Evaluation of the Physicochemical Properties and Antiaging Properties of Bitumen Mastic Modified by Layered Double Hydroxides
Abstract
:1. Introduction
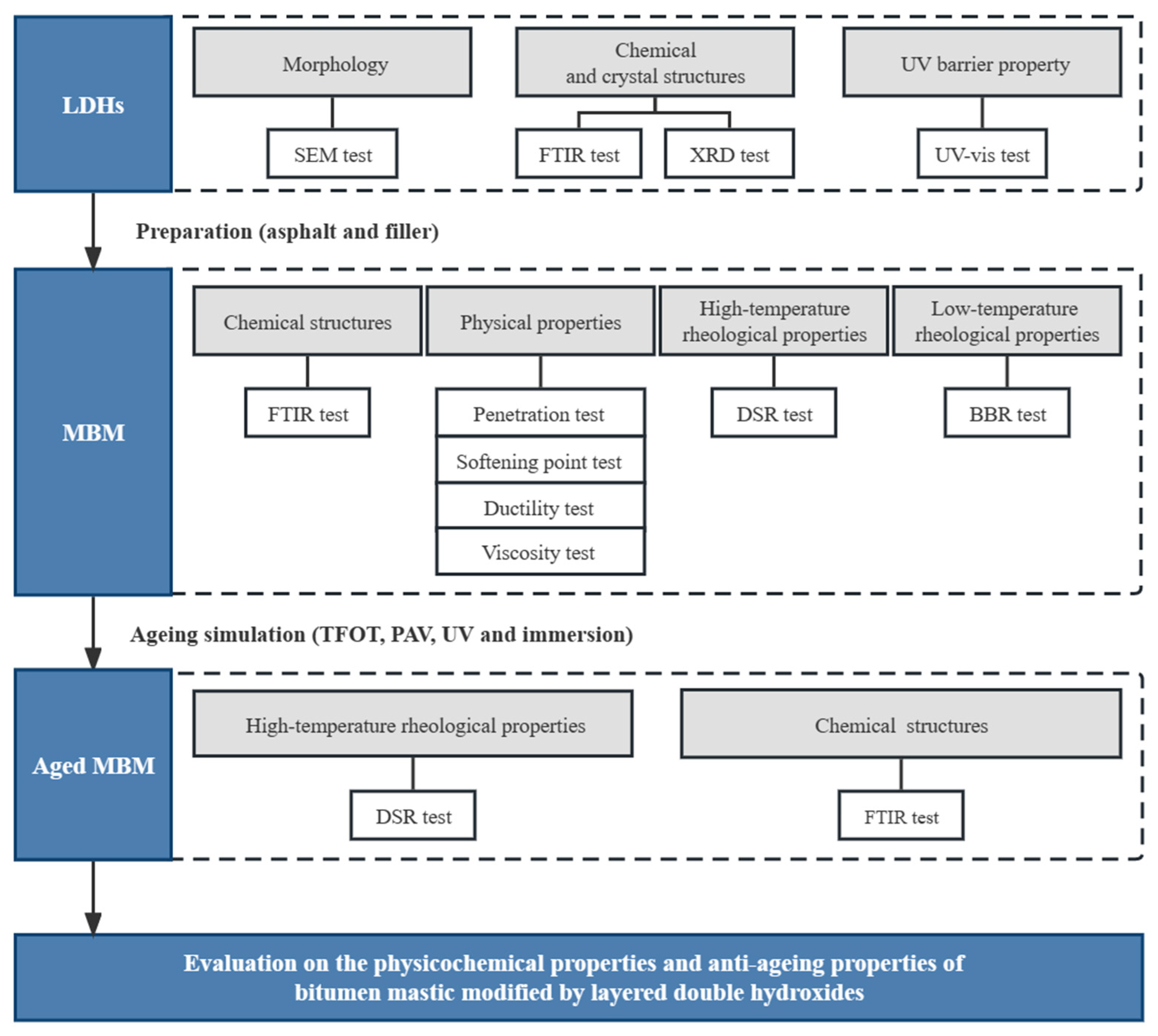
2. Materials and Experiment
2.1. Materials
2.1.1. Bitumen
2.1.2. LDHs
2.1.3. Filler
2.2. Characterization of LDHs
2.3. Preparation of BM
2.4. Aging Simulation of BM
2.5. Characterization of BM
3. Results and Discussion
3.1. Characterization of LDHs
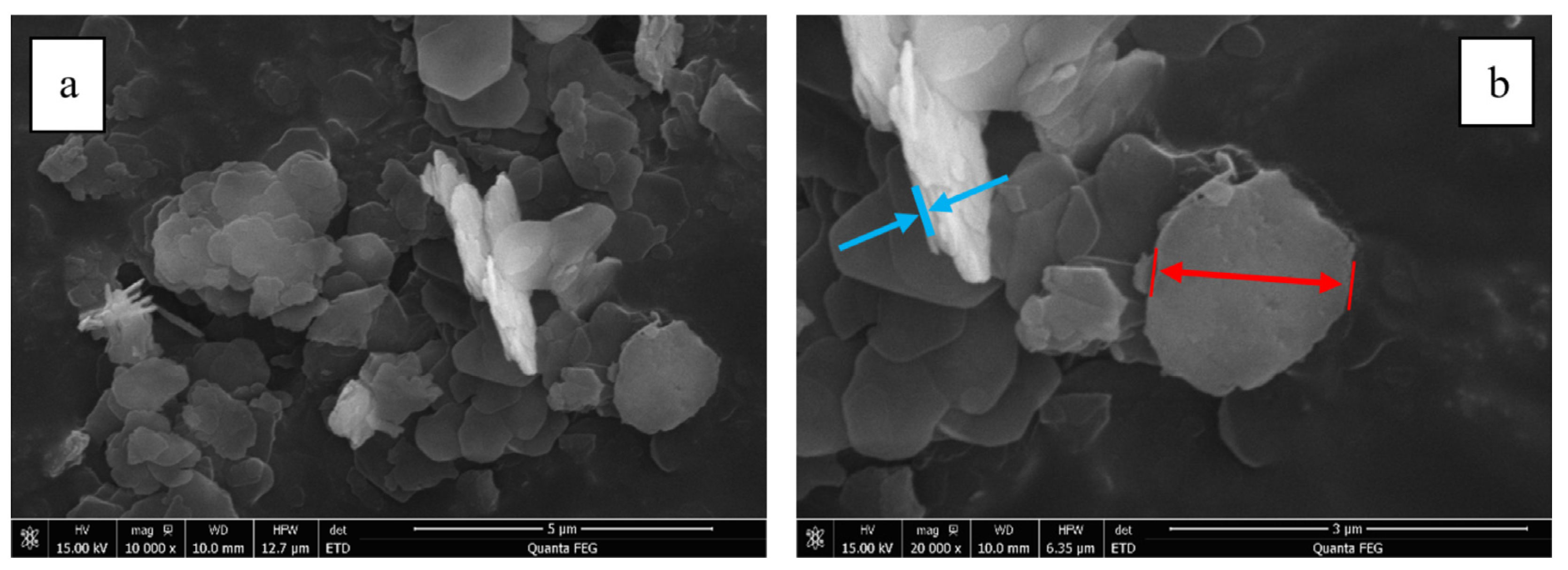
3.1.1. Morphology
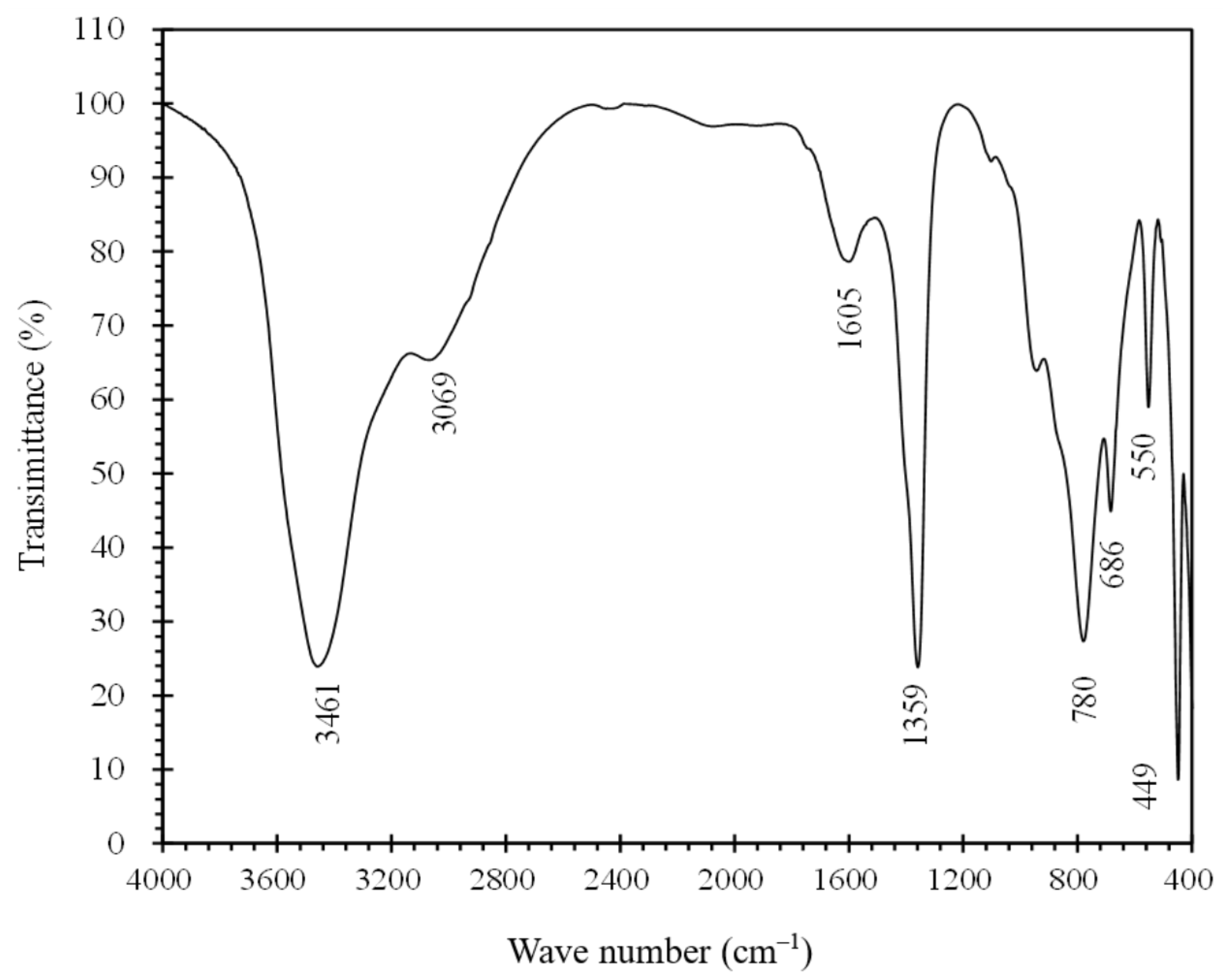
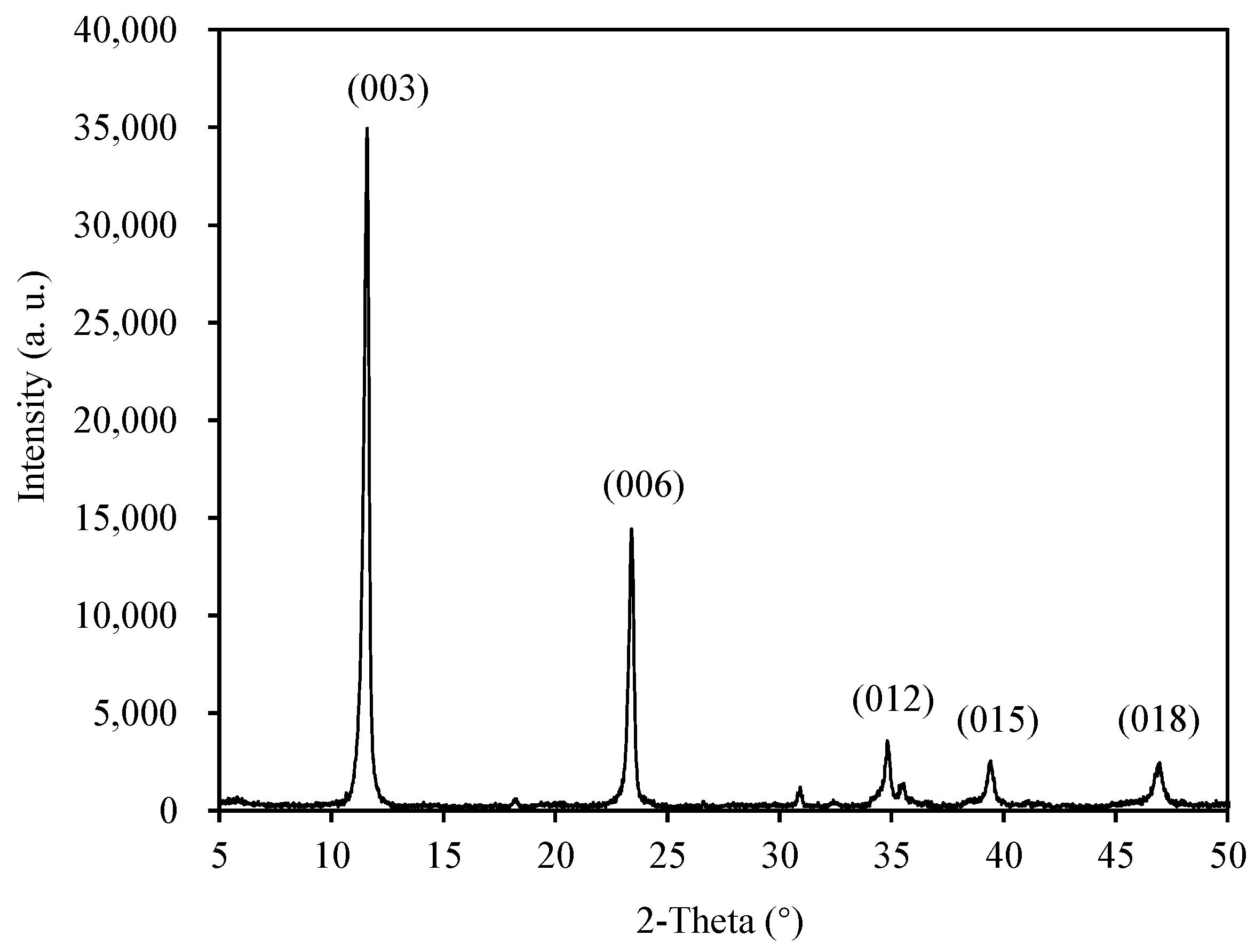
3.1.2. Chemical and Crystal Structures
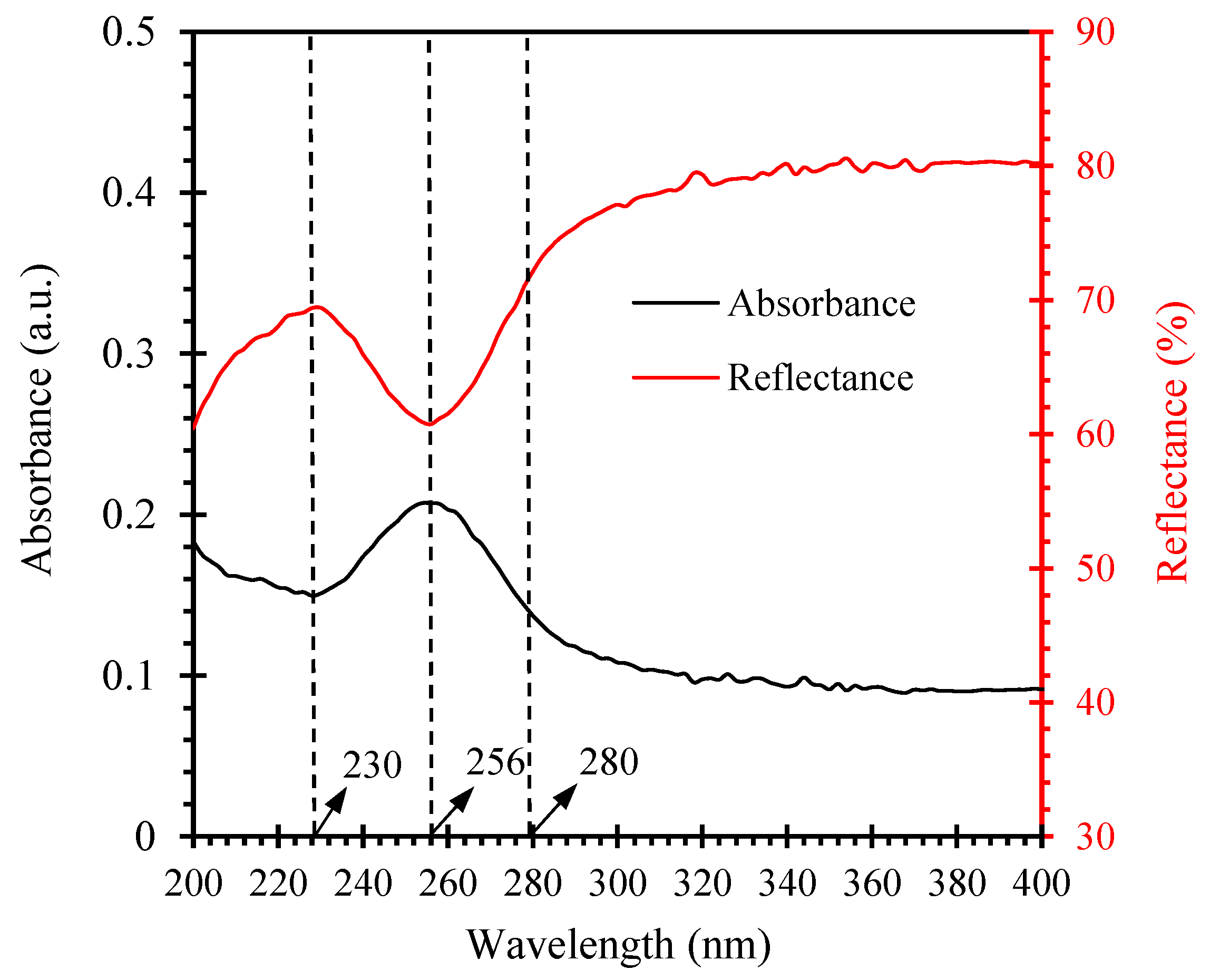
3.1.3. UV Barrier Property
3.2. Effect of LDHs on Physicochemical Properties of BM
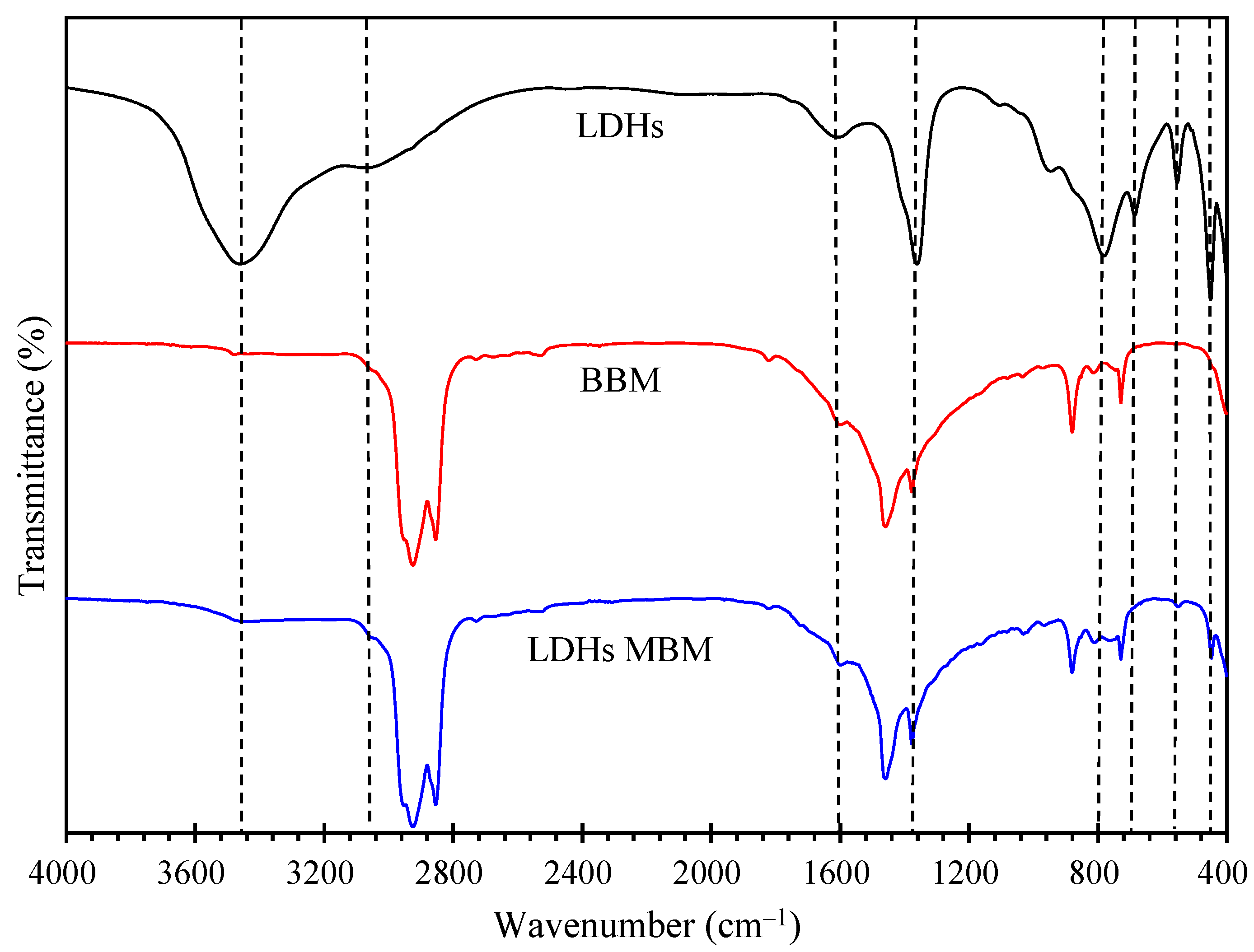
3.2.1. Chemical Structure
3.2.2. Physical Properties
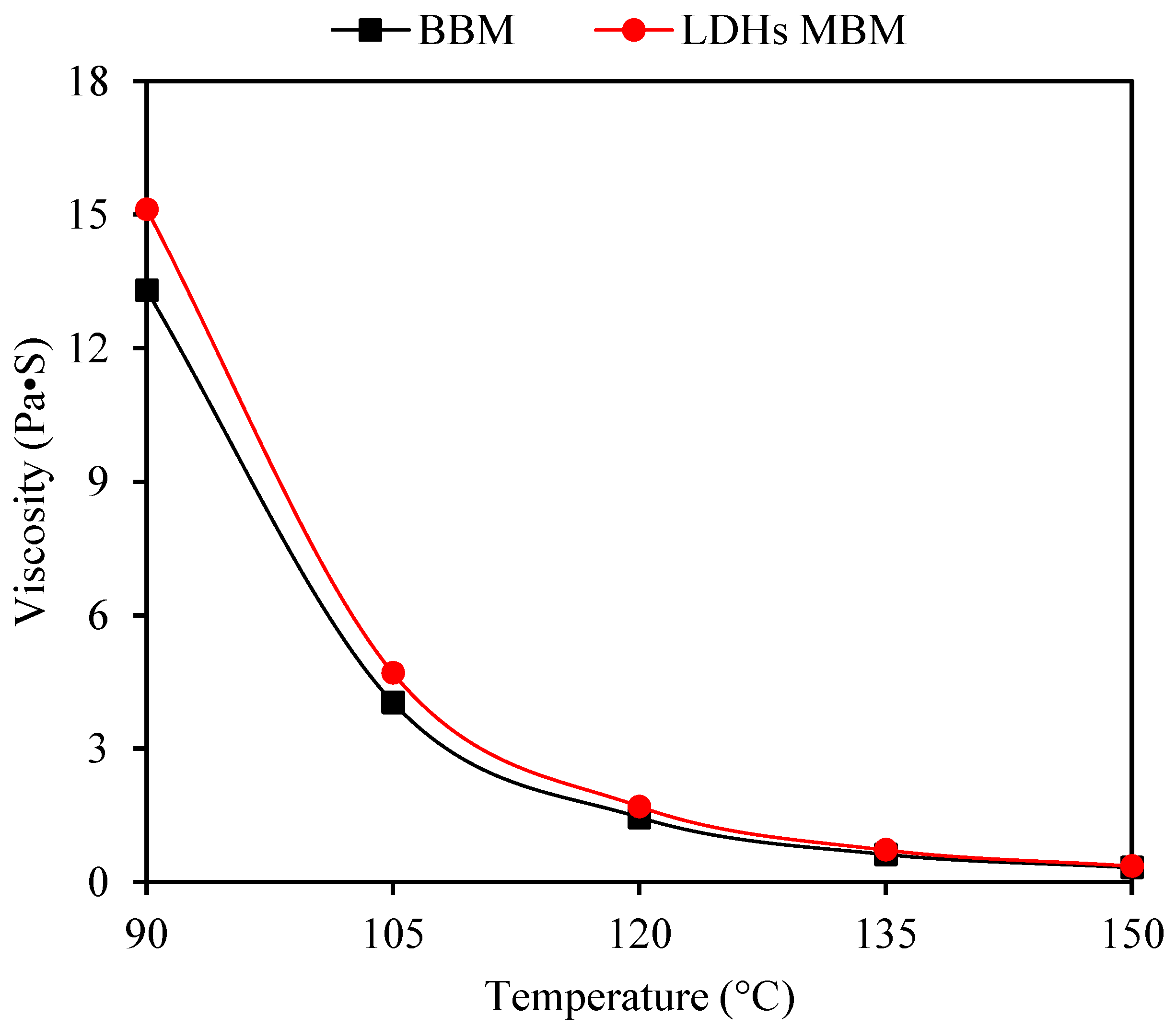
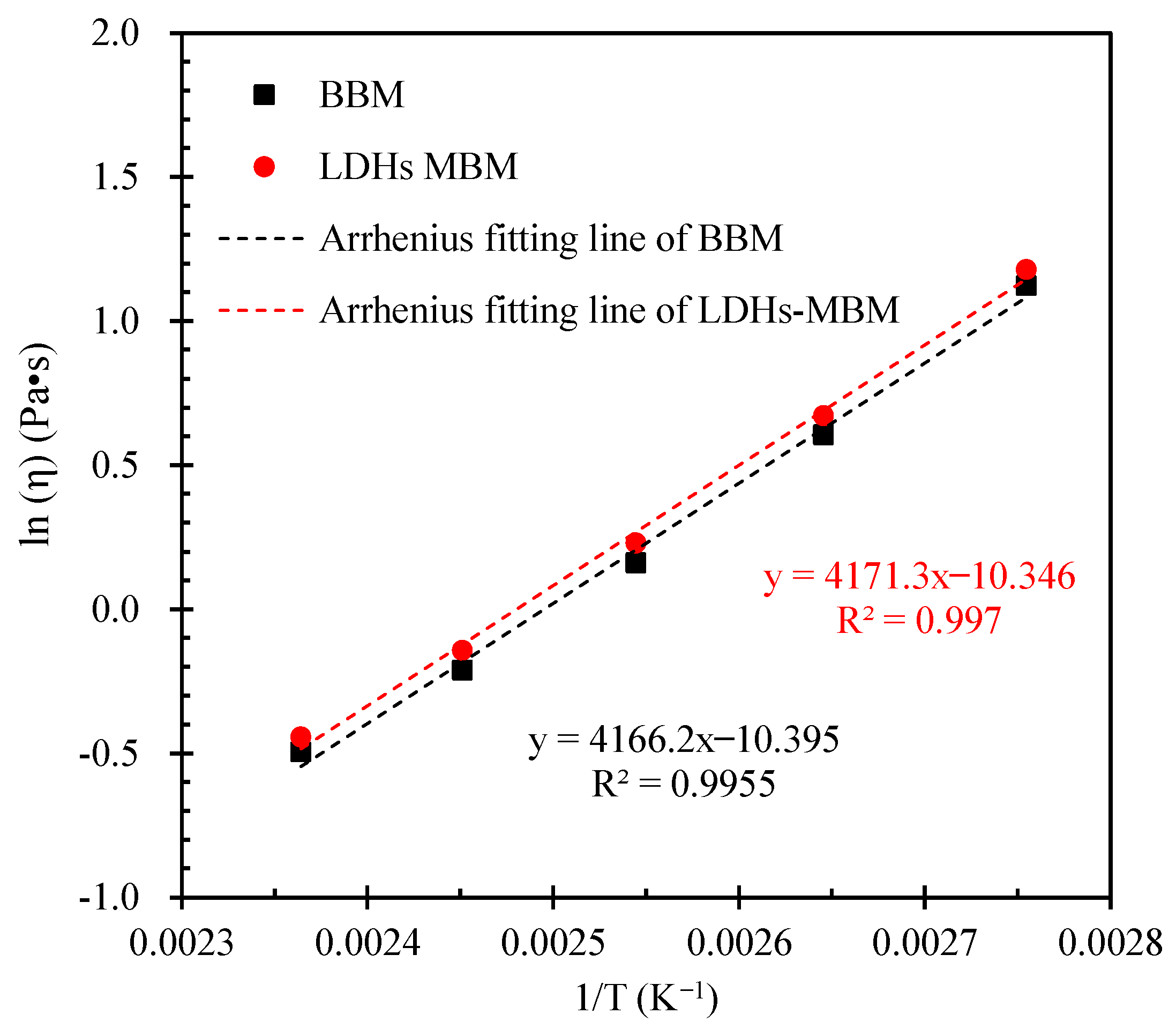
3.2.3. High-Temperature Rheological Properties
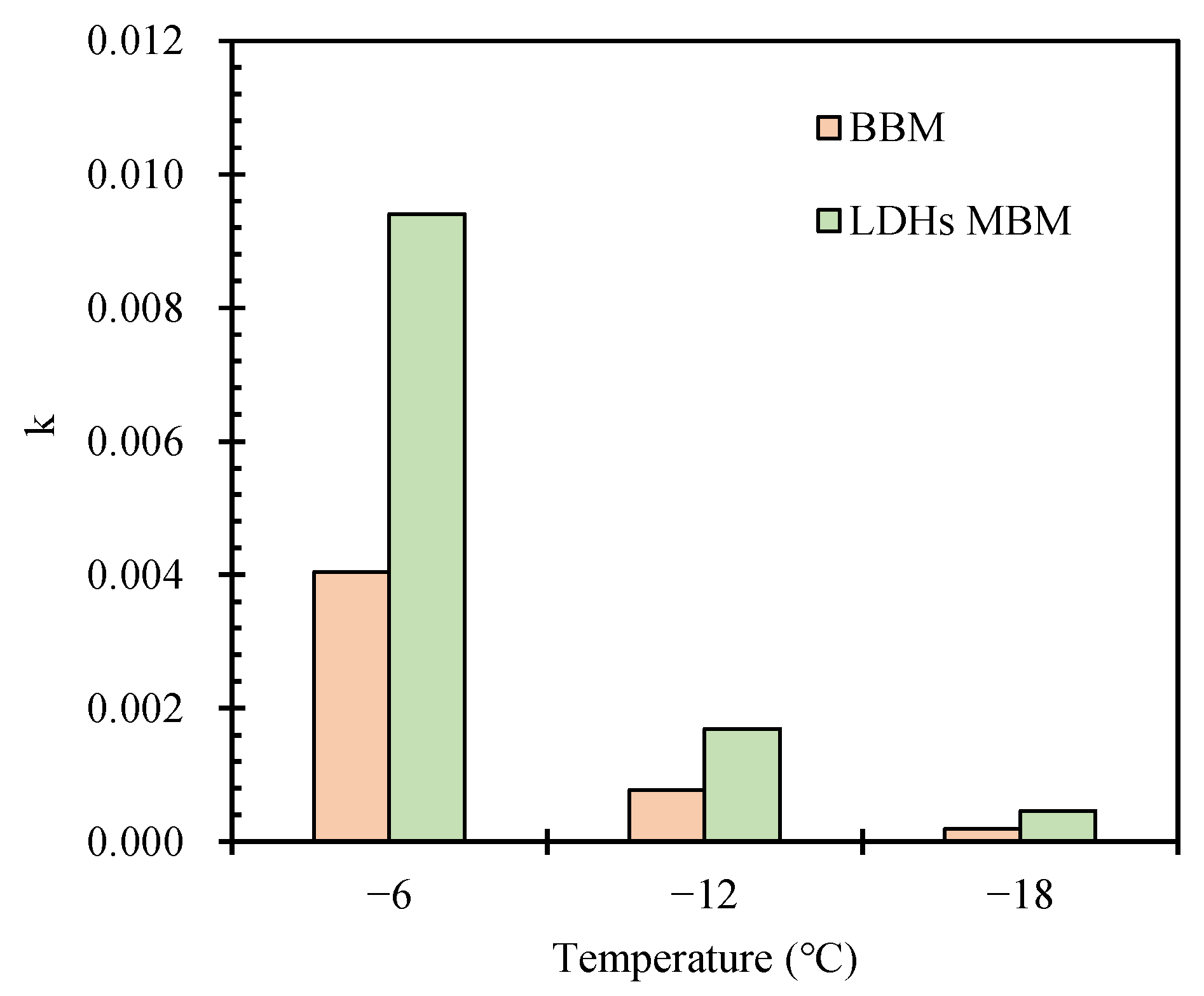
3.2.4. Low-Temperature Rheological Properties
3.3. Effect of LDHs on Antiaging Properties of BM
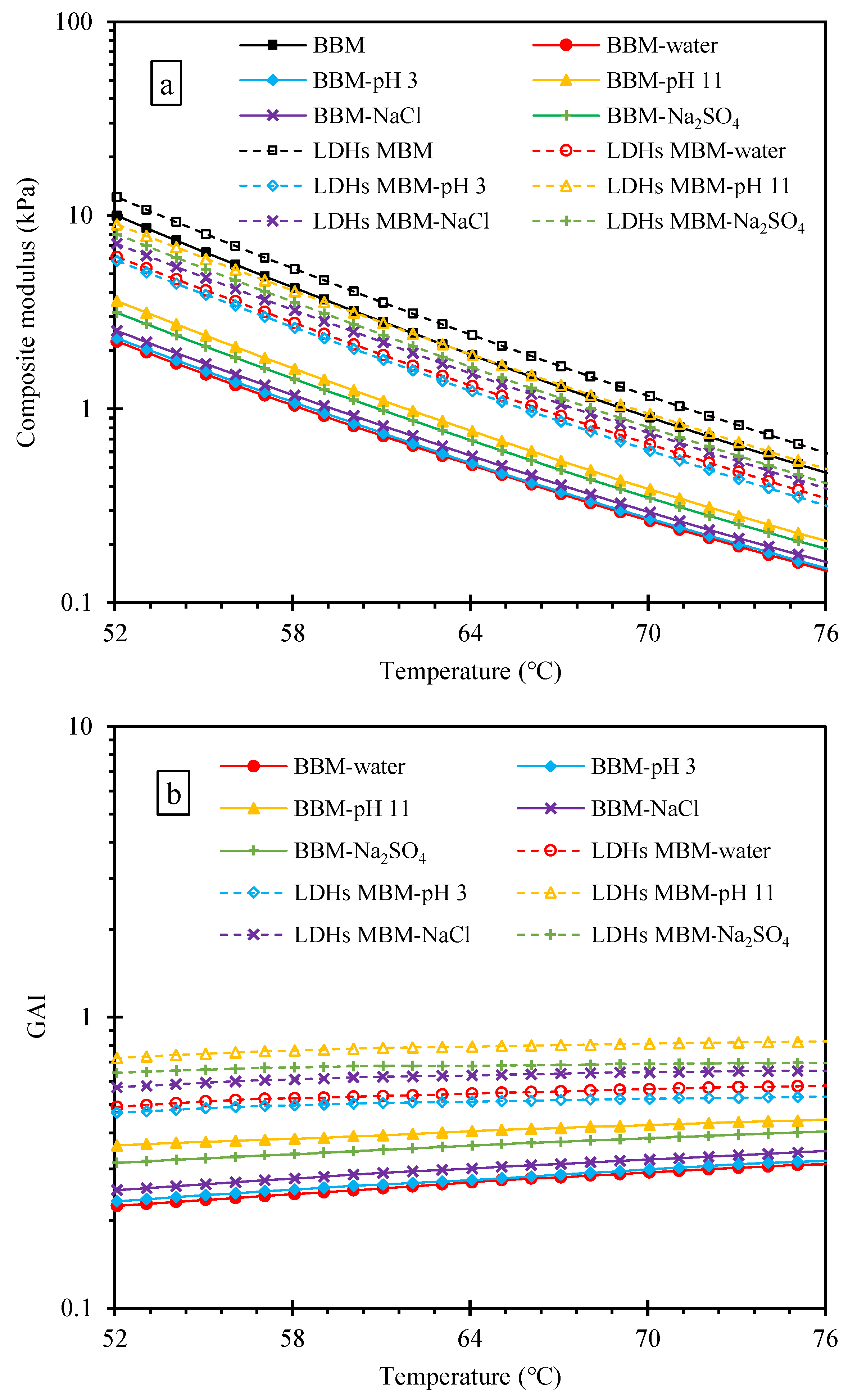
3.3.1. Effect of LDHs on GAI of BM
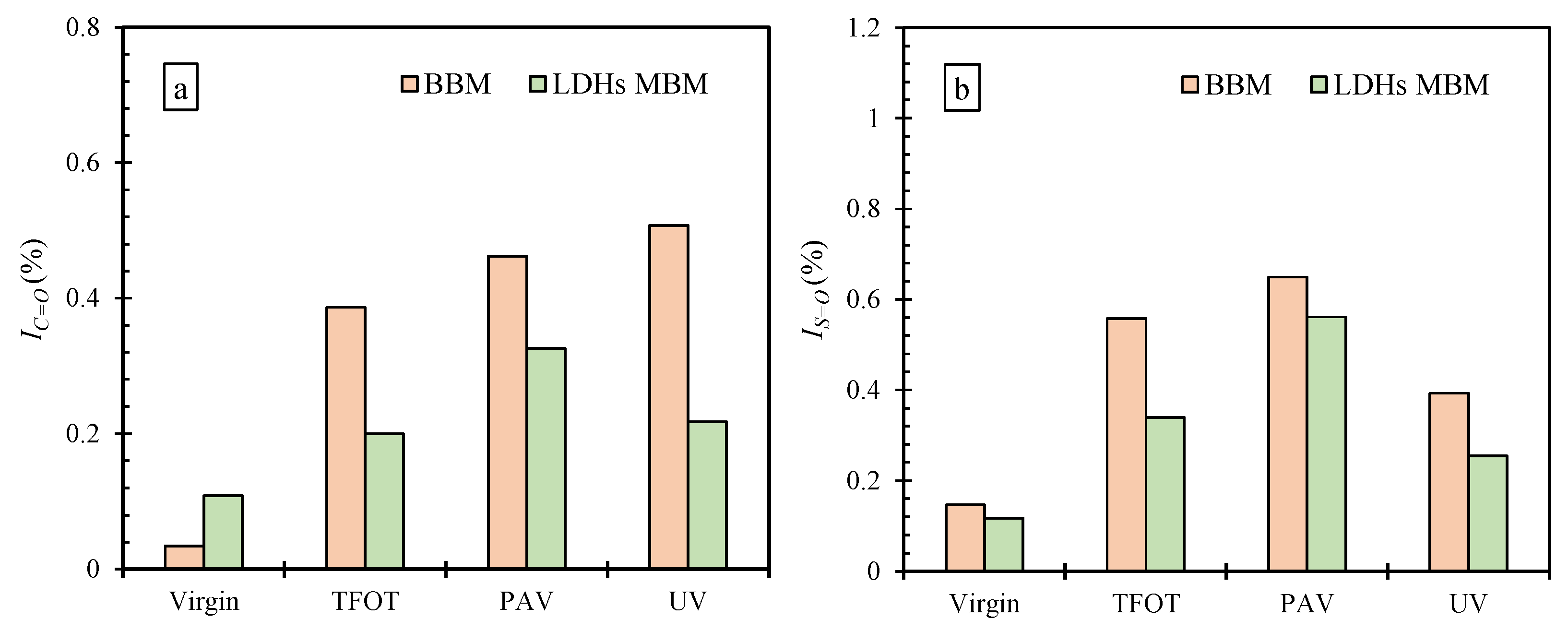
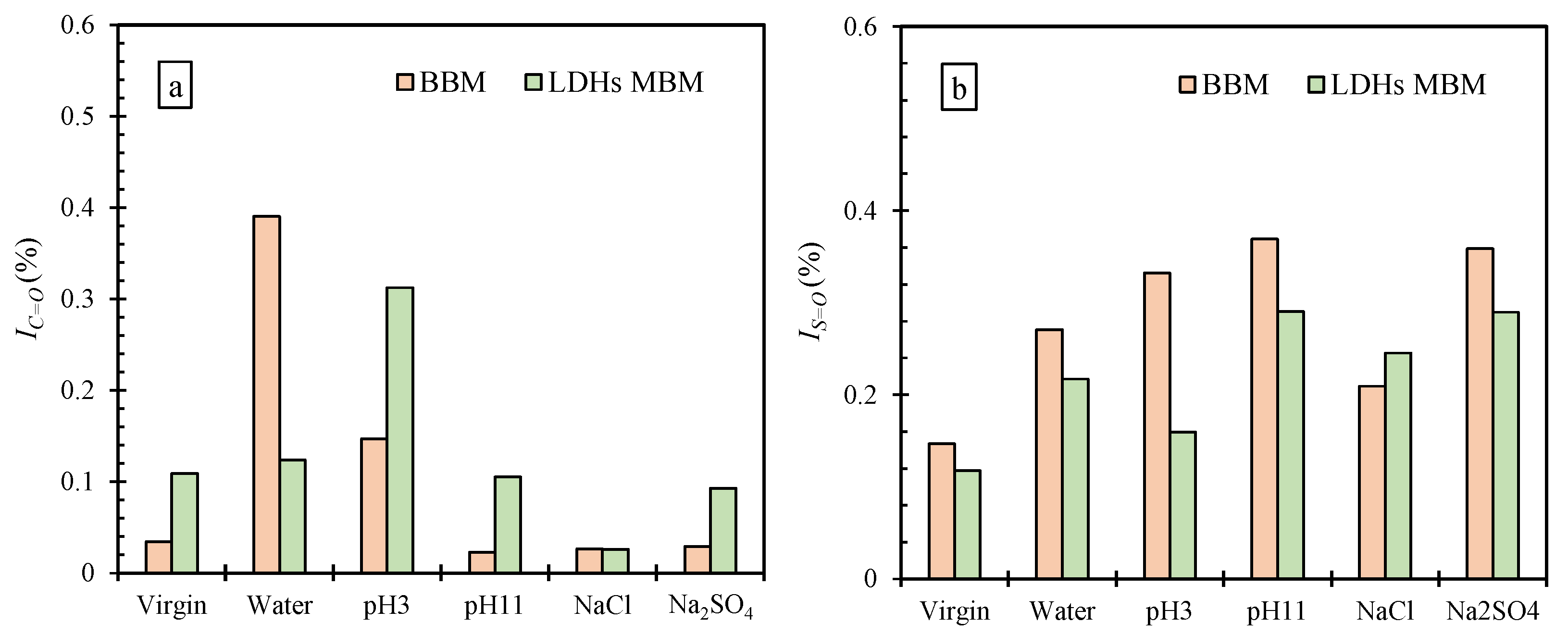
3.3.2. Effect of LDHs on IC=O and IS=O of BM
4. Conclusions
- There were few chemical reactions between the LDHs and BM; thus, the modification of BM by LDHs is a physical method.
- In terms of physical properties, LDHs could improve the high-temperature performance and weaken the low-temperature performance. Additionally, LDHs could increase the viscosity of BM, increasing η by 0.12%, indicating that LDHs could slightly reduce the temperature sensitivity of BM.
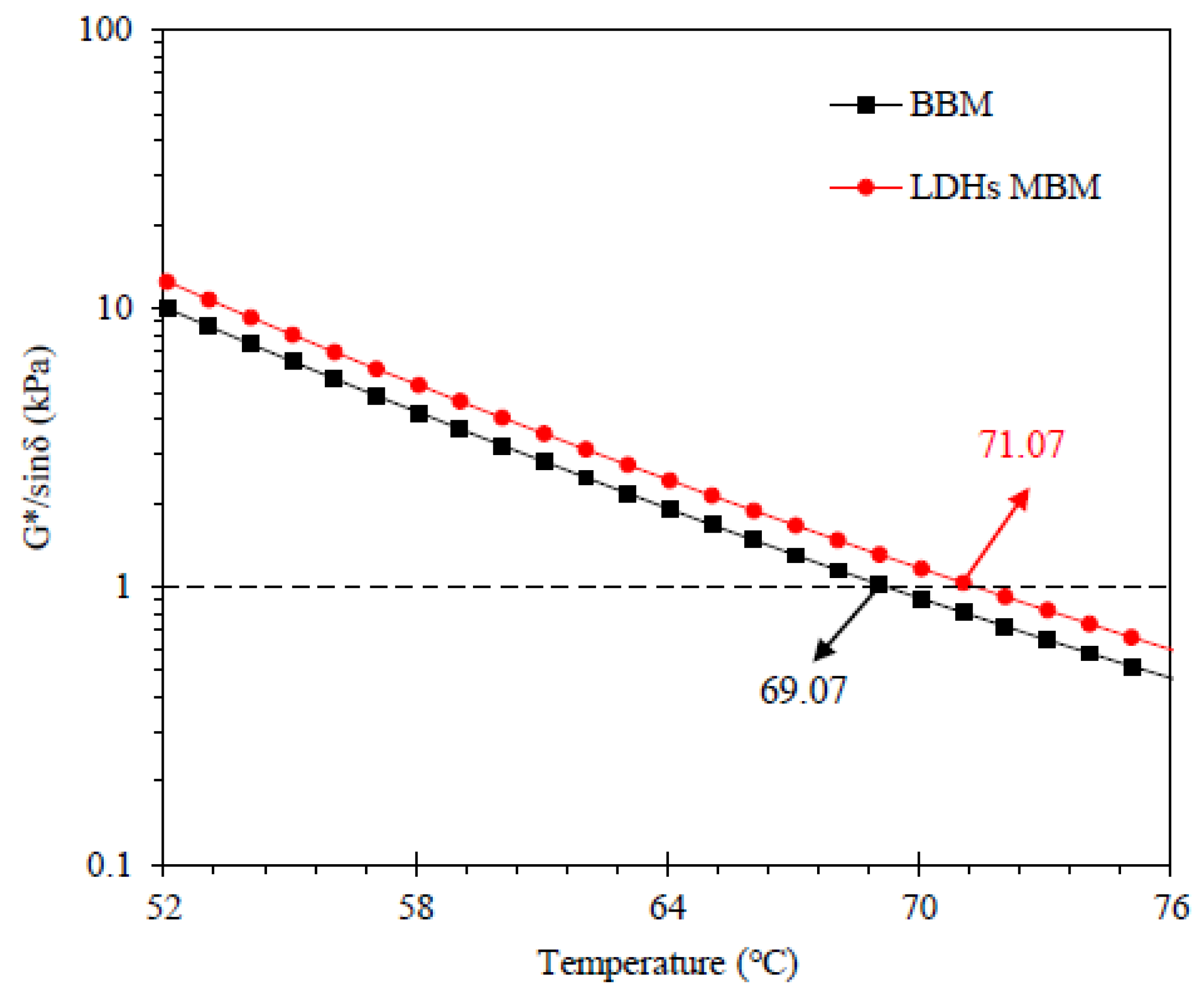
- LDHs enhanced the high failure temperature of BM from 69.07 °C to 71.07 °C and reduce the low failure temperature from −8.83 °C to −13.61 °C. Therefore, LDHs could improve the high-temperature and low-temperature rheological properties of BM.
- The addition of LDHs increased the G* of BM and hardened BM, making the rheological properties more susceptible to the effects of TFOT and PAV aging. Nevertheless, LDHs could slow the formation of carbonyl and sulfoxide groups during TFOT and PAV aging. This shows that LDHs could improve the resistance of BM to TFOT and PAV aging to a certain extent. Due to the UV barrier property of LDHs, the UV aging resistance of BM was successfully enhanced.
- The GAI of BBM decreased after immersion in the order of H2O < pH 3 < NaCl < Na2SO4 < pH 11. The addition of LDHs can enhance the GAI of BM immersed in various aqueous solutions. The erosion of different aqueous solutions on BM decreased in the order of erosion degree as follows: H2O > pH 3 > pH 11 > NaCl > Na2SO4. After the addition of LDHs, the change degree of IC=O and IS=O was greatly reduced after immersion in an aqueous solution except for NaCl solution. Combining the results of GAI, IC=O, and IS=O indicates that water and pH 3 had the greatest degree of erosion to BM, and LDHs could improve the resistance to five kinds of an aqueous solution.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Hoff, I.; Zhang, X.; Yang, C. Investigation of the self-healing and rejuvenating properties of aged asphalt mixture containing multi-cavity Ca-alginate capsules. Constr. Build. Mater. 2022, 361, 129685. [Google Scholar] [CrossRef]
- Wan, P.; Wu, S.; Liu, Q.; Wang, H.; Zhao, F.; Wu, J.; Niu, Y.; Ye, Q. Sustained-release calcium alginate diatomite capsules for sustainable self-healing asphalt concrete. J. Clean. Prod. 2022, 372, 133639. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, C.; Ouyang, J. Rejuvenation effect of waste cooking oil on the adhesion characteristics of aged asphalt to aggregates. Constr. Build. Mater. 2022, 327, 126907. [Google Scholar] [CrossRef]
- Xu, H.; Wu, S.; Chen, A.; Zou, Y. Influence of erosion factors (time, depths and environment) on induction heating asphalt concrete and its mechanism. J. Clean. Prod. 2022, 363, 132521. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Cui, P.; Amirkhanian, S.; Zhao, Z.; Wang, F.; Xie, J. Performance characterization and enhancement mechanism of recycled asphalt mixtures involving high RAP content and steel slag. J. Clean. Prod. 2022, 336, 130484. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Cao, J.; Chai, J.; Cao, C.; Si, Z.; Li, Y. Adhesion between asphalt molecules and acid aggregates under extreme temperature: A ReaxFF reactive molecular dynamics study. Constr. Build. Mater. 2021, 285, 122882. [Google Scholar] [CrossRef]
- Cui, P.; Wu, S.; Xiao, Y.; Hu, R.; Yang, T. Environmental performance and functional analysis of chip seals with recycled basic oxygen furnace slag as aggregate. J. Hazard. Mater. 2021, 405, 124441. [Google Scholar] [CrossRef]
- Xu, H.; Wu, S.; Chen, A.; Zou, Y.; Yang, C.; Cui, P. Study on preparation and characterization of a functional porous ultra-thin friction course (PUFC) with recycled steel slag as aggregate. J. Clean. Prod. 2022, 380, 134983. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Shi, J.; Yang, X.; Fang, Y. Comparative analysis of thermal aging behavior and comprehensive performance of high viscosity asphalt (HVA) from cohesion, adhesion and rheology perspectives. Constr. Build. Mater. 2022, 317, 125982. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Wu, S.; Xie, J. The mechanical resistance of asphalt mixture with steel slag to deformation and skid degradation based on laboratory accelerated heavy loading test. Materials 2022, 15, 911. [Google Scholar] [CrossRef]
- Xie, Q.; Ling, X.; Orji, J.; Onu], E. Evaluation of sustainable acid rain control options utilizing a fuzzy TOPSIS multi-criteria decision analysis model frame work. J. Clean. Prod. 2017, 141, 612–625. [Google Scholar]
- Zhang, J.; Lai, Y.; Li, J.; Zhao, Y. Study on the influence of hydro-thermal-salt-mechanical interaction in saturated frozen sulfate saline soil based on crystallization kinetics. Int. J. Heat Mass Tran. 2020, 146, 118868. [Google Scholar] [CrossRef]
- Xu, O.; Yang, X.; Xiang, S.; Zhang, H. Migration characteristic and model of chloride ions for NaCl-based salt storage asphalt mixtures. Constr. Build. Mater. 2021, 280, 122482. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Z.; Zhang, H.; Wei, C. Rheological and anti-aging performance of SBS modified asphalt binders with different multi-dimensional nanomaterials. Constr. Build. Mater. 2018, 188, 409–416. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Wang, H.; Fan, W.; Erkens, S. Evaluation of rheological behaviors and anti-aging properties of recycled asphalts using low-viscosity asphalt and polymers. J. Clean. Prod. 2020, 253, 120048. [Google Scholar] [CrossRef]
- Xu, E.; Chen, P.; Wang, G.; Li, K.; Lin, Y. A facile approach to enhancing the UV blocking properties of layered double hydroxides and potential application in asphalt. Mater. Today Sustain. 2021, 14, 100081. [Google Scholar] [CrossRef]
- He, B.; Xiao, Y.; Li, Y.; Fu, M.; Yu, J.; Zhu, L. Preparation and characterization of lignin grafted layered double hydroxides for sustainable service of bitumen under ultraviolet light. J. Clean. Prod. 2022, 350, 131536. [Google Scholar] [CrossRef]
- He, B.; Yu, J.; Yi, G.; Zhuang, R.; Sun, Y. Rheological properties of lignosulfonate intercalated layered double hydroxides modified bitumen before and after ultraviolet aging. Constr. Build. Mater. 2018, 180, 342–350. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, M.; He, B.; Han, X.; Yu, J.; Xue, L. Investigation of ultraviolet aging resistance of bitumen modified by layered double hydroxides with different particle sizes. Constr. Build. Mater. 2019, 196, 166–174. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Pang, L.; Liu, Q.; Wang, Z.; Zhang, A. Investigation of the effect of Mg-Al-LDH on pavement performance and aging resistance of styrene-butadiene-styrene modified asphalt. Constr. Build. Mater. 2018, 172, 584–596. [Google Scholar] [CrossRef]
- ASTM D-5; Standard Test Method for Penetration of Bituminous Materials. ASTM International: West Conshohocken, PA, USA, 1992.
- ASTM D-36; Standard Test Method for Softening Point of Bitumen (Ring-and-Ball Apparatus). ASTM International: West Conshohocken, PA, USA, 2009.
- ASTM D-113; Standard Test Method for Ductility of Bituminous Materials. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM D-2042; Standard Test Method for Solubility of Asphalt Materials in Trichloroethylene. ASTM International: West Conshohocken, PA, USA, 2015.
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. Ministry of Transport of the People’s Republic of China: Beijing, China, 2011.
- Vargas, M.A.; Moreno, L.; Montiel, R.; Manero, O.; Vázquez, H. Effects of montmorillonite (Mt) and two different organo-Mt additives on the performance of asphalt. Appl. Clay Sci. 2017, 139, 20–27. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Q.; Wu, H.; Yang, C.; Gong, X.; Wu, S.; Li, Y. Simultaneous enhancement of the thermal oxygen and ultraviolet aging resistance of asphalt by incorporating antioxidant intercalated layered double hydroxide. Constr. Build. Mater. 2022, 349, 128743. [Google Scholar] [CrossRef]
- ASTM D4402-06; Standard Test Method for Viscosity Determination of Asphaltat Elevated Temperatures Using a Rotational Viscometer. ASTM International: West Conshohocken, PA, USA, 2006.
- Yang, C.; Wu, S.; Xie, J.; Amirkhanian, S.; Zhao, Z.; Xu, H.; Wang, F.; Zhang, L. Development of blending model for RAP and virgin asphalt in recycled asphalt mixtures via a micron-Fe3O4 tracer. J. Clean. Prod. 2023, 383, 135407. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, H.; Zhang, D.; Chen, Z. Influence of base asphalt and SBS modifier on the weathering aging behaviors of SBS modified asphalt. J. Mater. Civ. Eng. 2018, 30, 04019184. [Google Scholar] [CrossRef]
- ASTM D6648-08; Standard Test Method for Determining the Flexural Creep Stiffness of Asphalt Binder Using the Bending Beam Rheometer (BBR). ASTM International: West Conshohocken, PA, USA, 2016.
- Ren, H.; Qian, Z.; Huang, W.; Li, H.; Liu, Y. Low-temperature thermal cracking performance of waterborne epoxy asphalt emulsion mastic based on bending beam rheometer (BBR). Constr. Build. Mater. 2022, 334, 127461. [Google Scholar] [CrossRef]
- Garilli, E.; Autelitano, F.; Giuliani, F. Use of bending beam rheometer test for Theological analysis of asphalt emulsion-cement mastics in cold in-place recycling. Constr. Build. Mater. 2019, 222, 484–492. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, Y.; Zhang, W.; Xie, G.; Li, Y.; Shen, Q.; Yu, X. Electrochemical properties of Zn-Al-La layered double hydroxides in Zn-Ni secondary batteries. ACS Omega 2021, 6, 35244–35249. [Google Scholar] [CrossRef]
- Quilez-Molina, A.I.; Marini, L.; Athanassiou, A.; Bayer, I.S. UV-blocking, transparent, and antioxidant polycyanoacrylate films. Polymers 2020, 12, 2011. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, J.; Xue, L.; Sun, Y. Investigation of γ-(2,3-epoxypropoxy) propyltrimethoxy silane surface modified layered double hydroxides improving UV ageing resistance of asphalt. Materials 2017, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, P.; Sun, C.; Liang, M.; Li, Y.; Yao, Z.; Zhang, X. Effects of composite warm mix additive (CAR) on the physical and rheological performance of bitumen and the pavement performance of its concrete. Materials 2019, 12, 3916. [Google Scholar] [CrossRef] [Green Version]
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.C. Polypropylene/Montmorillonite Nanocomposites. Review of the Synthetic Routes and Materials Properties. Am. Chem. Soc. 2001, 13, 3516–3523. [Google Scholar] [CrossRef]
- Saboo, N.; Singh, B.; Kumar, P.; Vikram, D. Study on viscosity of conventional and polymer modified asphalt binders in steady and dynamic shear domain. Mech. Time-Depend. Mat. 2018, 22, 67–78. [Google Scholar] [CrossRef]
- Ren, H.; Qian, Z.; Huang, W.; Li, H.; Liu, G. High-temperature rheological properties of waterborne epoxy asphalt emulsion mastic. Constr. Build. Mater. 2022, 325, 126796. [Google Scholar] [CrossRef]
- Liu, S.; Cao, W.; Shang, S.; Hui, Q.; Fang, J. Analysis and application of relationships between low-temperature rheological performance parameters of asphalt binders. Constr. Build. Mater. 2010, 24, 471–478. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Sun, H.; Tan, Y. Research on viscoelastic-plastic damage characteristics of cement asphalt composite binder. Constr. Build. Mater. 2021, 274, 122064. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, T.; Zhu, K.; Tang, D.; Ke, W.U. Effect of Various Metal Hydroxide Flame Retardants on the Rheological Properties of Asphalt Binder. Mater. Sci. 2019, 25, 348–355. [Google Scholar] [CrossRef]



| Physical Performance | Units | Result | Standards |
|---|---|---|---|
| Penetration (25 °C, 100 g, 5 s) | 0.1 mm | 85.3 | ASTM D-5 [21] |
| Softening point | °C | 45.2 | ASTM D-36 [22] |
| Ductility (10 °C) | cm | 392 | ASTM D-113 [23] |
| Solubility (trichloroethylene) | % | 99.8 | ASTM D-2042 [24] |
| Parameters | Units | LHDs |
|---|---|---|
| Ratio of MgO/Al2O3 | / | 4.2 |
| Content of LDHs | % | ≥99.5 |
| Mass loss (105 °C) | % | ≤0.5 |
| Specific surface area | m2/g | 1.34 |
| Average particle size | μm | 5.267 |
| Technical Performance | Result | Requirement [25] | |
|---|---|---|---|
| Apparent density/g∙cm−3 | 2.671 | ≥2.5 | |
| Gradation/wt.% | <0.6 mm | 100 | 100 |
| <0.15 mm | 94.2 | 90~100 | |
| <0.075 mm | 81.4 | 75~100 | |
| Hydrophilic coefficient | 0.62 | <1 | |
| Physical Properties | Units | BBM | LDHs MBM | Variation (%) |
|---|---|---|---|---|
| Penetration (25 °C, 100 g, 5 s) | 0.1 mm | 59.3 | 56.2 | −5.23 |
| Softening point | °C | 49.9 | 52.3 | 4.81 |
| Ductility (10 °C) | cm | 221 | 214 | −3.17 |
| Mastic | Fitting Line | R2 | Slope (−Eη/R) | |
|---|---|---|---|---|
| BBM | y = 4166.2x − 10.395 | 0.9955 | 4166.2 | 34.6378 |
| LDHs MBM | y = 4171.3x − 10.346 | 0.9970 | 4171.3 | 34.6802 |
| Sample | IC=O | IS=O | ||
|---|---|---|---|---|
| BBM | LDHs MBM | 90 M | LDHs MBM | |
| Water | 10.41 | 0.14 | 0.84 | 0.85 |
| pH 3 | 3.29 | 1.88 | 1.27 | 0.36 |
| pH 11 | −0.34 | −0.03 | 1.52 | 1.47 |
| NaCl | −0.24 | −0.76 | 0.43 | 1.09 |
| Na2SO4 | −0.15 | −0.14 | 1.45 | 1.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Pang, L.; Chen, S.; Xu, S.; Wu, S.; Amirkhanian, S.; Xu, H.; Zhao, Z. Evaluation of the Physicochemical Properties and Antiaging Properties of Bitumen Mastic Modified by Layered Double Hydroxides. Sustainability 2023, 15, 1546. https://doi.org/10.3390/su15021546
Zou Y, Pang L, Chen S, Xu S, Wu S, Amirkhanian S, Xu H, Zhao Z. Evaluation of the Physicochemical Properties and Antiaging Properties of Bitumen Mastic Modified by Layered Double Hydroxides. Sustainability. 2023; 15(2):1546. https://doi.org/10.3390/su15021546
Chicago/Turabian StyleZou, Yingxue, Ling Pang, Shuaichao Chen, Shi Xu, Shaopeng Wu, Serji Amirkhanian, Haiqin Xu, and Zenggang Zhao. 2023. "Evaluation of the Physicochemical Properties and Antiaging Properties of Bitumen Mastic Modified by Layered Double Hydroxides" Sustainability 15, no. 2: 1546. https://doi.org/10.3390/su15021546







