Systematic Review of Degradation Processes for Microplastics: Progress and Prospects
Abstract
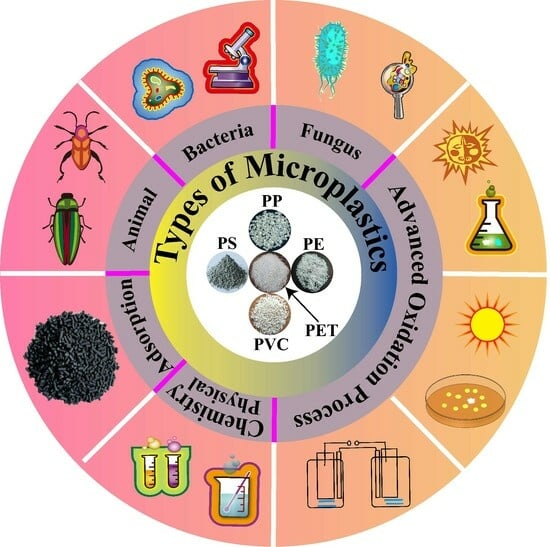
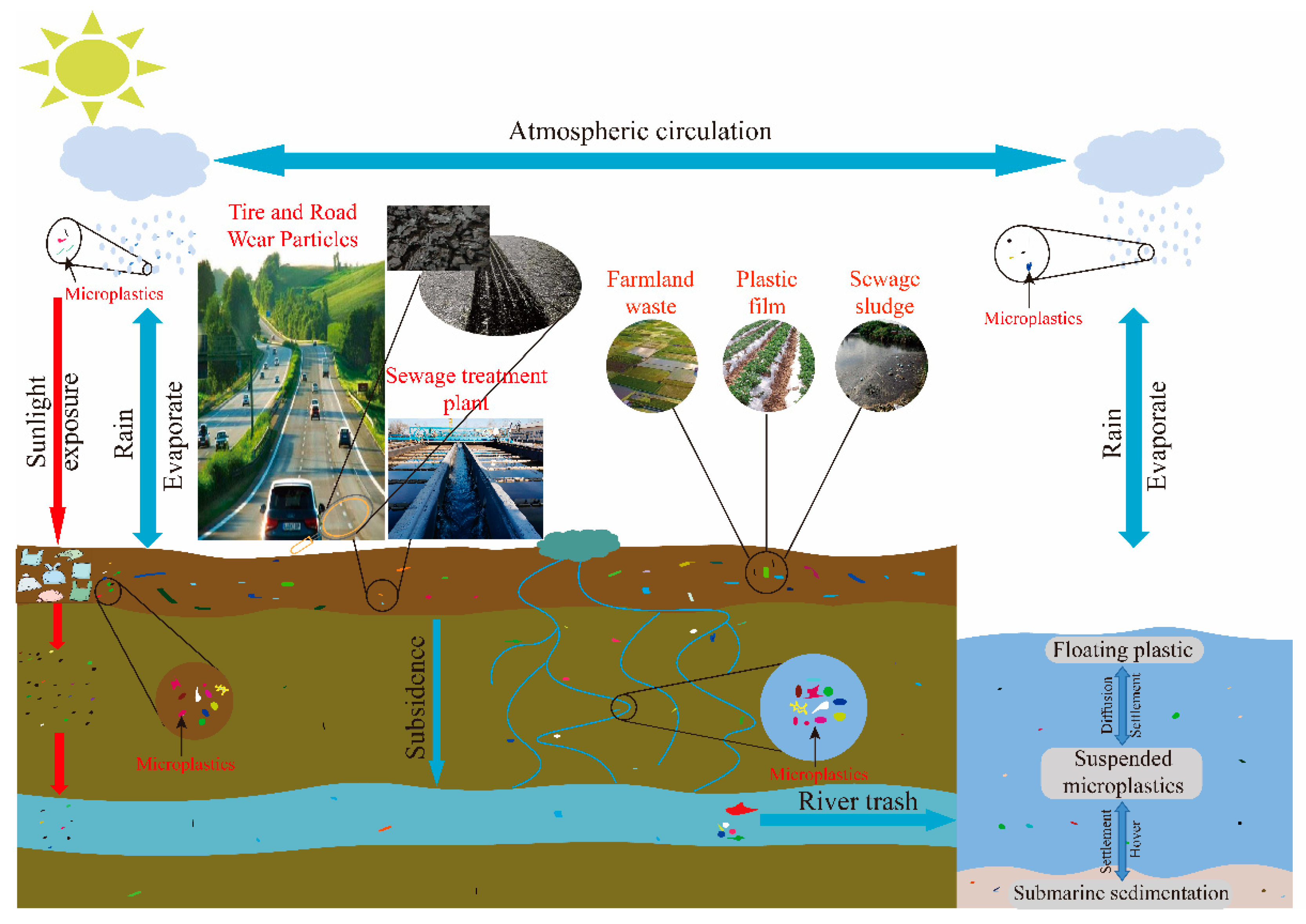
:1. Introduction
2. Physical and Chemical Processes
2.1. Physical Law
2.2. Advanced Oxidation Processes (AOPs) Degradation
2.2.1. Direct Photodegradation
2.2.2. Photocatalytic Oxidation
2.2.3. Electrochemical Oxidation
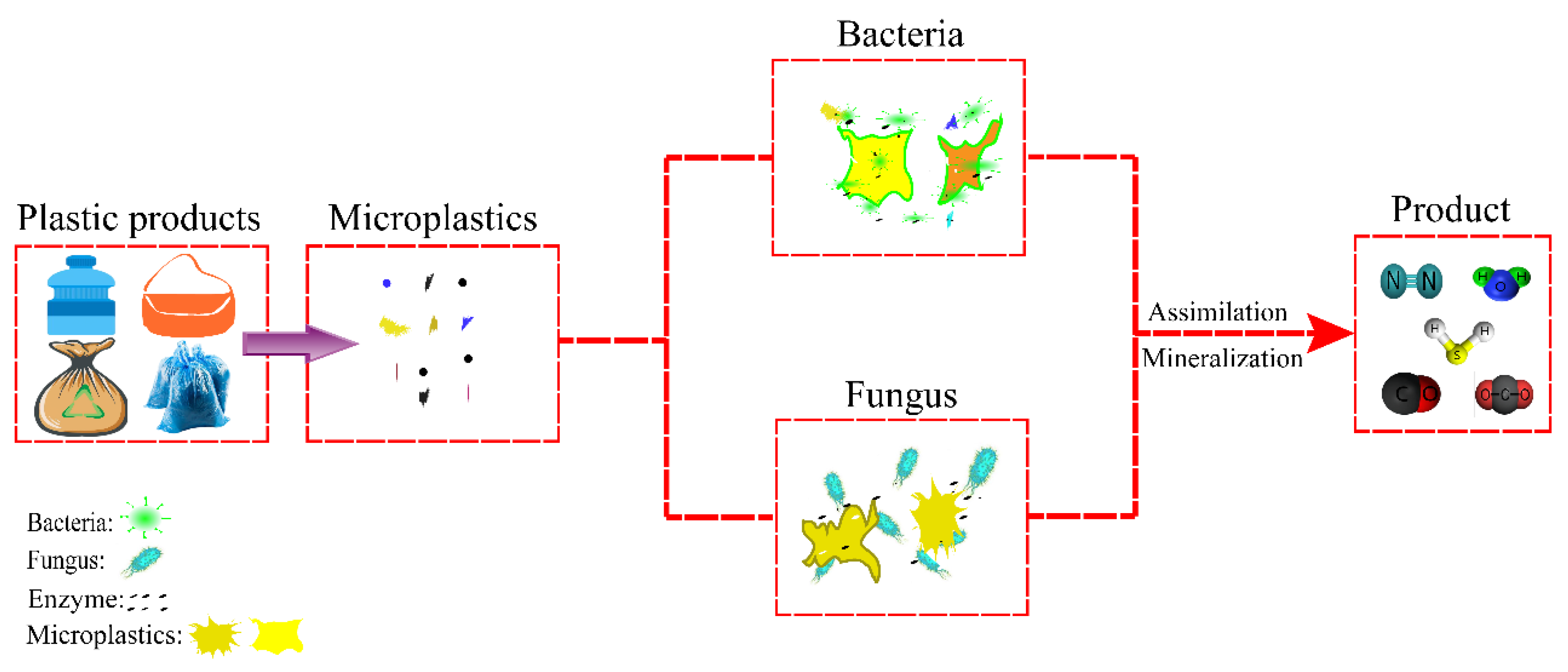
3. Biodegradation Processes
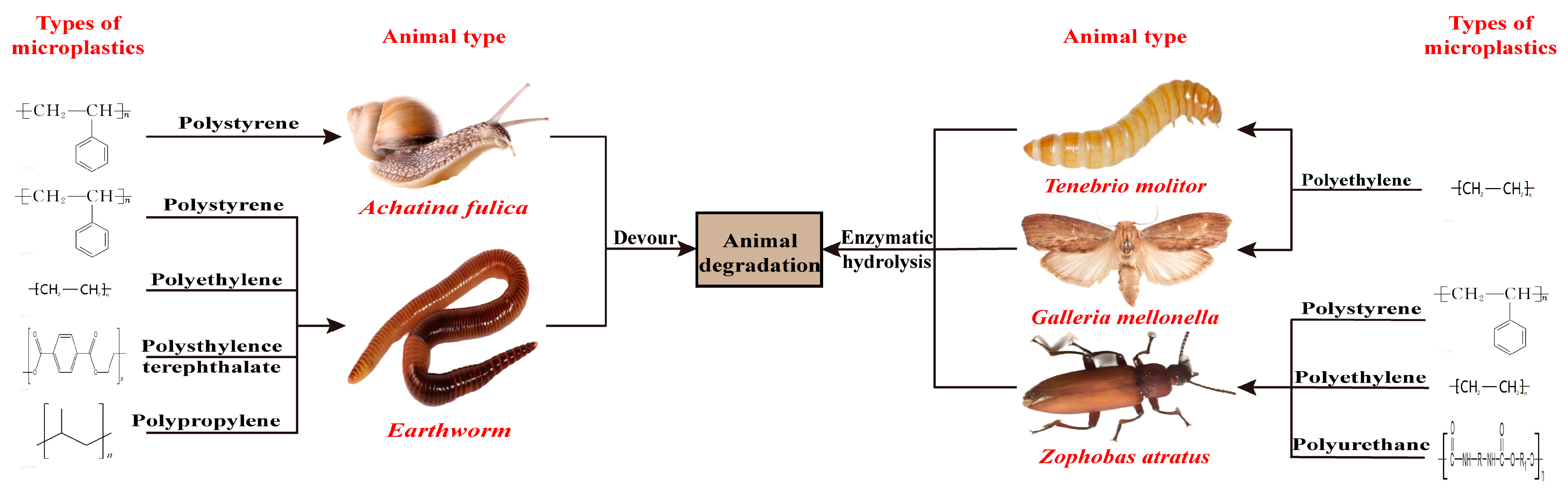
3.1. Animals
3.2. Bacteria
| The Bacteria Types | Types of MPs | Duration of Degradation | Weight Loss | References |
|---|---|---|---|---|
| Acinetobacter sp. NyZ450/Bacillus sp. NyZ451 | PE | 30 d | 18% | [93] |
| Exiguobacterium sp. YT2 | PS | 60 d | 7.4% | [94] |
| Bacillus simplex, and Bacillus sp. | LDPE | 21 d | [95] | |
| Bacillus subtilis | PE | 30 d | 9.26% | [96] |
| Bacillus sp. and Paenibacillus sp. | PE | 60 d | 14.7% | [97] |
| Bacillus cereus | PE/PET/PS | 40 d | 1.6%/6.6%/7.4% | [98] |
| Bacillus gottheilii | PE/PET/PP/PS | 40 d | 6.2%/3.0%/3.6%/5.8% | [98] |
| B. cereus, S. globispora and B. flexus and B. gottheilii | PS and PET | 90 d | 18% | [99] |
| Bacillus sp. 27 | PP | 40 d | 4.0% | [100] |
| Rhodococcus sp. 36 | PP | 40 d | 6.4% | [100] |
| Firmicutes, Bacteroidetes, Proteobacteria, Gemmatimonadetes, Deinococcus-Thermus | PE/PVC/PHA | 60 d | 13%/3%/29% | [103] |
| Thermus, Bacillus, and Geobacillus | PS | 56 d | 7.3% | [104] |
| Aneurinibacillus aneurinilyticus btDSCE01, Brevibacillus agri btDSCE02, Brevibacillus sp. btDSCE03, and Brevibacillus brevis btDSCE04 | LDPE/HDPE/PP | 140 d | 58.21%/46.6%/56.3% | [106] |
| Pseudomonas sp. ADL15 | PP | 40 d | 17.3% | [107] |
| Rhodococcus sp. | PP | 40 d | 7.3% | [107] |
| Bacillus licheniformis/Lysinibacillus mas-siliensis, Bacillus sp. and Delftia acidovarans | LDPE and PS | 22 d | [108] | |
| Bacillus paramycoides (BP) | PP | 21 d | 78.99% | [109] |
| Bacillus cereus (BC) | PE | 21 d | 63% | [109] |
| Bacillus paramycoides (BP) and Bacillus cereus (BC) | PP/PE | 21 d | 78.62%/72.50% | [109] |
| M. hydrolyticus IRE-31 | LDPE | 30 d | [110] | |
| Anaerobic bacteria | PVC | 210 d | 11.7% | [111] |
| Kocuria palustris M16 | PE | 30 d | 1% | [112] |
| Bacillus pumilus M27 | PE | 30 d | 1.5% | [112] |
| Bacillus subtilis H1584 | PE | 30 d | 1.75% | [112] |
| V. parahaemolyticus (BTTV4 and BTTN18) and V.alginolyticus (BTTC10 and BTTC27) | PVA-LLDPE | 105 d | [113] | |
| Exiguobacterium sp., Halomonas sp., and Ochrobactrum sp. | PET/PE | 28 d | 2.7%/19.6% | [114] |
3.3. Fungi
| Fungi Type | Types of MPs | Duration of Degradation | Weight Loss | References |
|---|---|---|---|---|
| Aspergillus flavus PEDX3 | HDPE | 28 d | 3.9% | [87] |
| Aspergillus clavatu JASK1 | LDPE | 90 d | 35% | [125] |
| A. flavus | LDPE | 120 d | 14.3% | [126] |
| A. terreus | LDPE | 120 d | 13.1% | [126] |
| A. flavus | LDPE | 270 d | 30.6% | [126] |
| A. terreus | LDPE | 270 d | 11.4% | [126] |
| Aspergillus terreus MF12. | HDPE | 30 d | 9.4% | [127] |
| Aspergillus flavus MCP5 | LDPE | 14 d | 1.67% | [128] |
| Alternaria alternata, Aspergillus caespitosus, Aspergillus terreus, Eupenicillium hirayamae, Paecilomyces variotii, and Phialophora alba | LDPE | 28 d | [129] | |
| Pestalotiopsis microspora | PUR | 14 d | [130] | |
| Zalerion maritimum | PE | 14 d | 43% | [131] |
| Aspergillus tubingensis VRKPT1 | HDPE | 30 d | 6.02% | [132] |
| Aspergillus flavus VRKPT2 | HDPE | 30 d | 8.51% | [132] |
3.4. Combined Degradation of MPs by Bacteria and Fungi
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sharuddin, S.; Abnisa, F.; Daud, W.; Aroua, M.K.J.E.C. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Kawecki, D.; Scheeder, P.; Nowack, B. Probabilistic Material Flow Analysis of Seven Commodity Plastics in Europe. Environ. Sci. Technol. 2018, 52, 9874–9888. [Google Scholar] [CrossRef]
- Meng, Y.; Kelly, F.J.; Wright, S.L. Advances and challenges of microplastic pollution in freshwater ecosystems: A UK perspective. Environ. Pollut. 2020, 256, 113445. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Abel, D.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2017, 24, 1405–1416. [Google Scholar]
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; Santillan, L. New plastic formations in the Anthropocene. Sci. Total Environ. 2022, 754, 142216. [Google Scholar] [CrossRef]
- Xiang, P.; Zhang, Y.; Zhang, T.; Wu, Q.; Zhao, C.; Li, Q. A novel bacterial combination for efficient degradation of polystyrene microplastics. J. Hazard. Mater. 2023, 458, 131856. [Google Scholar] [CrossRef]
- Shen, M.C.; Song, B.; Zeng, G.M.; Zhang, Y.X.; Huang, W.; Wen, X.F.; Tang, W.W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef]
- Yurtsever, M. Glitters as a Source of Primary Microplastics: An Approach to Environmental Responsibility and Ethics. J. Agric. Environ. Ethics 2019, 32, 459–478. [Google Scholar] [CrossRef]
- Yurtsever, M. Tiny, shiny, and colorful microplastics: Are regular glitters a significant source of microplastics? Mar. Pollut. Bull. 2019, 146, 678–682. [Google Scholar] [CrossRef]
- Qu, H.; Ma, R.X.; Barrett, H.; Wang, B.; Han, J.J.; Wang, F.; Chen, P.; Wang, W.; Peng, G.L.; Yu, G. How microplastics affect chiral illicit drug methamphetamine in aquatic food chain? From green alga (Chlorella pyrenoidosa) to freshwater snail (Cipangopaludian cathayensis). Environ. Int. 2020, 136, 105480. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.H.; Li, H.; Liu, P.; Zhang, Z.P.; Ouyang, Z.Z.; Guo, X.T. Review of the toxic effect of microplastics on terrestrial and aquatic plants. Sci. Total Environ. 2021, 791, 148333. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Cardenas, A.; Scott, D.T.; Seitmaganbetova, G.; van Pelt, F.; O’Halloran, J.; Jansen, M.A.K. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Watanuki, Y.; Takada, H.; Ishizuka, M.; Yamashita, R.; Kazama, M.; Hiki, N.; Kashiwada, F.; Mizukawa, K.; Mizukawa, H.; et al. In Vivo Accumulation of Plastic-Derived Chemicals into Seabird Tissues. Curr. Biol. 2020, 30, 723–728.e3. [Google Scholar] [CrossRef]
- Qiao, R.X.; Deng, Y.F.; Zhang, S.H.; Wolosker, M.B.; Zhu, Q.D.; Ren, H.Q.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of microplastics promotes their ingestion by marine zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef]
- Sathicq, M.B.; Sabatino, R.; Corno, G.; Di Cesare, A. Are microplastic particles a hotspot for the spread and the persistence of antibiotic resistance in aquatic systems? Environ. Pollut. 2021, 279, 116896. [Google Scholar] [CrossRef]
- Naik, R.K.; Naik, M.M.; D’Costa, P.M.; Shaikh, F. Microplastics in ballast water as an emerging source and vector for harmful chemicals, antibiotics, metals, bacterial pathogens and HAB species: A potential risk to the marine environment and human health. Mar. Pollut. Bull. 2019, 149, 110525. [Google Scholar] [CrossRef]
- Sharma, M.D.; Elanjickal, A.I.; Mankar, J.S.; Krupadam, R.J. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef] [PubMed]
- Tien, C.J.; Wang, Z.X.; Chen, C.S. Microplastics in water, sediment and fish from the Fengshan River system: Relationship to aquatic factors and accumulation of polycyclic aromatic hydrocarbons by fish. Environ. Pollut. 2020, 265, 114962. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Q.; Liu, Y.G.; Chen, Y.; Zhang, W.; Zhao, J.M.; He, S.Y.; Yang, C.P.; Zhang, T.; Tang, C.F.; Zhang, C.; et al. A review: Research progress on microplastic pollutants in aquatic environments. Sci. Total Environ. 2021, 766, 142572. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Song, B.A.; Liang, J.; Niu, Q.Y.; Zeng, G.M.; Shen, M.C.; Deng, J.Q.; Luo, Y.A.; Wen, X.F.; Zhang, Y.F. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Conlledo, N.; Larsen, B.K.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandia, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. Trac-Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Labrenz, M. Marine Microbial Assemblages on Microplastics: Diversity, Adaptation, and Role in Degradation. Annu. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef]
- Kesy, K.; Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Spatial Environmental Heterogeneity Determines Young Biofilm Assemblages on Microplastics in Baltic Sea Mesocosms. Front. Microbiol. 2019, 10, 1665. [Google Scholar] [CrossRef]
- Stabnikova, O.; Stabnikov, V.; Marinin, A.; Klavins, M.; Klavins, L.; Vaseashta, A. Microbial Life on the Surface of Microplastics in Natural Waters. Appl. Sci. 2021, 11, 11692. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.T.; Wang, D.D.; Yan, M.Q.; Zhang, J.; Zhang, P.Y.; Ding, T.G.; Chen, L.; Chen, C. Current technologies for plastic waste treatment: A review. J. Clean. Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Bao, Z.; Feng, H.; Tu, W.; Li, L.; Li, Q. Method and mechanism of chromium removal from soil: A systematic review. Environ. Sci. Pollut. Res. Int. 2022, 29, 35501–35517. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Xie, Y.Q.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Tan, Y.; Yang, Y.; Yang, J.; Tan, D. Fluid-induced vibration evolution mechanism of multiphase free sink vortex and the multi-source vibration sensing method. Mech. Syst. Signal Process. 2023, 189, 110058. [Google Scholar] [CrossRef]
- Zhu, K.C.; Jia, H.Z.; Sun, Y.J.; Dai, Y.C.; Zhang, C.; Guo, X.T.; Wang, T.C.; Zhu, L.Y. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Xu, L.; Crawford, K.; Gorman, C.B. Effects of Temperature and pH on the Degradation of Poly(lactic acid) Brushes. Macromolecules 2011, 44, 4777–4782. [Google Scholar] [CrossRef]
- Anand, U.; Dey, S.; Bontempi, E.; Ducoli, S.; Vethaak, A.D.; Dey, A.; Federici, S. Biotechnological methods to remove microplastics: A review. Environ. Chem. Lett. 2023, 21, 1787–1810. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M. Nanoplastics in aquatic systems—Are they more hazardous than microplastics? Environ. Pollut. 2021, 272, 115950. [Google Scholar] [CrossRef]
- Li, L.; Gu, Z.; Xu, W.; Tan, Y.; Fan, X.; Tan, D. Mixing mass transfer mechanism and dynamic control of gas-liquid-solid multiphase flow based on VOF-DEM coupling. Energy 2023, 272, 127015. [Google Scholar] [CrossRef]
- Lin, L.; Bin, L.; Wei-xin, X.; Ze-heng, G.; Yuan-shan, Y.; Da-peng, T. Multiphase coupling transport evolution mechanism of the free sink vortex. Acta Phys. Sin. 2023, 72, 034702. [Google Scholar] [CrossRef]
- Herbort, A.F.; Sturm, M.T.; Schuhen, K. A new approach for the agglomeration and subsequent removal of polyethylene, polypropylene, and mixtures of both from freshwater systems—A case study. Environ. Sci. Pollut. Res. 2018, 25, 15226–15234. [Google Scholar] [CrossRef]
- Leppänen, I.; Lappalainen, T.; Lohtander, T.; Jonkergouw, C.; Arola, S.; Tammelin, T. Capturing colloidal nano- and microplastics with plant-based nanocellulose networks. Nat. Commun. 2022, 13, 1814. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef]
- Regkouzas, P.; Diamadopoulos, E. Adsorption of selected organic micro-pollutants on sewage sludge biochar. Chemosphere 2019, 224, 840–851. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.X.; Chi, Y.; Yan, J.H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Application of Zn/Al layered double hydroxides for the removal of nanoscale plastic debris from aqueous systems. J. Hazard. Mater. 2020, 397, 122769. [Google Scholar] [CrossRef]
- Sun, C.Z.; Wang, Z.G.; Chen, L.Y.; Li, F.M. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Eng. J. 2021, 408, 127317. [Google Scholar] [CrossRef]
- Chen, J.L.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.P.; Zhang, W.X.; Yang, J.P. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef]
- Ricardo, I.A.; Alberto, E.A.; Silva, A.H.; Macuvele, D.; Padoin, N.; Soares, C.; Riella, H.G.; Starling, M.; Trovo, A.G. A critical review on microplastics, interaction with organic and inorganic pollutants, impacts and effectiveness of advanced oxidation processes applied for their removal from aqueous matrices. Chem. Eng. J. 2021, 424, 130282. [Google Scholar] [CrossRef]
- Hu, W.; Liu, D.D.; Su, S.H.; Ren, L.J.; Ren, H.; Wei, L.F.; Yue, S.Y.; Xie, Q.R.; Zhang, Z.M.; Wang, Z.H.; et al. Photochemical Degradation of Organic Matter in the Atmosphere. Adv. Sustain. Syst. 2021, 5, 2100027. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.Y.; Zhan, X.; Lu, K.; Gu, C.; Gao, S.X. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.Q.; Wang, J.D.; Peng, J.P.; Wu, Z.Q.; Tan, X.L. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628–629, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.C.; Jia, H.Z.; Zhao, S.; Xia, T.J.; Guo, X.T.; Wang, T.C.; Zhu, L.Y. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Cao, W.M.; Qu, R.J.; Zhou, D.M.; Sun, C.; Wang, Z.Y. Photochemical transformation of decachlorobiphenyl (PCB-209) on the surface of microplastics in aqueous solution. Chem. Eng. J. 2021, 420, 129813. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Ding, L.; Ouyang, Z.Z.; Liu, P.; Wang, T.C.; Jia, H.Z.; Guo, X.T. Photodegradation of microplastics mediated by different types of soil: The effect of soil components. Sci. Total Environ. 2022, 802, 149840. [Google Scholar] [CrossRef]
- Wang, C.; Xian, Z.; Jin, X.; Liang, S.; Chen, Z.; Pan, B.; Wu, B.; Ok, Y.S.; Gu, C. Photo-aging of polyvinyl chloride microplastic in the presence of natural organic acids. Water Res. 2020, 183, 116082. [Google Scholar] [CrossRef]
- Zhang, X.L.; Xia, M.L.; Su, X.J.; Yuan, P.; Li, X.K.; Zhou, C.Y.; Wan, Z.P.; Zou, W. Photolytic degradation elevated the toxicity of polylactic acid microplastics to developing zebrafish by triggering mitochondrial dysfunction and apoptosis. J. Hazard. Mater. 2021, 413, 125321. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.X.; Zhenyu, W.; Xing, B.S. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Chen, H.B.; Yang, Y.; Wang, C.; Hua, X.; Li, H.; Xie, D.L.; Xiang, M.D.; Yu, Y.J. Reproductive toxicity of UV-photodegraded polystyrene microplastics induced by DNA damage-dependent cell apoptosis in Caenorhabditis elegans. Sci. Total Environ. 2022, 811, 152350. [Google Scholar] [CrossRef]
- Addanki Tirumala, R.T. Exploiting the Optical Properties of Earth Abundant Cuprous Oxide Nanocatalysts for Energy and Health Applications. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2022. [Google Scholar]
- Li, L.; Tan, Y.; Xu, W.; Ni, Y.; Yang, J.; Tan, D. Fluid-induced transport dynamics and vibration patterns of multiphase vortex in the critical transition states. Int. J. Mech. Sci. 2023, 252, 108376. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Lim, G.D.; Kim, S.; Ryu, S.H.; Hwang, T.Y.; Park, K.R.; Choa, Y.H. Near-infrared absorbance properties of Cu2-xS/SiO2 nanoparticles and their PDMS-based composites. J. Mater. Chem. C 2018, 6, 754–760. [Google Scholar] [CrossRef]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.U.R.; Li, K.J.; Cheng, H.Y.; Wang, T.; Liu, Y.Y.; Ajmal, S.; Yang, Y.; Feng, Y.Q.; Zhang, L.W. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. Iscience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Luo, H.W.; Xiang, Y.H.; Tian, T.; Pan, X.L. An AFM-IR study on surface properties of nano-TiO2 coated polyethylene (PE) thin film as influenced by photocatalytic aging process. Sci. Total Environ. 2021, 757, 143900. [Google Scholar] [CrossRef]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced Visible Light Photodegradation of Microplastic Fragments with Plasmonic Platinum/Zinc Oxide Nanorod Photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Cao, B.Q.; Wan, S.P.; Wang, Y.A.; Guo, H.W.; Ou, M.; Zhong, Q. Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst. J. Colloid. Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef]
- Zhou, X.S.; Jin, B.; Luo, J.; Gu, X.X.; Zhang, S.Q. Photoreduction preparation of Cu2O@polydopamine nanospheres with enhanced photocatalytic activity under visible light irradiation. J. Solid. State Chem. 2017, 254, 55–61. [Google Scholar] [CrossRef]
- Zhu, B.J.; Jiang, G.F.; Lv, Y.; Liu, F.; Sun, J. Photocatalytic degradation of polyacrylamide by rGO@Fe3O4/Cu2O@ZnO magnetic recyclable composites. Mater. Sci. Semicond. Process. 2021, 131, 105841. [Google Scholar] [CrossRef]
- Pary, F.F.; Addanki Tirumala, R.T.; Andiappan, M.; Nelson, T.L. Copper(i) oxide nanoparticle-mediated C-C couplings for synthesis of polyphenylenediethynylenes: Evidence for a homogeneous catalytic pathway. Catal. Sci. Technol. 2021, 11, 2414–2421. [Google Scholar] [CrossRef]
- Tirumala, R.T.A.; Gyawali, S.; Wheeler, A.; Ramakrishnan, S.B.; Sooriyagoda, R.; Mohammadparast, F.; Khatri, N.; Tan, S.; Kalkan, A.K.; Bristow, A.D.; et al. Structure–Property–Performance Relationships of Cuprous Oxide Nanostructures for Dielectric Mie Resonance-Enhanced Photocatalysis. ACS Catal. 2022, 12, 7975–7985. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Liu, J.D.; Zhou, F.; Zhou, S.Y.; Wu, J.C.; Chen, D.Y.; Xu, Q.F.; Lu, J.M. Polymer-Coated Fe2O3 Nanoparticles for Photocatalytic Degradation of Organic Materials and Antibiotics in Water. Acs Appl. Nano Mater. 2020, 3, 9200–9208. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xia, L.; Liu, C.; Cheng, X.B.; Yang, Z. A direct Z-scheme heterojunction g-C3N4/alpha-Fe2O3 nanocomposite for enhanced polymer-containing oilfield sewage degradation under visible light. Environ. Sci.-Water Res. Technol. 2022, 8, 1965–1975. [Google Scholar] [CrossRef]
- Yin, X.N.; Wang, J.; Zhou, J.J.; Li, L. Mussel-inspired modification of Microporous polypropylene membranes for functional catalytic degradation. Chin. J. Polym. Sci. 2015, 33, 1721–1729. [Google Scholar] [CrossRef]
- Xu, X.X.; Cui, Z.P.; Qi, J.; Liu, X.X. Fabrication of Ag/CPs composite material, an effective strategy to improve the photocatalytic performance of coordination polymers under visible irradiation. Dalton Trans. 2013, 42, 13546–13553. [Google Scholar] [CrossRef]
- Xu, C.Y.; Chen, W.Q.; Wang, J.J.; Wu, Q.Y.; Wu, P.Y.; Tang, L. Two Cu(I\II) Coordination Polymers for Photocatalytic Degradation of Organic Dyes and Efficient Detection of Fe3+ Ions. J. Inorg. Organomet. Polym. Mater. 2023, 33, 885–894. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.F.; Gao, M.M.; Yu, X.; Xiao, P.W.; Wang, M.; Wang, S.G.; Wang, X.H. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874. [Google Scholar] [CrossRef]
- Yang, S.S.; Wu, W.M.; Brandon, A.M.; Fan, H.Q.; Receveur, J.P.; Li, Y.R.; Wang, Z.Y.; Fan, R.; McClellan, R.L.; Gao, S.H.; et al. Ubiquity of polystyrene digestion and biodegradation within yellow mealworms, larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Chemosphere 2018, 212, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Baeza, C.; Cifuentes, C.; Gonzalez, P.; Araneda, A.; Barra, R. Experimental Exposure of Lumbricus terrestristo Microplastics. Water Air Soil. Pollut. 2020, 231, 308. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, R.; Hu, J.N.; Li, X.Y.; Zhang, X.T.; Chen, Y.X.; Wu, W.M.; He, D.F. Biodegradation and disintegration of expanded polystyrene by land snails Achatina fulica. Sci. Total Environ. 2020, 746, 141289. [Google Scholar] [CrossRef] [PubMed]
- Billen, P.; Khalifa, L.; Van Gerven, F.; Tavernier, S.; Spatari, S. Technological application potential of polyethylene and polystyrene biodegradation by macro -organisms such as mealworms and wax moth larvae. Sci. Total Environ. 2020, 735, 139521. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Gao, D.L.; Li, Q.H.; Zhao, Y.X.; Li, L.; Lin, H.F.; Bi, Q.R.; Zhao, Y.C. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Luo, L.P.; Wang, Y.M.; Guo, H.Q.; Yang, Y.H.; Qi, N.; Zhao, X.; Gao, S.H.; Zhou, A.F. Biodegradation of foam plastics by Zophobas atratus larvae (Coleoptera: Tenebrionidae) associated with changes of gut digestive enzymes activities and microbiome. Chemosphere 2021, 282, 131006. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.C.; Sun, J.Z. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Othman, A.R.; Abu Hasan, H.; Muhamad, M.H.; Ismail, N.I.; Abdullah, S.R.S. Microbial degradation of microplastics by enzymatic processes: A review. Environ. Chem. Lett. 2021, 19, 3057–3073. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.C.; Mahmoud, Y.A.G.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J.Z. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef]
- Yuan, J.H.; Ma, J.; Sun, Y.R.; Zhou, T.; Zhao, Y.C.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Yin, C.F.; Xu, Y.; Zhou, N.Y. Biodegradation of polyethylene mulching films by a co-culture of Acinetobacter sp. strain NyZ450 and Bacillus sp. strain NyZ451 isolated from Tenebrio molitor larvae. Int. Biodeterior. Biodegrad. 2020, 155, 105089. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, J.; Wu, W.M.; Zhao, J.; Song, Y.L.; Gao, L.C.; Yang, R.F.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Lwanga, E.H.; Thapa, B.; Yang, X.M.; Gertsen, H.; Salanki, T.; Geissen, V.; Garbeva, P. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: A potential for soil restoration. Sci. Total Environ. 2018, 624, 753–757. [Google Scholar] [CrossRef]
- Vimala, P.P.; Mathew, L. Biodegradation of Polyethylene Using Bacillus Subtilis. Procedia Technol. 2016, 24, 232–239. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Auta, H.S.; Abioye, O.P.; Aransiola, S.A.; Bala, J.D.; Chukwuemeka, V.I.; Hassan, A.; Aziz, A.; Fauziah, S.H. Enhanced microbial degradation of PET and PS microplastics under natural conditions in mangrove environment. J. Environ. Manag. 2022, 304, 114273. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Jayanthi, B.; Fauziah, S.H. Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Mar. Pollut. Bull. 2018, 127, 15–21. [Google Scholar] [CrossRef]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Li, Q. Chinese Baijiu: The Perfect Works of Microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef]
- Wu, Q.; Li, L.; Xiang, P.; Zhang, T.; Peng, L.; Zou, L.; Li, Q. Phages in Fermented Foods: Interactions and Applications. Fermentation 2023, 9, 201. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, X.N.; Rene, E.R.; Wang, Z.; Zhou, L.N.; Zhang, Z.Q.; Wang, Q. The degradation performance of different microplastics and their effect on microbial community during composting process. Bioresour. Technol. 2021, 332, 125133. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, W.Q.; Xing, R.Z.; Xie, S.J.; Yang, X.G.; Cui, P.; Lu, J.; Liao, H.P.; Yu, Z.; Wang, S.H.; et al. Enhanced in situ biodegradation of microplastics in sewage sludge using hyperthermophilic composting technology. J. Hazard. Mater. 2020, 384, 121271. [Google Scholar] [CrossRef]
- Bao, Z.; Wang, X.; Wang, Q.; Zou, L.; Peng, L.; Li, L.; Tu, W.; Li, Q. A novel method of domestication combined with ARTP to improve the reduction ability of Bacillus velezensis to Cr(VI). J. Environ. Chem. Eng. 2023, 11, 109091. [Google Scholar] [CrossRef]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Habib, S.; Iruthayam, A.; Abd Shukor, M.Y.; Alias, S.A.; Smykla, J.; Yasid, N.A. Biodeterioration of Untreated Polypropylene Microplastic Particles by Antarctic Bacteria. Polymers 2020, 12, 2616. [Google Scholar] [CrossRef] [PubMed]
- Grgic, D.K.; Miloloza, M.; Lovrincic, E.; Kovacevic, A.; Cvetnic, M.; Bulatovic, V.O.; Prevaric, V.; Bule, K.; Ukic, S.; Markic, M.; et al. Bioremediation of MP-polluted Waters Using Bacteria Bacillus licheniformis, Lysinibacillus massiliensis, and Mixed Culture of Bacillus sp. and Delftia acidovorans. Chem. Biochem. Eng. Q. 2021, 35, 205–224. [Google Scholar] [CrossRef]
- Devi, K.N.; Raju, P.; Santhanam, P.; Kumar, S.D.; Krishnaveni, N.; Roopavathy, J.; Perumal, P. Biodegradation of low-density polyethylene and polypropylene by microbes isolated from Vaigai River, Madurai, India. Arch. Microbiol. 2021, 203, 6253–6265. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wei, R.; Gao, M.X.; Ren, Y.R.; Yu, B.; Nie, K.L.; Xu, H.J.; Liu, L. Biodegradation of low-density polyethylene by Microbulbifer hydrolyticus IRE-31. J. Environ. Manag. 2020, 263, 110402. [Google Scholar] [CrossRef] [PubMed]
- Giacomucci, L.; Raddadi, N.; Soccio, M.; Lotti, N.; Fava, F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020, 158, 104949. [Google Scholar] [CrossRef]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Raghul, S.S.; Bhat, S.G.; Chandrasekaran, M.; Francis, V.; Thachil, E.T. Biodegradation of polyvinyl alcohol-low linear density polyethylene-blended plastic film by consortium of marine benthic vibrios. Int. J. Environ. Sci. Technol. 2014, 11, 1827–1834. [Google Scholar] [CrossRef]
- Gao, R.R.; Sun, C.M. A marine bacterial community capable of degrading poly(ethylene terephthalate) and polyethylene. J. Hazard. Mater. 2021, 416, 125928. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiang, P.; Li, L.; Zhang, T.; Wu, Q.; Bao, Z.; Tu, W.; Zhao, C. Phosphorus mining activities alter endophytic bacterial communities and metabolic functions of surrounding vegetables and crops. Plant Soil. 2023. [Google Scholar] [CrossRef]
- Li, Q.; Xiang, P.; Zhang, T.; Wu, Q.; Bao, Z.; Tu, W.; Li, L.; Zhao, C. The effect of phosphate mining activities on rhizosphere bacterial communities of surrounding vegetables and crops. Sci. Total Environ. 2022, 821, 153479. [Google Scholar] [CrossRef]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus Attachment Patterns on Glass Surfaces with Nanoscale Roughness. Curr. Microbiol. 2009, 58, 268–273. [Google Scholar] [CrossRef]
- Skariyachan, S.; Prasanna, A.; Manjunath, S.P.; Karanth, S.S.; Nazre, A. Environmental assessment of the degradation potential of mushroom fruit bodies of Pleurotus ostreatus (Jacq.: Fr.) P. Kumm. towards synthetic azo dyes and contaminating effluents collected from textile industries in Karnataka, India. Environ. Monit. Assess. 2016, 188, 121. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; Kornaros, M.; Khalil, M.; Elsamahy, T.; El-Shetehy, M.; Sun, J.Z. Coupling azo dye degradation and biodiesel production by manganese-dependent peroxidase producing oleaginous yeasts isolated from wood-feeding termite gut symbionts. Biotechnol. Biofuels 2021, 14, 61. [Google Scholar] [CrossRef]
- Daly, P.; Cai, F.; Kubicek, C.P.; Jiang, S.Q.; Grujic, M.; Rahimi, M.J.; Sheteiwy, M.S.; Giles, R.; Riaz, A.; de Vries, R.P.; et al. From lignocellulose to plastics: Knowledge transfer on the degradation approaches by fungi. Biotechnol. Adv. 2021, 50, 107770. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; El-Naggar, A.H.; Kornaros, M.; Sun, J.Z. Valorizing lignin-like dyes and textile dyeing wastewater by a newly constructed lipid-producing and lignin modifying oleaginous yeast consortium valued for biodiesel and bioremediation. J. Hazard. Mater. 2021, 403, 123575. [Google Scholar] [CrossRef]
- Sanchez, C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qi, M.; Wu, Q.; Xiang, P.; Tang, D.; Li, Q. Recent research progress on the synthesis and biological effects of selenium nanoparticles. Front. Nutr. 2023, 10, 1183487. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, A.; Krishnamoorthy, S.; Abraham, J. Microbial degradation of low-density polyethylene (LDPE) by Aspergillus clavatus strain JASK1 isolated from landfill soil. 3 Biotech. 2016, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Gupta, S. Assessment of LDPE degrading potential Aspergillus species isolated from municipal landfill sites of Agra. SN Appl. Sci. 2019, 1, 701. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Natarajan, K.; Rajeshkannan, V.; Perumal, P. Enhancement of in vitro high-density polyethylene (HDPE) degradation by physical, chemical, and biological treatments. Environ. Sci. Pollut. Res. 2014, 21, 12549–12562. [Google Scholar] [CrossRef]
- Kunlere, I.O.; Fagade, O.E.; Nwadike, B.I. Biodegradation of low density polyethylene (LDPE) by certain indigenous bacteria and fungi. Int. J. Environ. Stud. 2019, 76, 428–440. [Google Scholar] [CrossRef]
- Ameen, F.; Moslem, M.; Hadi, S.; Al-Sabri, A.E. Biodegradation of Low Density Polyethylene (LDPE) by Mangrove Fungi From the Red Sea Coast. Prog. Rubber Plast. Recycl. Technol. 2015, 31, 125–144. [Google Scholar] [CrossRef]
- Russell, J.R.; Huang, J.; Anand, P.; Kucera, K.; Sandoval, A.G.; Dantzler, K.W.; Hickman, D.; Jee, J.; Kimovec, F.M.; Koppstein, D.; et al. Biodegradation of Polyester Polyurethane by Endophytic Fungi. Appl. Environ. Microbiol. 2011, 77, 6076–6084. [Google Scholar] [CrossRef]
- Paco, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.M.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Li, Q.; Luo, Y.; Sha, A.; Xiao, W.; Xiong, Z.; Chen, X.; He, J.; Peng, L.; Zou, L. Analysis of synonymous codon usage patterns in mitochondrial genomes of nine Amanita species. Front. Microbiol. 2023, 8, 1134228. [Google Scholar] [CrossRef]
- Devi, R.S.; Kannan, V.R.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A.R. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Pourbabaee, A.A.; Alikhani, H.A.; Shabani, F.; Esmaeili, E. Biodegradation of Low-Density Polyethylene (LDPE) by Mixed Culture of Lysinibacillus xylanilyticus and Aspergillus niger in Soil. PLoS ONE 2013, 8, e71720. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, P.; Zhang, T.; Wu, Q.; Li, Q. Systematic Review of Degradation Processes for Microplastics: Progress and Prospects. Sustainability 2023, 15, 12698. https://doi.org/10.3390/su151712698
Xiang P, Zhang T, Wu Q, Li Q. Systematic Review of Degradation Processes for Microplastics: Progress and Prospects. Sustainability. 2023; 15(17):12698. https://doi.org/10.3390/su151712698
Chicago/Turabian StyleXiang, Peng, Ting Zhang, Qian Wu, and Qiang Li. 2023. "Systematic Review of Degradation Processes for Microplastics: Progress and Prospects" Sustainability 15, no. 17: 12698. https://doi.org/10.3390/su151712698