A Bioleaching Process for Sustainable Recycling of Complex Structures with Multi-Metal Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Waste Electroformed Components
2.2. Acidophilic Chemolithotrophs and Cultivation Conditions
2.3. Bioleaching of Cu from Waste Electroformed Components
2.4. Electrowinning of Cu from Bioleachates
2.5. Analytical Methods
2.6. Microbiological Analyses
3. Results
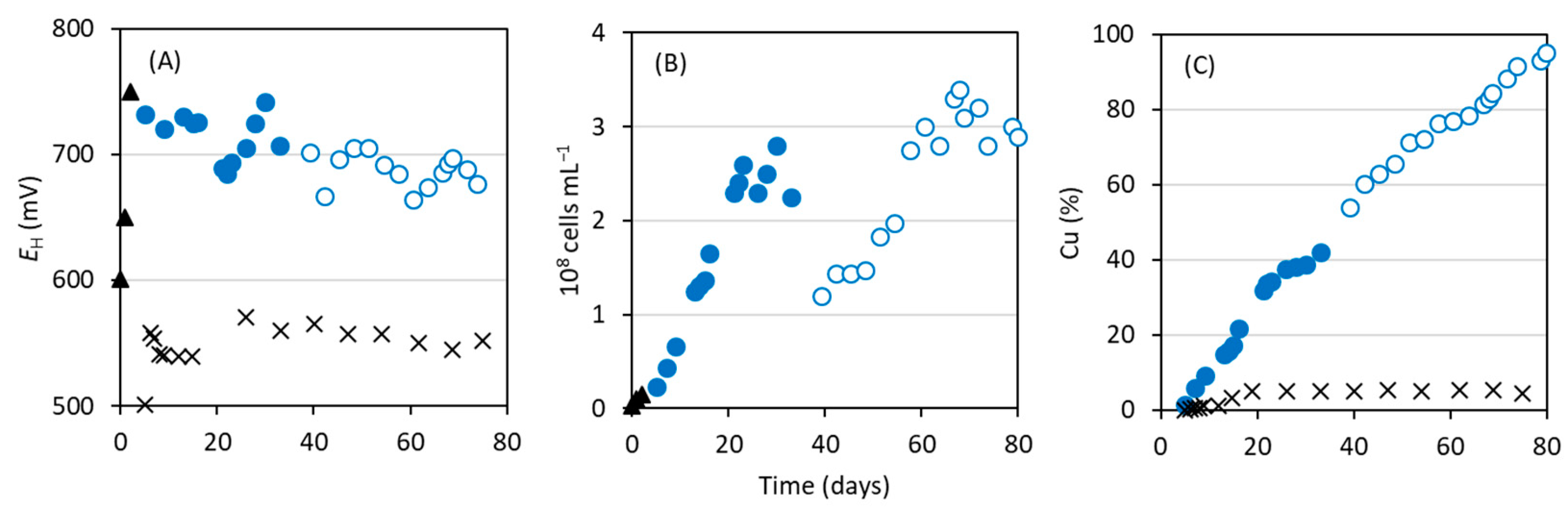
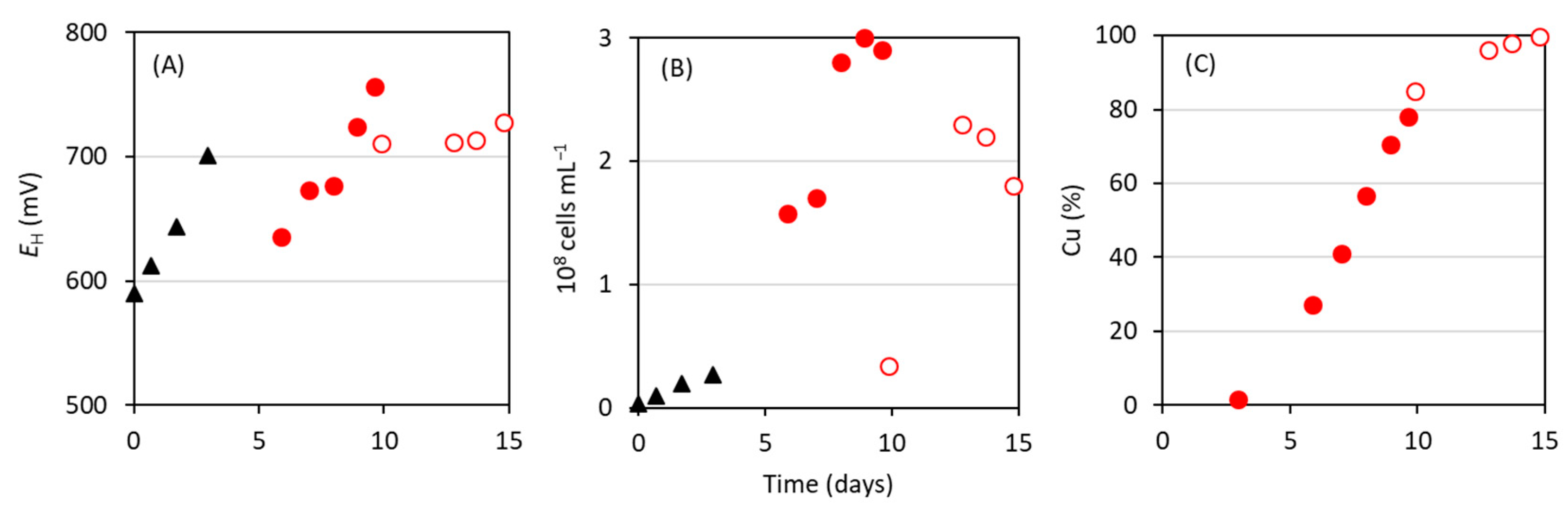
3.1. Bioleaching of Cu from Waste Electroformed Components Using At. ferrooxidans Strain CF3
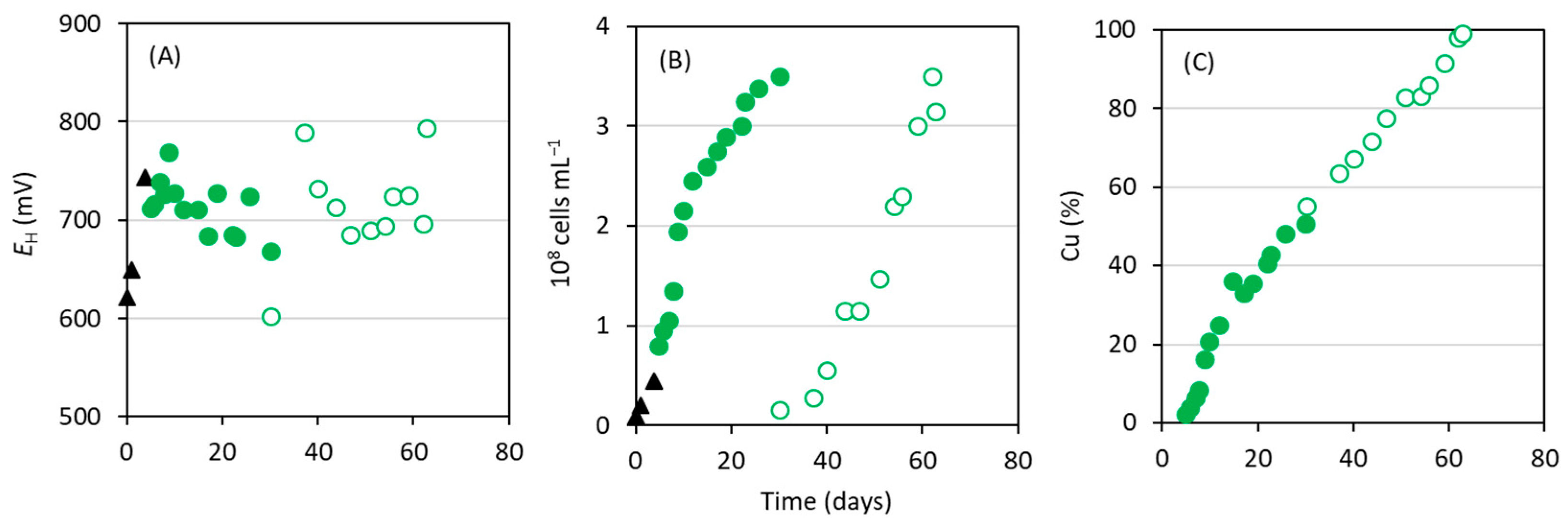
3.2. Bioleaching of Cu Using Acidophilic Fe2+-Oxidizing Consortium
3.3. Bioleaching of Cu in a Bioreactor Using Adapted Acidophilic Consortium
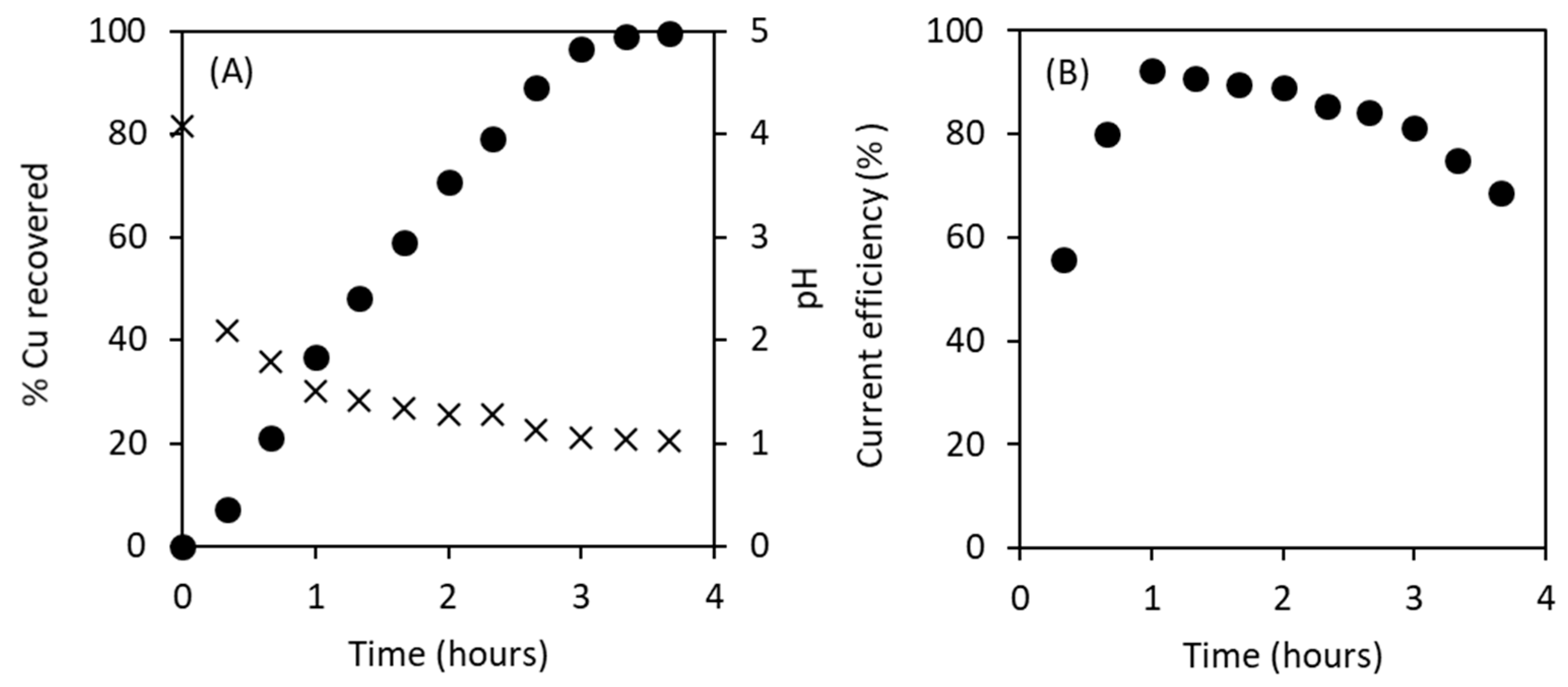
3.4. Cu Electrowinning from Bioleachates
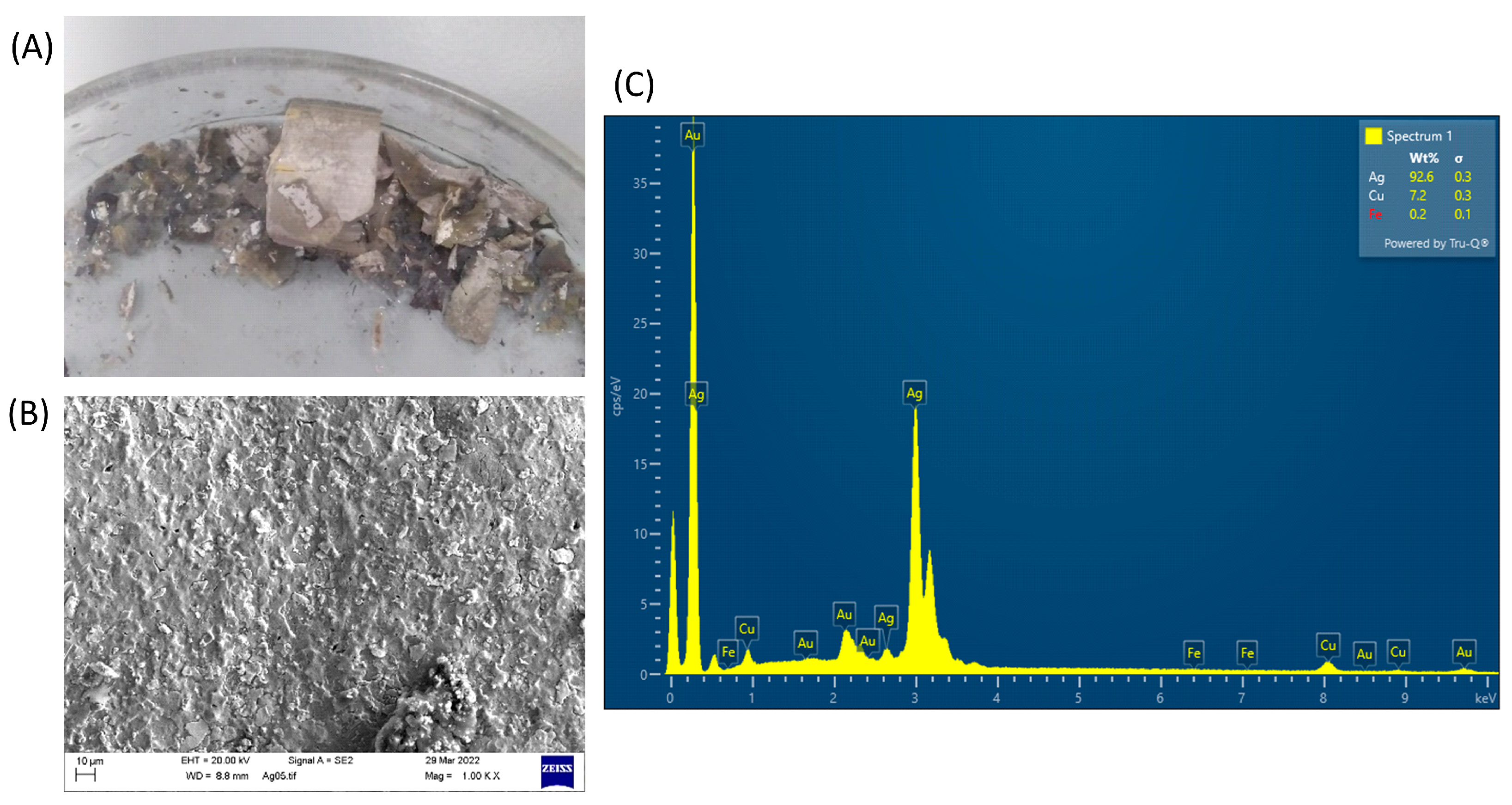
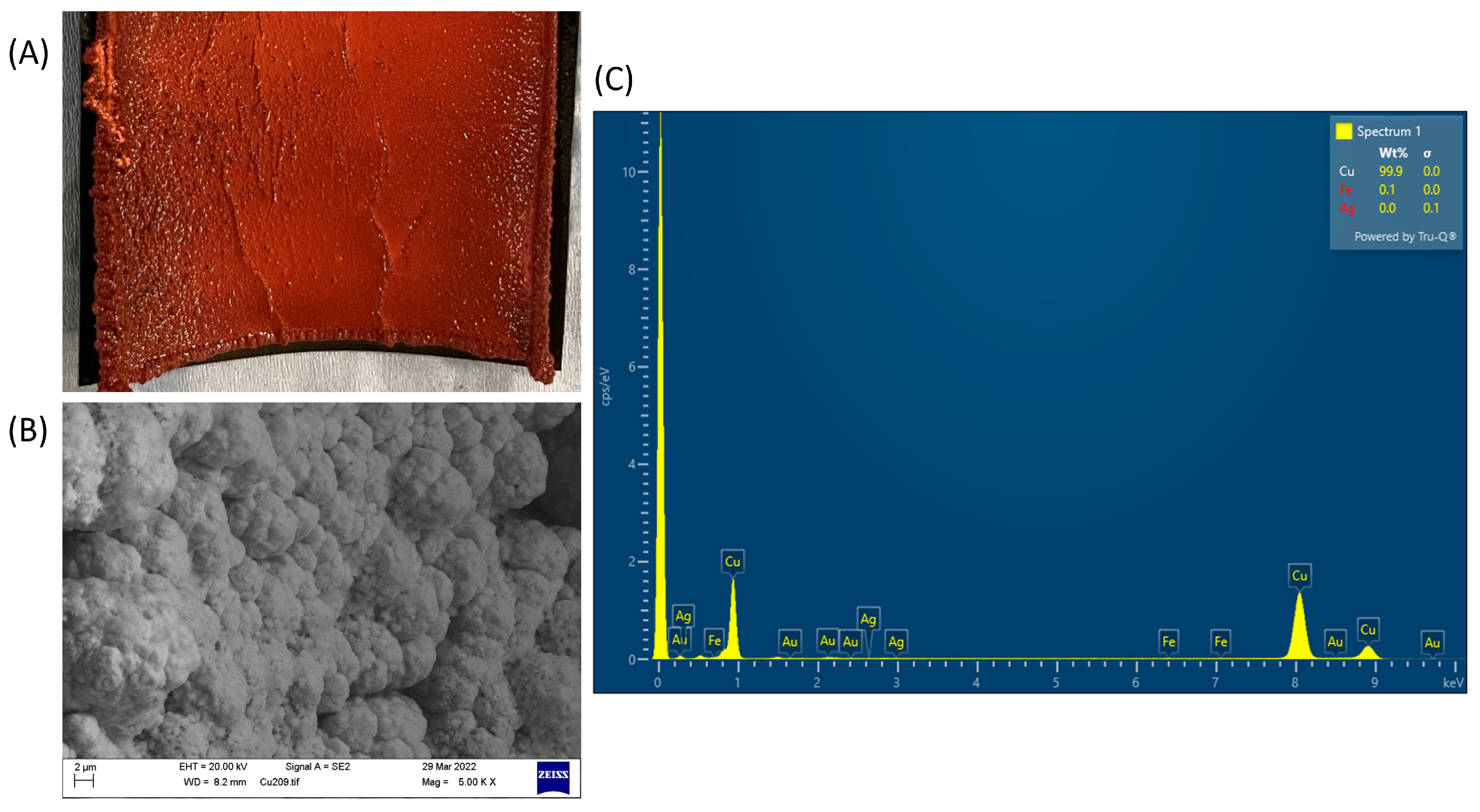
3.5. Solid Product Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandez, P.; Socas, A.; Benítez, A.; Díaz, N.; Marrero, M.; Ortega, F.; Monzon, M. Computer aided electroforming. Elecform3D™. Procedia Eng. 2013, 63, 532–539. [Google Scholar] [CrossRef][Green Version]
- McGeough, J.A.; Leu, M.C.; Rajurkar, K.P.; De Silva, A.K.M.; Liu, Q. Electroforming process and application to micro/macro manufacturing. CIRP Ann. Manuf. Technol. 2001, 50, 499–514. [Google Scholar] [CrossRef]
- Beukes, N.T.; Badenhorst, J. Copper electrowinning: Theoretical and practical design. J. South. Afr. Inst. Min. Metall. 2009, 109, 213–240. [Google Scholar]
- Zeng, X.; Zheng, L.; Xie, H.; Lu, B.; Xia, K.; Chao, K.; Li, W.; Yang, J.; Lin, S.; Li, J. Current status and future perspective of waste printed circuit boards recycling. Procedia Environ. Sci. 2012, 16, 590–597. [Google Scholar] [CrossRef]
- Forti, V.; Balde, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020: Quantities, Flows and the Circular Economy Potential; United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)—Co-hosted SCYCLE Programme; International Telecommunication Union (ITU) & International Solid Waste Association (ISWA): Bonn, Germany; Geneva, Switzerland; Rotterdam, The Netherlands, 2020. [Google Scholar]
- World Economic Forum. A New Circular Vision for Electronics Time for a Global Reboot; Platform for Accelerating the Circular Economy (PACE) and the UN E-Waste Coalition: Geneva, Switzerland, 2019. [Google Scholar]
- Li, J.; Lu, H.; Guo, J.; Xu, Z.; Zhou, Y. Recycle technology for recovering resources and products from waste printed circuit boards. Environ. Sci. Technol. 2007, 41, 1995–2000. [Google Scholar] [CrossRef]
- Duan, C.; Wen, X.; Shi, C.; Zhao, Y.; Wen, B.; He, Y. Recovery of metals from waste printed circuit boards by a mechanical method using a water medium. J. Hazard. Mater. 2009, 166, 478–482. [Google Scholar] [CrossRef]
- Zeng, X.; Mathews, J.A.; Li, J. Urban mining of e-waste is becoming more cost-effective than virgin mining. Environ. Sci. Technol. 2018, 52, 4835–4841. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Habashi, F. Pyro-versus hydrometallurgy or dry versus wet methods. JOJ Mater. Sci. 2017, 3, 555618. [Google Scholar]
- Johnson, D.B. The evolution, current status, and future prospects of using biotechnologies in the mineral extraction and metal recovery sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef]
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B, applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J. Biometallurgical recovery of metals from waste electrical and electronic equipment: A review. ChemBioEng Rev. 2014, 1, 148–169. [Google Scholar] [CrossRef]
- Ahmed, I.M.; El-Nadi, Y.A.; Daoud, J.A. Cementation of copper from spent copper-pickle sulfate solution by zinc ash. Hydrometallurgy 2011, 110, 62–66. [Google Scholar] [CrossRef]
- Cote, G. Hydrometallurgy of strategic metals. Solvent Extr. Ion. Exch. 2000, 18, 703–727. [Google Scholar] [CrossRef]
- Kordosky, G.A. Copper recovery using leach/solvent extraction/electrowinning technology: Forty years of innovation, 2.2 million tonnes of copper annually. J. South. Afr. Inst. Min. Metall. 2002, 102, 445–450. [Google Scholar]
- Fathima, A.; Tang, J.Y.B.; Giannis, A.; Ilankoon, I.M.S.K.; Chong, M.N. Catalysing electrowinning of copper from E-waste: A critical review. Chemosphere 2022, 298, 134340. [Google Scholar] [CrossRef]
- Ňancucheo, I.; Rowe, O.F.; Hedrich, S.; Johnson, D.B. Solid and liquid media for isolating and cultivating acidophilic and acid-tolerant sulfate-reducing bacteria. FEMS Microbiol. Lett. 2016, 363, fnw083. [Google Scholar] [CrossRef]
- Kelly, D.; Wood, A. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2000, 2, 511–516. [Google Scholar] [CrossRef]
- Moya-Beltran, A.; Beard, S.; Rojas-Villalobos, C.; Issotta, F.; Gallardo, Y.; Ulloa, R.; Giaveno, A.; Degli Esposti, M.; Johnson, D.B.; Quatrini, R. Genomic evolution of the class Acidithiobacillia: Deep-branching Proteobacteria living in extreme acidic conditions. ISME J. 2021, 15, 3221–3238. [Google Scholar] [CrossRef]
- Hallberg, K.B.; Gonzalez-Toril, E.; Johnson, D.B. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 2010, 14, 9–19. [Google Scholar] [CrossRef]
- Hedrich, S.; Johnson, D.B. Acidithiobacillus ferridurans sp. nov., an acidophilic iron-, sulfur- and hydrogen-metabolizing chemolithotrophic gammaproteobacterium. Int. J. Syst. Evol. Microbiol. 2013, 63, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Falagan, C.; Johnson, D.B. Acidithiobacillus ferriphilus sp. nov., a facultatively anaerobic iron- and sulfur-metabolizing extreme acidophile. Int. J. Syst. Evol. Microbiol. 2016, 66, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.R.; Falagan, C.; Moya-Beltran, A.; Castro, M.; Quatrini, R.; Johnson, D.B. Acidithiobacillus ferrianus sp. nov.: An ancestral extremely acidophilic and facultatively anaerobic chemolithoautotroph. Extremophiles 2020, 24, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Karavaiko, G.I.; Bulygina, E.S.; Tsaplina, I.A.; Bogdanova, T.I.; Chumakov, K.M. Sulfobacillus thermosulficooxidans: A new lineage of bacterial evolution? FEBS Lett. 1990, 261, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hippe, H. Leptospirillum gen. nov. (ex Markosyan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992). Int. J. Syst. Evol. Microbiol. 2000, 50, 501–503. [Google Scholar] [CrossRef]
- Coram, N.J.; Rawlings, D.E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40 °C. Appl. Environ. Microbiol. 2002, 68, 838–845. [Google Scholar] [CrossRef]
- Vergara, E.; Pakostova, E.; Johnson, D.B.; Holmes, D.S. Draft Genome Sequence of Firmicutes Strain S0AB, a Heterotrophic Iron/Sulfur-Oxidizing Extreme Acidophile. Microbiol. Resour. Announc. 2022, 11, e00271-22. [Google Scholar] [CrossRef]
- Johnson, D.B.; Holmes, D.S.; Vergara, E.; Holanda, R.; Pakostova, E. Sulfoacidibacillus ferrooxidans, gen. nov., sp. nov., Sulfoacidibacillus thermotolerans, gen. nov., sp. nov., and Ferroacidibacillus organovorans, gen. nov., sp. nov.: Extremely acidophilic chemolitho-heterotrophic firmicutes. Res. Microbiol. 2022, 174, 104008. [Google Scholar] [CrossRef]
- Stookey, L.L. Ferrozine—A new spectrophotometric reagent for iron. Anal. Chem. 1970, 42, 779–781. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Techniques for detecting and identifying acidophilic mineral-oxidising microorganisms. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Heidelberg, Germany, 2007; pp. 237–262. [Google Scholar]
- Distaso, M.A.; Bargiela, R.; Brailsford, F.L.; Williams, G.B.; Wright, S.; Lunev, E.A.; Toschakov, S.V.; Yakimov, M.M.; Jones, D.L.; Golyshin, P.N.; et al. High representation of archaea across all depths in oxic and low-pH sediment layers underlying an acidic stream. Front. Microbiol. 2020, 11, 576520. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 2015, 1, e00009-15. [Google Scholar] [CrossRef] [PubMed]
- Fadrosh, D.W.; Ma, B.; Gajer, P.; Sengamalay, N.; Ott, S.; Brotman, R.M.; Ravel, J. An improved dual-indexed approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Rystad Energy. Copper Supply Deficit of 6 Million Tons by 2030 Threatens Renewables, EVs, as Investment Lags Demand. 2022. Available online: https://www.rystadenergy.com/newsevents/news/press-releases/copper-supply-deficit-of-6-million-tons-by-2030-threatens-renewables-evs-as-investment-lags-demand/ (accessed on 6 July 2022).
- Silver Institute. Total Global Silver Demand Posts Record High of 1.24 Billion Ounces in 2022. 2023. Available online: https://www.silverinstitute.org/total-global-silver-demand-posts-record-high-1-24-billion-ounces-2022/ (accessed on 4 September 2023).
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Sajjad, W.; Zheng, G.; Din, G.; Ma, X.; Rafiq, M.; Xu, W. Metals extraction from sulfide ores with microorganisms: The bioleaching technology and recent developments. Tran. Indian. Inst. Met. 2019, 72, 559–579. [Google Scholar] [CrossRef]
- Ilyas, S.; Anwar, M.A.; Niazi, S.B.; Ghauri, M.A. Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 2007, 88, 180–188. [Google Scholar] [CrossRef]
- Ilyas, S.; Lee, J.; Chi, R. Bioleaching of metals from electronic scrap and its potential for commercial exploitation. Hydrometallurgy 2013, 131–132, 138–143. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Z.; Wen, J.; Yang, L. Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 97, 29–32. [Google Scholar] [CrossRef]
- Yang, C.Y.; Zhu, N.; Shen, W.; Zhang, T.; Wu, P. Bioleaching of copper from metal concentrates of waste printed circuit boards by a newly isolated Acidithiobacillus ferrooxidans strain Z1. J. Mater. Cycles Waste Manag. 2017, 19, 247–255. [Google Scholar] [CrossRef]
- Zhu, N.; Xiang, Y.; Zhang, T.; Wu, P.; Dang, Z.; Li, P.; Wu, J. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria. J. Hazard. Mater. 2011, 172, 1100–1105. [Google Scholar] [CrossRef]
- Nie, H.; Yang, C.; Zhu, N.; Wu, P.; Zhang, T.; Zhang, Y.; Xing, Y. Isolation of Acidithiobacillus ferrooxidans strain Z1 and its mechanism of bioleaching copper from waste printed circuit boards. J. Chem. Technol. Biotechnol. 2014, 90, 714–721. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Rodriguesc, M.L.M.; Leao, V.A.; Gomes, O.; Lambert, F.; Bastin, D.; Gaydardzhiev, S. Copper extraction from coarsely ground printed circuit boards using moderate thermophilic bacteria in a rotating-drum reactor. Waste Manag. 2015, 41, 148–158. [Google Scholar] [CrossRef]
- Isildar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, P.; Liu, W.P.; Wang, B. Enhanced bioleaching efficiency of copper from waste printed circuit boards (PCBs) by dissolved oxygen-shifted strategy in Acidithiobacillus ferrooxidans. J. Mater. Cycles Waste Manag. 2016, 18, 742–751. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Feasibility of bioleaching of selected metals from electronic waste by Acidiphilium acidophilum. Waste Biomass Valor. 2018, 9, 871–877. [Google Scholar] [CrossRef]
- Andrzejewska-Górecka, D.A.; Poniatowska, A.; Macherzyński, B.; Wojewódka, D.; Wszelaka-Rylik, M.E. Comparison of the effectiveness of biological and chemical leaching of copper, nickel and zinc from circuit boards. J. Ecol. Eng. 2019, 20, 62–69. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Tuncuk, A.; Deveci, H.; Yazici, E.Y.; Panda, S. A novel approach based on solvent displacement crystallisation for iron removal and copper recovery from solutions of semi-pilot scale bioleaching of WPCBs. J. Clean. Prod. 2021, 294, 126346. [Google Scholar] [CrossRef]
- Baniasadi, M.; Graves, J.E.; Ray, D.A.; De Silva, A.L.; Renshaw, D.; Farnaud, S. Closed-loop recycling of copper from waste printed circuit boards using bioleaching and electrowinning processes. Waste Biomass Valor. 2021, 12, 3125–3136. [Google Scholar] [CrossRef]
- Bas, A.D.; Deveci, H.; Yazici, E.Y. Bioleaching of copper from low grade scrap TV circuit boards using mesophilic bacteria. Hydrometallurgy 2013, 138, 65–70. [Google Scholar] [CrossRef]
- Morgan, B.; Lahav, O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution—Basic principles and a simple heuristic description. Chemosphere 2007, 68, 2080–2084. [Google Scholar] [CrossRef]
- Ilyas, S.; Ruan, C.; Bhatti, H.N.; Anwar, M.A. Column bioleaching of metals from electronic scrap. Hydrometallurgy 2010, 101, 135–140. [Google Scholar] [CrossRef]
- Huang, K.; Guo, J.; Xu, Z. Recycling of waste printed circuit boards: A review of current technologies and treatment status in China. J. Hazard. Mater. 2009, 164, 399–408. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Imre-Lucaci, A.; Ilea, P. Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation. J. Hazard. Mater. 2014, 273, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wu, P.; Zhu, N.; Zhang, T.; Liu, W.; Wu, J.; Li, P. Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage. J. Hazard. Mater. 2010, 184, 812–818. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Pakostova, E.; Grail, B.M.; Johnson, D.B. Indirect oxidative bioleaching of a polymetallic black schist sulfide ore. Miner. Eng. 2017, 106, 102–107. [Google Scholar] [CrossRef]
- Pakostova, E.; Grail, B.M.; Johnson, D.B. Bio-processing of a saline, calcareous copper sulfide ore by sequential leaching. Hydrometallurgy 2018, 179, 36–43. [Google Scholar] [CrossRef]
- Orellana, L.H.; Jerez, C.A. A genomic island provides Acidithiobacillus ferrooxidans ATCC 53993 additional copper resistance: A possible competitive advantage. Appl. Microbiol. Biotechnol. 2011, 92, 761–767. [Google Scholar] [CrossRef]
- Martinez-Bussenius, C.; Navarro, C.A.; Jerez, C.A. Microbial copper resistance: Importance in biohydrometallurgy. Microb. Biotechnol. 2017, 10, 279–295. [Google Scholar] [CrossRef]
- Rai, V.; Liu, D.; Xia, D.; Jayaraman, Y.; Gabriel, J.C.P. Electrochemical approaches for the recovery of metals from electronic waste: A critical review. Recycling 2021, 6, 53. [Google Scholar] [CrossRef]
- Veglio, F.; Quaresima, R.; Fornari, P.; Ubaldini, S. Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning. Waste Manag. 2003, 23, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A novel eco-friendly hybrid approach for recovery and reuse of copper from electronic waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Dini, J.W.; Snyder, D.D. Electrodeposition of copper. In Modern Electroplating, 5th ed.; Schlesingher, M., Paunovic, M., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 33–78. [Google Scholar]
- Bartos, P.J. SX-EW copper and the technology cycle. Resour. Policy 2002, 28, 85–94. [Google Scholar] [CrossRef]
- Zhang, C.L.; Fang, W.D.; Chen, Y.; Wang, J.W.; Bai, J.F. Recovery of copper from bio-leaching solutions of waste printed circuit boards waste by solvent extraction using RE609 and N902. Adv. Mater. Res. 2010, 113–116, 2228–2231. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, J.W.; Bai, J.F.; Wu, W.J. Life cycle assessment of the bio- hydrometallurgical process of recycling copper from printed circuit boards scraps. Adv. Manuf. Technol. 2011, 156–157, 929–932. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Enhancement of simultaneous gold and copper extraction from computer printed circuit boards waste using Bacillus megaterium. Bioresour. Technol. 2014, 175, 315–324. [Google Scholar] [CrossRef]
- Sethurajan, M.; Lens, P.N.L.; Rene, E.R.; van de Vossenberg, J.; Huguenot, D.; Horn, H.A.; Figueiredo, L.H.A.; van Hullenbusch, E.D. Bioleaching and selective biorecovery of zinc from zinc metallurgical leach residues from the Três Marias zinc plant (Minas Gerais, Brazil). J. Chem. Technol. Biotechnol. 2016, 92, 512–521. [Google Scholar] [CrossRef]
- Ferrier, J.; Csetenyi, L.; Gadd, G.M. Selective fungal bioprecipitation of cobalt and nickel for multiple-product metal recovery. Microb. Biotechnol. 2021, 14, 1747–1756. [Google Scholar] [CrossRef]
- Wang, J.L. Biosorption of copper(II) by chemically modified biomass of Saccharomyces cerevisiae. Process Biochem. 2002, 37, 847–850. [Google Scholar]
- Garcia-Garcia, J.D.; Sanchez-Thomas, R.; Moreno-Sanchez, R. Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol. Adv. 2016, 34, 859–873. [Google Scholar] [CrossRef] [PubMed]
- London Metal Exchange. 2023. Available online: https://www.lme.com/Metals/Non-ferrous/LME-Copper (accessed on 5 September 2023).
- Coin Price Forecast. 2023. Available online: https://coinpriceforecast.com/silver (accessed on 5 September 2023).


| Bacterium | Temperature Optimum (Range) (°C) | pH Optimum (Range) | Fe2+ Oxidation (Fe3+ Reduction) | S Oxidation | References |
|---|---|---|---|---|---|
| Acidithiobacillus (At.) ferrooxidans T | 28 to 30 (10 to 37) | 2.0 to 3.0 (1.3 to 5.5) | +(+) | + | [20] |
| At. ferrooxidans (‘ferruginosis’) strain CF3 | 28 to 30 (10 to 37) | ~2.5 (min. 1.3) | +(+) | + | [21] |
| At. ferrivorans T | 25 to 32 (min. 4) | 2.5 (min. 1.9) | +(+) | + | [22] |
| At. ferridurans T | 29 (10 to 37) | 2.1 (1.4 to 3.0) | +(+) | + | [23] |
| At. ferriphilus T | 30 (5 to 33) | 2.0 (min. 1.5) | +(+) | + | [24] |
| At. ferrianus T | 28 to 30 (20 to 32) | 1.7 to 2.0 (1.5 to 3.0) | +(+) | + | [25] |
| Sulfobacillus (Sb.) thermosulfidooxidans T | 50 (20 to 58) | 1.9 to 2.4 (0.8 to 5.5) | +(+) | + | [26] |
| Leptospirillum (L.) ferrooxidans T | 35 (10 to 45) | 1.6 to 2.0 (min. ~1.0) | +(-) | - | [27] |
| L. ferriphilum T | 30 to 37 (14 to 40) | 1.4 to 1.8 (1.3 to 2.0) | +(-) | - | [28] |
| Sulfoacidibacillus (S.) ferrooxidans T | 33 (max. 37.5) | 1.7 (min. 0.9) | +(+) | + | [29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakostova, E.; Herath, A. A Bioleaching Process for Sustainable Recycling of Complex Structures with Multi-Metal Layers. Sustainability 2023, 15, 14068. https://doi.org/10.3390/su151914068
Pakostova E, Herath A. A Bioleaching Process for Sustainable Recycling of Complex Structures with Multi-Metal Layers. Sustainability. 2023; 15(19):14068. https://doi.org/10.3390/su151914068
Chicago/Turabian StylePakostova, Eva, and Anuradha Herath. 2023. "A Bioleaching Process for Sustainable Recycling of Complex Structures with Multi-Metal Layers" Sustainability 15, no. 19: 14068. https://doi.org/10.3390/su151914068
APA StylePakostova, E., & Herath, A. (2023). A Bioleaching Process for Sustainable Recycling of Complex Structures with Multi-Metal Layers. Sustainability, 15(19), 14068. https://doi.org/10.3390/su151914068







