Abstract
Callus elicitation is advantageous for metabolite production due to its ability to increase yield, provide controllable conditions, and allow for genetic manipulation, offering a sustainable and scalable alternative to traditional plant-based extraction methods for the production of bioactive substances. In this research, in vitro callus cultures (CCs) of the wild medicinal plant Aerva sanguinolenta were used to evaluate the efficacy of various elicitation regimes of silver nanoparticles (AgNPs) and salicylic acid (SA) to evoke an increased production of secondary metabolites, such as aervine and antioxidant metabolites. Three concentrations of SA (i.e., 20, 50, and 100 µM) and three concentrations of AgNPs (i.e., 30, 60, and 90 µg/L) were used on shoot explant cultures using MS (Murashige and Skoog) media. All the SA and AgNP elicitation treatments significantly increased the production of antioxidant metabolites, total phenolic contents (TPCs), and total flavonoid contents (TFCs) compared to the control treatment experiments. The contents of aervine were increased significantly upon elicitation compared to the control trial. Furthermore, the antioxidant potential of the test extract was enhanced compared to the control treatment. Comparatively, the AgNPs were more beneficial as elicitors than the SA treatments. The elicitation treatments with about 90 µg/L AgNPs and 100 µM SA were the best among all elicitation regimes. Callus elicitation with SA and AgNPs can stimulate increased metabolite production and be used as a sustainable practice in the welfare and service industries for drug development and drug discovery.
1. Introduction
Aerva sanguinolenta (L.) Blume has many medicinal uses in indigenous cultures. It is a small perennial herb, bearing vernacular names such as “chiti booti”, mountain knotgrass, or kapok bush [1]. It belongs to the family Amaranthaceae, with its bushy form being sub-shrubby shaped. In traditional ethnomedicines (TEMs), various parts of Aerva sanguinolenta have been used to treat a variety of ailments, such as kidney stones, urinary tract infections, diarrhea, dysentery, asthma, and diabetes [2]. The plant contains a number of active compounds, including alkaloids, flavonoids, saponins, and tannins, that are believed to be responsible for its phototherapeutic effects. The plant is often used in various Ayurvedic preparations, including herbal teas, powders, and capsules, due to the presence of a vast variety of secondary metabolites in its various parts [3].
Aerva sanguinolenta contains several alkaloids, including aervine, aervoside, and lanthionine [4], which are potential sources of TEMs and known for having analgesic, anti-inflammatory, and antispasmodic properties. Similarly, the plant is rich in flavonoids, including quercetin, kaempferol, and rutin [5]. These compounds are known for their antioxidant properties. Additionally, Aerva sanguinolenta contains saponins, which are compounds that can form a foam-like substance when mixed with water and are potentially medicinal in their properties. Generally, in wild medicinal plants, the presence of various phytochemicals is limited and variable due to climate changes, altitude, and other parameters. Hence, this shows that there is a dire need to increase the contents of these botanicals and medicinal phytochemicals for their potential use in TEMs and the production of various allopathic drugs [6].
Antioxidant enzymes are naturally occurring enzymes in the body that work to neutralize free radicals and prevent them from causing damage due to ROS [7]. Plants produce these antioxidant metabolites to survive in and combat against harsh environments. A better antioxidant system helps the plant to cope under harsh environments and changing climates, and it ultimately effects the concentration of phytochemicals in the plant [8].
Elicitation typically involves the activation of signaling pathways within the plant that ultimately result in the production of a particular compound in a better quantity [9]. Researchers are interested in understanding the mechanisms underlying elicitation in order to develop new strategies for crop sustainability, drug discovery, and other applications. For this purpose, several protocols are being used worldwide, and an enormous number of research studies are being conducted on the use of elicitation [10]. For this purpose, in vitro callus cultures (CCs) provide a useful tool for studying the production of bioactive compounds in plants and developing new strategies for their sustainable and enhanced production. They offer a controlled and scalable system for elicitation studies and can be used to explore the potential of various plant species for the production of metabolic compounds [11]. In the current study, we used in vitro CCs of the A. sanguinolenta plant for the production of several antioxidant metabolites and useful botanicals, such as aervine.
Nanotechnology has emerged as a potential field of interest in recent times due to the large number of applications of nanomaterials in food, medicine, and agriculture [12]. Among nanomaterials, AgNPs have been reportedly used in agriculture, food, and pharmacy [13,14]. AgNPs have reportedly enhanced the capsaicin contents in suspension cultures of Capsicum [15]. Additionally, the use of Ag_NPs in hydroponic cultures of Silybum marianum has reportedly enhanced the contents of flavonolignans [16]. Like AgNPs, salicylic acid has been used as an elicitor to increase the production of metabolic compounds in plants, such as Arthrospira platensis [17], Nigella sativa [18], and Catharanthus roseus [19].
While the use of nanoparticles and salicylic acid in the production of secondary compounds by plants has been extensively studied, little research has been conducted on the use of elicitors in in vitro callus cultures of Aerva sanguinolenta. Additionally, there has been limited attention given to the production of aervine in CCs using elicitors. Therefore, this study aimed to investigate the effects of different concentrations of AgNPs and salicylic acid on the production of aervine and bioactive antioxidants in a callus culture of Aerva sanguinolenta. This study also explored the roles of these elicitors in callus morphology. The hypothesis was that the use of AgNPs and salicylic acid as elicitors may lead to an enhanced production of antioxidants and secondary metabolites, ultimately increasing the plant’s medicinal potential for curing different infirmities. This study assessed the total phenolic contents, total flavonoid contents, anthocyanin, tannins, and aervine as major secondary metabolites. The objective was to determine the effect of these elicitors on callus morphological traits and, subsequently, on the production of metabolites.
2. Materials and Methods
2.1. Establishment of Callus Cultures
To cultivate callus cultures (CCs) and obtain shoot explants, potted plantlets of Aerva sanguinolenta were used. Fresh apical shoot tips measuring 0.5 cm were injected onto the solid surface of Murashige and Skoog [20] media. The media were supplemented with 8 g/L of Oxoid agar (manufactured by Oxoid Limited, which is based in Basingstoke, UK) and 30 g/L of sucrose to serve as a carbon source, and the pH was adjusted to 5.7 using a pH meter (pH 510, Eutech Instruments, Singapore). The medium-filled flasks were then autoclaved for 20 min at 121 °C using a Systec (Germany) machine for disinfection purposes. The cultures were grown in a growth chamber with a temperature of 25 °C and a light intensity of 40–50 mmol m−2S−1. The photoperiod was set to 16/8 h to encourage the formation of callus cultures [21].
2.2. Elicitor Treatments and Determination of Callus Characteristics
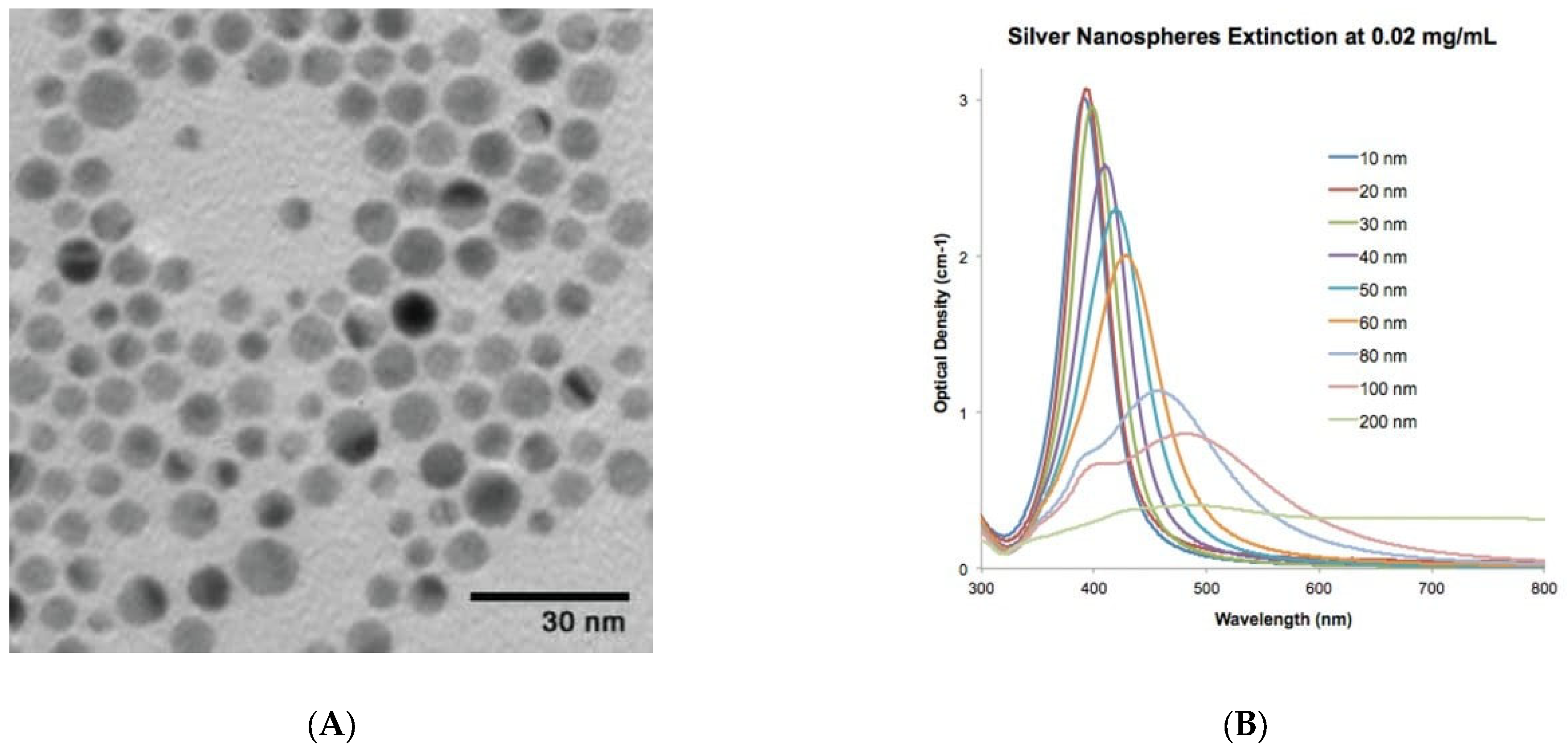
Faintly yellow to dark yellow silver nanoparticles (AgNPs) were supplied by Sigma Aldrich, product number 730785. According to the suppliers, the product was characterized using an inductively coupled plasma mass spectrometry (ICP-MS) analysis, which confirmed the presence of elemental silver. According to the suppliers, the particles were 10 nm in size, and their absorbance was between 380 nm and 405 nm with a mass concentration of 0.02 mg/mL. Figure 1 explains the characterization of the supplied AgNPs as described by Sigma Aldrich, Steinheim am Albuch, Germany.
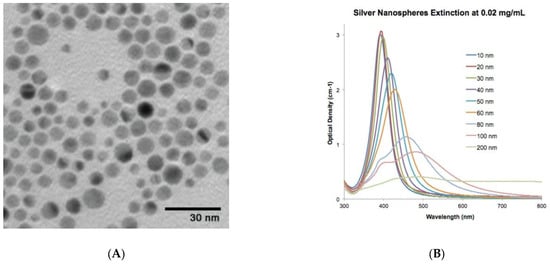
Figure 1.
Product description of AgNPs as provided by chemical supplier Sigma Aldrich: (A) TEM image and (B) optical density of the AgNPs. The image was “reproduced with permission from Merck KGaA, Darmstadt, Germany and/or its affiliates” and https://nanocomposix.com/.
Salicylic acid product number L7401 was also purchased from Sigma Aldrich. The product was supplied as a white powder, and, according to the supplier, the product is approved for practice in plant cell cultures. Three different elicitation regimes of SA were tested on the callus cultures in the experiment, based on the literature reports by Singh et al., Largia et al., and Gadzovska et al. [22,23,24].
AgNP stock solution was manufactured using the Rahman et al. [25] procedure. Based on the preliminary literature by Ali et al. [21], several concentrations of the nanoparticles (30, 60, and 90 μg/L) were evaluated.
After a period of 34 days, the calli were dissected into small fragments and transferred onto sterile filter paper prior to being cultured on MS media. For elicitation purposes, three different treatment concentrations of the silver nanoparticles (AgNPs) were selected (30 µg/L, 60 µg/L, and 90 µg/L), along with three treatment concentrations of salicylic acid (SA) (20 µM, 50 µM, and 100 µM). The effects of these elicitation treatments were compared to those of a control treatment consisting of MS media alone (MS0). When maintaining the in vitro callus cultures, certain precautions needed to be taken to ensure their success and overall health. It was crucial to maintain a sterile environment by ensuring that all instruments, culture media, and containers were properly sterilized before use. Working in a laminar flow hood or on a clean bench provided the necessary controlled environment. Aseptic techniques were followed diligently to prevent contamination, which could have had detrimental effects on the callus cultures. Regular subculturing was another important precaution that was prioritized. The callus was transferred to fresh media at regular intervals, typically every 2–4 weeks. This process prevented issues such as overgrowth, nutrient depletion, and the accumulation of toxic metabolites. Maintaining a consistent subculturing schedule ensured that the callus cultures received the optimal growth conditions. The pH of the culture media was also carefully monitored and adjusted within the recommended range. Typically, a pH range between 5.5 and 6.5 was considered optimal, although the specific requirements for the species were confirmed. Regular pH level monitoring ensured that the environment was conducive to callus growth.
By diligently adhering to these precautions, such as maintaining sterility, regularly subculturing, ensuring appropriate culture media composition, and monitoring and adjusting pH levels, healthy and thriving in vitro callus cultures were successfully maintained. Valuable insights into plant development and tissue culture techniques were gained through the effective study and manipulation of the callus cultures.
Seven test tubes were sterilized using an autoclave for the culture experiment. Approximately 9 mL of medium was added to each tube, which was then sealed with a cotton plug. All cultures were maintained at a temperature of 25 °C with a 16/8 h photoperiod in a growth chamber to encourage callus proliferation. After an incubation period of 50 days, data were collected on the fresh weight (FW), dry weight (DW), moisture content (CMC), and morphological traits of the callus. To determine the FW, calli from each treatment were rinsed with sterile distilled water, placed on filter paper to remove excess moisture, and weighed after being squeezed with forceps. Subsequently, each callus was dried in an oven at 50 °C for 24 h and weighed again to obtain the DW. The FW and DW measurements were converted to g/L [21]. The moisture content of the callus was calculated as a percentage using the FW and DW values, following the formula provided by Rashmi and Trivedi [26].
where A, B, and C denote the weights of the Petri dishes being either empty, containing a fresh callus, or a dry callus, respectively.
Percentage moisture contents = [(B − A) − (C − A)]/(B − A) × 100
2.3. Preparation of Callus Sample Extract for Phytochemical Tests
To perform a phytochemical analysis, a callus sample weighing around 300 mg was mixed with 10 mL of 50% methanol and agitated at a temperature of 25–30 °C for 24 h while shaking at 24 rpm. After vertexing for 30 min and sonicating for another 30 min, the mixture was subjected to an additional 15 min of sonication. The mix was then centrifuged at 6500 rpm for 10 min, and the supernatant was gathered and transferred to new Eppendorf tubes. The final volume was raised to 10 mg/mL and stored at 4 °C for the phytochemical analysis [27].
2.3.1. Determination of Total Phenolic Content (TPC)
For this test, 20 mL of the sample’s supernatant from a 10 mg/mL sample of each treatment was obtained and placed in the wells of a 96-well plate. Each well received 90 mL of the 10-times-diluted Folin–Ciocalteu reagent (Sigma Aldrich product: 1.09001), which was then left at room temperature for 5 min. Moreover, 90 mL of sodium carbonate was added to the mixture to bring the total amount to 200 mL. Methanol (20 mL) and gallic acid (1 mg/mL) were utilized in this technique as the negative and positive controls, respectively. The reaction was incubated for 90 min before the absorbance at 630 nm was measured. Furthermore, we calculated the tannin content of the sample based on a calibration curve generated from a known tannin standard following Swain and Goldstein [28].
2.3.2. Determination of Total Flavonoid Content (TFC)
To conduct the test, 20 mL of the supernatant from each treatment’s 10 mg/mL sample was taken and assayed for TFC using the process outlined by Chang et al. [29]. Negative and positive controls were also included in the test, with methanol (20 mL) and gallic acid (1 mg/mL), respectively, being used for this purpose. The reaction was incubated for 90 min, after which the absorbance at 630 nm was measured [29].
2.3.3. Determination of Aervine Content
The bioactive compound aervine was isolated from the extracts obtained from the callus of A. sanguinolenta using a high-performance liquid chromatography (HPLC) system manufactured by Shimadzu Corporation (Kyoto, Japan). The separation process was conducted on a C18 column (5 mm, 250 × 4.6 mm i.d.), with the column temperature being maintained at 35 °C. To achieve separation, a linear gradient elution method was employed, utilizing acetonitrile (A) and aqueous (B) solvents, at a flow rate of 0.5 mL/min. The gradient profile transitioned from A to B with the following proportions (v/v): 5–25% (0–5 min), 25–55% (5–10 min), and 55–100% (10–15 min). Detection was performed at a wavelength of 254 nm using a UV detector. External instrumental facilities were utilized for the HPLC analysis of the plant samples [30]. The samples were stored at −80 °C for this purpose.
2.3.4. Determination of Anthocyanin Contents
First, the callus samples were dried using a paper towel and subsequently frozen. The extraction of pigments was performed following the method outlined by Simoes et al. [31]. Chromatography was carried out using BAW 4:1:5, Bu-HCI 1:1, and 1% HCI, following the procedure described by Yashin et al. [32]. The absorbance of anthocyanin was measured at a wavelength of 525 nm.
2.4. Determination of DPPH Scavenging Percentage
The IC50 value in DPPH (2,2-diphenyl-1-picrylhydrazyl) refers to the half-maximal inhibitory concentration. In order to determine the IC50 value, various volumes of a sample (10 µL, 5 µL, 2.5 µL, and 1 µL) were added to the wells of a 96-well plate, along with a negative control (methanol) and a positive control (ascorbic acid). The volume of each sample was adjusted to 1000, 750, 500, and 250 µg/mL by adding 190, 195, 197.5, and 199 µL of a DPPH solution (4.8 mg/50 mL), respectively. The absorbance was measured at 515 nm, and the IC50 value was calculated using ascorbic acid as the positive control with an equal amount of extract in mg/mg. To determine the amount of DPPH discoloration resulting from free radical scavenging activity, a formula was employed.
DPPH Scavenging % = 100 × (1 − AE/AD)
AE represents the absorbance of the DPPH solution when a particular amount of test extract is added. AD represents the absorbance of DPPH when nothing is added to this solution [33].
2.5. Determination of Antioxidant Functions
The antioxidant functions were studied using the method described by Khan et al. [27]. Each sample was combined with 2 mL of phosphate buffer (50 mM, 1% PVP, 0.1 mM EDTA, pH 7.8) and homogenized to ensure a uniform mixture. The resulting mixture was then subjected to two rounds of centrifugation at 4 °C and 12,000 rpm for a total of 15 min to obtain a homogeneous reaction buffer. The supernatants obtained from each sample were collected for further analysis of antioxidant enzyme activities, including superoxide dismutase (SOD; EC 1.15.1.1), peroxidase (POD; EC 1.11.1.7), catalase (CAT; EC 1.11.1.6), and ascorbate peroxidase (APX; EC 1.11.1.11). The activity of these enzymes was measured using the methods developed by Giannopolitis and Ries [34], Abeles and Biles [35], Arrigoni et al. [36], and Miyake et al. [37], respectively. For phenylalanine ammonia lyase (PAL), the protocol of Khan et al. [27] was employed.
2.6. Statistical Analysis and Experimental Layout
The experimental scheme employed in this study followed a completely randomized approach. The collected data underwent a statistical analysis through the separation of means using Duncan’s multiple range test (DMRT) at a significance level of p < 0.05 in Costat software version 6.3 (developed by Cohort software, Berkeley, CA, USA).
3. Results
Callus growth, in terms of callus fresh weights (CFWs), callus dry weights (CDWs), and callus moisture contents (CMCs), was recorded as presented in Table 1. All the elicitation treatments significantly enhanced callus growth in terms of CFW, CDW, and CMC. A pictorial presentation of the calli is shown in Figure 2. The highest elicitation treatments of both elicitors (i.e., 90 µg/L AgNPs and 100 µM SA) were found to be the best among all the elicitation regimes. Furthermore, the LSD test interpretation was added to the table to comprehend the elicitation effects induced by the AgNPs and SA.

Table 1.
Effect of different concentrations of AgNPs and salicylic acid on callus proliferation in Aerva sanguinolenta.
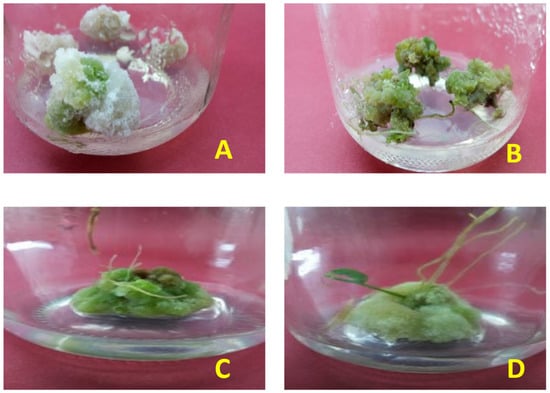
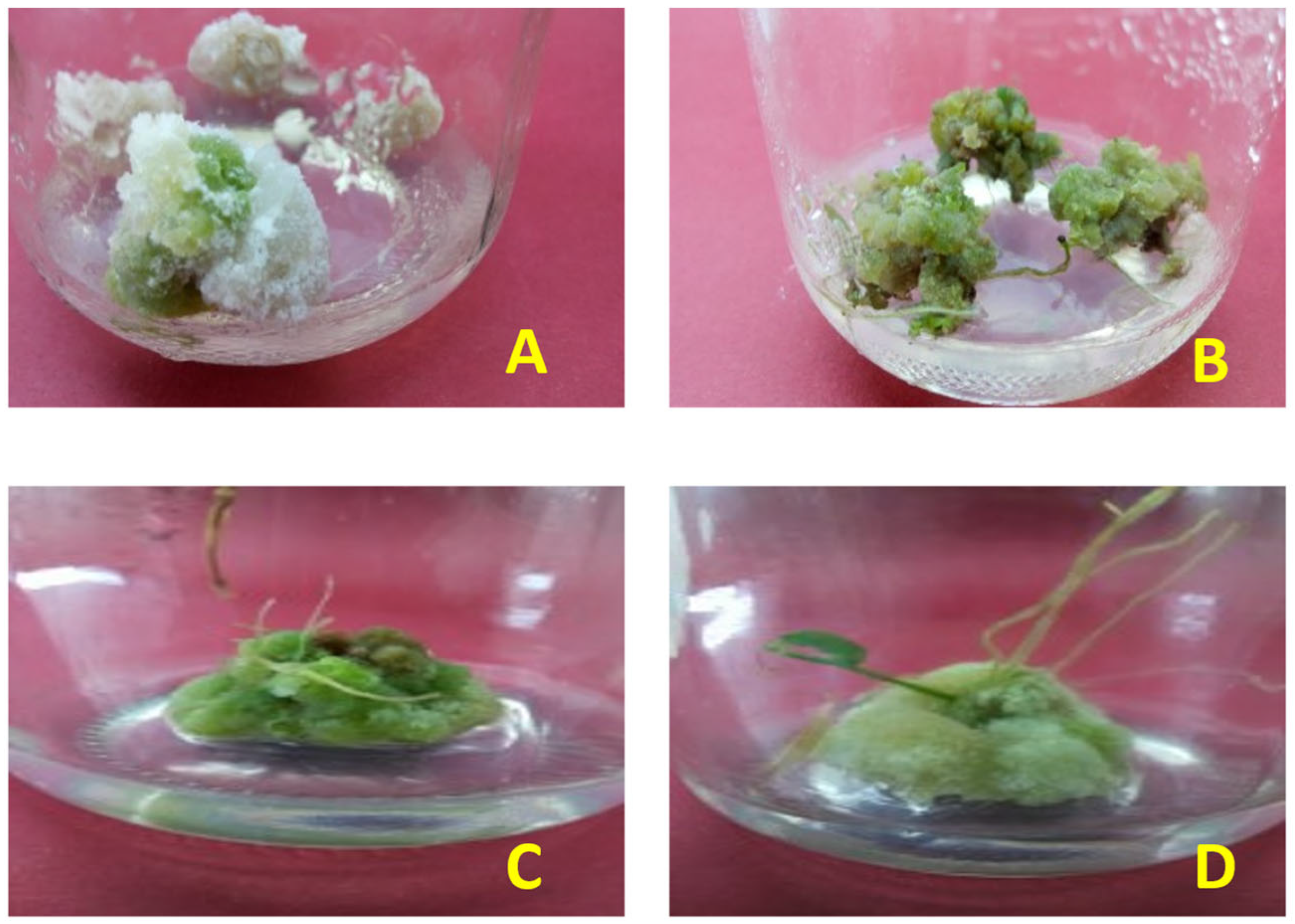
Figure 2.
Pictorial presentation of callus (A) before elicitation treatments, showing significantly lower greenish tinge and compactness than (B) callus after silver nanoparticle application. (C) Response of callus treated with salicylic acid 100 µM concentration and (D) response of callus treated with silver nanoparticles at 90 µg/L concentration.
The callus nature in terms of callus response, callus texture, and callus color was examined, and the results are presented in Table 2. An excellent callus formation response was shown after applying the 100 µM SA and higher doses of AgNPs. Similarly, the elicitation treatments also improved the callus texture and color. The application of higher doses of AgNPs and the highest dose of SA induced a greenish tinge on the callus, and the texture was also significantly improved.

Table 2.
Morphological attributes of CC of Aerva sanguinolenta as affected by different concentrations of AgNPs and salicylic acid.
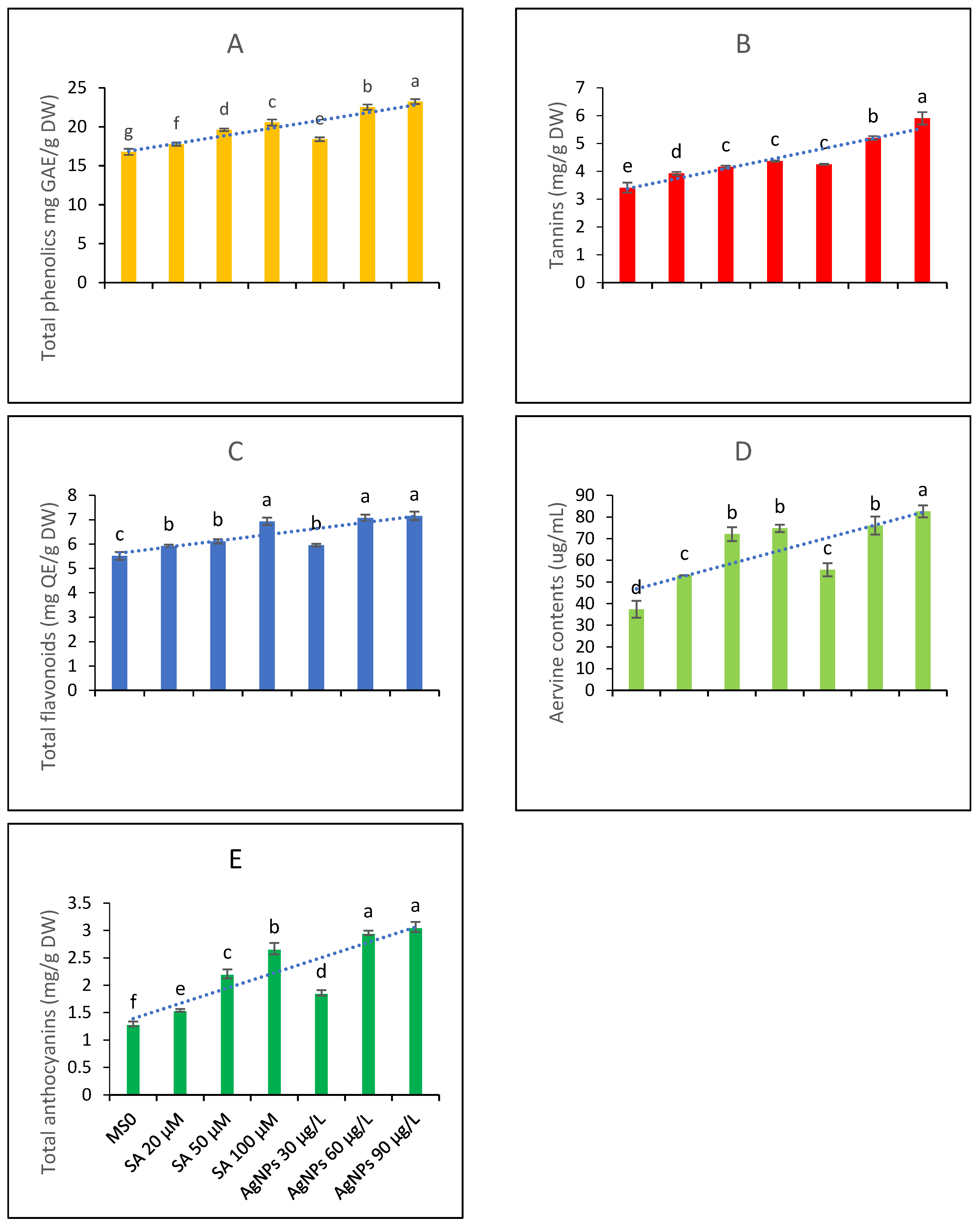
The data presented in Figure 3A represent the total phenolic contents assayed from the test samples subjected to various levels of treatment with SA and AgNPs. According to the LSD test values placed on the bars, the highest mean value for the total phenolic content was achieved through higher doses of AgNPs. The elicitation with SA was also found to be beneficial in boosting the total phenolic profile, and the 100 µM concentration proved to be the best elicitation treatment in enhancing the TPC.
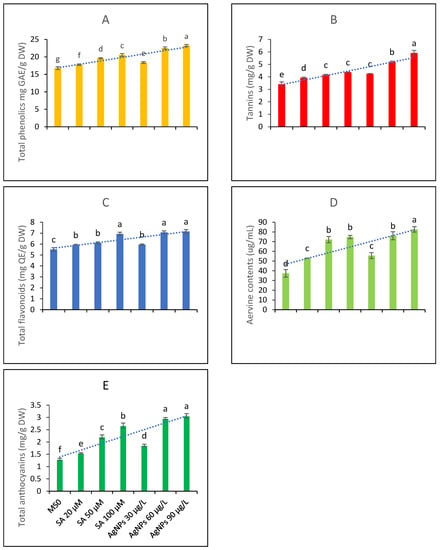
Figure 3.
Contents of bioactive metabolites from the in vitro callus culture of Aerva sanguinolenta (A) total phenolics contents (B) Tannins contents (C) total flavonoids contents (D) aervine contents and (E) total anthocyanin contents elicited with various elicitation regimes of AgNPs and SA. The bars with different letters contain data that differ significantly at alpha 0.05.
Among phenolics, tannins are key metabolites with important pharmacogenetic applications. Therefore, the tannin contents of the test extracts were also determined. The data presented in Figure 3B show that all the elicitation treatments significantly affected the tannin contents. The higher doses of AgNPs were found to be the most effective among all elicitation treatments. Elicitation with SA was also beneficial; however, the LSD test predicted that 100 µM SA and 30 µg/L AgNPs would not differ significantly in increasing the tannin profile. Overall, elicitation was found to be useful in enhancing the tannin profile.
The data presented in Figure 3C interpret the total flavonoid contents of an A. sanguinolenta plant exposed to various elicitation regimes of SA and AgNPs grown in vitro. The data suggest that higher doses of AgNPs and the highest dose of SA did not differ significantly in raising the total flavonoid contents and were thus equally effective. A lower elicitation concentration of SA and the lowest treatment concentration of AgNPs, i.e., 30 µg/L, proved to be the least beneficial in terms of increasing the plant’s flavonoid pool.
Aervine is a key alkaloid produced by Aerva plants. The contents of aervine were examined as a key metabolite and were found to increase with the elicitation treatments. The highest elicitation dose of AgNPs was found to be the best when raising the aervine contents in the in vitro CC of the A. sanguinolenta plant. The LSD score predicted that the higher doses of SA and 60 µg/L of AgNPs are equally effective as elicitors in raising the aervine contents, while the lowest doses of both abiotic elicitors are the least beneficial but significantly differ from the control treatment in boosting aervine production (Figure 3D). The HPLC analysis indicated that the peak area corresponding to aervine (10-hydroxycanthin-6-one) constituted 80% of the total peak area; thus, the extracted aervine was 80% pure when considering the average of all replicates.
The total anthocyanin contents were also examined in the test extracts. The data presented in Figure 3E show that anthocyanin contents might be elicited upon treating the CC with SA and AgNPs. Comparing the individual elicitors, the AgNPs as elicitors produced results comparable to those of SA, and the higher elicitation doses were more effective in terms of increasing the anthocyanin contents.
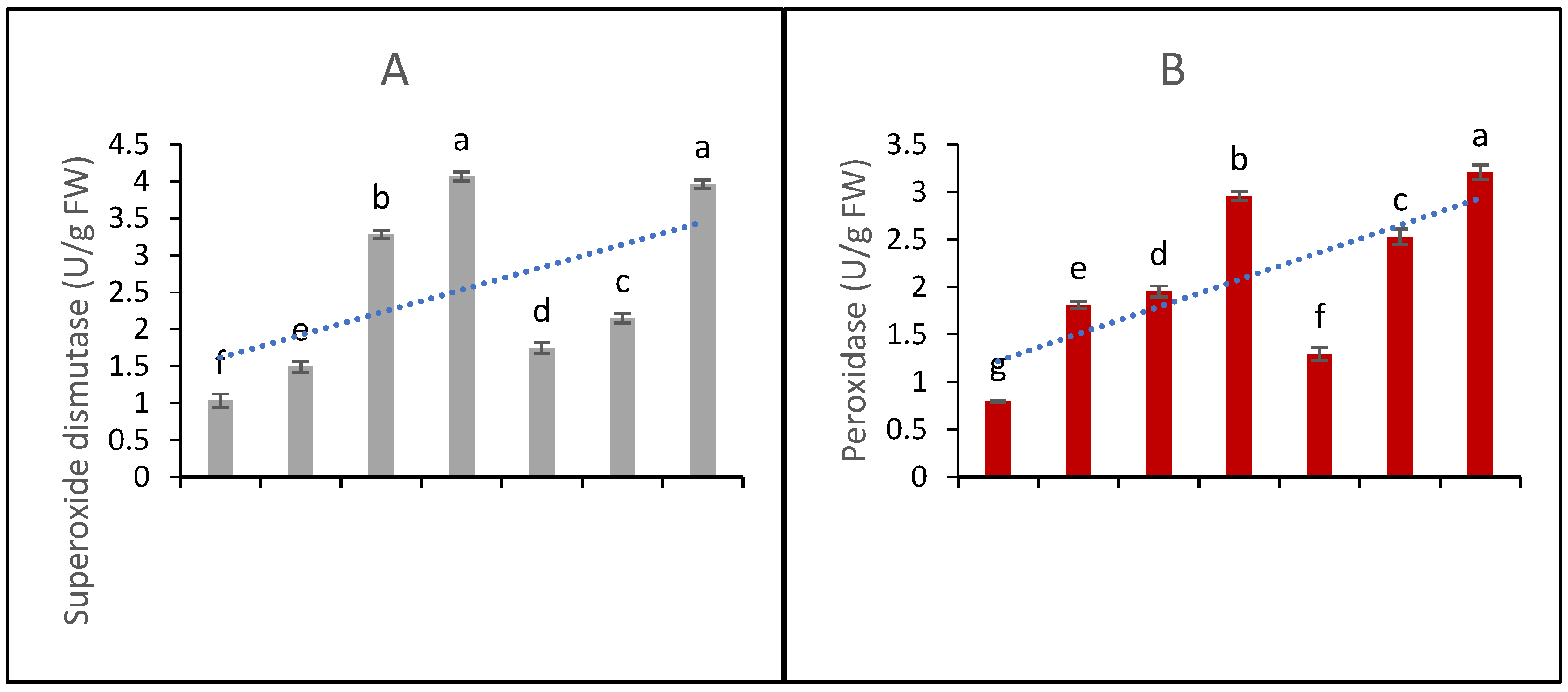
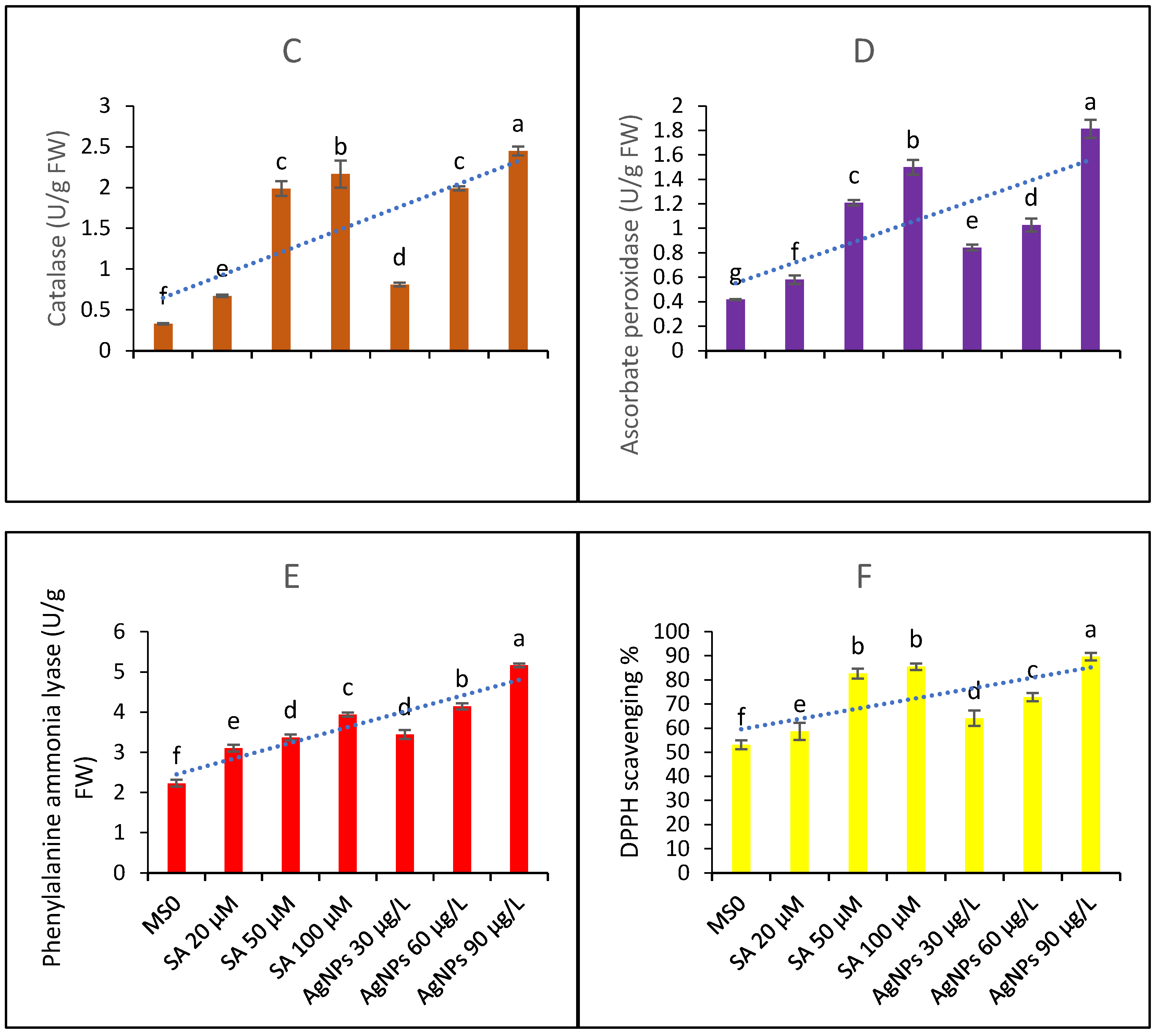
The functions of five major antioxidant metabolites, i.e., SOD, POD, CAT, APX, and GPX, were studied in the in vitro CC. The data presented in Figure 4A show that elicitation significantly affects the production and activities of SOD. The highest elicitation doses, i.e., 90 µg/L of AgNPs and 100 µM SA, produced effects that did not differ significantly; hence, it might be inferred that both of these doses are equally beneficial in terms of raising SOD functions and contents.
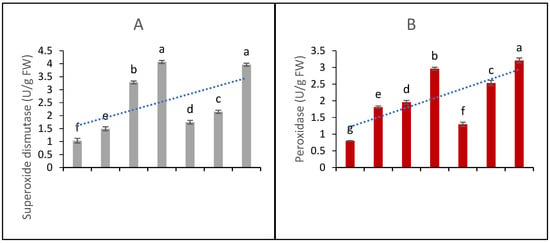
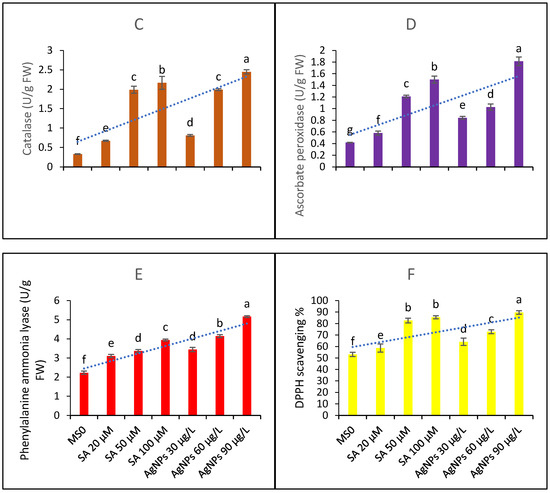
Figure 4.
Contents of antioxidant metabolites and DPPH scavenging activity (A) superoxide dismutase functions (B) peroxidase functions (C) catalase functions (D) ascorbate peroxidase functions (E) phenylalanine ammonia lyase contents and (F) DPPH scavenging percentage ability from the in vitro callus culture of Aerva sanguinolenta elicited with various elicitation regimes of AgNPs and SA. The bars with different letters contain data that differ significantly at alpha 0.05.
The data presented in Figure 4B represent peroxidase functions. The data show that all the elicitation treatments differ significantly in their efficacy. Similarly, the CAT contents (4C) were significantly affected by the elicitation treatments and found to increase upon elicitation with SA and AgNPs. The AgNP doses produced better efficacy than the SA elicitation doses. The functions of APX and PAL (as shown in Figure 4D,E, respectively) were significantly elicited upon the treatments with SA and AgNPs. Again, the higher doses proved to be more beneficial, and the AgNPs proved to be a better elicitor than SA. In all cases, the 20 µM SA treatment proved to be the least effective; however, all the treatments significantly enhanced the antioxidant activities compared to the control with MS0.
The DPPH scavenging percentage activity is shown in Figure 4F. The highest elicitation doses of both of the elicitors most effectively boosted the antioxidant abilities of the sample extracts taken from the CC under examination.
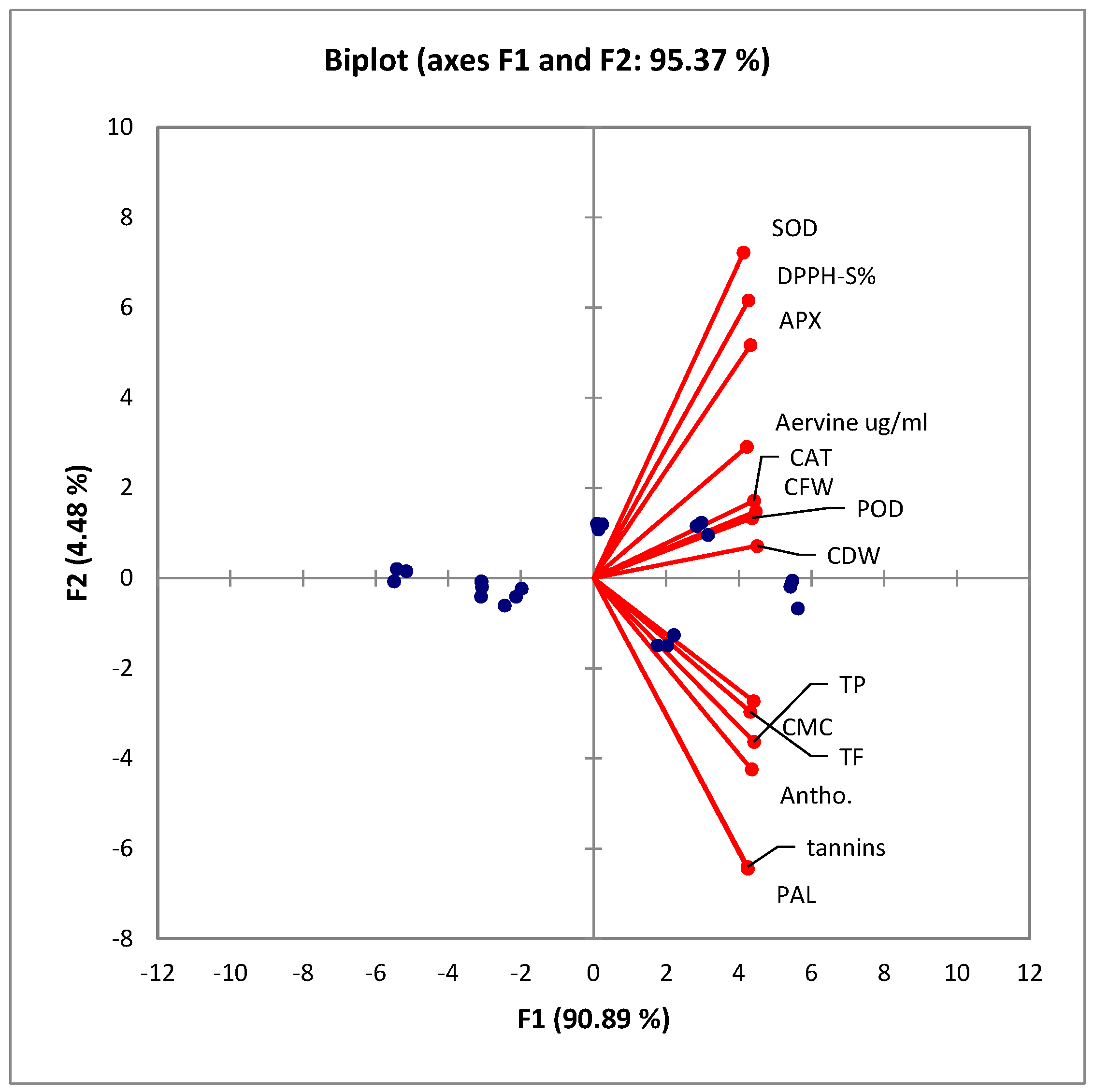
Figure 5 describes a PCA biplot diagram showing that all the variables significantly correlate. Under the elicitation treatments with both SA and AgNPs, the contents of each assayed metabolite improved significantly. Furthermore, the callus nature, texture, color, and response parameters improved significantly. These observations are further endorsed by the Spearman correlation matrix, as presented in Table 3.
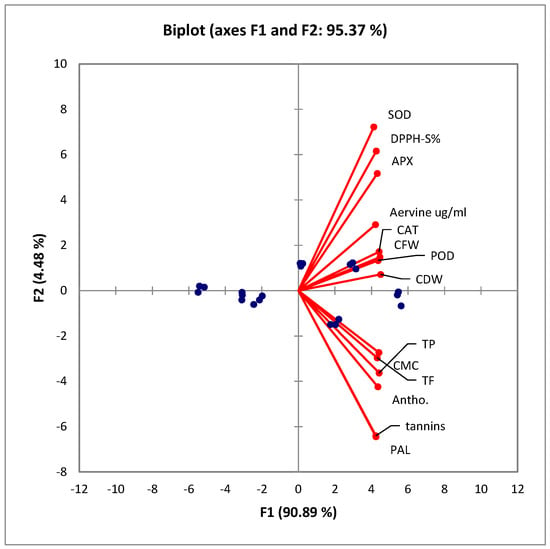
Figure 5.
PCA biplot showing aggregation of variables in the positive quarter. All of these variables significantly correlate with each other. CFW: callus fresh weight; CDW: callus dry weight; CMC: callus moisture content; TP: total phenolics; TF: total flavonoids; DPPH-S%: 2,2-diphenyl-1-picrylhydrazyl scavenging percentage; PAL: phenylalanine ammonium lyase; SOD: superoxide dismutase; POD: peroxidase; CAT: catalase; APX: ascorbate peroxidase; antho: total anthocyanins. The arrows or vectors represent the variables or features used in the analysis. The blue dots represent the data points or observations from the original dataset.

Table 3.
Spearman correlation matrix of the studied variables of in vitro grown callus culture of Aerva sanguinolenta affected by various elicitation regimes of AgNPs and SA.
4. Discussion
The use of medicinal plants in TEMs by indigenous communities is well known, and it is also globally recognized that plant-based allopathic drugs are much more effective than synthetic medicines. There is one issue regarding the variable concentrations of different compounds in medicinal plants, and their efficacy can be consistent or better only if the targeted compound conc. is reasonable in the medicine. So, the use of NP and SA treatment protocols can be better alternative modes to mitigate the fear of fluctuating concentrations of phytoconstituents in selected plants.
4.1. AgNP and SA Application Improved Callus Texture, Color, Compactness, and Growth
In the present study, we noticed a better callus proliferation and nature upon the elicitation treatments with AgNPs and SA. The addition of AgNPs in in vitro callus cultures can improve the nature and color traits of the callus [21]. AgNPs positively affect callus biomass and growth. In addition, AgNPs have been found to improve the color of the callus, making it greener and healthier [38]. This effect is believed to be due to the ability of AgNPs to stimulate the production of chlorophyll and other pigments in the callus tissues. Furthermore, the use of AgNPs has been shown to increase the levels of secondary metabolites, such as flavonoids and phenols, which have antioxidant properties and potential therapeutic applications. Overall, the use of AgNPs in the in vitro callus culture improved the quality of the callus tissue [39].
CMCs were improved significantly upon the elicitation with AgNPs. AgNPs may improve the water-holding capacity of callus tissue, which could lead to an increased moisture content [40]. One possible mechanism for this effect is that AgNPs can upregulate aquaporins, which can help to maintain the water balance in cells [41]. Additionally, the use of AgNPs may improve the structural integrity of cell membranes, reducing water loss and improving water retention in the callus tissue [42].
Like the AgNPs, the SA-mediated elicitation also improved callus biomass and longevity. SA treatment has been shown to improve callus traits in in vitro cultures [43]. SA can induce various physiological and biochemical fluctuations in plants, including an increased synthesis of secondary metabolites and antioxidants, enhanced defense responses, and improved tolerance to stresses. Various research studies have shown that the application of SA to in vitro CCs can lead to an increase in fresh and dry weights and chlorophyll content [44]. SA can also induce the production of molecules that have important medicinal and commercial value. In addition, SA can enhance the activity of antioxidant enzymes and can improve the stress tolerance and longevity of the CC [45]. SA can also induce changes in gene expression and signal transduction pathways, which can lead to the activation of various stress-responsive genes and the synthesis of stress-related proteins. These changes can improve the resilience of the CC to external stressors, such as salt, drought, or pathogen attack [46]. The above discussion reveals that SA and AgNPs might be biorational if used in the CC for growth and longevity. This overall situation leads to a better internal homeostasis culminating into a better phytochemical profile. Similar results have been reported by previous researchers [15,16,17,18,19] in terms of better callus proliferation and callus growth parameters upon elicitation in in vitro culture systems.
4.2. Efficacy of AgNPs and SA in Improving Total Phenolics and Flavonoids
The elicitation with the AgNPs and SA boosted the TFC and TPC in the CC of A. sanguinolenta. These results support previously published studies where AgNPs were found to stimulate the production of flavonoids and phenolics in in vitro CCs [47,48]. AgNPs can induce oxidative stress in cells, which can trigger the upregulation of genes involved in the biosynthesis of secondary metabolic compounds, such as flavonoids and phenolics. It might be assumed that the upregulation of these genes can lead to an increased production of total phenolics, tannins, and flavonoids in CCs [47,48]. Furthermore, AgNPs can increase the activity of antioxidants, which can help to scavenge free radicals. This causes a reduction in oxidative stress and can redirect metabolic resources for the production of cell metabolites. Mubeen et al. [16] reported similar results for a higher accumulation of flavonoids and phenolics in callus cultures of Silybum marianum by exposing them to silver nanoparticles. Similarly, Bhat and Bhat [15] reported the benefits of elicitation in phenolic accumulation in in vitro cultures of Capsicum sp. Overall, the use of AgNPs in in vitro CCs appears to be a promising approach for enhancing the production of bioactive compounds, such as flavonoids and phenolics. Along with phenolics and flavonoids, the CC of A. sanguinolenta had better anthocyanin contents upon elicitation. These results are supported by a large pool of studies [49,50]. Likewise, SA has been shown to act as an elicitor, inducing the production of bioactive compounds in plants [51]. When applied to in vitro CCs, SA can increase the contents of flavonoids, phenolic compounds, alkaloids, and terpenoids. In addition to its direct effects at the molecular level, SA can also enhance the production of bioactive compounds by inducing stress responses [52]. SA can activate a plant’s defense mechanisms, leading to an increase in the production of bioactive compounds that have antimicrobial and antifungal properties. Overall, the application of SA as an elicitor can increase the quantity of bioactive compounds in in vitro CCs, with important implications for the pharmaceutical, nutraceutical, and cosmetic industries [53].
4.3. Elicitation with AgNPs and SA Enhanced Aervine Production
Aervine production and enhancement were among the major objectives of the current study. The elicitation with the AgNPs and SA enhanced aervine production. Aervine is an alkaloid compound that has been shown to have a range of pharmacological properties, including antioxidant, anti-inflammatory, analgesic, and diuretic effects [54,55]. An enhanced production of alkaloids upon elicitation has been previously documented by several researchers [15,16,17,18,19]. Hadizadeh et al. [17] reported enhanced alkaloid production upon elicitation in marine algae Arthrospira platensis. Similarly, Ibrahim et al. [18] reported a higher accumulation of essential metabolites in callus cultures of Nigella sativa upon elicitation with silver salts and salicylic acid. A possible mechanism behind the increased aervine biosynthesis might be due to the AgNPs’ role in improving nutrient uptake in cells, which can enhance metabolic activity and lead to an increased production of secondary metabolites [56]. SA acts as a signaling molecule, inducing changes in gene expression and metabolic pathways. It can activate the expressions of genes involved in the biosynthesis of secondary metabolites, leading to an increase in their production [57]. SA can also upregulate the expressions of antioxidant enzymes, leading to a reduction in oxidative stress and an increase in the accumulation of bioactive compounds. Furthermore, SA can modulate the activity of the enzymes involved in the biosynthesis of bioactive compounds. For example, SA has been shown to enhance the activity of phenylalanine ammonia-lyase (PAL), which is an enzyme involved in the production of phenolic compounds. SA can also enhance the activity of chalcone synthase (CHS), an enzyme involved in the biosynthesis of flavonoids [58].
4.4. Elicitation Boosted the Antioxidant Metabolite Functions and Production
In the present research, we noted an enhanced production of antioxidant metabolites, such as SOD, POD, CAT, APX, and PAL. SOD is an enzyme that converts the superoxide radical (O−2) into hydrogen peroxide (H2O2), which is then converted into water and oxygen by other antioxidant enzymes [59]. An enhanced production of SOD upon elicitation with silver nanoparticles has been previously demonstrated by several researchers [17,21]. POD catalyzes the breakdown of H2O2 into water and oxygen. POD helps to protect cells against the damaging effects of H2O2, which can cause oxidative stress if it accumulates in cells [60]. CAT converts H2O2 into water and oxygen [61]. PAL is an enzyme that plays a role in the synthesis of phenolic compounds, which are important secondary metabolites in plants. PAL operates in the phenylpropanoid pathway, which is concerned with the production of secondary metabolites. The increased aervine production and secondary metabolites that we discussed above might also be due to the upregulation of PAL functions [62]. Ascorbate peroxidase (APX) is an enzyme that converts ascorbate (vitamin C) into its oxidized form (dehydroascorbate) while simultaneously breaking down H2O2 into water and oxygen. APX is important in plants and algae, where it helps to protect chloroplasts against the oxidative damage caused by photosynthesis [63]. It might be assumed that the greenish hue on the CC upon elicitation might be due to the upregulation of APX. A possible mechanism behind elicitation-mediated better antioxidant defense is that both AgNPs and SA can stimulate the production of reactive oxygen species (ROS) [64] in cells, which can then activate the expressions of genes encoding antioxidant enzymes. This upregulation of antioxidant enzymes is useful for a biological system. Another possible mechanism is that SA and AgNPs can directly interact with antioxidant enzymes, altering their structure and function. Studies have shown that AgNPs can bind to antioxidant proteins, increasing their activity and stability [65,66]. Additionally, AgNPs can act as electron acceptors and transfer electrons to antioxidant enzymes, leading to increased activity. This electron transfer mechanism has been observed in studies of AgNPs in bacterial systems, where they have been shown to enhance the activity of antioxidant enzymes, such as glutathione peroxidase. Additionally, PAL activity is often used as a biochemical marker for the biosynthesis of phenolics in plants, and it can be induced by various biotic and abiotic stresses [67].
4.5. Elicitation Mediated More DPPH Scavenging Ability
In the present research, we noted an enhanced DPPH scavenging percentage upon elicitation. The application of AgNPs to in vitro CCs can enhance the biosynthesis of secondary compounds with antioxidant properties, such as phenolic compounds and flavonoids [47,48]. These compounds scavenge free radicals, such as DPPH, and protect the plant from oxidative stress [68]. AgNPs can also increase the activity of antioxidant enzymes, which can further enhance the antioxidant potential of the CC. Studies [69,70] have shown that salicylic acid can increase the DPPH scavenging percentage by directly reacting with DPPH and donating an electron or hydrogen radical to reduce its absorbance. The antioxidant activity of salicylic acid is dose-dependent, meaning that higher concentrations of salicylic acid can result in a higher DPPH scavenging percentage. These observations are according to our results, where it was shown that a higher concentration of SA 100 µM promised better DPPH scavenging [70].
Apart from chemically synthesized AgNPs, the green synthesis of nanoparticles may serve as another useful strategy for inducing oxidative stress in callus cultures. For example, in a previous study [71], the biosynthesis of AgNPs mediated by the fungus Rhizoctonia solani was explained. This approach is considered environmentally friendly and cost-effective. Similarly, another study [72] presented the fabrication and antibacterial properties of AgNPs on the surface of graphene oxide (GO) nanosheets. The researchers utilized a biomass extract of Pseudomonas aeruginosa to synthesize the nanocomposites. These novel strategies have the potential to produce cost-effective nanoparticles for their application in elicitation.
5. Conclusions
In conclusion, this study evaluated the effects of various elicitation regimes of AgNPs and salicylic acid (SA) on an in vitro callus culture of Aerva sanguinolenta. The results show that all the elicitation treatments significantly enhanced the production of antioxidant metabolites, total phenolics, flavonoids, and aervine. Upon callus elicitation with salicylic acid and silver nanoparticles, the plant cells in the callus cultures experienced an increase in oxidative stress. This can be attributed to the generation of ROS, such as superoxide radicals, as a result of cellular responses to the elicitors. To counteract the oxidative stress, the callus cells of A. sanguinolenta responded by inducing antioxidative defense mechanisms. The induction of antioxidative defense resulted in the upregulation or the induction of antioxidative enzymes, such as SOD, PAL, and APX, as shown by our results. Additionally, the antioxidant potential of the test extract was also enhanced. Among the elicitation regimes, the Ag_NP elicitation was found to be more effective than the SA treatments in increasing secondary metabolite production. This mechanism helps to counteract oxidative stress and maintain cellular homeostasis, highlighting the adaptive response of the callus cultures to the elicitors. Elicitation mediated the increased activity of PAL, which resulted in the synthesis of phytochemicals, such as aervine. Obtaining phytochemicals from plants in vivo can be challenging due to their low concentration and the complex matrix of plant tissues, which may interfere with extraction and purification processes. Additionally, seasonal and environmental variability can further complicate the process by influencing the composition of phytochemicals in plants. Therefore, the authors recommend the exploitation of in vitro callus cultures for the production of plant metabolites.
Author Contributions
Conceptualization, M.I. and M.M.; methodology, E.A.M. and M.M.; software, validation, R.C., H.O.E. and M.I.; formal analysis, R.C. and E.A.M.; investigation, E.A.M.; resources, M.I.; data curation, M.W.M.; writing—original draft preparation, M.W.M.; writing—review and editing, M.I., M.M. and M.W.M.; visualization, R.C.; supervision, M.I. and M.M.; project administration, H.O.E.; funding acquisition, M.I., H.O.E., R.C. and E.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers Supporting Project number (RSP2023R118), King Saud University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available within this publication.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2023R118), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goyal, M.; Pareek, A.; Nagori, B.; Sasmal, D. Aerva sanguinolenta: A review on phytochemistry and pharmacological aspects. Pharmacogn. Rev. 2011, 5, 195. [Google Scholar] [CrossRef]
- Singh, S.A.; Gowri, K.; Chitra, V. A Review on Phytochemical Constituents and Pharmacological Activities of the plant: Aerva sanguinolenta. Res. J. Pharm. Technol. 2020, 13, 1580–1586. [Google Scholar] [CrossRef]
- Khan, B.; Abdukadir, A.; Qureshi, R.; Mustafa, G. Medicinal uses of plants by the inhabitants of Khunjerab National Park, Gilgit, Pakistan. Pak. J. Bot. 2011, 43, 2301–2310. [Google Scholar]
- Yamunadevi, M.; Wesely, E.G.; Johnson, M. Phytochemical studies on the terpenoids of medicinally important plant Aerva sanguinolenta L. using HPTLC. Asian Pac. J. Trop. Biomed. 2011, 1, S220–S225. [Google Scholar] [CrossRef]
- Gujjeti, R.P.; Mamidala, E. Phytochemical screening and thin layer chromatographic studies of Aerva sanguinolenta root extract. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 5725–5730. [Google Scholar]
- Murugan, M.; Mohan, V.R. Phytochemical, FT-IR and antibacterial activity of whole plant extract of Aerva sanguinolenta (L.) Juss. Ex. Schult. J. Med. Plants Stud. 2014, 4, 51–57. [Google Scholar]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Ajaib, M.; Hussain, I.; Hussain, T.; Parveen, A.; Thind, S.; Sardar, T.; Akram, R.; et al. Synergistic application of calcium oxide nanoparticles and farmyard manure induces cadmium tolerance in mung bean (Vigna radiata L.) by influencing physiological and biochemical parameters. PLoS ONE 2023, 18, e0282531. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. S. Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Eder, J.; Cosio, E.G. Elicitors of plant defense responses. Int. Rev. Cytol. 1994, 148, 1–36. [Google Scholar]
- Patel, H.; Krishnamurthy, R. Elicitors in plant tissue culture. J. Pharmacogn. Phytochem. 2013, 2, 60–65. [Google Scholar]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A tool for improving fruit phenolic content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with zinc oxide nanoparticles improves growth, osmolyte accumulation, antioxidant defence and yield quality of water-stressed mung bean plants. Arid. Land Res. Manag. 2022, 37, 222–246. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ramalingam, C. Silver nanoparticle antimicrobial activity explained by membrane rupture and reactive oxygen generation. Environ. Chem. Lett. 2016, 14, 477–485. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef]
- Bhat, P.; Bhat, A. Silver nanoparticles for enhancement of accumulation of capsaicin in suspension culture of Capsicum sp. J. Exp. Sci. 2016, 7, 1–6. [Google Scholar]
- Mubeen, B.; Hasnain, A.; Mehboob, R.; Rasool, R.; Riaz, A.; Elaskary, S.A.; Shah, M.M.; Faridi, T.A.; Ullah, I. Hydroponics and elicitation, a combined approach to enhance the production of designer secondary medicinal metabolites in Silybum marianum. Front. Plant Sci. 2022, 13, 897795. [Google Scholar] [CrossRef]
- Hadizadeh, M.; Ofoghi, H.; Kianirad, M.; Amidi, Z. Elicitation of pharmaceutical alkaloids biosynthesis by salicylic acid in marine microalgae Arthrospira platensis. Algal Res. 2019, 42, 101597. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Arafa, N.M.; Matter, M.A. Effect of some elicitors on chemicals composition for Nigella sativa callus cultures. World J. Pharm. Sci. 2015, 3, 2160–2166. [Google Scholar]
- Vázquez-Flota, F.; Hernández-Domínguez, E.; de Lourdes Miranda-Ham, M.; Monforte-González, M. A differential response to chemical elicitors in Catharanthus roseus in vitro cultures. Biotechnol. Lett. 2009, 31, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. J. Pharmacogn. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Largia, M.J.V.; Pothiraj, G.; Shilpha, J.; Ramesh, M. Methyl jasmonate and salicylic acid synergism enhances bacoside A content in shoot cultures of Bacopa monnieri (L.). Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 9–20. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 113, 25–39. [Google Scholar] [CrossRef]
- Rahman, L.U.; Qureshi, R.; Yasinzai, M.M.; Shah, A. Synthesis and spectroscopic characterization of Ag-Cu alloy nanoparticles prepared in various ratios. C. R. Chim. 2012, 15, 533–538. [Google Scholar] [CrossRef]
- Rashmi, R.; Trivedi, M.P. Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Appl. Biochem. Biotechnol. 2014, 172, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Abbasi, B.H.; Ahmed, N.; Ali, H. Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind. Crops Prod. 2013, 46, 105–110. [Google Scholar] [CrossRef]
- Swain, T.; Goldstein, J.L. The quantitative analysis of phenolic compounds. In Methods in Polyphenol Chemistry; Pridham, J.B., Ed.; Permagon: New York, NY, USA, 1964. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Boobalan, S.; Kamalanathan, D. Tailoring enhanced production of aervine in Aerva sanguinolenta (L.) Juss. Ex Schult by Agrobacterium rhizogenes-mediated hairy root cultures. Ind. Crops Prod. 2020, 155, 112814. [Google Scholar] [CrossRef]
- Simoes, C.; Bizarri, C.H.B.; da Silva Cordeiro, L.; de Castro, T.C.; Coutada, L.C.M.; da Silva, A.J.R.; Albarello, N.; Mansur, E. Anthocyanin production in callus cultures of Cleome rosea: Modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol. Biochem. 2009, 47, 895–903. [Google Scholar] [CrossRef]
- Yashin, Y.I.; Yashin, A.Y. Analytical chromatography. Methods, instrumentation and applications. Russ. Chem. Rev. 2006, 75, 329. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Khan, M.A.; Mahmood, T.; Ahmad, M.; Chaudhary, M.F.; Khan, M.A. Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 371–376. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Abeles, F.B.; Biles, C.L. Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol. 1991, 95, 269–273. [Google Scholar] [CrossRef]
- Arrigoni, O.; De Gara, L.; Tommasi, F.; Liso, R. Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol. 1992, 99, 235–238. [Google Scholar] [CrossRef]
- Miyake, C.; Shinzaki, Y.; Nishioka, M.; Horiguchi, S.; Tomizawa, K.I. Photoinactivation of ascorbate peroxidase in isolated tobacco chloroplasts: Galdieria partita APX maintains the electron flux through the water–water cycle in transplastomic tobacco plants. Plant Cell Physiol. 2006, 47, 200–210. [Google Scholar] [CrossRef]
- Golkar, P.; Moradi, M.; Garousi, G.A. Elicitation of stevia glycosides using salicylic acid and silver nanoparticles under callus culture. Sugar Tech 2019, 21, 569–577. [Google Scholar] [CrossRef]
- Begum, S.; Zahid, A.; Khan, T.; Khan, N.Z.; Ali, W. Comparative analysis of the effects of chemically and biologically synthesized silver nanoparticles on biomass accumulation and secondary metabolism in callus cultures of Fagonia indica. Physiol. Mol. Biol. Plants 2020, 26, 1739–1750. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V. Chitosan/gelatin/silver nanoparticles composites films for biodegradable food packaging applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.M.; Siddiqui, M.K.J.; AlSalhi, M.S.; Alrokayan, S.A. Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 1266–1271. [Google Scholar] [CrossRef]
- Nazir, S.; Jan, H.; Zaman, G.; Ahmed, N.; Drouet, S.; Hano, C.; Abbasi, B.H. Synergistic effects of salicylic acid and light stress on bioactive metabolites in basil callus cultures. Biocatal. Agric. Biotechnol. 2021, 37, 102176. [Google Scholar] [CrossRef]
- Golkar, P.; Taghizadeh, M.; Yousefian, Z. The effects of chitosan and salicylic acid on elicitation of secondary metabolites and antioxidant activity of safflower under in vitro salinity stress. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 137, 575–585. [Google Scholar] [CrossRef]
- Nechaeva, T.L.; Nikolaeva, T.N.; Zagoskina, N.V. Salicylic and hydroxybenzoic acids affect the accumulation of phenolic compounds in tea-plant cultures in vitro. Biol. Bull. 2020, 47, 374–380. [Google Scholar] [CrossRef]
- Miclea, I.; Suhani, A.; Zahan, M.; Bunea, A. Effect of jasmonic acid and salicylic acid on growth and biochemical composition of in-vitro-propagated Lavandula angustifolia Mill. Agronomy 2020, 10, 1722. [Google Scholar] [CrossRef]
- Anjum, S.; Abbasi, B.H. Thidiazuron-enhanced biosynthesis and antimicrobial efficacy of silver nanoparticles via improving phytochemical reducing potential in callus culture of Linum usitatissimum L. Int. J. Nanomed. 2016, 11, 715. [Google Scholar]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef]
- Siatka, T. Effects of growth regulators on production of anthocyanins in callus cultures of Angelica archangelica. Nat. Prod. Commun. 2019, 14, 1934578–19857344. [Google Scholar] [CrossRef]
- Nazir, M.; Ullah, M.A.; Younas, M.; Siddiquah, A.; Shah, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 142, 107–120. [Google Scholar] [CrossRef]
- Khan, T.; Khan, T.; Hano, C.; Abbasi, B.H. Effects of chitosan and salicylic acid on the production of pharmacologically attractive secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2019, 129, 525–535. [Google Scholar] [CrossRef]
- Woch, N.; Laha, S.; Gudipalli, P. Salicylic acid and jasmonic acid induced enhanced production of total phenolics, flavonoids, and antioxidant metabolism in callus cultures of Givotia moluccana (L.) Sreem. In Vitro Cell. Dev. Biol.-Plant 2023, 59, 227–248. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid: An efficient elicitor of secondary metabolite production in plants. Biocatal. Agric. Biotechnol. 2021, 31, 101884. [Google Scholar] [CrossRef]
- Srinivasan, R.; Kamalanathan, D.; Rathinavel, T.; Iqbal, M.N.; Shanmugam, G. Anti-cancer potentials of aervine validated through in silico molecular docking, dynamics simulations, pharmacokinetic prediction and in vitro assessment of caspase–3 in SW480 cell line. Mol. Simul. 2023, 49, 799–815. [Google Scholar] [CrossRef]
- Devi, B.; Jeet, K.; Nigam, V. A Review study over: Pharmacognostic study, Phytochemical and Pharmacological studies over whole plant of Aerva sanguinolenta (L.). In Biological Trace Element Research; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Fouad, A.; Hegazy, A.E.; Azab, E.; Khojah, E.; Kapiel, T. Boosting of antioxidants and alkaloids in Catharanthus roseus suspension cultures using silver nanoparticles with expression of CrMPK3 and STR genes. Plants 2021, 10, 2202. [Google Scholar] [CrossRef]
- Prakash, V.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Corpas, F.J. Nitric oxide (NO) and salicylic acid (SA): A framework for their relationship in plant development under abiotic stress. Plant Biol. 2021, 23, 39–49. [Google Scholar] [CrossRef]
- Khattab, S.; Yap, Y.K.; El Sherif, F. Salicylic Acid Foliar Spray Enhanced Silybum marianum Growth and Yield, as Well as Its Chemical Constituents and Chalcone Synthase Gene Activity. Horticulturae 2022, 8, 556. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozymes: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Fall, I.; Czerwiec, Q.; Abdellaoui, S.; Doumèche, B.; Ochs, M.; Rémond, C.; Rakotoarivonina, H. A thermostable bacterial catalase-peroxidase oxidizes phenolic compounds derived from lignins. Appl. Microbiol. Biotechnol. 2023, 107, 201–217. [Google Scholar] [CrossRef]
- Jannesari, M.; Akhavan, O.; Hosseini, H.R.M.; Bakhshi, B. Oxygen-Rich Graphene/ZnO2-Ag Nanoframeworks with pH-Switchable Catalase/Peroxidase Activity as O2 Nanobubble-Self Generator for Bacterial Inactivation. J. Colloid Interface Sci. 2023, 637, 237–250. [Google Scholar] [CrossRef]
- Medda, S.; Dessena, L.; Mulas, M. Monitoring of the PAL enzymatic activity and polyphenolic compounds in leaves and fruits of two myrtle cultivars during maturation. Agriculture 2020, 10, 389. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chen, H.; Liu, F.; Fu, Z.Q. PTI and ETI: Convergent pathways with diverse elicitors. Trends Plant Sci. 2021, 27, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Saleeb, N.; Robinson, B.; Cavanagh, J.; Ross, J.; Munir, K.; Gooneratne, R. Antioxidant enzyme activity and lipid peroxidation in Aporrectodea caliginosa earthworms exposed to silver nanoparticles and silver nitrate in spiked soil. Environ. Toxicol. Chem. 2020, 39, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Shaikhaldein, H.O.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Tarroum, M.; Salih, A.M. Biosynthesis and characterization of silver nanoparticles using Ochradenus arabicus and their physiological effect on Maerua oblongifolia raised in vitro. Sci. Rep. 2020, 10, 17569. [Google Scholar] [CrossRef] [PubMed]
- Reichert, A.I.; He, X.Z.; Dixon, R.A. Phenylalanine ammonia-lyase (PAL) from tobacco (Nicotiana tabacum): Characterization of the four tobacco PAL genes and active heterotetrameric enzymes. Biochem. J. 2009, 424, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Sharifi, Y.; Ghasemi Omran, V.; Tavabe Ghavami, T.S.; Nematzadeh Gharakhili, G.A.; Ebrahimzadeh, M.A. Effect of Salicylic acid on Phenols and flavonoids content and DPPH scavenging activity in cell suspension culture of Iranian sodab (Ruta graveolens). Tabari Biomed. Stud. Res. J. 2019, 1, 18–21. [Google Scholar] [CrossRef]
- Mahdavian, K. Effect of salicylic acid and calcium chloride on lipid peroxidation and scavenging capacity of radical of red bean (Phaseolus calcaratus L.) under salt stress. Int. J. Hortic. Sci. Technol. 2022, 9, 55–72. [Google Scholar]
- Potbhare, A.K.; Chouke, P.B.; Mondal, A.; Thakare, R.U.; Mondal, S.; Chaudhary, R.G.; Rai, A.R. Rhizoctonia solani assisted biosynthesis of silver nanoparticles for antibacterial assay. Mater. Today Proc. 2020, 29, 939–945. [Google Scholar] [CrossRef]
- Potbhare, A.K.; Umekar, M.S.; Chouke, P.B.; Bagade, M.B.; Aziz, S.T.; Abdala, A.A.; Chaudhary, R.G. Bioinspired graphene-based silver nanoparticles: Fabrication, characterization and antibacterial activity. Mater. Today Proc. 2020, 29, 720–725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).