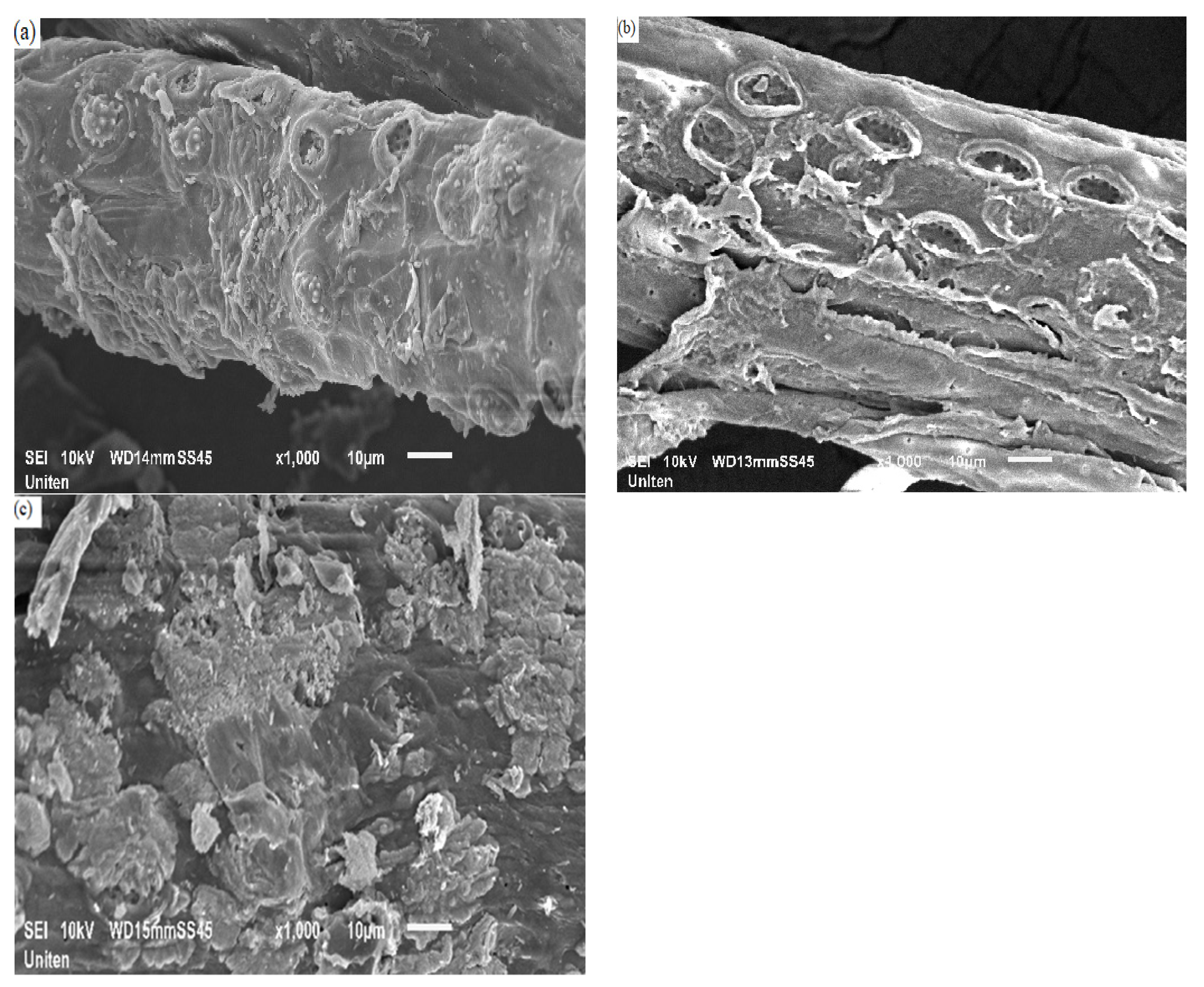
3.3. Morphology and Elemental Analysis
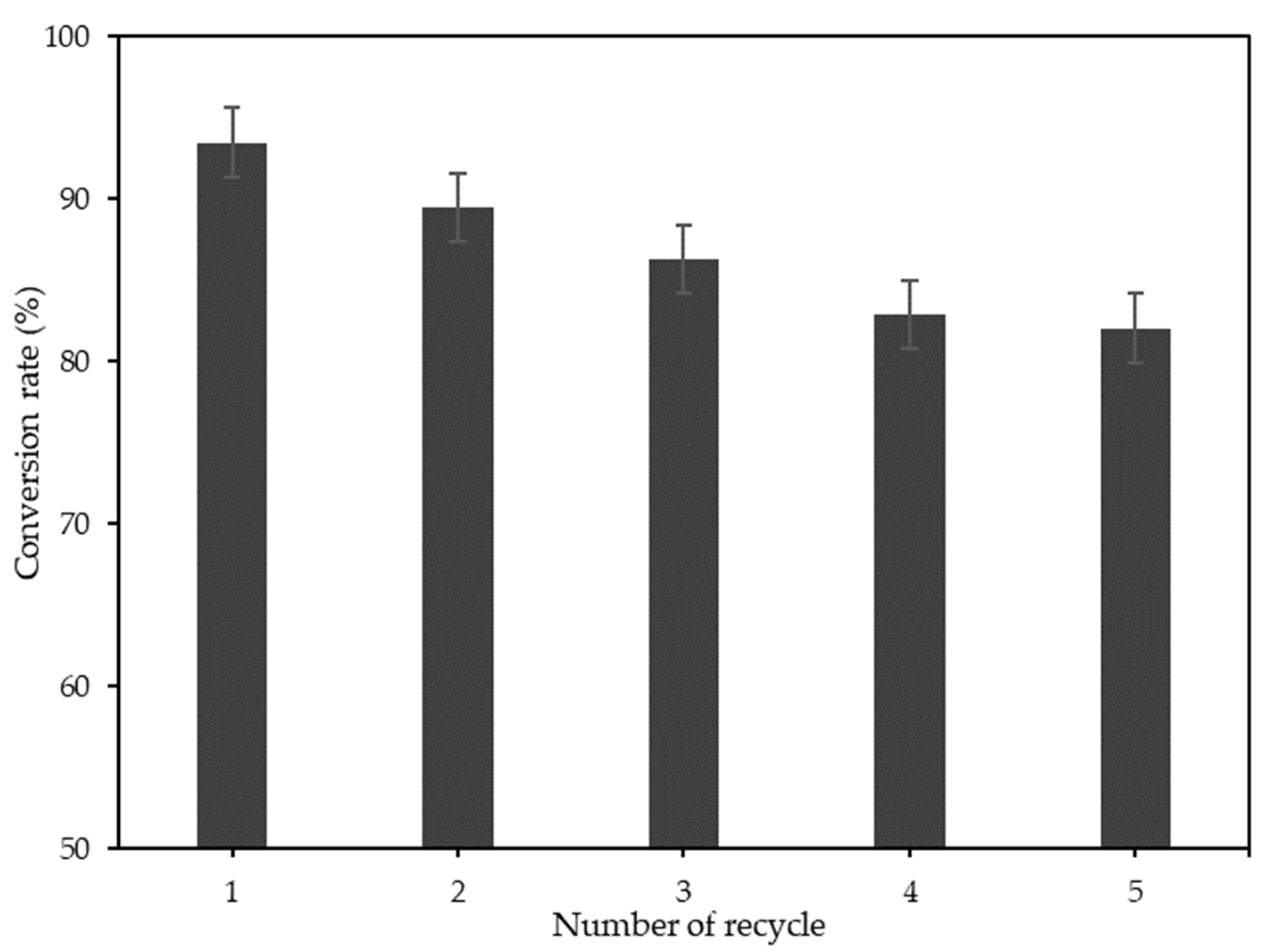
3.4. Catalyst Reusability
3.5. Comparison of Surface Acidity on the Catalytic Activity of Biomass Derived Solid Catalysts
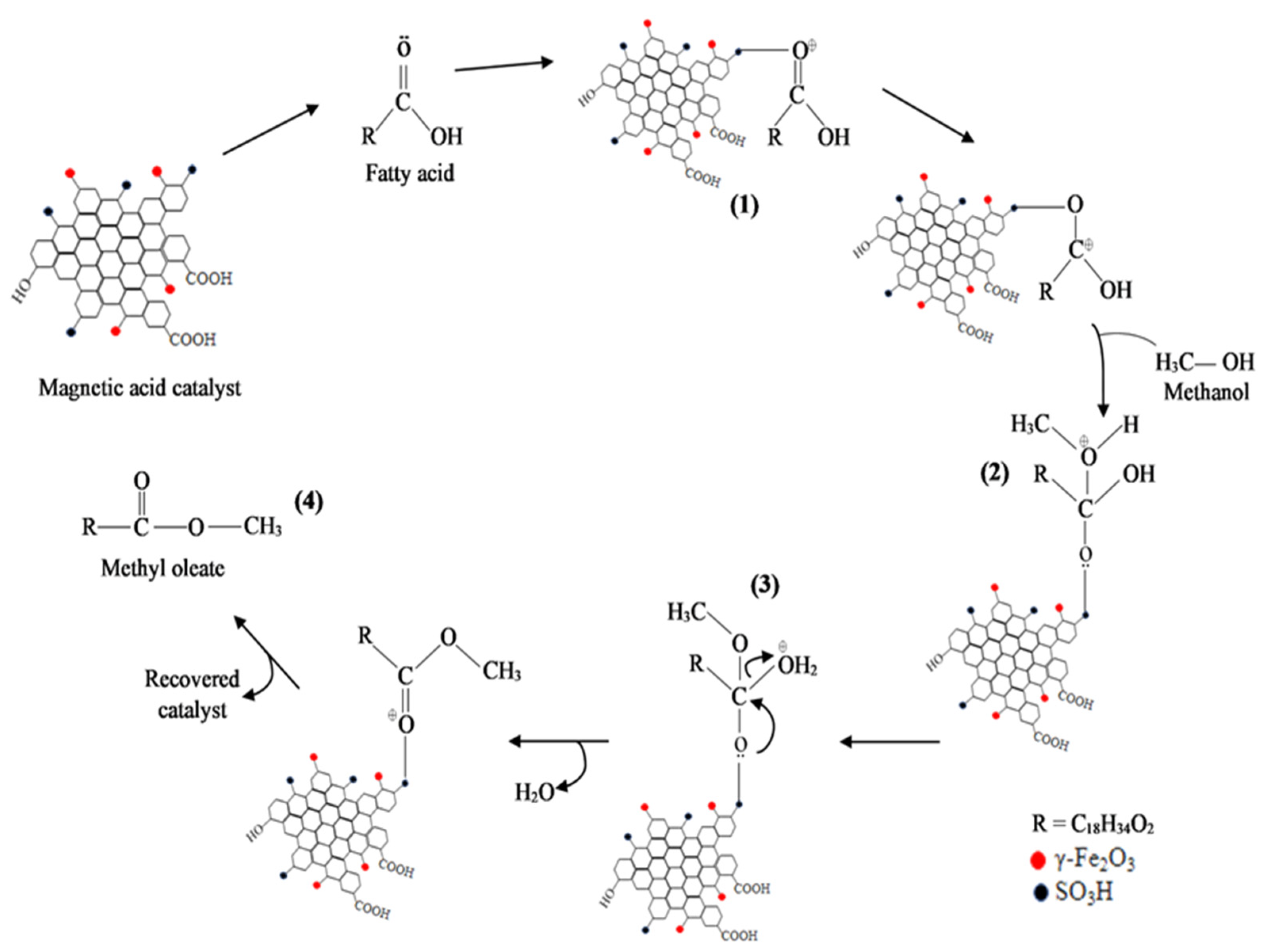
3.6. Reaction Mechanism Using Na2CO3-T-EFBC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rath, B.N.; Akram, V.; Bal, D.P.; Mahalik, M.K. Do Fossil Fuel and Renewable Energy Consumption Affect Total Factor Productivity Growth? Evidence from Cross-Country Data with Policy Insights. Energy Policy 2019, 127, 186–199. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of Biodiesel Production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Faruque, M.O.; Razzak, S.A.; Hossain, M.M. Application of Heterogeneous Catalysts for Biodiesel Production from Microalgal Oil—A Review. Catalysts 2020, 10, 1025. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, S.A.; Hsieh, P.H.; Hou, S.S. Optimized Conversion of Waste Cooking Oil to Biodiesel Using Modified Calcium Oxide as Catalyst via a Microwave Heating System. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Ullah, F.; Dong, L.; Bano, A.; Peng, Q.; Huang, J. Current Advances in Catalysis toward Sustainable Biodiesel Production. J. Energy Inst. 2016, 89, 282–292. [Google Scholar] [CrossRef]
- Chen, M.N.; Mo, L.P.; Cui, Z.S.; Zhang, Z.H. Magnetic Nanocatalysts: Synthesis and Application in Multicomponent Reactions. Curr. Opin. Green Sustain. Chem. 2019, 15, 27–37. [Google Scholar] [CrossRef]
- Tang, X.; Niu, S. Preparation of Carbon-Based Solid Acid with Large Surface Area to Catalyze Esterification for Biodiesel Production. J. Ind. Eng. Chem. 2019, 69, 187–195. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Nguyen, M.H.; Nguyen, M.D. Synthesis of Acidic Heterogeneous Catalysts with High Stability Based on Graphene Oxide/Activated Carbon Composites for the Esterification of Lactic Acid. J. Chem. 2019, 7815697, 1–7. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A Magnetically Separable SO4/Fe-Al-TiO2 Solid Acid Catalyst for Biodiesel Production from Waste Cooking Oil. Appl. Catal. B 2018, 234, 268–278. [Google Scholar] [CrossRef]
- Kristiani, A.; Sembiring, K.C.; Aulia, F.; Abimanyu, H. Sulfated Zirconia Catalyst for Hydrolysis of Palm Oil Lignocellulosic Wastes. Energy Procedia 2015, 65, 8–13. [Google Scholar] [CrossRef]
- Iwanow, M.; Gärtner, T.; Sieber, V.; König, B. Activated Carbon as Catalyst Support: Precursors, Preparation, Modification and Characterization. Beilstein J. Org. Chem. 2020, 16, 1188–1202. [Google Scholar] [CrossRef]
- Feyzi, M.; Norouzi, L. Preparation and Kinetic Study of Magnetic Ca/Fe3O4@SiO2 Nanocatalysts for Biodiesel Production. Renew Energy 2016, 94, 579–586. [Google Scholar] [CrossRef]
- Gardy, J.; Nourafkan, E.; Osatiashtiani, A.; Lee, A.F.; Wilson, K.; Hassanpour, A.; Lai, X. A Core-Shell SO4/Mg-Al-Fe3O4 Catalyst for Biodiesel Production. Appl. Catal. B 2019, 259, 118093. [Google Scholar] [CrossRef]
- Loh, S.K. The Potential of the Malaysian Oil Palm Biomass as a Renewable Energy Source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Thushari, I.; Babel, S. Sustainable Utilization of Waste Palm Oil and Sulfonated Carbon Catalyst Derived from Coconut Meal Residue for Biodiesel Production. Bioresour. Technol. 2018, 248, 199–203. [Google Scholar] [CrossRef]
- Jafri, N.H.S.; Jimat, D.N.; Azmin, N.F.M.; Sulaiman, S.; Nor, Y.A. The Potential of Biomass Waste in Malaysian Palm Oil Industry: A Case Study of Boustead Plantation Berhad. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 12028. [Google Scholar] [CrossRef]
- Dolah, R.; Karnik, R.; Hamdan, H. A Comprehensive Review on Biofuels from Oil Palm Empty Bunch (Efb): Current Status, Potential, Barriers and Way Forward. Sustainability 2021, 13, 10210. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, J.; Ma, P.; Cai, J.; Zhang, Y. Acid Value Determination and Pre-Esterification of Crude Euphorbia Lathyris L. Oil. World J. Eng. Technol. 2015, 3, 70–75. [Google Scholar] [CrossRef]
- Zhou, Y.; Niu, S.; Li, J. Activity of the Carbon-Based Heterogeneous Acid Catalyst Derived from Bamboo in Esterification of Oleic Acid with Ethanol. Energy Convers. Manag. 2016, 114, 188–196. [Google Scholar] [CrossRef]
- Az-Zahraa, B.; Zakaria, S.; Daud, M.F.B.; Jaafar, S.N.S. Removal of Oil Palm Trunk Lignin in Ammonium Hydroxide Pretreatment. AIP Conf. Proc. 2018, 1940, 20017. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Yang, Y.; Ge, B.; Meng, F. Research and Application Progress of Lignin-Based Composite Membrane. J. Polym. Eng. 2021, 41, 245–258. [Google Scholar] [CrossRef]
- Ariffin, H.; Hassan, M.; Umi Kalsom, M.; Abdullah, N.; Shirai, Y. Effect of Physical, Chemical and Thermal Pretreatments on the Enzymatic Hydrolysis of Oil Palm Empty Fruit Bunch (OPEFB) (Kesan Prarawatan Fizikal, Kimia Dan Termal Terhadap Hidrolisis Enzimitik Tandan Kosong Kelapa Sawit). J. Trop. Agric. Food Sci. 2008, 36, 259–268. [Google Scholar]
- Ismail, S.; Saharuddin, M.Q.; Zahari, M.S.M. Upgraded Seawater-Alkaline Pre-Treatment of Lignocellulosic Biomass for Bio-Methane Production. Energy Procedia 2017, 138, 372–379. [Google Scholar] [CrossRef]
- Chin, S.X.; Chia, C.H.; Zakaria, S.; Ahmad, S.H.; Tasirin, S.M. Combination of Gamma Irradiation and Sodium Carbonate Pretreatment on Oil Palm Empty Fruit Bunch (EFB) for High Acidic Hydrolysis Yield. Sains Malays. 2017, 46, 167–173. [Google Scholar] [CrossRef]
- Guo, F.; Xiu, Z.L.; Liang, Z.X. Synthesis of Biodiesel from Acidified Soybean Soapstock Using a Lignin-Derived Carbonaceous Catalyst. Appl. Energy 2012, 98, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.T.; Yang, X.X.; Xu, J.; Wang, H.L.; Wang, Z.B.; Zhang, L.; Wang, S.L.; Liang, J.L. Biodiesel Production from Esterification of Oleic Acid by a Sulfonated Magnetic Solid Acid Catalyst. Renew Energy 2019, 139, 688–695. [Google Scholar] [CrossRef]
- Jing, H.; Wang, X.; Liu, Y.; Wang, A. Preparation of Magnetic Nanocomposites of Solid Acid Catalysts and Their Applicability in Esterification. Chin. J. Catal. 2015, 36, 244–251. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal Conversion of Biomass Waste to Activated Carbon with High Porosity: A Review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Chellappan, S.; Aparna, K.; Chingakham, C.; Sajith, V.; Nair, V. Microwave Assisted Biodiesel Production Using a Novel BrØnsted Acid Catalyst Based on Nanomagnetic Biocomposite. Fuel 2019, 246, 268–276. [Google Scholar] [CrossRef]
- Eterigho, E.J.; Alueshima, B.M.; Ejejigbe, S.E. Optimization of Process Parameters for the Synthesis of Locally Sourced Alumina-Supported Eggshell Catalyst. Lect. Notes Eng. Comput. Sci. 2018, 2238, 523–527. [Google Scholar]
- Hussain, Z.; Kumar, R. Synthesis and Characterization of Novel Corncob-Based Solid Acid Catalyst for Biodiesel Production. Ind. Eng. Chem. Res. 2018, 57, 11645–11657. [Google Scholar] [CrossRef]
- Liu, K.; Wang, R.; Yu, M. An Efficient, Recoverable Solid Base Catalyst of Magnetic Bamboo Charcoal: Preparation, Characterization, and Performance in Biodiesel Production. Renew Energy 2018, 127, 531–538. [Google Scholar] [CrossRef]
- Abdedayem, A.; Guiza, M.; Ouederni, A. Copper Supported on Porous Activated Carbon Obtained by Wetness Impregnation: Effect of Preparation Conditions on the Ozonation Catalyst’s Characteristics. Comptes Rendus Chim. 2015, 18, 100–109. [Google Scholar] [CrossRef]
- Amadine, O.; Essamlali, Y.; Fihri, A.; Larzek, M.; Zahouily, M. Effect of Calcination Temperature on the Structure and Catalytic Performance of Copper-Ceria Mixed Oxide Catalysts in Phenol Hydroxylation. RSC Adv. 2017, 7, 12586–12597. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, C.; Li, Y.; Ma, Z.; Lv, X. Effect of Calcination Temperature on the Structure and Catalytic Performance of the Cu-Mcm-41 Catalysts for the Synthesis of Dimethyl Carbonate. Quim. Nova 2018, 41, 1156–1161. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Duan, A.; Zhang, P. Effects of Calcination Temperature on the Acidity and Catalytic Performances of HZSM-5 Zeolite Catalysts for the Catalytic Cracking of n-Butane. J. Nat. Gas Chem. 2005, 14, 213–220. [Google Scholar] [CrossRef]
- Zainol, M.M.; Amin, N.A.S.; Asmadi, M. Synthesis and Characterization of Porous Microspherical Ionic Liquid Carbon Cryogel Catalyst for Ethyl Levulinate Production. Diam. Relat. Mater. 2019, 95, 154–165. [Google Scholar] [CrossRef]
- Zainol, M.M.; Amin, N.A.S.; Asmadi, M. Effects of Thermal Treatment on Carbon Cryogel Preparation for Catalytic Esterification of Levulinic Acid to Ethyl Levulinate. Fuel Process. Technol. 2017, 167, 431–441. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced Synthesis Strategies of Mesoporous SBA-15 Supported Catalysts for Catalytic Reforming Applications: A State-of-the-Art Review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- He, B.; Fu, X.; Lian, X.; Jiang, S.; Xu, P.; Deng, X.; He, C.; Chen, C. Catalytic Deacidification of Vacuum Gas Oil by Zno/Al2o3 and Its Modification with Fe2O3. Catalysts 2019, 9, 499. [Google Scholar] [CrossRef]
- Rosli, N.S.; Harun, S.; Jahim, J.M.; Othaman, R. Chemical and Physical Characterization of Oil Palm Empty Fruit Bunch. Malays. J. Anal. Sci. 2017, 21, 188–196. [Google Scholar] [CrossRef]
- Agapay, R.C.; Liu, H.C.; Ju, Y.H.; Go, A.W.; Angkawijaya, A.E.; Nguyen, P.L.T.; Truong, C.T.; Quijote, K.L. Synthesis and Initial Evaluation of Solid Acid Catalyst Derived from Spent Coffee Grounds for the Esterification of Oleic Acid and Methanol. Waste Biomass Valorization 2021, 12, 4387–4397. [Google Scholar] [CrossRef]
- Bureros, G.M.A.; Tanjay, A.A.; Cuizon, D.E.S.; Go, A.W.; Cabatingan, L.K.; Agapay, R.C.; Ju, Y.H. Cacao Shell-Derived Solid Acid Catalyst for Esterification of Oleic Acid with Methanol. Renew Energy 2019, 138, 489–501. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Kristiani, A.; Sudiyarmanto; Khaerudini, D.S.; Takeishi, K. Sulfonated Magnetic Nanobiochar as Heterogeneous Acid Catalyst for Esterification Reaction. J. Environ. Chem. Eng. 2020, 8, 103912. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, M.; Geng, J.; Cheng, Y.; Wang, X.; Wu, C.; Wang, Q.; Liu, S.; Cheung, S.M. Catalytic Performance and Deactivation Mechanism of a One-Step Sulfonated Carbon-Based Solid-Acid Catalyst in an Esterification Reaction. Renew Energy 2021, 164, 824–832. [Google Scholar] [CrossRef]
- Huang, M.; Luo, J.; Fang, Z.; Li, H. Biodiesel Production Catalyzed by Highly Acidic Carbonaceous Catalysts Synthesized via Carbonizing Lignin in Sub- and Super-Critical Ethanol. Appl. Catal. B 2016, 190, 103–114. [Google Scholar] [CrossRef]
- Rechnia-Gorący, P.; Malaika, A.; Kozłowski, M. Acidic Activated Carbons as Catalysts of Biodiesel Formation. Diam. Relat. Mater. 2018, 87, 124–133. [Google Scholar] [CrossRef]

| Factors | Unit | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| EFB fibre loading | g | 1 | 3 | 5 | 7 | 9 |
| Reaction time | h | 1 | 2 | 3 | 4 | 5 |
| Calcination temperature | °C | 300 | 400 | 500 | 600 | 700 |
| Calcination time | h | 1 | 2 | 3 | 4 | 5 |
| Samples | Elemental Composition (wt%) | |||
|---|---|---|---|---|
| Carbon (C) | Oxygen (O) | Iron (Fe) | Sulphur (S) | |
| Treated EFB fibres | 57.76 | 42.24 | n.d a | n.d a |
| Na2CO3-T-EFB magnetic catalyst | 37.88 | 34.78 | 21.82 | 5.52 |
| Catalysts | Surface Acidity | Esterification Performance | Refs. | ||||
|---|---|---|---|---|---|---|---|
| By S Content (mmol SO3H/g) a | By Base Titration (mmol/g) | By NH3-TPD (mmol/g) | Conditions b | Conversion (%) | Reusability | ||
| Spent coffee grounds-derived solid acid catalyst | 3.36 | 4.22 | - | CL of 10 wt%, MeOH:OA of 10:1, 80 °C/7 h | >90.0 | ~70% after 4 cycles | [42] |
| Cacao shell-derived solid acid catalyst | 1.48 | 4.56 | - | CL of 0.05 wt%, MeOH:OA of 7:1, 42 °C/4 h | 78.0 | 48.0% after 3 cycles | [43] |
| EFB derived MBC02-SO3H | - | - | 0.28 | CL of 5 wt%, MeOH:OA of 8:1, 150 °C/1.5 h | 81.0 | Not reported | [44] |
| Bamboo derived solid acid catalyst (S150-4) | 0.82 | - | - | CL of 10 wt%, MeOH:OA of 8:1, 65 °C/8 h | 98.0 | 79.2% after 4 cycles | [45] |
| Dealkaline lignin derived E−260-20-SO3H (Supercritical ethanol) | 1.41 | 5.05 | - | CL of 5 wt%, MeOH:OA of 12:1, 80 °C/7 h | 95.4 | ≥81.9% after 5 cycles | [46] |
| Dealkaline lignin derived E-P400−2-SO3H (Subcritical ethanol) | 1.06 | 5.35 | - | CL of 5 wt%, MeOH:OA of 15:1, 80 °C/5 h | 95.5 | ≥84.6% after 3 cycles | |
| Na2CO3-T-EFBC magnetic catalyst | 1.72 | 3.50 | - | CL of 7 wt%, MeOH:OA of 10:1, 60 °C/2 h | 93.5 | 82.1% after 5 cycles | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, S.G.; Pua, F.L.; Fan, Z. Effect of Chemical Pre-Treatment on the Catalytic Performance of Oil Palm EFB Fibre Supported Magnetic Acid Catalyst. Sustainability 2023, 15, 8637. https://doi.org/10.3390/su15118637
Krishnan SG, Pua FL, Fan Z. Effect of Chemical Pre-Treatment on the Catalytic Performance of Oil Palm EFB Fibre Supported Magnetic Acid Catalyst. Sustainability. 2023; 15(11):8637. https://doi.org/10.3390/su15118637
Chicago/Turabian StyleKrishnan, Shamala Gowri, Fei Ling Pua, and Zhang Fan. 2023. "Effect of Chemical Pre-Treatment on the Catalytic Performance of Oil Palm EFB Fibre Supported Magnetic Acid Catalyst" Sustainability 15, no. 11: 8637. https://doi.org/10.3390/su15118637
APA StyleKrishnan, S. G., Pua, F. L., & Fan, Z. (2023). Effect of Chemical Pre-Treatment on the Catalytic Performance of Oil Palm EFB Fibre Supported Magnetic Acid Catalyst. Sustainability, 15(11), 8637. https://doi.org/10.3390/su15118637




