Characteristic of Molecular Weight-Fractions of Soil Organic Matter from Calcareous Soil and Yellow Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Extraction and Purification of HA
2.2. Fractionation of HA by Ultrafiltration
2.3. Titration of the Size Fractions of HAs and the Bulk HA
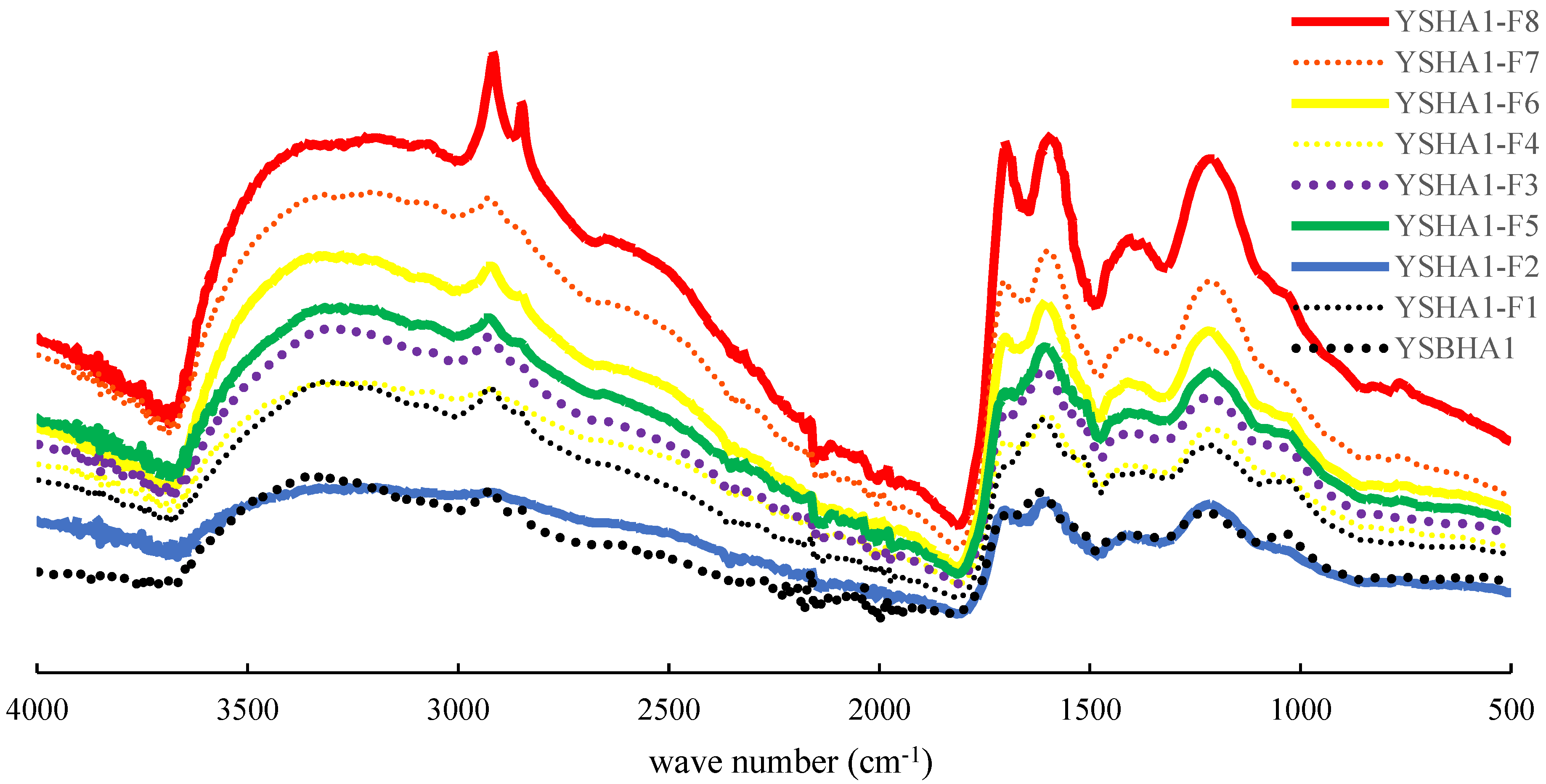
2.4. The Fourier Transform Infrared (FTIR) Spectroscopy of the Size Fractions of HAs and the Bulk HA
2.5. The Solid 13C-NMR of the Size Fractions of HAs and the Bulk HA
3. Results and Discussion
3.1. The Acidic Functional Groups
3.2. Fourier Transform Infrared Spectra
3.3. Carbon-13-Nuclear Resonance Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Macalady, D.L.; Ranville, J.F. The chemistry and geochemistry of natural organic matter. In Perspectives in Environmental Chemistry; Macalady, D.L., Ed.; Oxford University Press: New York, NY, USA, 1998; pp. 94–137. [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, E.A.; Janssens, I.A. temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 65–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile-Doelsch, I.; Balesdent, J.; Pellerin, S. The mechanisms underlying carbon storage. Biogeosciences 2020, 17, 5223–5242. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Ding, F.; Gao, X.; Li, S.; Sun, L.; An, T.; Pei, J.; Li, M.; Wang, Y.; et al. Process of Plant Residue Transforming into Soil Organic Matter and Mechanism of Its Stabilization: A Review. Acta Pedol. Sin. 2019, 56, 528–540. (In Chinese) [Google Scholar]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Kogel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter: Fourteen years on. Soil Biol. Biochem. 2017, 105, A3–A8. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Thevenot, M.; Dignac, M.-F.F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Sugars in soil and sweets for microorganisms: Review of origin, content, composition and fate. Soil Biol. Biochem. 2015, 90, 87–100. [Google Scholar] [CrossRef]
- Jansen, B.; Wiesenberg, G.L.B. Opportunities and limitations related to the application of plant-derived lipid molecular proxies in soil science. Soil 2017, 3, 211–234. [Google Scholar] [CrossRef] [Green Version]
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Tonneijck, F.H.; Jansen, B.; Nierop, K.G.J.; Verstraten, J.M.; Sevink, J.; De Lange, L. Towards understanding of carbon stocks and stabilization in volcanic ash soils in natural Andean ecosystems of northern Ecuador. Eur. J. Soil Sci. 2010, 61, 392–405. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efciency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, G.; Cao, J.; He, S.; Tao, Y.; Shun, Y.; Teng, Y. Soil carbon as dynamic mechanism for epikarstification in humid subtropical region: Evidence of carbon reservoirs and transfer in the system. J. Nanjing Agric. Univ. 1999, 22, 49–52. (In Chinese) [Google Scholar]
- Burdon, J. Are the traditional concepts of the structures of humic substances realistic? Soil Sci. 2001, 166, 752–769. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some concepts of soil organic carbon characteristics and mineral interaction from a review of literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Kleber, M. What is recalcitrant soil organic matter? Environ. Chem. 2010, 7, 320–332. [Google Scholar] [CrossRef]
- Kleber, M.; Lehmann, J. Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novelunderstanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- 23. Galicia-Andrés, E.; Escalona, Y.; Oostenbrink, C.; Tunega, D.; Gerzabek, M.H. Soil organic matter stabilization at molecular scale: The role of metal cations and hydrogen bonds. Geoderma 2021, 401, 1–13. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Hayes, M.H.B.; MacCarthy, P.; Malcolm, R.L.; Swift, R.S. Humic substances II. In Search of Structure; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Agnelli, A.; Celi, L.; Corti, G.; Degl’Innocenti, A.; Ugolini, F.C. The changes with depth of humic and fulvic acids extracted from the fine earth and rock fragments of a forest soil. Soil Sci. 2002, 167, 524–538. [Google Scholar] [CrossRef]
- Swift, R.S.; Posner, A.M. Nitrogen, phosphorus and sulfur contents of humic acids fractionated with respect to molecular weight. J. Soil Sci. 1972, 23, 50–56. [Google Scholar] [CrossRef]
- Piccolo, A.; Mirabella, A. Molecular-weight distribution of peat humic substances extracted with different inorganic and organic solutions. Sci. Total Environ. 1987, 62, 39–46. [Google Scholar] [CrossRef]
- Tonelli, D.; Seeber, R.; Ciavatta, C.; Gessa, C. Extraction of humic acids from a natural matrix by alkaline pyrophosphate. Evaluation of the molecular weight of fractions obtained by ultrafiltration. Fresenius J. Ana.l Chem. 1997, 359, 555–560. [Google Scholar] [CrossRef]
- Francioso, O.; Sanchez-Cortes, S.; Casarini, D.; Garcia-Ramos, J.V.; Ciavatta, C.; Gessa, C. Spectroscopic study of humic acids fractionated by means of tangential ultrafiltration. J. Mol. Struct. 2002, 609, 137–147. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Peng, P.; Sheng, G.; Fu, J. Chemical and molecular heterogeneity of humic acids repetitively extracted from a peat. Soil Sci. Soc. Am. J. 2003, 67, 740–746. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Huang, W.; Peng, P.; Sheng, G.; Fu, J. Characterization of humic acids fractionated by ultrafiltration. Org. Geochem. 2004, 35, 1025–1037. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, B.; Di, X.; Huang, W.; Wang, S. Characteristics and distributions of humic acids in two soil profiles of the southwest China Karst area. Acta Geochim. 2016, 35, 85–94. [Google Scholar] [CrossRef]
- Kilduff and Weber, Transport and separation of organic macromolecules in ultrafiltration processes. Environ. Sci. Technol. 1992, 26, 569–577. [CrossRef]
- Stevenson,1976, Stability-constants of Cu2+, Pb2+, and Cd2+ complexes with humic acids. Soil Sci. Soc. Am. J. 1976, 40, 665–672. [CrossRef]
- Takamatsu, T.; Yoshida, T. Determination of stability-constants of metal humic acid complexes by potentiometric titration and ion-selective electrodes. Soil Sci. 1978, 125, 377–386. [Google Scholar] [CrossRef]
- Aleixo, L.M.; Godinho, O.E.S.; da Costa, W.F. Potentiometric study of acid-base properties of humic-acid using linear functions for treatment of titration data. Anal. Chim. Acta 1992, 257, 35–39. [Google Scholar] [CrossRef]
- Barak, P.; Chen, Y.N. Equivalent radii of humic macromolecules from acid-base titration. Soil Sci. 1992, 154, 184–195. [Google Scholar] [CrossRef]
- McCallum, C.; Midgley, D. Linear titration plots for the potentiometric determiantion of mixtures of strong and weak acids. Anal. Chim. Acta 1975, 78, 171–181. [Google Scholar] [CrossRef]
- Gran, G. Determination of the equivalence point in potentiometric titrations. PartAnalist Lond. 1952, 77, 661–671. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, K.; Yao, S.; Zhang, Y.; Zhang, X.; He, H.; Feng, W.; Ndzana, G.M.; Chenu, C.; Olk, D.C.; et al. Formation efficiency of soil organic matter from plant litter is governed by clay mineral type more than plant litter quality. Geoderma 2022, 412, 115727. [Google Scholar] [CrossRef]
- Preston, C.M. Application of NMR to soil organic matter analysis: History and prospects. Soil Sci. 1996, 161, 144–166. [Google Scholar] [CrossRef]
- Cook, R.L.; Langford, C.H. Structural characterization of a fulvic acid and a humic acid using solid-state ramp-CP-MAS 13C nuclear magnetic resonance. Environ. Sci. Technol. 1998, 32, 719–725. [Google Scholar] [CrossRef]
- Wilson, M.A. NMR Techniques and Applications in Geochemistry and Soil Chemistry; Pergamon: Oxford, UK, 1987. [Google Scholar]
- Mao, J.-D.; Xing, B.; Schmidt-Rohr, K. New structural information on a humic acid from two-dimensional 1H-13C correlation solid-state nuclear magnetic resonance. Environ. Sci. Technol. 2001, 35, 1928–1934. [Google Scholar] [CrossRef] [PubMed]
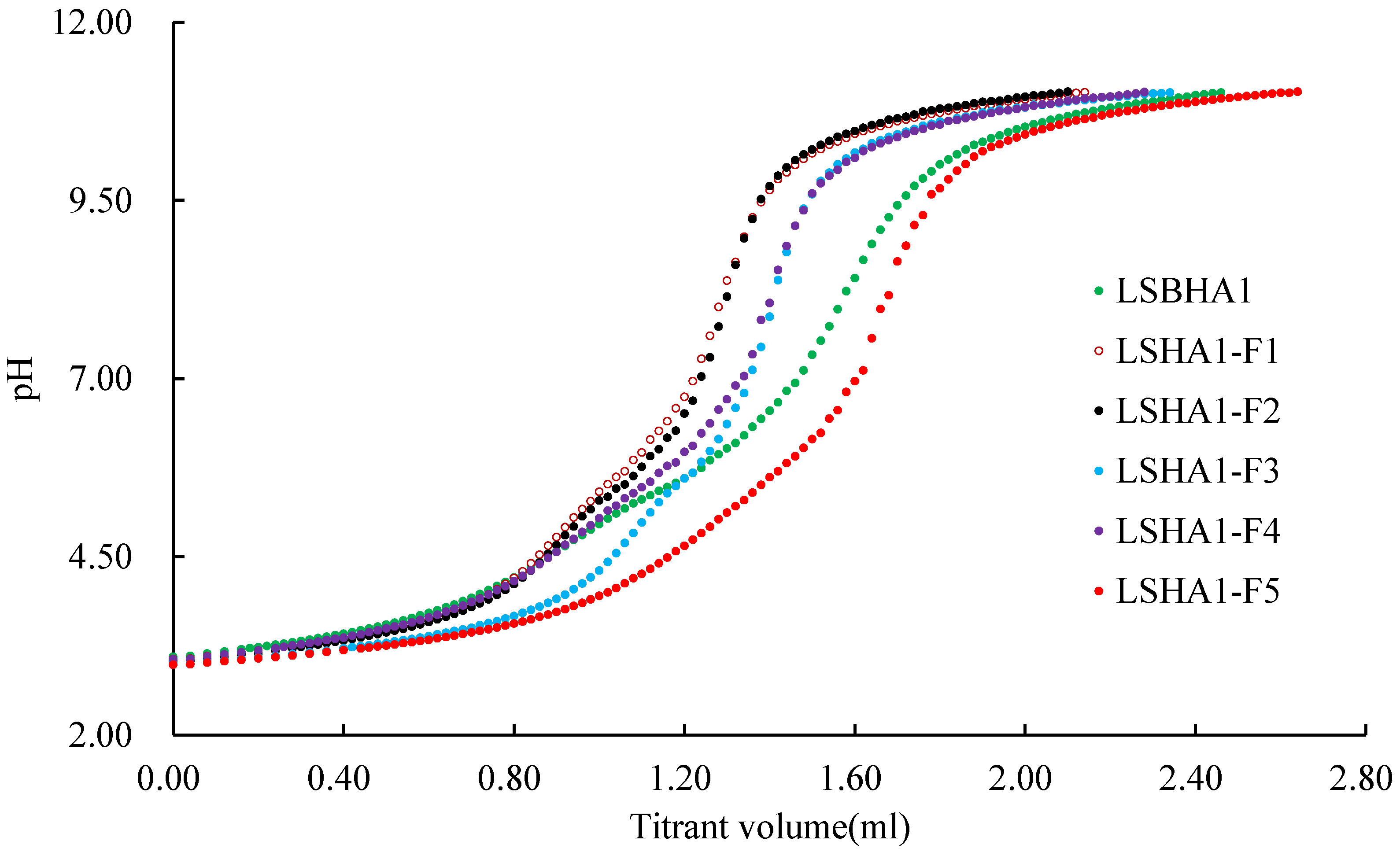
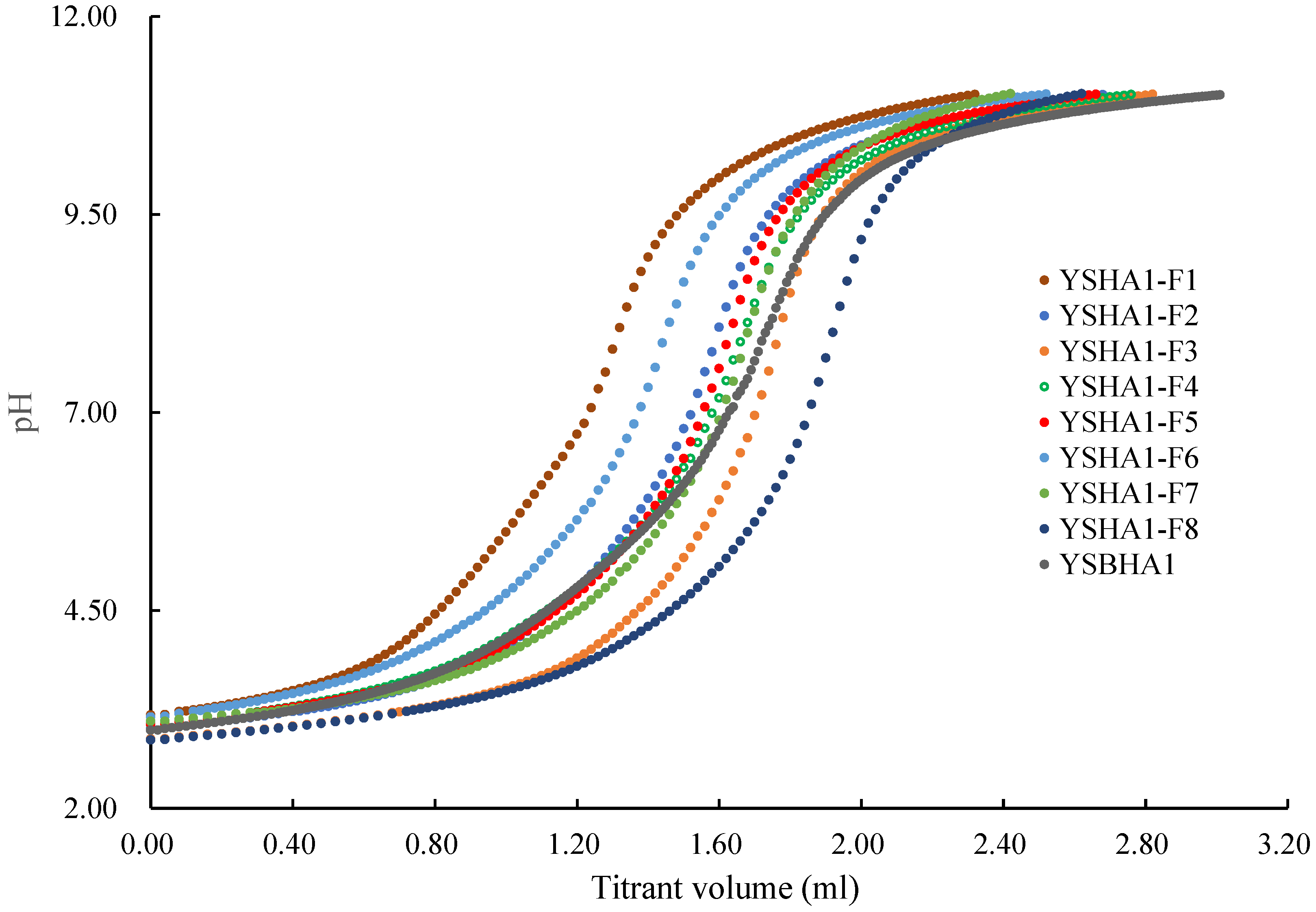

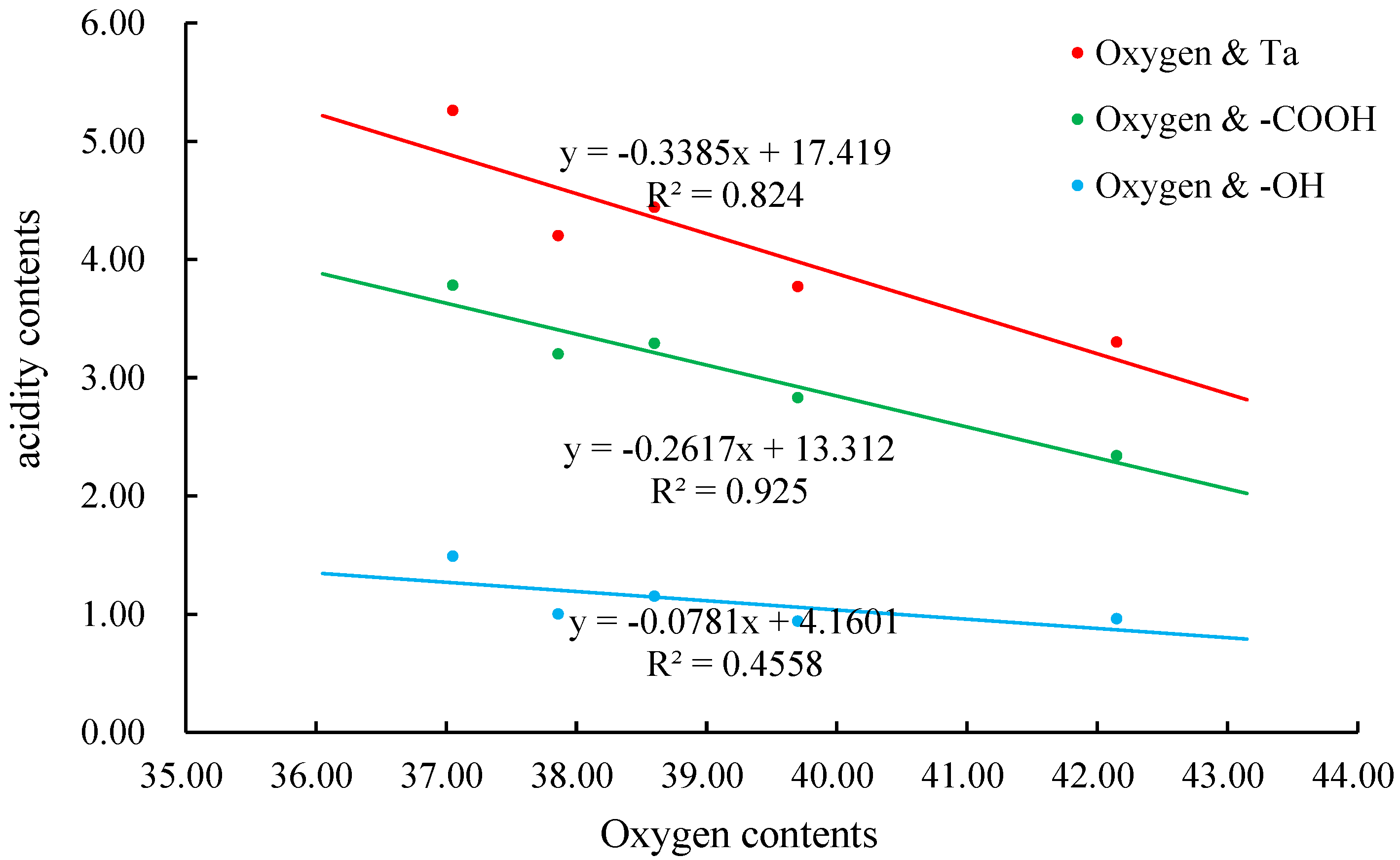




| Sample Name | pHVS | pHVN | pHVE | Ta | -COOH | -OH | pKα1 α1 = 0.5 | pKα2 α2 = 0.5 |
|---|---|---|---|---|---|---|---|---|
| mol/kg | ||||||||
| LSHA1-F1 | 4.27 | 8.15 | 10.08 | 3.30 | 2.34 | 0.96 | 5.70 | 9.59 |
| LSHA1-F2 | 4.31 | 8.15 | 10.05 | 3.77 | 2.83 | 0.94 | 5.56 | 9.40 |
| LSHA1-F3 | 4.10 | 8.16 | 10.06 | 4.20 | 3.20 | 1.00 | 5.47 | 9.40 |
| LSHA1-F4 | 4.10 | 8.18 | 10.06 | 4.44 | 3.29 | 1.15 | 5.41 | 9.54 |
| LSHA1-F5 | 4.18 | 8.12 | 10.12 | 5.26 | 3.78 | 1.49 | 5.59 | 9.49 |
| LSBHA1 | 4.06 | 7.90 | 10.00 | 3.84 | 2.56 | 1.28 | 5.50 | 9.22 |
| YSHA1-F1 | 4.10 | 7.55 | 9.65 | 3.04 | 2.11 | 0.93 | 5.70 | 8.78 |
| YSHA1-F2 | 4.05 | 7.60 | 9.67 | 3.31 | 2.50 | 0.81 | 5.53 | 8.85 |
| YSHA1-F3 | 4.10 | 7.50 | 9.69 | 3.69 | 2.77 | 0.92 | 5.50 | 8.74 |
| YSHA1-F4 | 4.05 | 7.59 | 9.67 | 4.02 | 3.20 | 0.82 | 5.40 | 8.74 |
| YSHA1-F5 | 3.99 | 7.65 | 9.61 | 4.30 | 3.51 | 0.79 | 5.30 | 8.74 |
| YSHA1-F6 | 4.09 | 7.53 | 9.64 | 4.54 | 3.65 | 0.89 | 5.35 | 8.73 |
| YSHA1-F7 | 4.10 | 7.63 | 9.61 | 4.54 | 3.63 | 0.91 | 5.42 | 8.74 |
| YSHA1-F8 | 3.97 | 7.59 | 9.61 | 4.42 | 3.67 | 0.85 | 5.31 | 8.71 |
| YSBHA1 | 4.06 | 7.55 | 9.75 | 3.66 | 2.83 | 0.84 | 5.24 | 8.90 |
| Shift/ppm | 0–45 | 45–63 | 63–110 | 110–165 | 165–187 | 187–220 | Aromaticity |
|---|---|---|---|---|---|---|---|
| C Type | Alkyl C | O-CH3 C | O-alkyl C | Aromatic C | Carboxyl C | Carbonyl C | |
| LSHA1-F1 | 22.4 | 13.6 | 30.9 | 18.3 | 11.6 | 3.0 | 0.21 |
| LSHA1-F2 | 22.9 | 13.8 | 24.3 | 21.7 | 13.4 | 3.6 | 0.26 |
| LSHA1-F3 | 22.5 | 13.8 | 22.7 | 23.3 | 13.8 | 3.7 | 0.28 |
| LSHA1-F4 | 20.4 | 11.8 | 20.5 | 27.9 | 14.4 | 4.6 | 0.35 |
| LSHA1-F5 | 23.5 | 9.1 | 19.6 | 29.4 | 13.7 | 4.5 | 0.36 |
| LSBHA1 | 24.1 | 13.6 | 26.7 | 20.4 | 12.4 | 2.8 | 0.24 |
| YSHA1-F1 | 34.4 | 11.6 | 20.5 | 19.6 | 10.5 | 3.1 | 0.23 |
| YSHA1-F2 | 26.7 | 11.1 | 20.9 | 24.7 | 14.6 | 3.7 | 0.30 |
| YSHA1-F3 | 23.9 | 10.9 | 21.4 | 27.6 | 16.1 | 4.3 | 0.33 |
| YSHA1-F4 | 21.7 | 10.6 | 20.7 | 28.6 | 17.2 | 4.9 | 0.35 |
| YSHA1-F5 | 20.1 | 9.9 | 20.1 | 30.5 | 18.1 | 5.4 | 0.38 |
| YSHA1-F6 | 20.2 | 9.0 | 19.1 | 31.1 | 18.5 | 5.6 | 0.39 |
| YSHA1-F7 | 21.5 | 9.0 | 19.2 | 31.4 | 18.2 | 5.1 | 0.39 |
| YSHA1-F8 | 23.0 | 8.1 | 19.0 | 31.1 | 17.2 | 4.7 | 0.38 |
| YSBHA1 | 29.6 | 10.3 | 19.7 | 24.3 | 12.1 | 3.9 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Xiao, B. Characteristic of Molecular Weight-Fractions of Soil Organic Matter from Calcareous Soil and Yellow Soil. Sustainability 2023, 15, 1537. https://doi.org/10.3390/su15021537
Ma L, Xiao B. Characteristic of Molecular Weight-Fractions of Soil Organic Matter from Calcareous Soil and Yellow Soil. Sustainability. 2023; 15(2):1537. https://doi.org/10.3390/su15021537
Chicago/Turabian StyleMa, Liangang, and Baohua Xiao. 2023. "Characteristic of Molecular Weight-Fractions of Soil Organic Matter from Calcareous Soil and Yellow Soil" Sustainability 15, no. 2: 1537. https://doi.org/10.3390/su15021537
APA StyleMa, L., & Xiao, B. (2023). Characteristic of Molecular Weight-Fractions of Soil Organic Matter from Calcareous Soil and Yellow Soil. Sustainability, 15(2), 1537. https://doi.org/10.3390/su15021537






