Effect of Magnetized Water-Based Alkaline Activator on Geopolymer Concrete Mechanical Performance and Durability
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
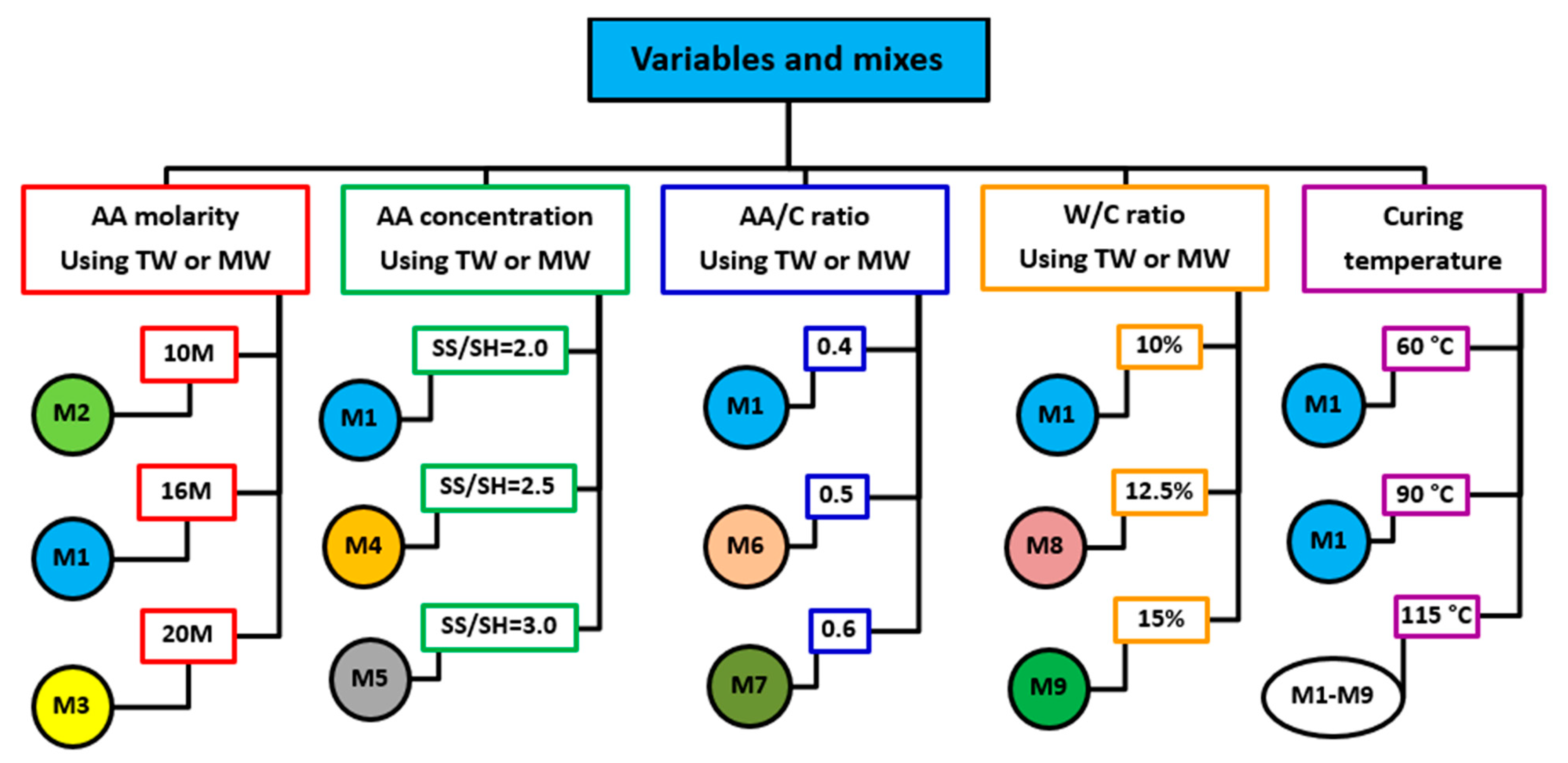
2.2. Variables and Mixes
2.3. Specimens Preparation and Testing
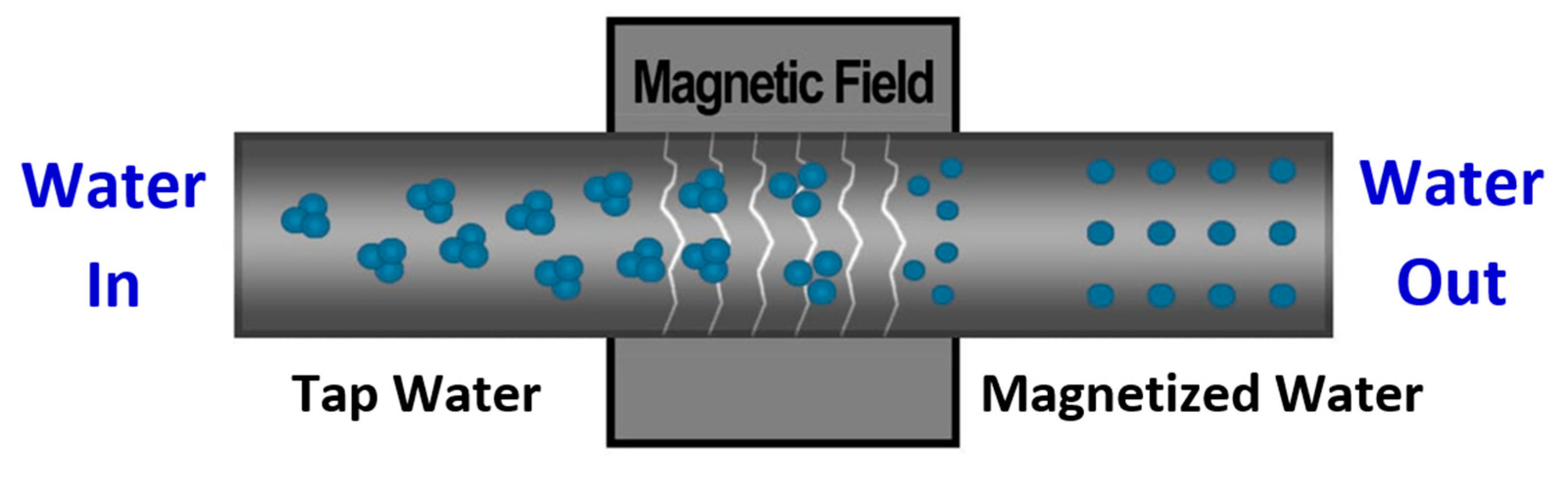
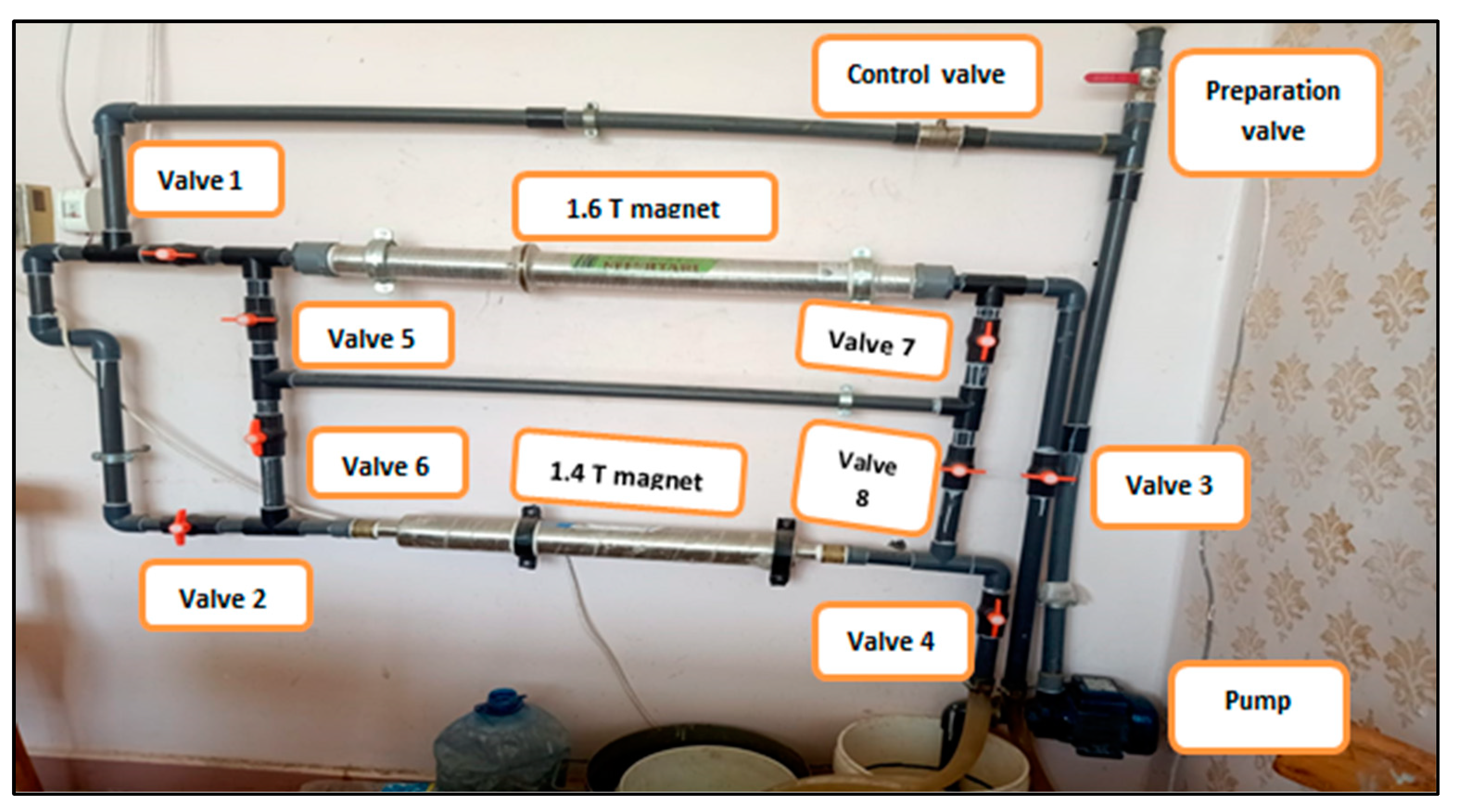
2.3.1. Water and Alkaline Activator
2.3.2. Fresh, Hardened, and Durability Properties
2.3.3. Water Permeability
2.3.4. Microstructural Analysis
3. Results and Discussion
3.1. Water and Alkaline Activator
3.2. Fresh and Hardened Mechanical and Durability Properties
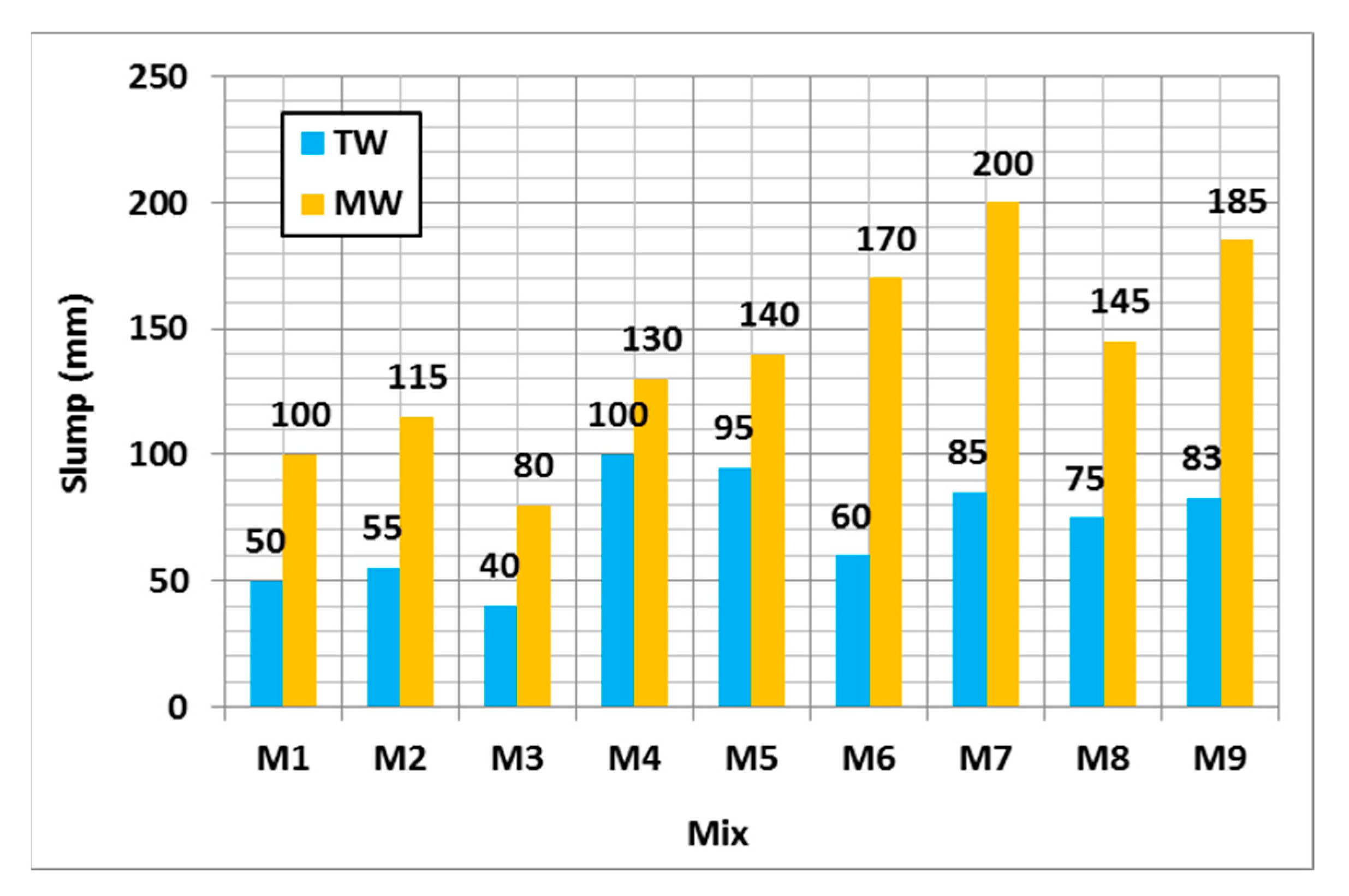
3.2.1. Workability
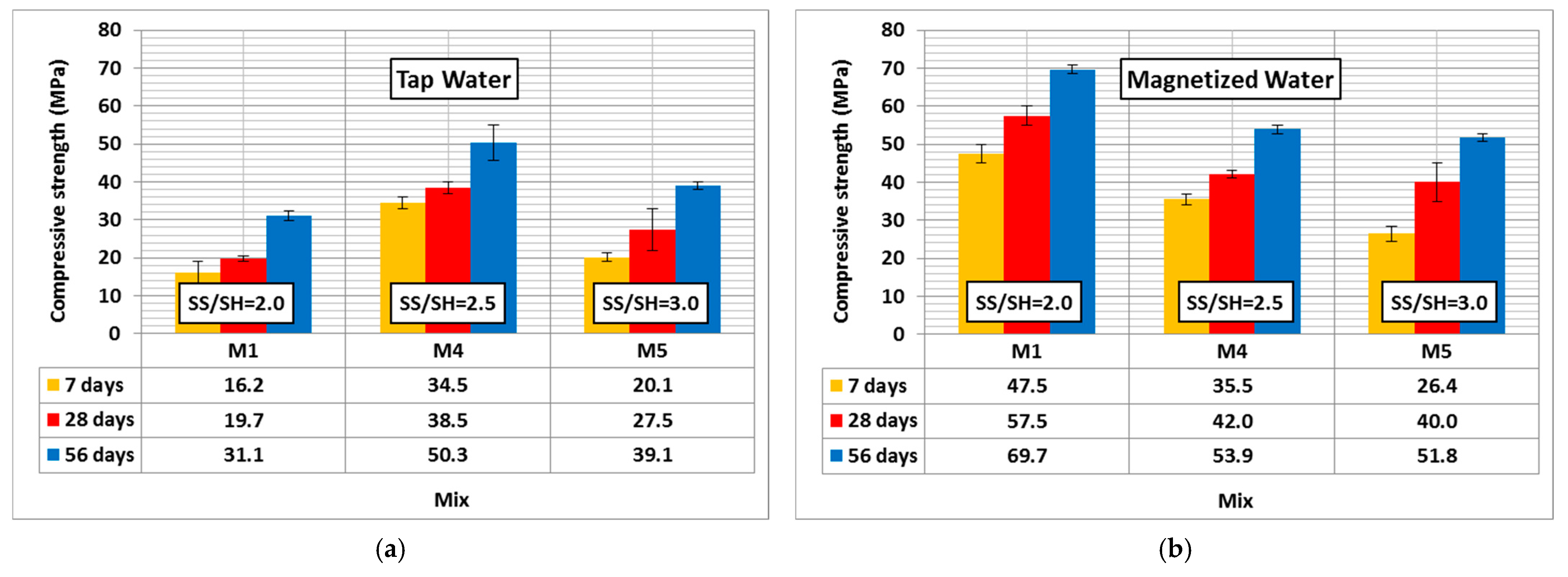
3.2.2. Compressive Strength
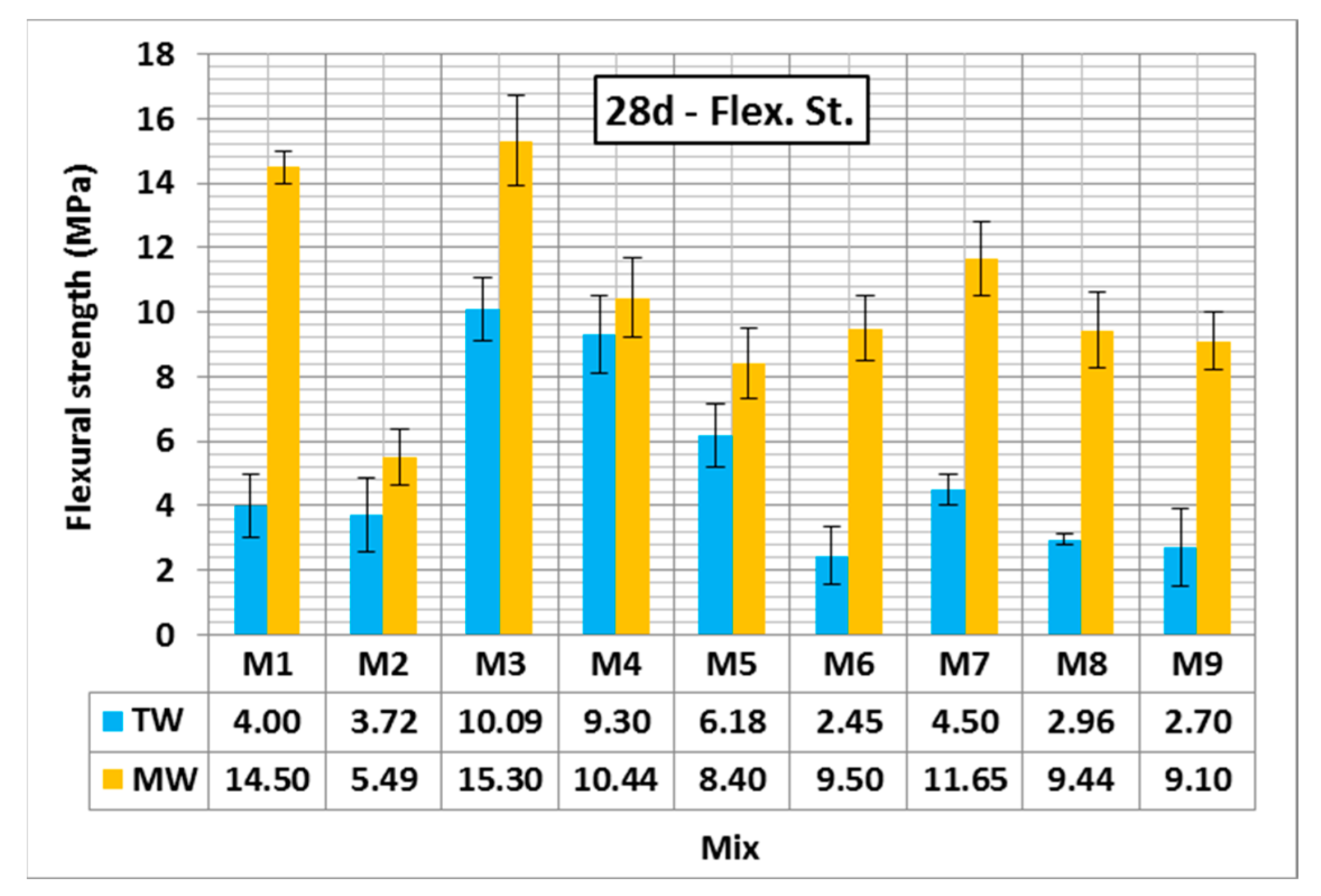
3.2.3. Flexural Strength
3.2.4. Splitting Tensile Strength
3.3. Water Permeability
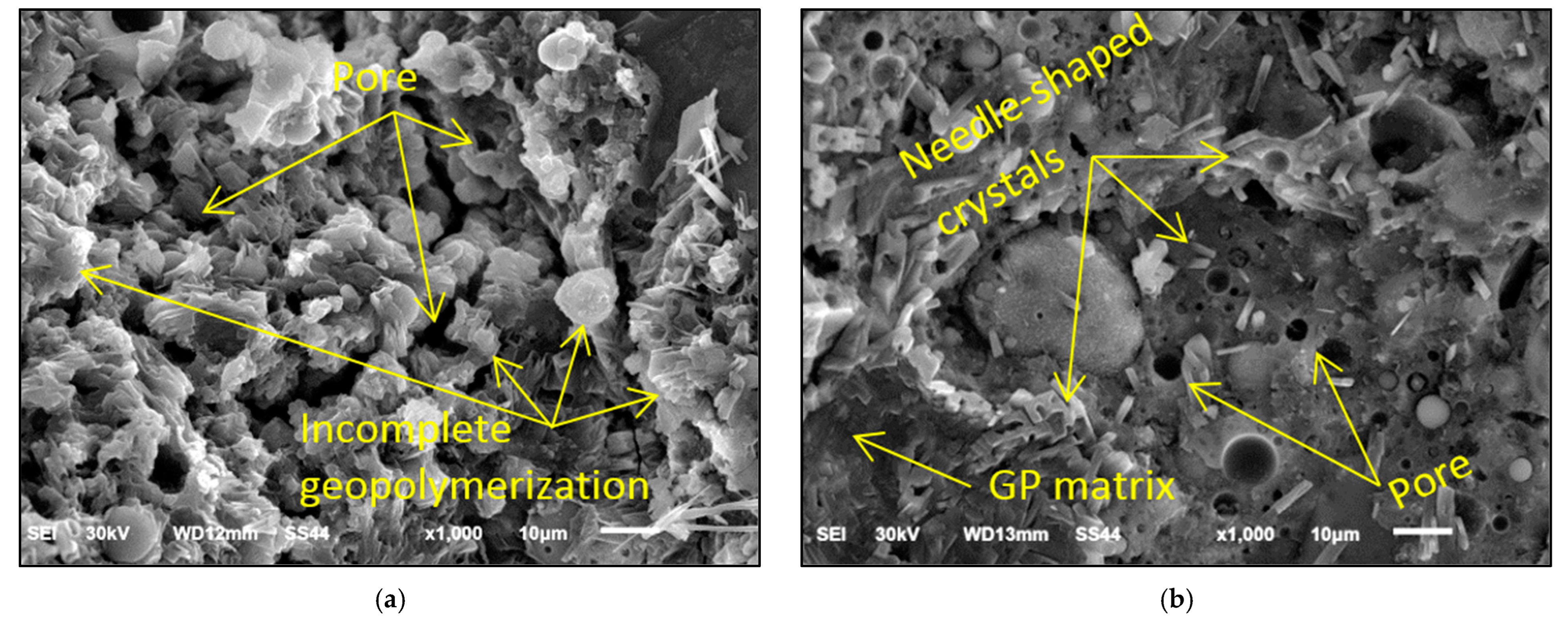
3.4. Microstructural Analysis
4. Conclusions
- The outcomes of hardened concrete tests demonstrated that using an SH molarity of 16 M, an SS/SH ratio of 2, an AA/C ratio of 0.4, a W/C ratio of 10%, and a curing temperature of 115 °C could display the best outcomes in this study when used in geopolymer concrete made from MW, regardless of the concrete age.
- MW could increase the slump of geopolymer concrete by up to 100% compared to TW. The geopolymer concrete compressive strength was enhanced by up to 193%, 192%, and 124% after 7, 28, and 56 days using MW, respectively. The compressive strength was slightly enhanced by up to 5% when the SH molarity increased up to 20 M compared to the control mixture. MW could reduce the water permeability of geopolymer concrete by up to 13%.
- The microstructural analysis showed that the MW was able to enhance the surface morphology of geopolymer concrete. The geopolymer concrete mixtures made with MW showed a better geopolymerization reaction, and the geopolymer matrix was denser with fewer voids, which explains the improved compressive strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aïtcin, P.-C.; Mindess, S. Sustainability of Concrete; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Youssf, O.; Swilam, A.; Tahwia, A.M. Performance of Crumb Rubber Concrete Made with High Contents of Heat Pre-treated Rubber and Magnetized Water. J. Mater. Res. Technol. 2023, 23, 2160–2176. [Google Scholar] [CrossRef]
- Keshta, M.M.; Elshikh, M.M.Y.; Abd Elrahman, M.; Youssf, O. Utilizing of Magnetized Water in Enhancing of Volcanic Concrete Characteristics. J. Compos. Sci. 2022, 6, 320. [Google Scholar] [CrossRef]
- Elkerany, A.M.; Keshta, M.M.; Elshikh, M.M.Y.; Elshami, A.A.; Youssf, O. Characteristics of Sustainable Concrete Containing Metakaolin and Magnetized Water. Buildings 2023, 13, 1430. [Google Scholar] [CrossRef]
- Barham, W.S.; Albiss, B.; Latayfeh, O. Influence of magnetic field treated water on the compressive strength and bond strength of concrete containing silica fume. J. Build. Eng. 2021, 33, 101544. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Z.B. The New Technology of Concrete Engineering; The Publishing House of Chinese Architectural: Beijing, China, 1994. [Google Scholar]
- Su, N.; Lee, K.C. Effect of magnetic water on mechanical properties and micro-structures of concrete. J. Chin. Inst. Civil Hydraulic Eng. 1999, 11, 30–38. [Google Scholar]
- Rashad, A.M.; Ezzat, M. A Preliminary study on the use of magnetic, Zamzam, and sea water as mixing water for alkali-activated slag pastes. Constr. Build. Mater. 2019, 207, 672–678. [Google Scholar] [CrossRef]
- Su, N.; Wu, C.-F. Effect of magnetic field treated water on mortar and concrete containing fly ash. Cem. Concr. Compos. 2003, 25, 681–688. [Google Scholar] [CrossRef]
- Su, N.; Wu, Y.-H.; Mar, C.-Y. Effect of magnetic water on the engineering properties of concrete containing granulated blast-furnace slag. Cem. Concr. Res. 2000, 30, 599–605. [Google Scholar] [CrossRef]
- Weilin, S.; Yun, L.; Hanzhao, H.; Quingwang, L. Effects of magnetic treatment on properties of cement slurry. Soc. Pet. Eng. AIME (Pap.) SPE 1992, 8, 122–130. [Google Scholar]
- Ahmed, S.M. Effect of magnetic water on engineering properties of concrete. Al-Rafidain Eng. 2009, 17, 71–82. [Google Scholar]
- Afshin, H.; Gholizadeh, M.; Khorshidi, N. Improving mechanical properties of high strength concrete by magnetic water technology. Sci. Iran. 2010, 17, 74–79. [Google Scholar]
- Abdel-Magid, T.I.M.; Hamdan, R.M.; Abdelgader, A.A.B.; Omer, M.E.A. Effect of magnetized water on workability and compressive strength of concrete. Procedia Eng. 2017, 193, 494–500. [Google Scholar] [CrossRef]
- Gholhaki, M.; Hajforoush, M.; Kazemi, M. An investigation on the fresh and hardened properties of self-compacting concrete incorporating magnetic water with various pozzolanic materials. Constr. Build. Mater. 2018, 158, 173–180. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Imtiaz, L.; Kashif-ur-Rehman, S.; Alaloul, W.S.; Nazir, K.; Javed, M.F.; Aslam, F.; Musarat, M.A. Life cycle impact assessment of recycled aggregate concrete, geopolymer concrete, and recycled aggregate-based geopolymer concrete. Sustainability 2021, 13, 13515. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Hu, S.; Wang, J.; Wang, T. High-temperature behavior of polyvinyl alcohol fiber-reinforced metakaolin/fly ash-based geopolymer mortar. Compos. Part B Eng. 2022, 244, 110171. [Google Scholar] [CrossRef]
- Malhotra, V.M. CANMET Investigations Dealing with High-Volume Fly Ash Concrete, Advances in Concrete Technology; CANMET, MSL 62-6(R); Canada Communication Group—Publishing: Ottawa, ON, Canada, 1992; pp. 433–470. [Google Scholar]
- Cao, V.D.; Pilehvar, S.; Salas-Bringas, C.; Szczotok, A.M.; Rodriguez, J.F.; Carmona, M.; Al-Manasir, N.; Kjøniksen, A.-L. Microencapsulated phase change materials for enhancing the thermal performance of Portland cement concrete and geopolymer concrete for passive building applications. Energy Convers. Manag. 2017, 133, 56–66. [Google Scholar] [CrossRef]
- Şevin, E.; Mermerdaş, K.; Alğın, Z.; Işıker, Y. Response surface optimization of geopolymer mix parameters in terms of key engineering properties. Rev. Construcción J. Constr. 2022, 21, 631–644. [Google Scholar] [CrossRef]
- Köksal, F.; Kaya, M. Physical and mechanical properties of C class fly ash based lightweight geopolymer mortar produced with expanded vermiculite aggregate. Revista De La Construcción. J. Constr. 2022, 21, 21–35. [Google Scholar] [CrossRef]
- Sevim, O.; Alakara, E.H.; Demir, I.; Bayer, I.R. Effect of magnetic water on properties of slag-based geopolymer composites incorporating ceramic tile waste from construction and demolition waste. Arch. Civ. Mech. Eng. 2023, 23, 107. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: Conshohocken, PA, USA, 2019.
- Committee, E.-P. ECP-203: 2007-Egyptian Code for Design and Construction of Concrete Structures; HBRC: Giza, Egypt, 2007. [Google Scholar]
- Ahmed, A.S.; Elshikh, M.M.Y.; Elemam, W.E.; Youssf, O. Influence of Mixing-Water Magnetization Method on the Performance of Silica Fume Concrete. Buildings 2022, 13, 44. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J. Am. Concr. Inst. 2001, 98, 3–9. [Google Scholar]
- ASTM, D 1125-14; Standard Test Methods for Electrical Conductivity and Resistivity of Water. ASTM International: Conshohocken, PA, USA, 2014.
- ASTM, D 1293-12; Standard Test Methods for pH of Water. ASTM International: Conshohocken, PA, USA, 2012.
- ASTM, D 5907-10; Standard Test Methods for Filterable Matter (Total Dissolved Solids) and Nonfilterable Matter (Total Suspended Solids) in Water. ASTM International: Conshohocken, PA, USA, 2010.
- ASTM, D 1590; Standard Test Method for Surface Tension of Water. ASTM International: Conshohocken, PA, USA, 2010.
- A. C143; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: Conshohocken, PA, USA, 2015.
- Aravinna, A.P.; Officer, S.Q. Estimation on Measurement Uncertainty in Determination of Compressive Strength of Hardened Concrete (Test Method: BSEN 12390-3: 2009); Technical Report; CECB Laboratory Services, CECB: Colombo, Sri Lanka, 2018. [Google Scholar]
- Quinn, G.D.; Morrell, R. Design data for engineering ceramics: A review of the flexure test. J. Am. Ceram. Soc. 1991, 74, 2037–2066. [Google Scholar] [CrossRef]
- Raphael, J.M. Tensile strength of concrete. J. Proc. 1984, 81, 158–165. [Google Scholar]
- EN 12390-8; Testing Hardened Concrete—Part 8: Depth of Penetration of Water under Pressure. European Standard: Brussels, Belgium, 2009.
- Karkush, M.O.; Ahmed, M.D.; Al-Ani, S. Magnetic field influence on the properties of water treated by reverse osmosis. Eng. Technol. Appl. Sci. Res. 2019, 9, 4433–4439. [Google Scholar] [CrossRef]
- Indrasari, W.; Budi, E.; Alayya, S.R.; Ramli, R. Measurement of water polluted quality based on turbidity, pH, magnetic property, and dissolved solid. J. Phys. Conf. Ser. 2019, 1317, 012060. [Google Scholar] [CrossRef]
- Niu, L. Experimental Research on the Influence of Magnetized Water on the Compressive Strength of Concrete. Int. Core J. Eng. 2022, 8, 176–180. [Google Scholar]
- Atiş, C.D.; Karahan, O. Properties of steel fiber reinforced fly ash concrete. Constr. Build. Mater. 2009, 23, 392–399. [Google Scholar] [CrossRef]
- Memon, F.A.; Nuruddin, M.F.; Khan, S.; Shafiq, N.; Ayub, T. Effect of sodium hydroxide concentration on fresh properties and compressive strength of self-compacting geopolymer concrete. J. Eng. Sci. Technol 2013, 8, 44–56. [Google Scholar]
- Sathonsaowaphak, A.; Chindaprasirt, P.; Pimraksa, K. Workability and strength of lignite bottom ash geopolymer mortar. J. Hazard. Mater. 2009, 168, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Al Bakri, A.M.; Kamarudin, H.; Bnhussain, M.; Rafiza, A.; Zarina, Y. Effect of Na2SiO3/NaOH Ratios and NaOH Molarities on Compressive Strength of Fly-Ash-Based Geopolymer. ACI Mater. J. 2012, 109, 503. [Google Scholar]
- Feng, Y.; Zhang, Q.; Chen, Q.; Wang, D.; Guo, H.; Liu, L.; Yang, Q. Hydration and strength development in blended cement with ultrafine granulated copper slag. PLoS ONE 2019, 14, e0215677. [Google Scholar] [CrossRef]
- BS EN 12390-8 (2012); Testing Hardened Concrete. Depth of Penetration of Water under Pressure. BSI: London, UK, 2009.













| Oxide | CaO | MgO | LOI | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) by mass | 52.42 | 29.59 | 4.99 | 1.19 | 0.52 | 0.5 | 1.09 | 2.42 | <1.5 | 2–3 | <2 | 100 |
| Mix No. | Mix ID | CA | S | FA | SH * | SS | Water (TW or MW) ** |
|---|---|---|---|---|---|---|---|
| M1 | 16 M | 1170 | 630 | 428 | 57 | 114 | 42.8 |
| M2 | 10 M | 1170 | 630 | 428 | 57 | 114 | 42.8 |
| M3 | 20 M | 1170 | 630 | 428 | 57 | 114 | 42.8 |
| M4 | SS/SH 2.5 | 1170 | 630 | 428 | 50 | 125 | 42.8 |
| M5 | SS/SH 3.0 | 1170 | 630 | 428 | 44 | 132 | 42.8 |
| M6 | AA/C 0.5 | 1233 | 667 | 342 | 57 | 114 | 42.8 |
| M7 | AA/C 0.6 | 1276 | 690 | 285 | 57 | 114 | 42.8 |
| M8 | W/C 12.5 | 1150 | 622 | 428 | 57 | 114 | 53.5 |
| M9 | W/C 15 | 1134 | 613 | 428 | 57 | 114 | 64.2 |
| Property | Water | Activator | ||||
|---|---|---|---|---|---|---|
| TW | MW | Change (%) | TW | MW | Change (%) | |
| T (°C) | 25.4 | 29.2 | 15% | 24.4 | 26.9 | 10% |
| pH | 7.2 | 8.2 | 14% | 11.6 | 12.5 | 8% |
| TDS (ppm) | 195 | 222 | 14% | 211 | 195 | −7% |
| EC (µs) | 394 | 419 | 6% | 519 | 367 | −29% |
| ST (mN/m) | 70.7 | 68.1 | −4% | 58.9 | 48.6 | −17% |
| D (gm/cm3) | 0.977 | 0.970 | ≈0% | 1.016 | 1.014 | ≈0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattab, S.A.; Elshikh, M.M.Y.; Elemam, W.E.; Elshami, A.A.; Youssf, O. Effect of Magnetized Water-Based Alkaline Activator on Geopolymer Concrete Mechanical Performance and Durability. Sustainability 2023, 15, 16315. https://doi.org/10.3390/su152316315
Khattab SA, Elshikh MMY, Elemam WE, Elshami AA, Youssf O. Effect of Magnetized Water-Based Alkaline Activator on Geopolymer Concrete Mechanical Performance and Durability. Sustainability. 2023; 15(23):16315. https://doi.org/10.3390/su152316315
Chicago/Turabian StyleKhattab, Sarah A., Mohamed M. Yousry Elshikh, Walid E. Elemam, Ahmed A. Elshami, and Osama Youssf. 2023. "Effect of Magnetized Water-Based Alkaline Activator on Geopolymer Concrete Mechanical Performance and Durability" Sustainability 15, no. 23: 16315. https://doi.org/10.3390/su152316315






