Monitoring of Phosphorus Compounds in the Influence Zone Affected by Nuclear Power Plant Water Discharge in the Styr River (Western Ukraine): Case Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Estimation of Phosphorus Input from Discharge
3.2. Results of Chemical Control of Phosphorus Compounds
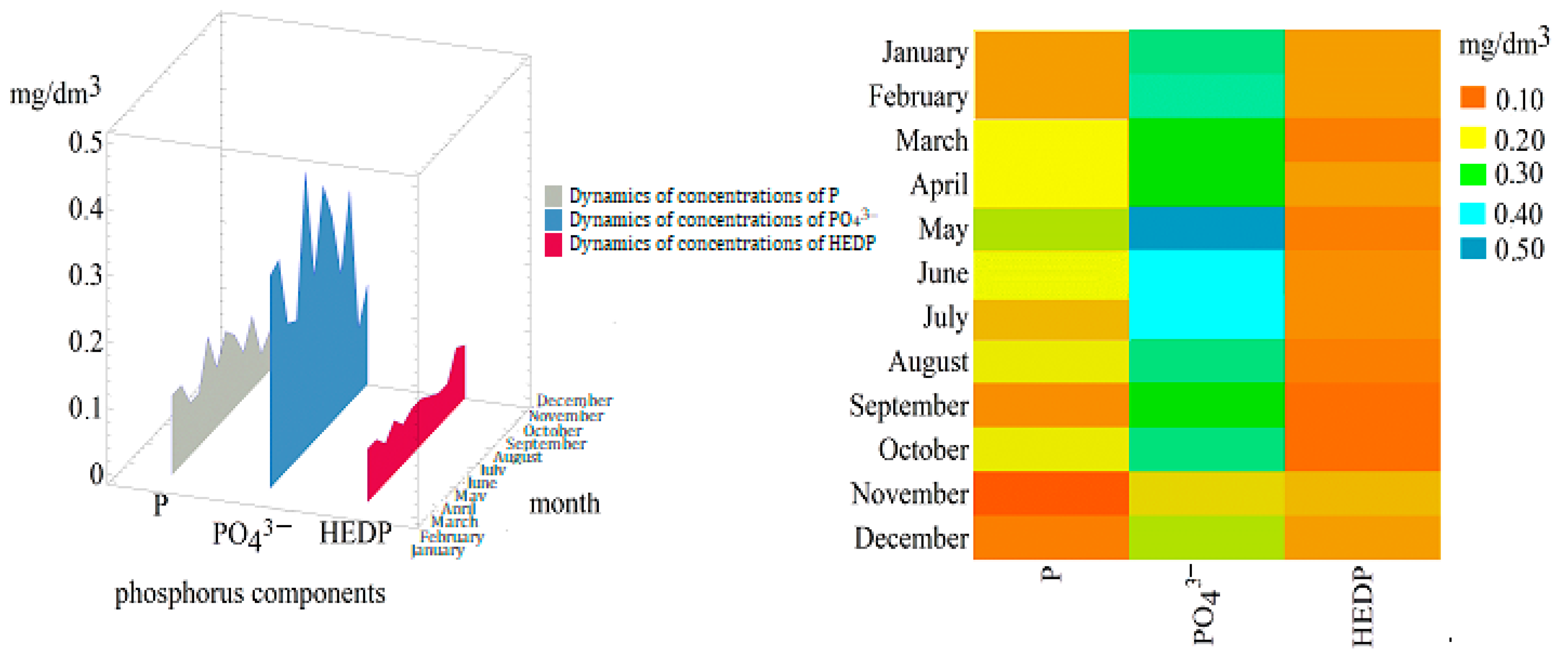
3.3. Form Distribution and Seasonal Variability of Phosphorus Content
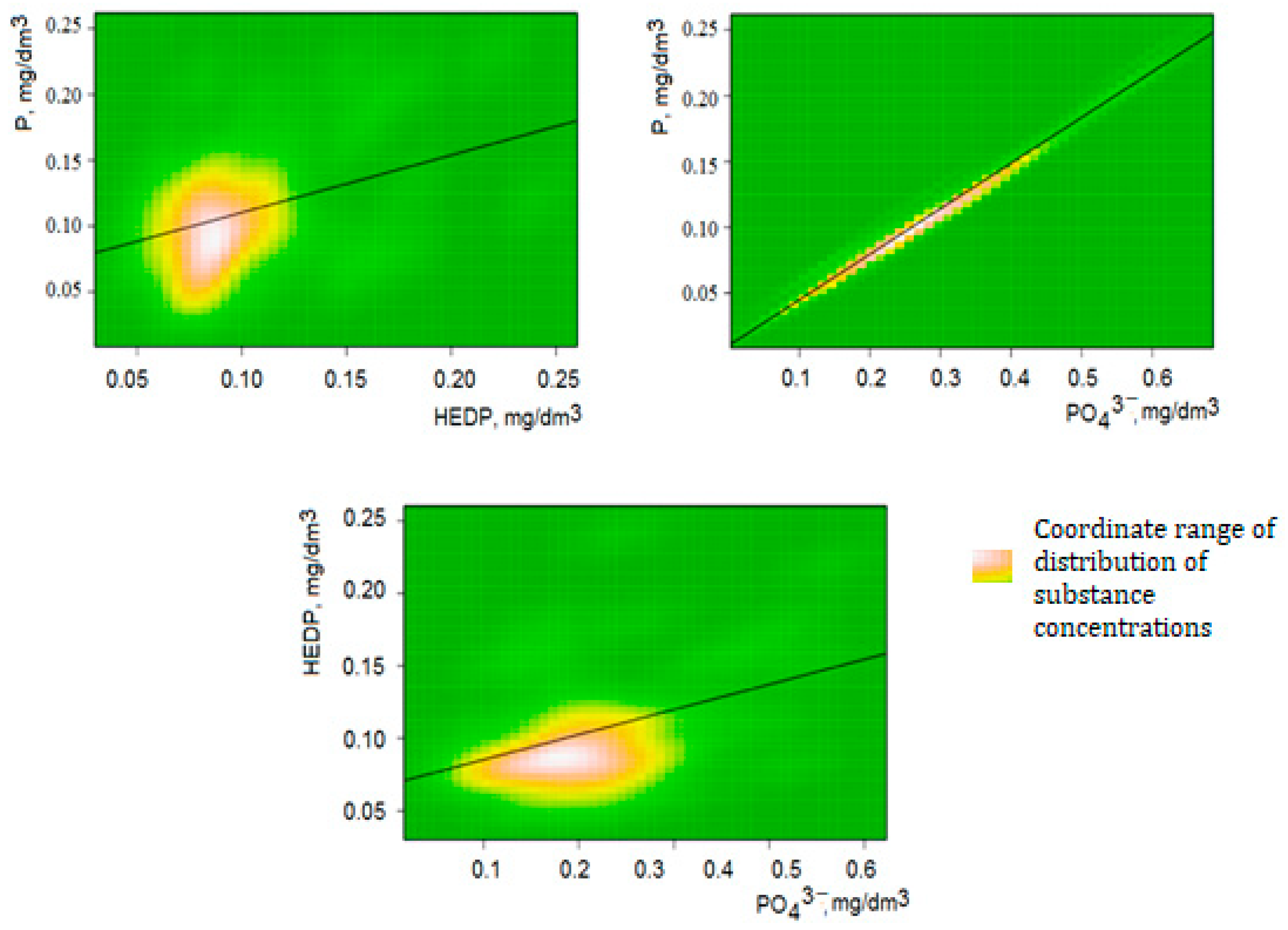
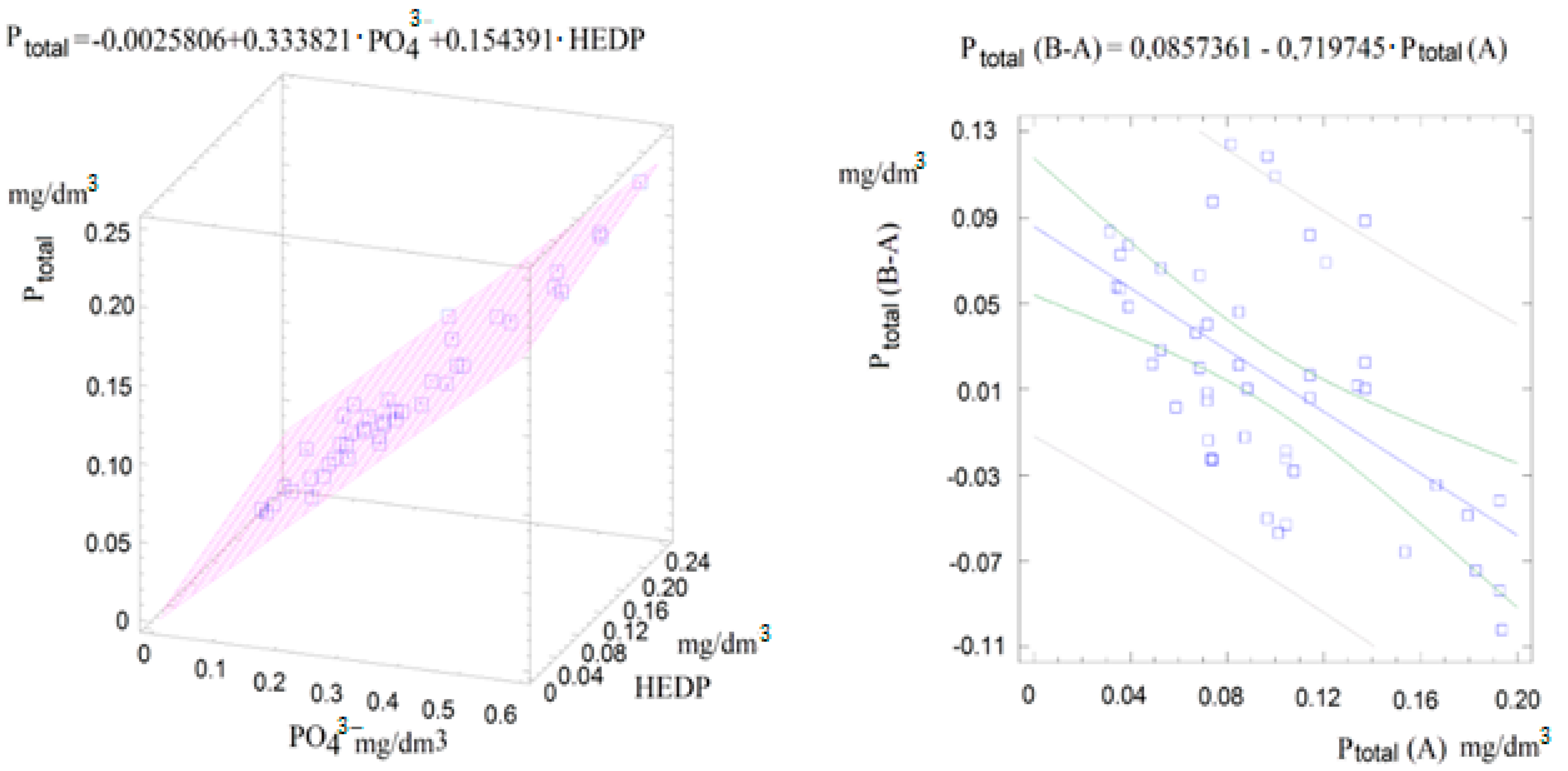
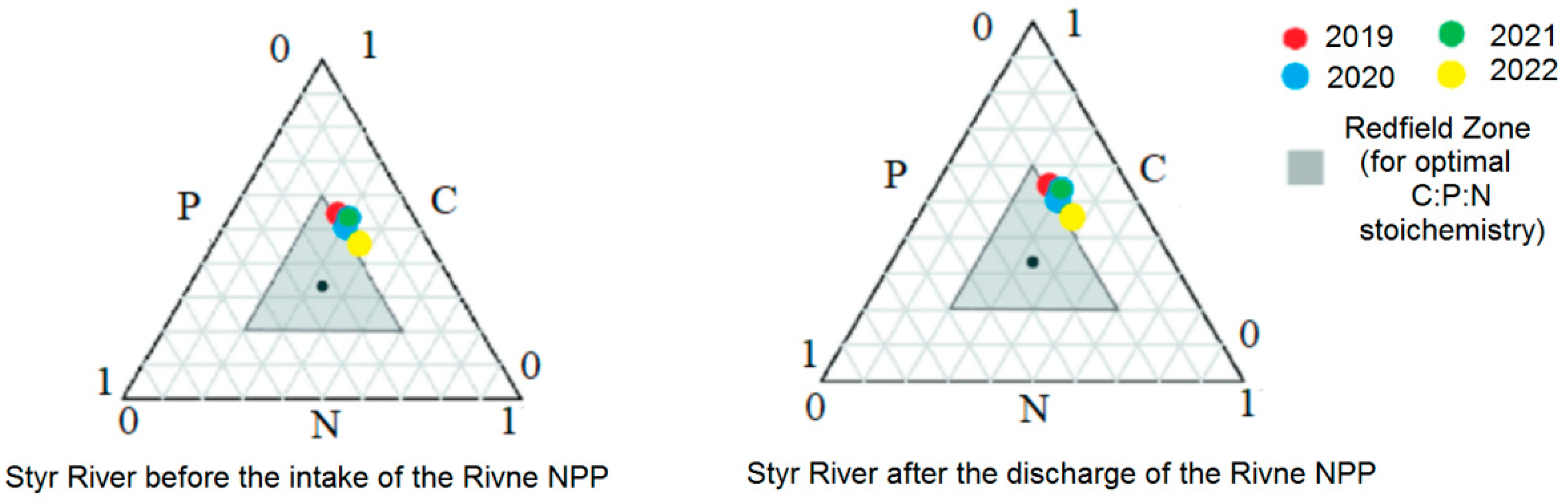
3.4. Correlation of the Content of Phosphorus Compounds
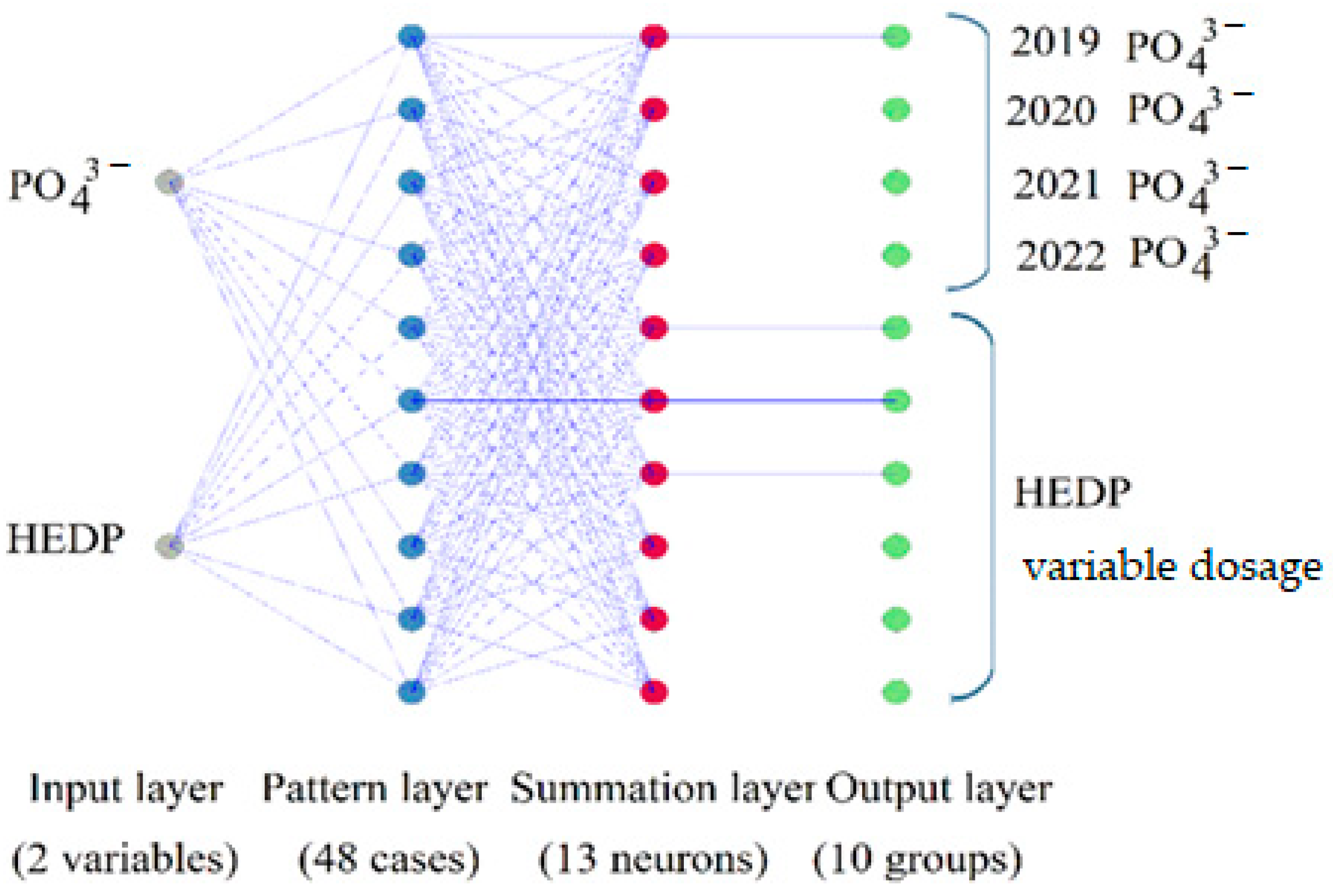
3.5. Environmental Assessment of Water Quality by Phosphorus Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickens, C.; Smakhtin, V.; McCartney, M.; O’Brien, G.; Dahir, L. Defining and Quantifying National-Level Targets, Indicators and Benchmarks for Management of Natural Resources to Achieve the Sustainable Development Goals. Sustainability 2019, 11, 462. [Google Scholar] [CrossRef]
- Withers, P.; Elser, J.; Hilton, J.; Ohtake, H.; Schipper, W.; Van, D. ChemInform Abstract: Greening the Global Phosphorus Cycle: How Green Chemistry Can Help Achieve Planetary P Sustainability. Green Chem. 2015, 17, 2087–2099. [Google Scholar] [CrossRef]
- Giri, S. Water quality prospective in Twenty First Century: Status of water quality in major river basins, contemporary strategies and impediments: A review. Environ. Pollut. 2021, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ma, J. Recent advances in the determination of phosphate in environmental water samples: Insights from practical perspectives. TrAC Trends Anal. Chem. 2020, 127, 115908. [Google Scholar] [CrossRef]
- Kuznietsov, P.M.; Biedunkova, O.O. Technological and environmental problems in the stabilization treatment of the main condenser cooling circuit by sulfuric acid. J. Eng. Sci. 2023, 10, H1–H8. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, G.; Qu, X.; Yang, X.; Wang, J.; Wang, P. Influence of Cooling Water Parameters on the Thermal Performance of the Secondary Circuit System of a Modular High-Temperature Gas-Cooled Reactor Nuclear Power Plant. Energies 2023, 16, 6560. [Google Scholar] [CrossRef]
- Kuznietsov, P.; Tykhomyrov, A.; Biedunkova, O.; Zaitsev, S. Improvement of methods for controlling power oil of cooling tower recycling water supply units at Rivne nuclear power plant. Sci. Horiz. 2022, 12, 69–79. [Google Scholar] [CrossRef]
- Chen, W.; Hui, K.; Wang, B.; Zhao, Q.; Chong, D.; Yan, J. Review of the tube external condenstion heat transfer characteristic of the passive containment cooling system in nuclear power plant. Ann. Nucl. Energy 2021, 157, 108226. [Google Scholar] [CrossRef]
- Gosse, P.; Samie, R. Water evaporation at wet-cooled nuclear power plants on river banks: Application to the French Rhône river. Water-Energy Nexus 2020, 3, 155–169. [Google Scholar] [CrossRef]
- Kuznietsov, P.N.; Biedunkova, O.O.; Yaroshchuk, O.V. Experimental study of transformation of carbonate system components cooling water of Rivne Nuclear Power Plant during water treatment by liming. Probl. At. Sci. Technol. 2022, 2, 69–73. [Google Scholar] [CrossRef]
- Environment Agency. Chemical Discharges from Nuclear Power Stations: Historical Releases and Implications for Best Available Techniques; Annex Report SC090012/R2; Environment Agency: Bristol, UK, 2011. Available online: https://www.gov.uk/government/organisations/environment-agency (accessed on 1 October 2023).
- Anh, N.T.; Can, L.D.; Nhan, N.T.; Schmalz, B.; Luu, T.L. Influences of key factors on river water quality in urban and rural areas: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100424. [Google Scholar] [CrossRef]
- Tang, L.; Pan, X.; Feng, J.; Pu, X.; Liang, R.; Li, R.; Li, K. Experimental Investigation on the Relationship Between COD Degradation and Hydrodynamic Conditions in Urban Rivers. Int. J. Environ. Res. Public Health 2019, 16, 3447. [Google Scholar] [CrossRef]
- Egbueri, J.C. Predicting and analysing the quality of water resources for industrial purposes using integrated data-intelligent algorithms. Groundw. Sustain. Dev. 2022, 18, 100794. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, T.; Li, J.; Li, Z.; Xu, G.; Yang, R. Reducing Nitrogen and Phosphorus Losses from Different Crop Types in the Water Source Area of the Danjiang River, China. Int. J. Environ. Res. Public Health 2019, 16, 3442. [Google Scholar] [CrossRef]
- Varol, M. Spatio-temporal changes in surface water quality and sediment phosphorus content of a large reservoir in Turkey. Environ. Pollut. 2020, 259, 113860. [Google Scholar] [CrossRef]
- Zheng, J.; Cao, X.; Chunzi, M.; Weng, Y.; Huo, S. What drives the change of nitrogen and phosphorus loads in the Yellow River Basin during 2006–2017. J. Environ. Sci. 2023, 126, 17–28. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, H.; Ma, Y.; Li, Y.; Tie, C.; Zhao, Q. Effects of Dissolved Organic Matter on the Release of Soluble Phosphorus and Fluoride Ion from Phosphate Ore. Separations 2023, 10, 425. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, S.; Xu, M.; Luo, X.; Peng, X.; Ren, C.; Zhang, J. Spatiotemporal Nutrient Patterns, Stoichiometry, and Eutrophication Assessment in the Tieshan Bay Coastal Water, China. J. Mar. Sci. Eng. 2023, 11, 1602. [Google Scholar] [CrossRef]
- Huang, C.-L.; Kang, W.; Xu, S.; Gao, B.; Huang, W.; Li, Z.; Cui, S. Growing phosphorus dilemma: The opportunity from aquatic systems’ secondary phosphorus retention capacity. Sci. Total Environ. 2021, 796, 148938. [Google Scholar] [CrossRef]
- Feng, M.; Cheng, H.; Zhang, P.; Wang, K.; Wang, T.; Zhang, H.; Wang, H.; Zhou, L.; Xu, J.; Zhang, M. Stoichiometric stability of aquatic organisms increases with trophic level under warming and eutrophication. Sci. Total Environ. 2022, 858, 160106. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. The Global Phosphorus Cycle. Treatise Geochem. 2003, 8, 585–643. [Google Scholar] [CrossRef]
- Hu, S.; Wang, T.; Xu, S.; Ma, L.; Sun, A.X. Seasonal Release Potential of Sediments in Reservoirs and its Impact on Water Quality Assessment. Int. J. Environ. Res. Public Health 2019, 16, 3303. [Google Scholar] [CrossRef]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef]
- Mutke, A.M.; Tavichaiyuth, K.; Drees, F.; Lutze, H.V.; Schmidt, T.C. Oxidation of the nitrogen-free phosphonate antiscalants HEDP and PBTC in reverse osmosis concentrates: Reaction kinetics and degradation rate. Water Res. 2023, 233, 119571. [Google Scholar] [CrossRef]
- Han, C.; Dai, Y.; Sun, N.; Wu, H.; Tang, Y.; Dai, T. Algae Bloom and Decomposition Changes the Phosphorus Cycle Pattern in Taihu Lake. Water 2022, 14, 3607. [Google Scholar] [CrossRef]
- Panja, A.K.; Vasavdutta, S.; Choudhary, M.; Thiyagarajan, I.; Shinde, A.H.; Ray, S.; Sahoo, T.P.; Chatterjee, S.; Thorat, R.B.; Madhava, A.K.; et al. Interaction of physico-chemical parameters with Shannon-Weaver Diversity Index based on phytoplankton diversity in coastal water of Diu, India. Mar. Pollut. Bull. 2023, 190, 114839. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Yang, S. Response of phytoplankton community structure to environmental changes in the coastal areas of northern China. Mar. Pollut. Bull. 2023, 195, 115300. [Google Scholar] [CrossRef]
- Jia, J.; Gao, Y.; Zhou, F.; Shi, K.; Johnes, P.J.; Dungait, J.A.J.; Ma, M.; Lu, Y. Identifying the main drivers of change of phytoplankton community structure and gross primary productivity in a river-lake system. J. Hydrol. 2020, 583, 124633. [Google Scholar] [CrossRef]
- Marazgioui, S.; Fadar, A. Impact of cooling tower technology on performance and cost-effectiveness of CSP plants. Energy Convers. Manag. 2022, 258, 115448. [Google Scholar] [CrossRef]
- Klymenko, M.O.; Biedunkova, O.O.; Klymenko, O.M.; Statnyk, I.I. Influence of river water quality on homeostasis characteristics of cypriniform and perciform fish. Biosyst. Divers. 2018, 26, 16–23. [Google Scholar] [CrossRef]
- Bilous, O.; Afanasyev, S.; Lietytska, O.; Manturova, O.; Polishchuk, O.; Nezbrytska, I.; Pohorielova, M.; Barinova, S. Preliminary Assessment of Ecological Status of the Siversky Donets River Basin (Ukraine) Based on Phytoplankton Parameters and Its Verification by Other Biological Data. Water 2021, 13, 3368. [Google Scholar] [CrossRef]
- Kuznietsov, P.; Biedunkova, O. Experimental Tests of Biocidal Treatment for Cooling Water of Safety Systems at Rivne NPP. Nucl. Radiat. Saf. 2023, 1, 30–40. [Google Scholar] [CrossRef]
- Zakonodavstvo Ukraini. Methodology for Assigning a Body of Surface Water to One of the Classes of Ecological and Chemical State of the Body of Surface Water, As Well As Assigning an Artificial or Significantly Altered Body of Surface Water to One of the Classes of Ecological Potential of an Artificial or Significantly Altered body of Surface Water. 2019. Available online: https://zakon.rada.gov.ua/laws/show/z0127-19#Tex (accessed on 1 October 2023).
- Directive 2006/44/EC of the European Parliament and of the Council of 6 September 2006 on the Quality of Fresh Waters Needing Protection or Improvement in order to Support Fish Life. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32006L0044 (accessed on 1 October 2023).
- Vagheei, H.; Laini, A.; Vezza, P.; Palau-Salvador, G.; Boano, F. Climate change impact on the ecological status of rivers: The case of Albaida Valley (SE Spain). Sci. Total Environ. 2023, 893, 164645. [Google Scholar] [CrossRef] [PubMed]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.l.; Topa, C.; Timofti, M.; Pintilie, V.M. Lower Danube Water Quality Quantified through WQI and Multivariate Analysis. Water 2019, 11, 1305. [Google Scholar] [CrossRef]
- Jo, C.D.; Kwon, H.G. Temporal and spatial evaluation of the effect of river environment changes caused by climte change on water quality. Environ. Technol. Innov. 2023, 30, 103066. [Google Scholar] [CrossRef]
- Yin, S.; Gao, G.; Li, Y.; Xu, Y.J.; Turner, R.E.; Ran, L.; Wang, X.; Fu, B. Long-term trends of streamflow, sediment load and nutrient fluxes from the Mississippi River Basin: Impacts of climate change and human activities. J. Hydrol. 2023, 616, 128822. [Google Scholar] [CrossRef]
- Whitehead, P.G.; Voepel, H.E.; Darby, S.E.; Vasilopoulos, G.; Hutton, C.; Bussi, G.; Jin, L.; Manley, R.; Rodda, H.; Tri, V.P.D.; et al. Water quality modelling of the Mekong River basin: Climate change and socioeconomics drive flow and nutrient flux changes to the Mekong Delta. Sci. Total Environ. 2019, 673, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Jarvie, H.P.; Smith, D.R.; Norton, L.R.; Edwards, F.K.; Bowes, M.J.; King, S.M.; Scarlett, P.; Davies, S.; Dils, R.M.; Bachiller-Jareno, N. Phosphorus and nitrogen limitation and impairment of headwater streams relative to rivers in Great Britain: A national perspective on eutrophication. Sci. Total Environ. 2018, 621, 849–862. [Google Scholar] [CrossRef]
- Bardsley, J.M.; Howard, M.; Lorang, M. Matlab Software for Supervised Habitat Mapping of Freshwater Systems Using Image Processing. Remote Sens. 2021, 13, 4906. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance). Available online: http://data.europa.eu/eli/dir/2020/2184/oj (accessed on 1 October 2023).
- DSanPiN 2.2.4-171-10. Hygienic Requirements for Drinking Water Intended for Human Consumption. Approved by Order No. 400 of the Ministry of Health of Ukraine Dated 2010 May 12. 2010. Available online: https://zakon.rada.gov.ua/laws/show/z0452-10#Text (accessed on 1 October 2023).
- Zakonodavstvo Ukraini. Hygienic Water Quality Standards of Water Bodies to Meet Drinking, Household and Other Needs of the Population. Order of the Ministry of Health of Ukraine Dated May 2, 2022 No. 721. 2022. Available online: https://zakon.rada.gov.ua/laws/show/z0524-22#Text (accessed on 1 October 2023).
- Zakonodavstvo Ukraini. Rules for the Protection of Surface Water from Pollution by Return Water. Resolution of March 25, 1999 N 465, Kyiv. 1999. Available online: https://zakon.rada.gov.ua/laws/show/465-99-%D0%BF#Text (accessed on 1 October 2023).
- Zakonodavstvo Ukraini. Generalized List of Maximum Permissible Concentrations (MPC) and Tentatively Safe Levels of Exposure (VZUV) of Harmful Substances for the Water of Fishing Reservoirs. Ministry of Agriculture (Order of the State Emergency Service of Ukraine dated 31.08.2017 No. 47 On Approval of the List of Industry Standards, Valid until 01.01.2025). 1990. Available online: http://online.budstandart.com/ua/catalog/ (accessed on 1 October 2023).
- UKTAG. Updated Recommendations on Environmental Standards; (Phase 3); River Basin Management (2015–21); Final; November 2012. Available online: http://www.wfduk.org/stakeholders/stakeholder-review-2012-response-submissions (accessed on 1 October 2023).
- MVV 081/12-0005-01. Surface and Treated Wastewater. Methodology for Measuring the Mass Concentration of Dissolved Orthophosphates by the Photometric Method (2001). Kyiv, Ministry of Ecology and Natural Resources of Ukraine. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=76321 (accessed on 1 October 2023).
- MVV 081/12-0878-13. Return, Surface Waters. Surface and treated wastewater. The method of measuring the mass concentration of dissolved orthophosphates by the photometric method, (2013). Kyiv, Ministry of Ecology and Natural Resources of Ukraine. Available online: https://online.budstandart.com/ua/catalog/doc-page.html?id_doc=90473 (accessed on 1 October 2023).
- Wessa, P. Free Statistics Software, Version 1.2.1; Office for Research Development and Education: 2023. Available online: https://www.wessa.net/ (accessed on 1 October 2023).
- Wang, Q. Multivariate Kernel Smoothing and Its Applications. J. Am. Stat. Assoc. 2020, 115, 486. [Google Scholar] [CrossRef]
- Omer, N.; Samak, A.H.; Taloba, A.I.; Abd El-Aziz, R.M. A novel optimized probabilistic neural network approach for intrusion detection and categorization. Alex. Eng. J. 2023, 72, 351–361. [Google Scholar] [CrossRef]
- Permit for Special Water Use of VP RAEP No. 53/RV/49d-20. 2020. Available online: https://e-services.davr.gov.ua (accessed on 1 October 2023).
- McLaughlin, K.; Cade-Menun, B.J.; Paytan, A. The oxygen isotopic composition of phosphate in Elkhorn Slough, California: A tracer for phosphate sources. Estuar. Coast. Shelf Sci. 2006, 70, 499–506. [Google Scholar] [CrossRef]
- Schneider, S.C.; Skarbøvik, E. Ecological status assessment of clay rivers with naturally enhanced water phosphorus concentrations. Environ. Adv. 2022, 9, 100279. [Google Scholar] [CrossRef]
- Tappin, A.D.; Navarro-Rodriguez, A.; Combera, S.D.W.; Worsfold, P.J. The role of alkalinity in setting water quality metrics: Phosphorus standards in United Kingdom rivers. Environ. Sci. Process. Impacts 2018, 20, 1361–1372. [Google Scholar] [CrossRef]
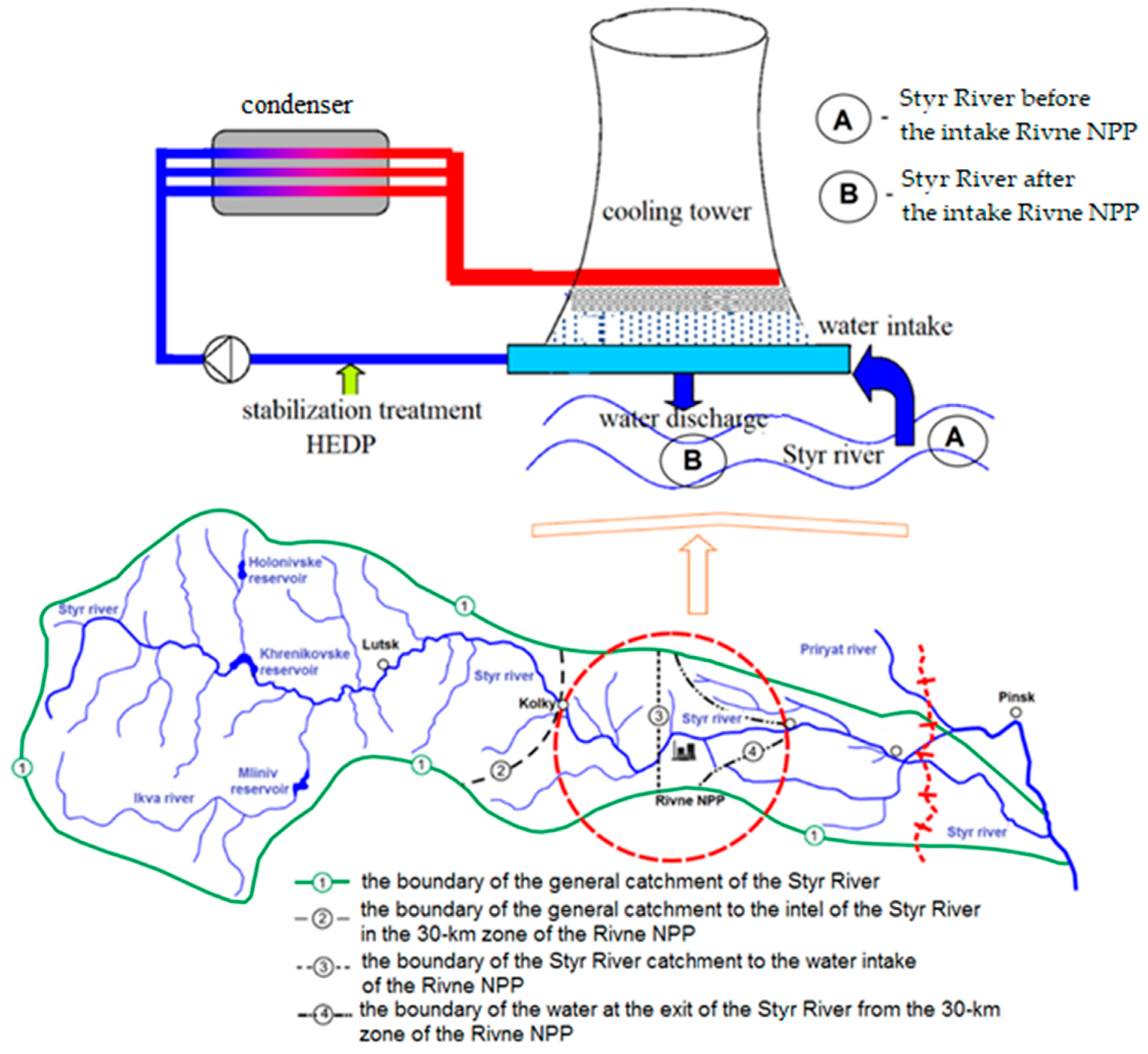
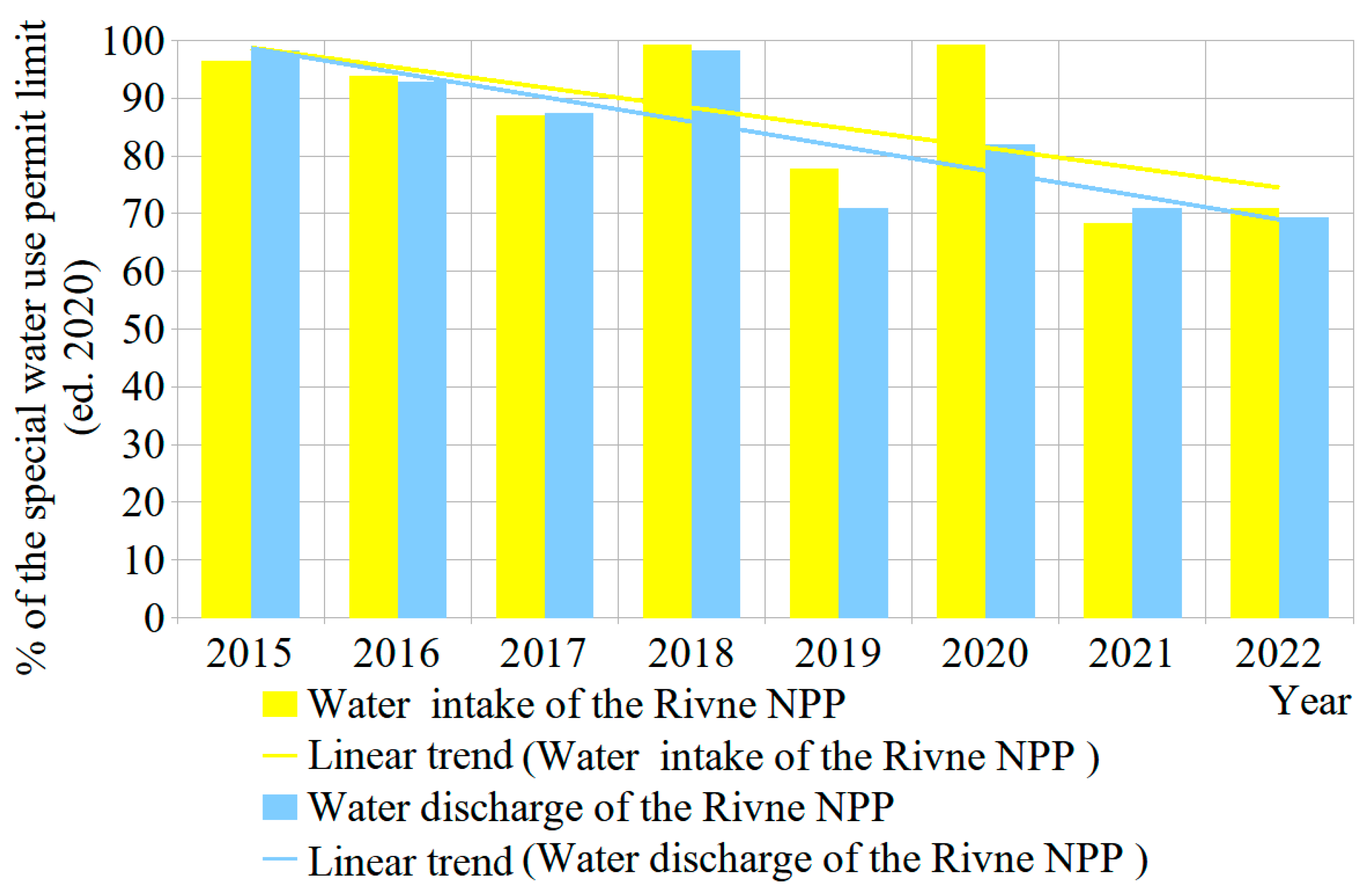

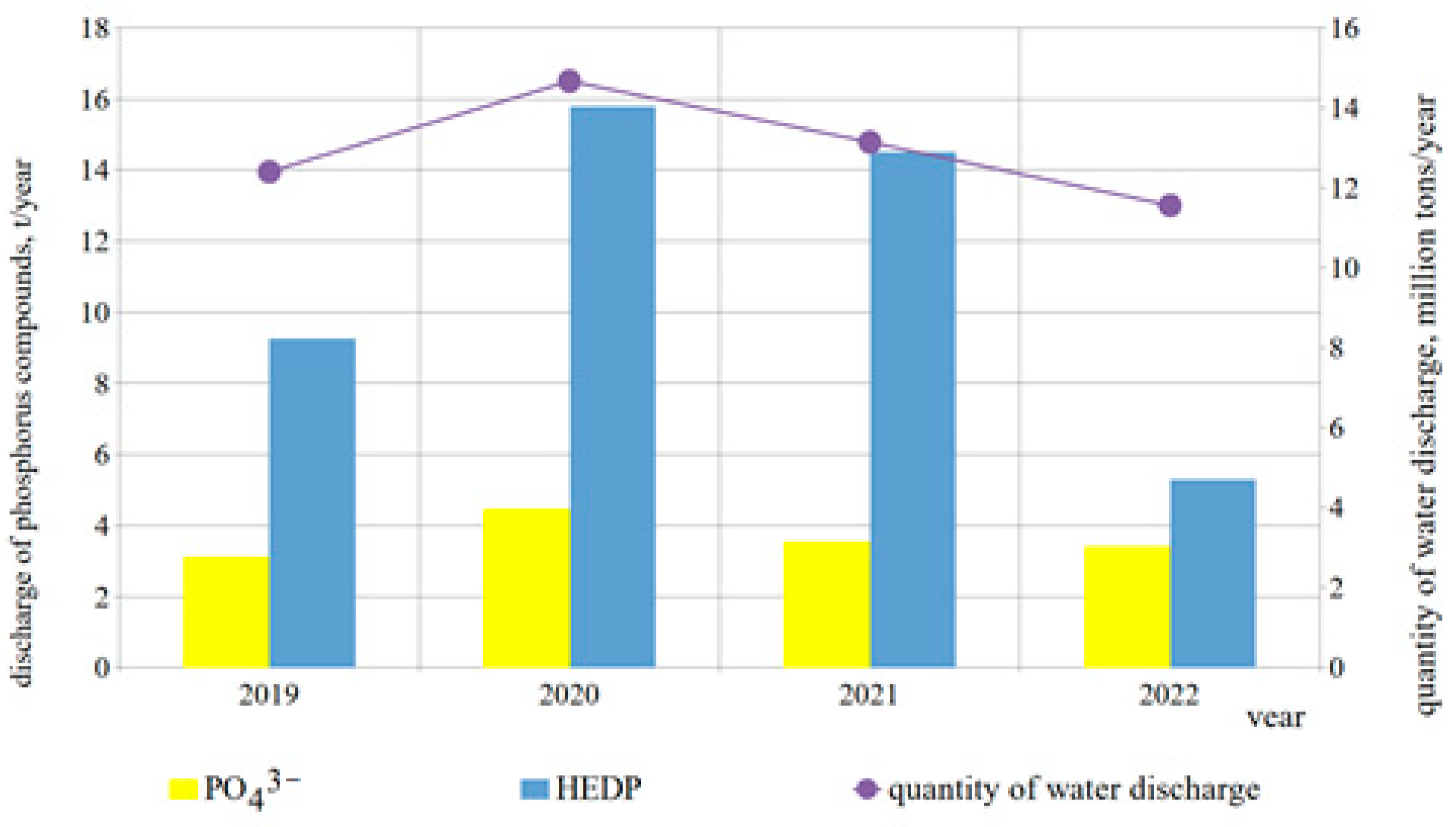
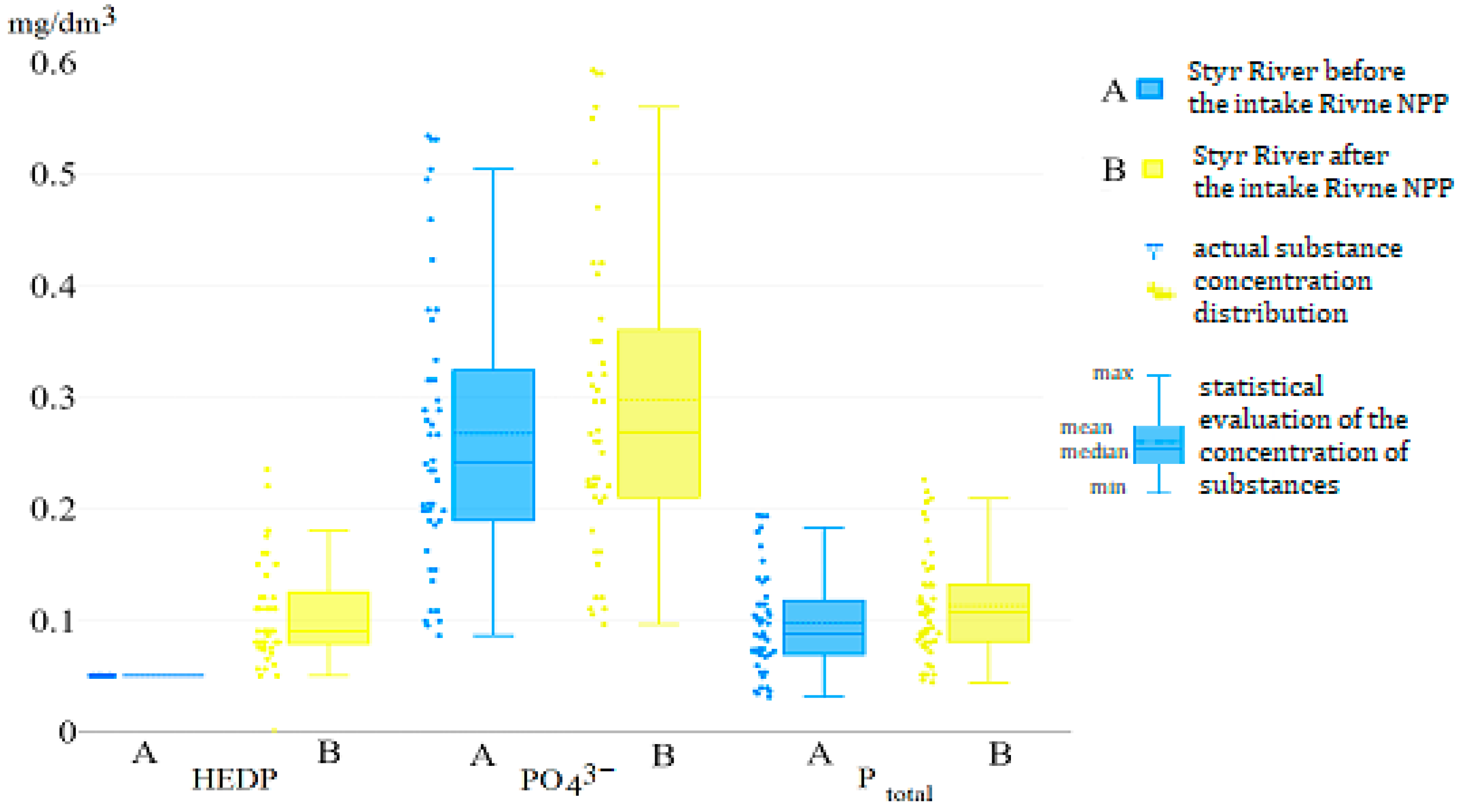

| Parameter | Ecological Indicator | Measurement Methods | ||||||
|---|---|---|---|---|---|---|---|---|
| EA | Ukrainian | EC | Cmin–Cmax (3), mg/dm3 | δ (4), % | ||||
| HA | WMA | HA | FW | |||||
| DW | FW | |||||||
| Phosphates, mg PO43−/dm3 | <0.015–0.10 mg P/dm3 (I, II, III Classes) | 3.5 | 3.5 | 2.14 | - | 0.2–0.4 (2) | 0.05–100 | ± 15 (5) ± 10 (5) |
| Pure phosphorus, mg P/dm3 | - | - | 0.7 | - | - | |||
| HEDP 1, mg/dm3 | - | 0.3 (1) | 0.3 (1) | 0.9 | - | - | 0.06–4.0 | ± 18 |
| Chemical Parameters | Parameter (1) | Year | |||
|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | ||
| Phosphates, mg/dm3 | M | 0.264 | 0.303 | 0.291 | 0.327 |
| ±m | 0.096 | 0.167 | 0.131 | 0.170 | |
| min | 0.080 | 0.120 | 0.105 | 0.110 | |
| max | 0.420 | 0.495 | 0.590 | 0.590 | |
| Cv | 35.33 | 34.55 | 35.86 | 38.60 | |
| HEDP, mg/dm3 | M | 0.07 | 0.12 | 0.13 | 0.17 |
| ±m | 0.03 | 0.08 | 0.15 | 0.10 | |
| min | 0.05 | 0.08 | 0.08 | 0.07 | |
| max | 0.09 | 0.18 | 0.24 | 0.15 | |
| Cv | 18.50 | 30.51 | 40.20 | 33.30 | |
| Total Phosphorus, mg/dm3 | M | 0.097 | 0.118 | 0.116 | 0.120 |
| ±m | 0.030 | 0.050 | 0.040 | 0.050 | |
| min | 0015 | 0.051 | 0.051 | 0.046 | |
| max | 0.145 | 0.209 | 0.226 | 0.215 | |
| Cv | 18.30 | 30.32 | 35.23 | 34.76 | |
| Month for 2019–2022 | Water from the Styr River to the Rivne NPP Water Intake | River Styr after Discharge of Return Water from the Rivne NPP | ||
|---|---|---|---|---|
| Environmental Threshold for Phosphorus without Alkalinity | Environmental Threshold for Phosphorus with Alkalinity | Environmental Threshold for Phosphorus without Alkalinity | With Alkalinity | |
| January | III «satisfactory, contaminated» | «good» | III «satisfactory, contaminated» | «good» |
| February | III «satisfactory, contaminated» | «good» | III «satisfactory, contaminated» | «good» |
| March | IV «bad, dirty» | «moderate» | IV «bad, dirty» | «moderate» |
| April | IV «bad, dirty» | «moderate» | IV «bad, dirty» | «moderate» |
| May | V «very bad, very dirty» | «moderate» | V «very bad, very dirty» | «moderate» |
| June | IV «bad, dirty» | «moderate» | IV «bad, dirty» | «moderate» |
| July | IV «bad, dirty» | «moderate» | IV «bad, dirty» | «moderate» |
| August | III «satisfactory, contaminated» | «good» | III «satisfactory, contaminated» | «good» |
| September | III «satisfactory, contaminated» | «good» | III «satisfactory, contaminated» | «good» |
| October | III «satisfactory, contaminated» | «good» | III «satisfactory, contaminated» | «good» |
| November | II «good, clean» | «good» | II «good, clean» | «good» |
| December | III «satisfactory, contaminated» | «good» | III «satisfactory, contaminated» | «good» |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznietsov, P.; Biedunkova, O.; Trach, Y. Monitoring of Phosphorus Compounds in the Influence Zone Affected by Nuclear Power Plant Water Discharge in the Styr River (Western Ukraine): Case Study. Sustainability 2023, 15, 16316. https://doi.org/10.3390/su152316316
Kuznietsov P, Biedunkova O, Trach Y. Monitoring of Phosphorus Compounds in the Influence Zone Affected by Nuclear Power Plant Water Discharge in the Styr River (Western Ukraine): Case Study. Sustainability. 2023; 15(23):16316. https://doi.org/10.3390/su152316316
Chicago/Turabian StyleKuznietsov, Pavlo, Olha Biedunkova, and Yuliia Trach. 2023. "Monitoring of Phosphorus Compounds in the Influence Zone Affected by Nuclear Power Plant Water Discharge in the Styr River (Western Ukraine): Case Study" Sustainability 15, no. 23: 16316. https://doi.org/10.3390/su152316316
APA StyleKuznietsov, P., Biedunkova, O., & Trach, Y. (2023). Monitoring of Phosphorus Compounds in the Influence Zone Affected by Nuclear Power Plant Water Discharge in the Styr River (Western Ukraine): Case Study. Sustainability, 15(23), 16316. https://doi.org/10.3390/su152316316









