Valorization of Grape Pomace for Trametes versicolor Mycelial Mass and Polysaccharides Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Growth Conditions
2.2. Fermentation Supplements and Submerged Fermentations (SmFs)
2.3. Analytical Methods
2.3.1. Determination of Total Dry Weight (TDW)
2.3.2. Evaluation of Sugar and Free Amino Nitrogen Concentration
2.3.3. Preparation of Extracellular Polysaccharide Extracts (EPSs)
2.3.4. Preparation of Intracellular Polysaccharide Extracts (IPSs)
2.3.5. Compositional Characterization and Antioxidant Activity of Crude IPSs and EPSs
2.3.6. Amino Acid Analysis in the Crude IPS Extracts
2.3.7. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.8. Statistical Analysis
3. Results
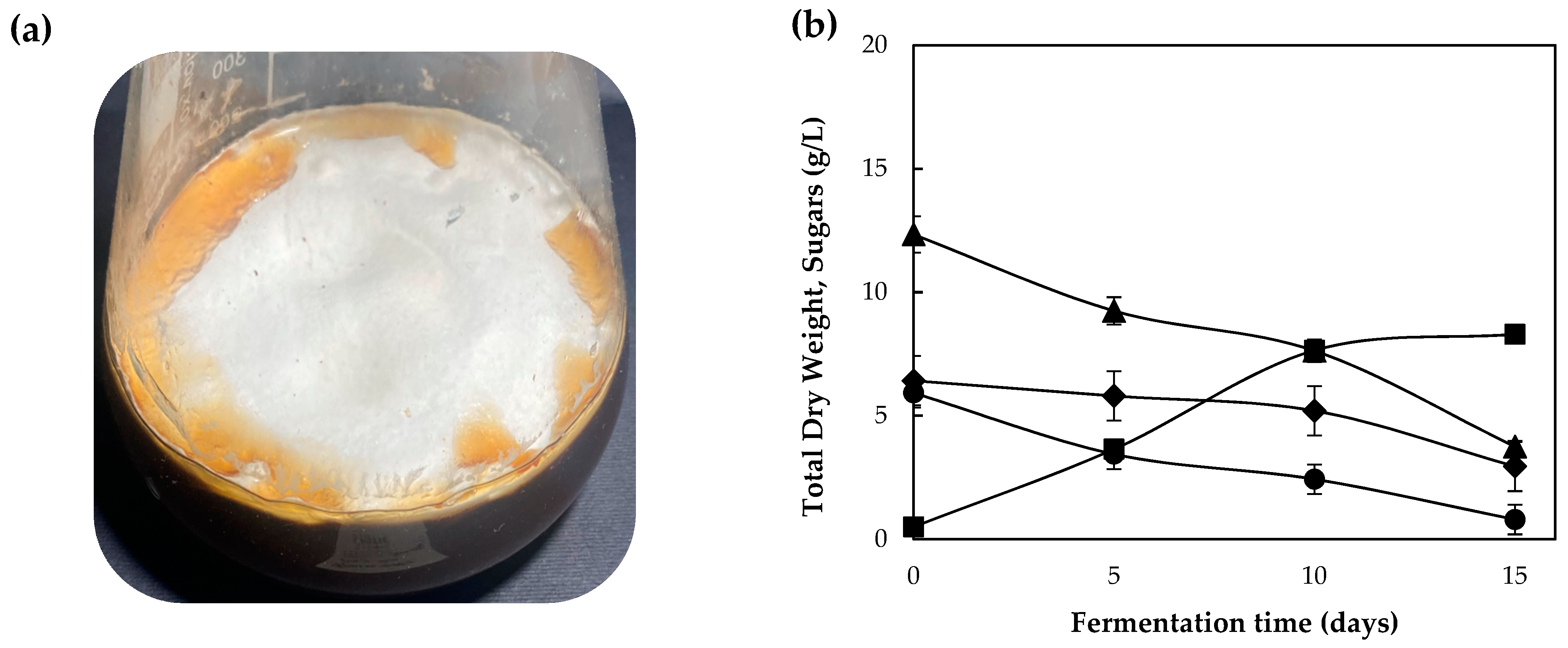
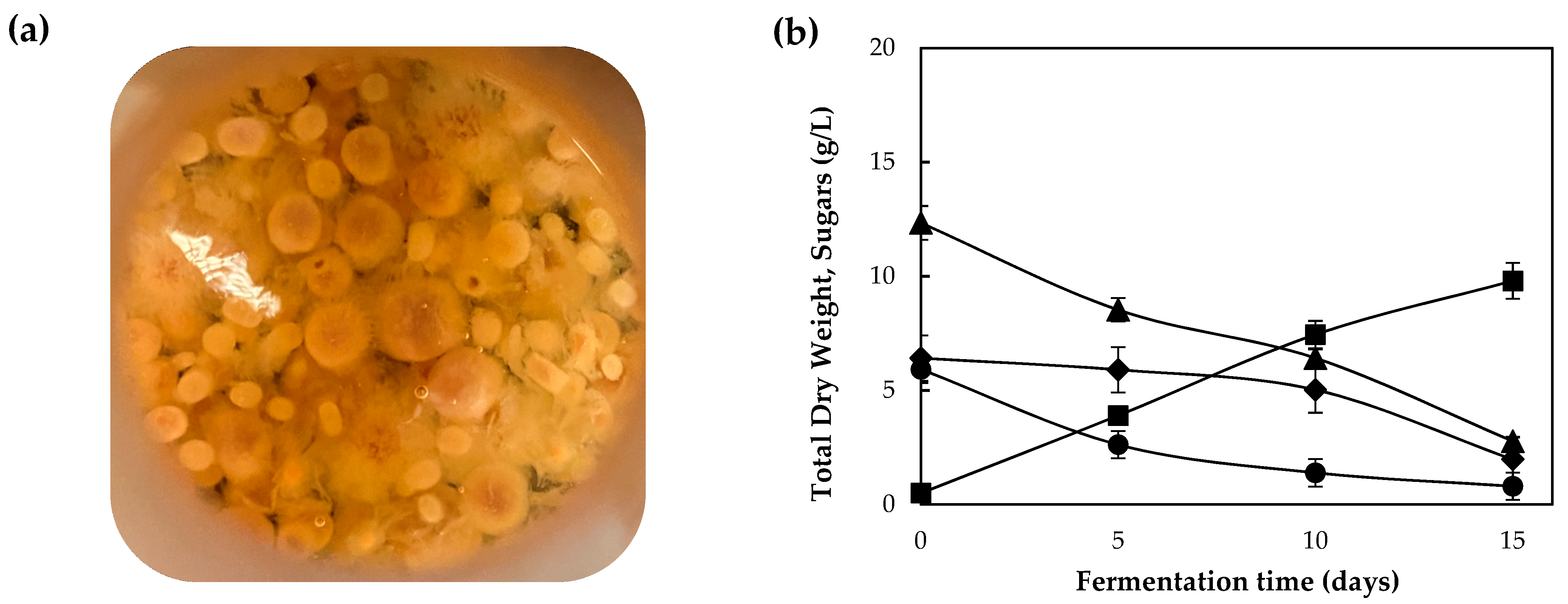
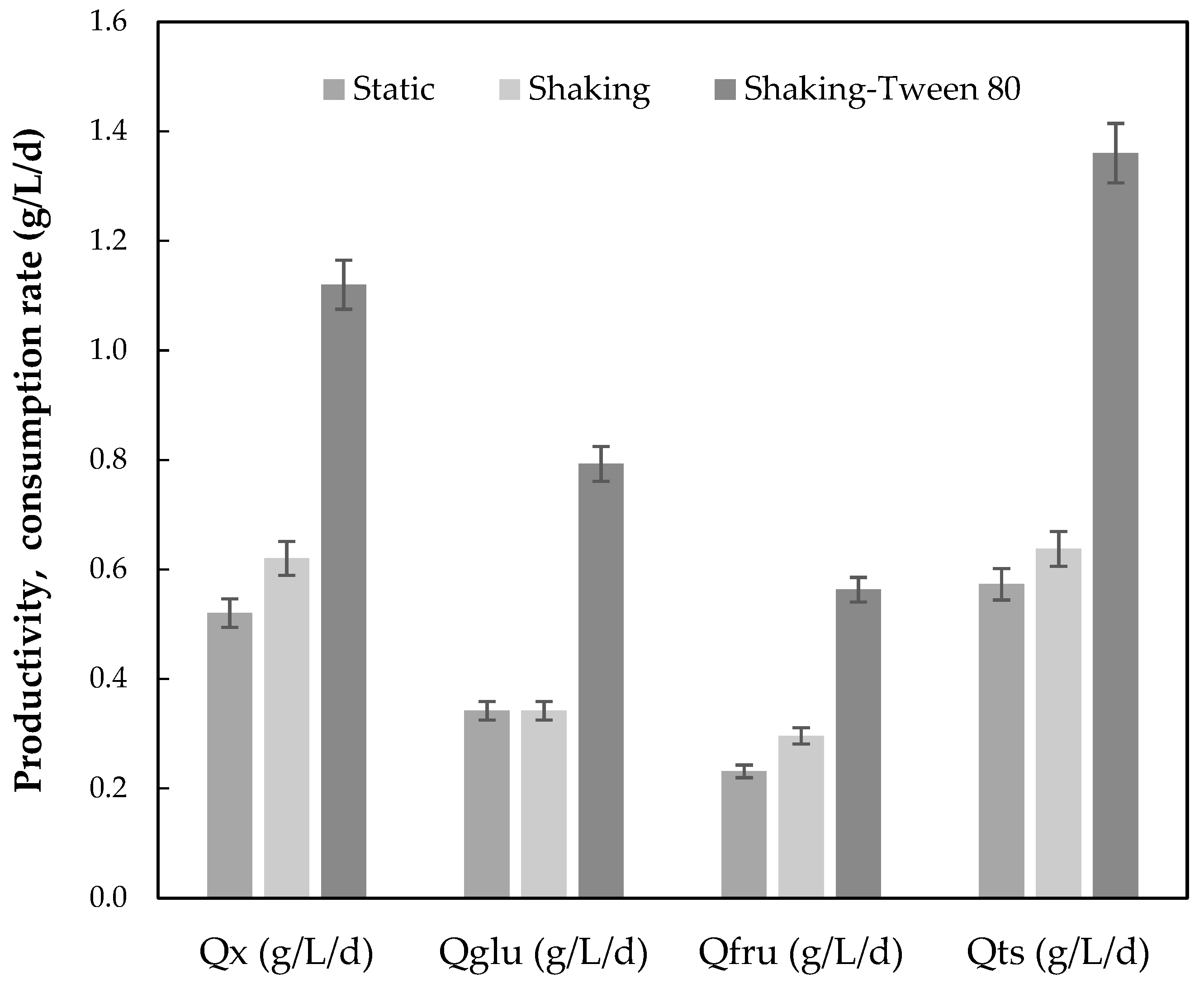
3.1. Growth Performance on Commercial Carbon Sources and Synthetic Media
3.2. Assessment of Grape Pomace Extract (GPE) as Fermentation Substrate
3.3. Evaluation of Crude Intracellular (IPS) and Extracellular (EPS) Polysaccharide Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The Mycelium of the Trametes Versicolor (Turkey Tail) Mushroom and Its Fermented Substrate Each Show Potent and Complementary Immune Activating Properties In Vitro. BMC Complement. Altern. Med. 2019, 19, 342. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal Importance of Mushroom Mycelium: Mechanisms and Applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Habtemariam, S. Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent Trends in Submerged Cultivation of Mushrooms and Their Application as a Source of Nutraceuticals and Food Additives. Futur. Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of Utilizing Mushroom Polysaccharides (MPs) as Potent Nutraceutical Components in Food and Medicine: A Comprehensive Review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Angelova, G.; Brazkova, M.; Mihaylova, D.; Slavov, A.; Petkova, N.; Blazheva, D.; Deseva, I.; Gotova, I.; Dimitrov, Z.; Krastanov, A. Bioactivity of Biomass and Crude Exopolysaccharides Obtained by Controlled Submerged Cultivation of Medicinal Mushroom Trametes Versicolor. J. Fungi 2022, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Rau, U.; Kuenz, A.; Wray, V.; Nimtz, M.; Wrenger, J.; Cicek, H. Production and Structural Analysis of the Polysaccharide Secreted by Trametes (Coriolus) Versicolor ATCC 200801. Appl. Microbiol. Biotechnol. 2009, 81, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y. Polysaccharide-Protein Complexes from Edible Fungi and Applications. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–10. ISBN 978-3-319-03751-6. [Google Scholar]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes Versicolor in Lignocellulose-Based Bioeconomy: State of the Art, Challenges and Opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Gonou-Zagou, Z.; Kopsahelis, N. Trametes Versicolor as a Natural Source of Bioactive Compounds for the Production of Whey Protein Films with Functional Properties: A Holistic Approach to Valorize Cheese Whey. Waste Biomass Valorization 2022, 13, 3989–3998. [Google Scholar] [CrossRef]
- Mikkilä, M.; Utanun, P.; Luhas, J.; Horttanainen, M.; Linnanen, L. Sustainable Circular Bioeconomy—Feasibility of Recycled Nutrients for Biomass Production within a Pulp and Paper Integration in Indonesia, Southeast Asia. Sustainability 2021, 13, 10169. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Papadaki, A.; Alexandri, M.; Poulios, V.; Gonou-Zagou, Z.; Kopsahelis, N. Sepedonium sp. and Phellinus sp. Novel Isolates: Growth Pattern and Production of Polysaccharide-Protein Complexes on Conventional and Grape Pomace Substrates. Waste Biomass Valorization 2023, 14, 3315–3326. [Google Scholar] [CrossRef]
- Dedousi, M.; Fourtaka, K.; Melanouri, E.-M.; Argyropoulos, D.; Psallida, C.; Diamantis, I.; Papanikolaou, S.; Diamantopoulou, P. Detoxification of Molasses and Production of Mycelial Mass and Valuable Metabolites by Morchella Species. Appl. Sci. 2021, 11, 9481. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Alexandri, M.; Nascimento, M.F.; Alimpoumpa, D.; Torres Faria, N.; Papadaki, A.; Castelo Ferreira, F.; Kopsahelis, N. Lactobacilli and Moesziomyces Biosurfactants: Toward a Closed-Loop Approach for the Dairy Industry. Fermentation 2022, 8, 517. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Alimpoumpa, D.; Papadaki, A.; Lappa, I.; Alexopoulos, K.; Kopsahelis, N. Cheese Whey Utilization for Biosurfactant Production: Evaluation of Bioprocessing Strategies Using Novel Lactobacillus Strains. Biomass Convers. Biorefinery 2022, 12, 4621–4635. [Google Scholar] [CrossRef]
- Yang, L.; Kang, X.; Dong, W.; Wang, L.; Liu, S.; Zhong, X.; Liu, D. Prebiotic Properties of Ganoderma Lucidum Polysaccharides with Special Enrichment of Bacteroides Ovatus and B. Uniformis In Vitro. J. Funct. Foods 2022, 92, 105069. [Google Scholar] [CrossRef]
- Lin, S.Y.; Chien, S.C.; Wang, S.Y.; Mau, J.L. Nonvolatile Taste Components and Antioxidant Properties of Fruiting Body and Mycelium with High Ergothioneine Content from the Culinary-Medicinal Golden Oyster Mushroom Pleurotus Citrinopileatus (Agaricomycetes). Int. J. Med. Mushrooms 2016, 18, 689–698. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom Polysaccharides and Lipids Synthesized in Liquid Agitated and Static Cultures. Part I: Screening Various Mushroom Species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Nguyen, B.T.T.; Van Le, V.; Nguyen, H.T.T.; Nguyen, L.T.; Tran, T.T.T.; Ngo, N.X. Nutritional Requirements for the Enhanced Mycelial Growth and Yield Performance of Trametes Versicolor. J. Appl. Biol. Biotechnol. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Jo, W.-S.; Kang, M.-J.; Choi, S.-Y.; Yoo, Y.-B.; Seok, S.-J.; Jung, H.-Y. Culture Conditions for Mycelial Growth of Coriolus Versicolor. Mycobiology 2010, 38, 195–202. [Google Scholar] [CrossRef]
- Tavares, A.P.A.; Agapito, M.S.M.; Coelho, M.A.M.; Lopes Da Silva, J.A.; Barros-Timmons, A.; Coutinho, J.A.J.; Xavier, A.M.R.B. Selection and Optimization of Culture Medium for Exopolysaccharide Production by Coriolus (Trametes) Versicolor. World J. Microbiol. Biotechnol. 2005, 21, 1499–1507. [Google Scholar] [CrossRef]
- Ergül, F.E.; Sargın, S.; Öngen, G.; Sukan, F.V. Dephenolisation of Olive Mill Wastewater Using Adapted Trametes Versicolor. Int. Biodeterior. Biodegrad. 2009, 63, 1–6. [Google Scholar] [CrossRef]
- Lueangjaroenkit, P.; Teerapatsakul, C.; Chitradon, L. Morphological Characteristic Regulation of Ligninolytic Enzyme Produced by Trametes Polyzona. Mycobiology 2018, 46, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus Ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295. [Google Scholar] [CrossRef]
- Lorenzo, M.; Moldes, D.; Rodríguez Couto, S.; Sanromán, A. Improving Laccase Production by Employing Different Lignocellulosic Wastes in Submerged Cultures of Trametes Versicolor. Bioresour. Technol. 2002, 82, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Santos Arteiro, J.M.; Martins, M.R.; Salvador, C.; Candeias, M.F.; Karmali, A.; Caldeira, A.T. Protein-Polysaccharides of Trametes Versicolor: Production and Biological Activities. Med. Chem. Res. 2012, 21, 937–943. [Google Scholar] [CrossRef]
- Kudahettige, R.L.; Holmgren, M.; Imerzeel, P.; Sellstedt, A. Characterization of Bioethanol Production from Hexoses and Xylose by the White Rot Fungus Trametes Versicolor. Bioenergy Res. 2012, 5, 277–285. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.-X.; Wang, Y.; Huang, B. Transcriptomic Profile of Lignocellulose Degradation from Trametes Versicolor on Poplar Wood. BioResources 2017, 12, 2507–2527. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Papadaki, A.; Papapostolou, H.; Alexandri, M.; Gonou-Zagou, Z.; Kopsahelis, N. Ganoderma Lucidum Mycelia Mass and Bioactive Compounds Production through Grape Pomace and Cheese Whey Valorization. Molecules 2023, 28, 6331. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wu, J.-Y. Effects of Tween 80 and PH on Mycelial Pellets and Exopolysaccharide Production in Liquid Culture of a Medicinal Fungus. J. Ind. Microbiol. Biotechnol. 2012, 39, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Zhang, Y.; He, J.; Xie, Y. Enhanced Exopolysaccharide Production in Submerged Fermentation of Ganoderma Lucidum by Tween 80 Supplementation. Bioprocess Biosyst. Eng. 2021, 44, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-F.; Sui, K.; Guo, C.; Liu, C.-Z. Improved Production and Antitumor Activity of Intracellular Protein-Polysaccharide from Trametes Versicolor by the Quorum Sensing Molecule-Tyrosol. J. Funct. Foods 2017, 37, 90–96. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous Fungus Trametes Versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Duvnjak, D.; Pantić, M.; Pavlović, V.; Nedović, V.; Lević, S.; Matijašević, D.; Sknepnek, A.; Nikšić, M. Advances in Batch Culture Fermented Coriolus Versicolor Medicinal Mushroom for the Production of Antibacterial Compounds. Innov. Food Sci. Emerg. Technol. 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P.; Gkatzionis, K.; Ioannou, Z.; Sarris, D. Submerged Cultivation of Selected Macro-Fungi to Produce Mycelia Rich in β-Glucans and Other Bioactive Compounds, Valorizing Side Streams of the Food Industry. Carbon Resour. Convers. 2023. [Google Scholar] [CrossRef]
- Cui, J.; Goh, K.K.T.; Archer, R.; Singh, H. Characterisation and Bioactivity of Protein-Bound Polysaccharides from Submerged-Culture Fermentation of Coriolus Versicolor Wr-74 and ATCC-20545 Strains. J. Ind. Microbiol. Biotechnol. 2007, 34, 393–402. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and Pitfalls in Measuring Antioxidant Efficacy: A Critical Evaluation of ABTS, DPPH, and ORAC Assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Knežević, A.; Stajić, M.; Sofrenić, I.; Stanojković, T.; Milovanović, I.; Tešević, V.; Vukojević, J. Antioxidative, Antifungal, Cytotoxic and Antineurodegenerative Activity of Selected Trametes Species from Serbia. PLoS ONE 2018, 13, e0203064. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative Activities and Chemical Characterization of Polysaccharide Extracts from the Widely Used Mushrooms Ganoderma Applanatum, Ganoderma Lucidum, Lentinus Edodes and Trametes Versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, S.; Li, M.; Ma, Y.; Zheng, Y.; Zhang, D.; Wu, L. Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor. Foods 2022, 11, 2126. [Google Scholar] [CrossRef]
- Miletić, D.; Turło, J.; Podsadni, P.; Sknepnek, A.; Szczepańska, A.; Lević, S.; Nedović, V.; Nikšić, M. Turkey Tail Medicinal Mushroom, Trametes Versicolor (Agaricomycetes), Crude Exopolysaccharides with Antioxidative Activity. Int. J. Med. Mushrooms 2020, 22, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Li, Z.; Wu, W.; Tang, X. Analysis and Evaluation of the Characteristic Taste Components in Portobello Mushroom. J. Food Sci. 2018, 83, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T.; Ikemoto, N.; Terashima, M. Designing Antioxidant Peptides Based on the Antioxidant Properties of the Amino Acid Side-Chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef]
- Cerig, S. A Safety Assessment of Hot Aqueous Mycelium Extracts from Trametes Versicolor and Lepista Nuda as a Food Supplement. Biologia 2021, 76, 2381–2391. [Google Scholar] [CrossRef]
- Montoya, S.; Sánchez, Ó.J.; Levin, L. Polysaccharide Production by Submerged and Solid-State Cultures from Several Medicinal Higher Basidiomycetes. Int. J. Med. Mushrooms 2013, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cai, M.; Hu, H.; Jiao, C.; Zhang, Z.; Liu, Y.; Chen, J.; Xiao, C.; Li, X.; Gao, X.; et al. Whole-Genome Sequencing and Transcriptome Analysis of Ganoderma Lucidum Strain Yw-1-5 Provides New Insights into the Enhanced Effect of Tween80 on Exopolysaccharide Production. J. Fungi 2022, 8, 1081. [Google Scholar] [CrossRef]
- Srour, B.; Bruechert, S.; Andrade, S.L.A.; Hellwig, P. Secondary Structure Determination by Means of ATR-FTIR Spectroscopy BT. In Membrane Protein Structure and Function Characterization: Methods and Protocols; Lacapere, J.-J., Ed.; Springer: New York, NY, USA, 2017; pp. 195–203. ISBN 978-1-4939-7151-0. [Google Scholar]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Biswas, J.; Ganguly, J.; Paul, A.K. Partial Characterization of an Extracellular Polysaccharide Produced by the Moderately Halophilic Bacterium Halomonas Xianhensis SUR308. Biofouling 2015, 31, 735–744. [Google Scholar] [CrossRef]
- Fraga, I.; Coutinho, J.; Bezerra, R.M.; Dias, A.A.; Marques, G.; Nunes, F.M. Influence of Culture Medium Growth Variables on Ganoderma Lucidum Exopolysaccharides Structural Features. Carbohydr. Polym. 2014, 111, 936–946. [Google Scholar] [CrossRef]



| SmF Conditions | IPS Production (g/L) | IPS Content (mg/g DW *) | IPS Productivity (g/L/day) | Protein (%) | Polysaccharides (%) |
|---|---|---|---|---|---|
| Mixed sugars | |||||
| static | 1.13 ± 0.05 | 132.97 ± 0.14 | 0.11 ± 0.01 | 10.48 ± 0.01 | 14.07 ± 0.01 |
| shaking | 0.83 ± 0.04 | 107.50 ± 0.32 | 0.17 ± 0.01 | 8.96 ± 0.03 | 6.61 ± 0.02 |
| GPE | |||||
| static | 2.59 ± 0.11 | 268.21 ± 0.41 | 0.13 ± 0.01 | 13.61 ± 0.54 | 18.52 ± 0.71 |
| shaking | 2.63 ± 0.08 | 257.81 ± 0.38 | 0.17 ± 0.01 | 14.43 ± 0.52 | 15.93 ± 0.48 |
| shaking–Tween 80 | 2.96 ± 0.13 | 139.40 ± 0.51 | 0.19 ± 0.01 | 7.54 ± 0.34 | 29.10 ± 0.37 |
| SmF Conditions | IDPPH (%) | μg Trolox/mg Extract | IABTS (%) | μg Trolox/mg Extract |
|---|---|---|---|---|
| Synthetic media | ||||
| mixed–static | 18.76 ± 0.74 | 4.81 ± 0.74 | 81.35 ± 1.92 | 3.96 ± 0.18 |
| mixed–shaking | 8.44 ± 0.30 | 2.82 ± 0.30 | 79.73 ± 4.27 | 3.35 ± 0.41 |
| GPE | ||||
| static | 57.56 ± 0.42 | 11.24 ± 0.34 | 91.67 ± 0.12 | 4.68 ± 0.18 |
| shaking | 42.75 ± 0.21 | 8.71 ± 0.25 | 84.14 ± 0.19 | 4.17 ± 0.21 |
| shaking–Tween 80 | 35.27 ± 0.36 | 5.44 ± 0.29 | 90.05 ± 0.09 | 4.06 ± 0.10 |
| Amino Acid Content (mg/g) | Mixed Sugars Static | Mixed Sugars Shaking | GPE Static | GPE Shaking | GPE-Tween 80 Shaking |
|---|---|---|---|---|---|
| L-aspartic acid | 1.73 | 2.76 | 0.65 | 0.83 | 0.78 |
| L-glutamic acid | 5.24 | 4.11 | 3.12 | 2.06 | 1.49 |
| L-serine | 3.83 | 1.90 | 0.47 | 0.44 | n.d |
| Glutamine | 2.58 | 2.58 | 4.19 | 0.89 | n.d |
| L-histidine | 1.14 | 2.03 | 0.59 | 0.22 | 0.76 |
| Glycine | 1.78 | 1.32 | 0.45 | 0.29 | 0.35 |
| L-threonine | 2.63 | 2.65 | 0.55 | 0.34 | 0.61 |
| L-arginine | 7.11 | 6.22 | 2.67 | 4.69 | 1.48 |
| L-alanine | 2.53 | 1.99 | 1.12 | 0.44 | n.d |
| L-tyrosine | 1.27 | 1.81 | 0.42 | 0.50 | n.d |
| L-cystine | 1.82 | n.d | 0.32 | n.d. | n.d |
| L-valine | n.d | n.d | 0.42 | 0.64 | n.d |
| L-methionine | 0.47 | n.d | n.d | n.d | n.d |
| L-tryptophan | n.d | n.d | 0.11 | 0.13 | n.d |
| L-phenylalanine | n.d | 1.83 | 0.19 | 0.35 | n.d |
| L-isoleucine | n.d | 1.07 | 0.20 | 0.39 | 0.89 |
| L-leucine | 2.87 | 2.01 | n.d. | 0.51 | 0.82 |
| L-lysine | 4.32 | 2.13 | 0.52 | 0.46 | n.d |
| L-proline | 4.79 | 1.36 | n.d. | 0.58 | n.d |
| GPE | EPS (g/L) | IDPPH (%) | μg Trolox/mg Extract | Protein (%) | Polysaccharides (%) |
|---|---|---|---|---|---|
| Static | 5.35 ± 0.28 | 15.12 ± 0.32 | 2.89 ± 0.10 | 8.14 ± 0.41 | 7.78 ± 0.23 |
| Shaking | 5.32 ± 0.32 | 10.91 ± 0.13 | 2.51 ± 0.09 | 7.05 ± 0.28 | 12.12 ± 0.16 |
| Shaking–Tween 80 | 12.54 ± 0.09 | 7.64 ± 0.11 | 1.84 ± 0.03 | 8.12 ± 0.03 | 21.05 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachrimanidou, V.; Alexandri, M.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Valorization of Grape Pomace for Trametes versicolor Mycelial Mass and Polysaccharides Production. Sustainability 2023, 15, 15080. https://doi.org/10.3390/su152015080
Kachrimanidou V, Alexandri M, Papapostolou H, Papadaki A, Kopsahelis N. Valorization of Grape Pomace for Trametes versicolor Mycelial Mass and Polysaccharides Production. Sustainability. 2023; 15(20):15080. https://doi.org/10.3390/su152015080
Chicago/Turabian StyleKachrimanidou, Vasiliki, Maria Alexandri, Harris Papapostolou, Aikaterini Papadaki, and Nikolaos Kopsahelis. 2023. "Valorization of Grape Pomace for Trametes versicolor Mycelial Mass and Polysaccharides Production" Sustainability 15, no. 20: 15080. https://doi.org/10.3390/su152015080





