Biochar Amendment in Vermi-Wetland for Enhancing Nitrification during Excess Sludge Recycling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
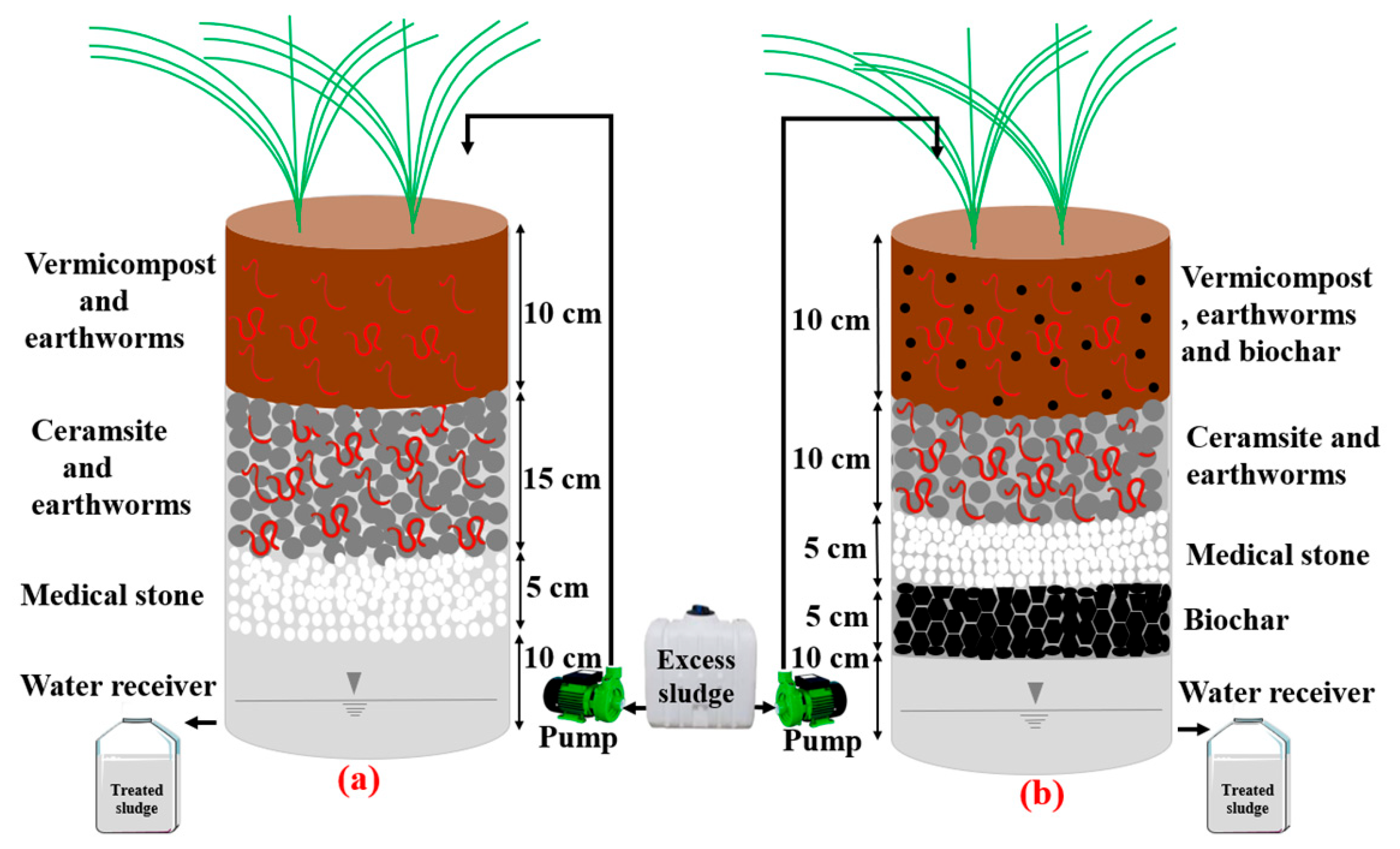
2.2. Experimental Set-Up
2.3. Methods
2.3.1. Physicochemical Properties Analysis
2.3.2. DNA Extraction and Real-Time PCR
2.3.3. High-Throughput Sequencing
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Earthworms and Plants Biomass for Promoting AOA and AOB
3.2. Effect of BC on the Nitrification Mechanisms inside Vermi-Wetland
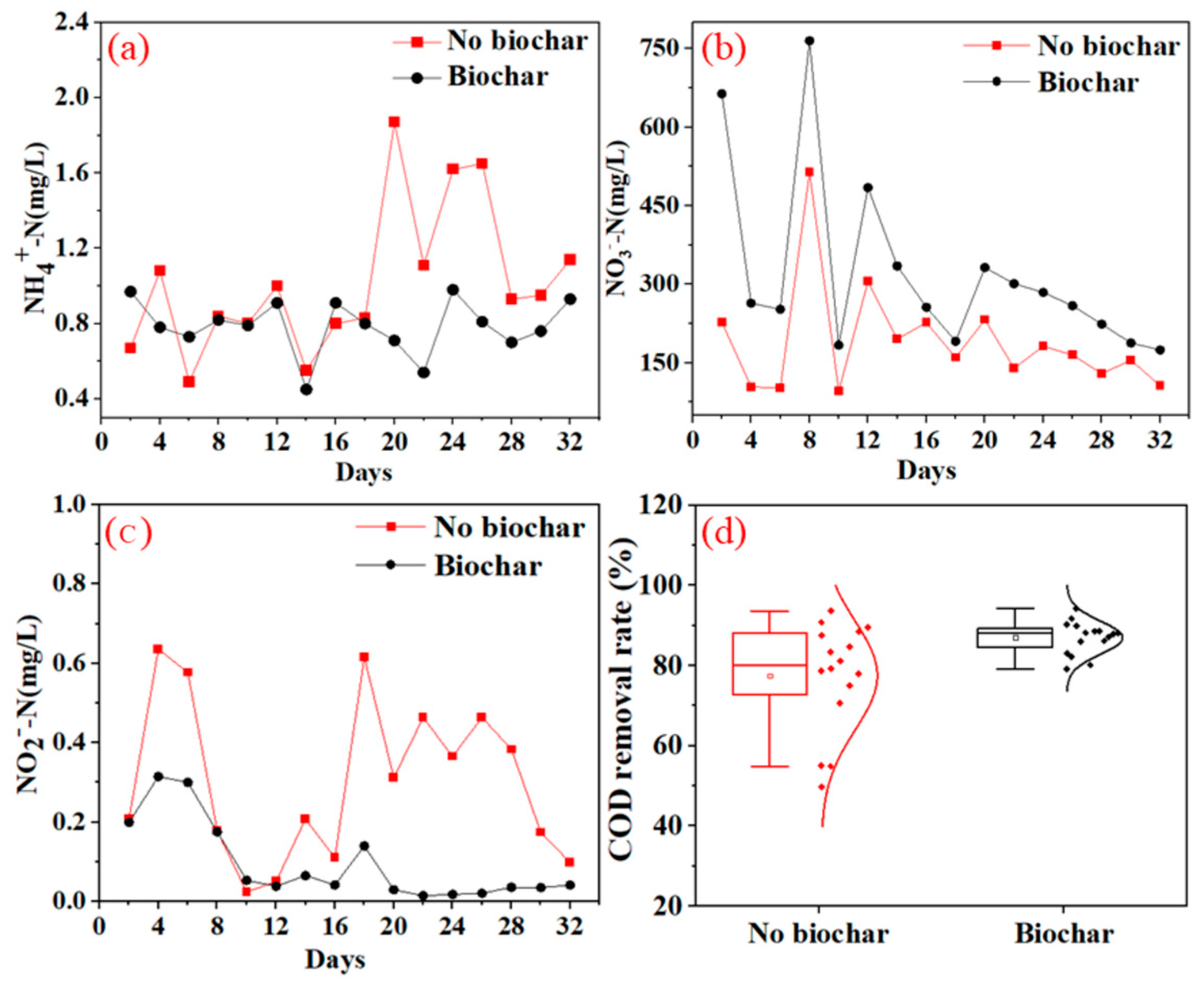
3.2.1. Effect of BC on the Nitrification Process during Excess Sludge Recycling
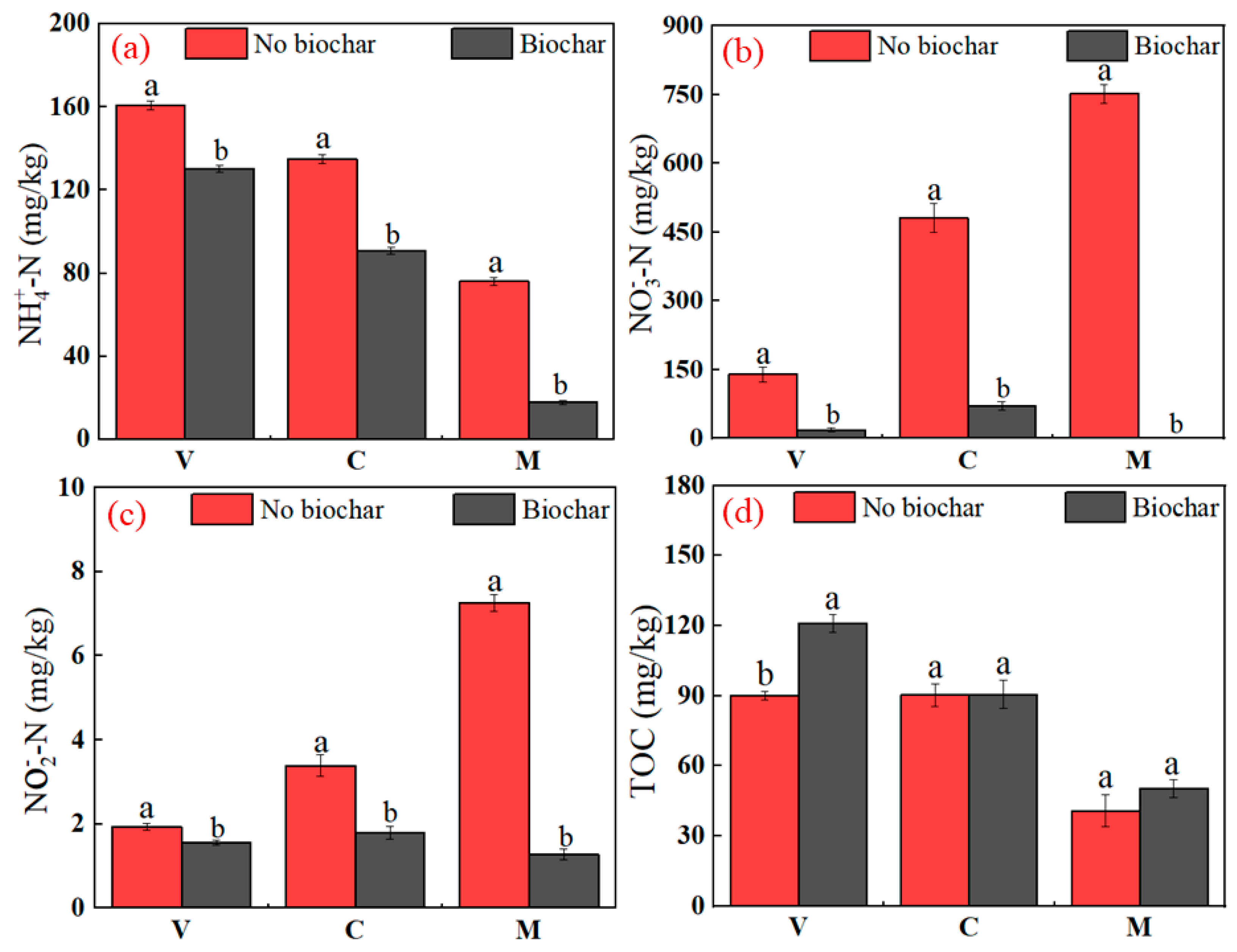
3.2.2. Effect of Biochar on the Nitrification Process inside Vermi-Wetlands
3.2.3. Effect of Biochar on Numbers of Nitrification Genes inside Vermi-Wetland
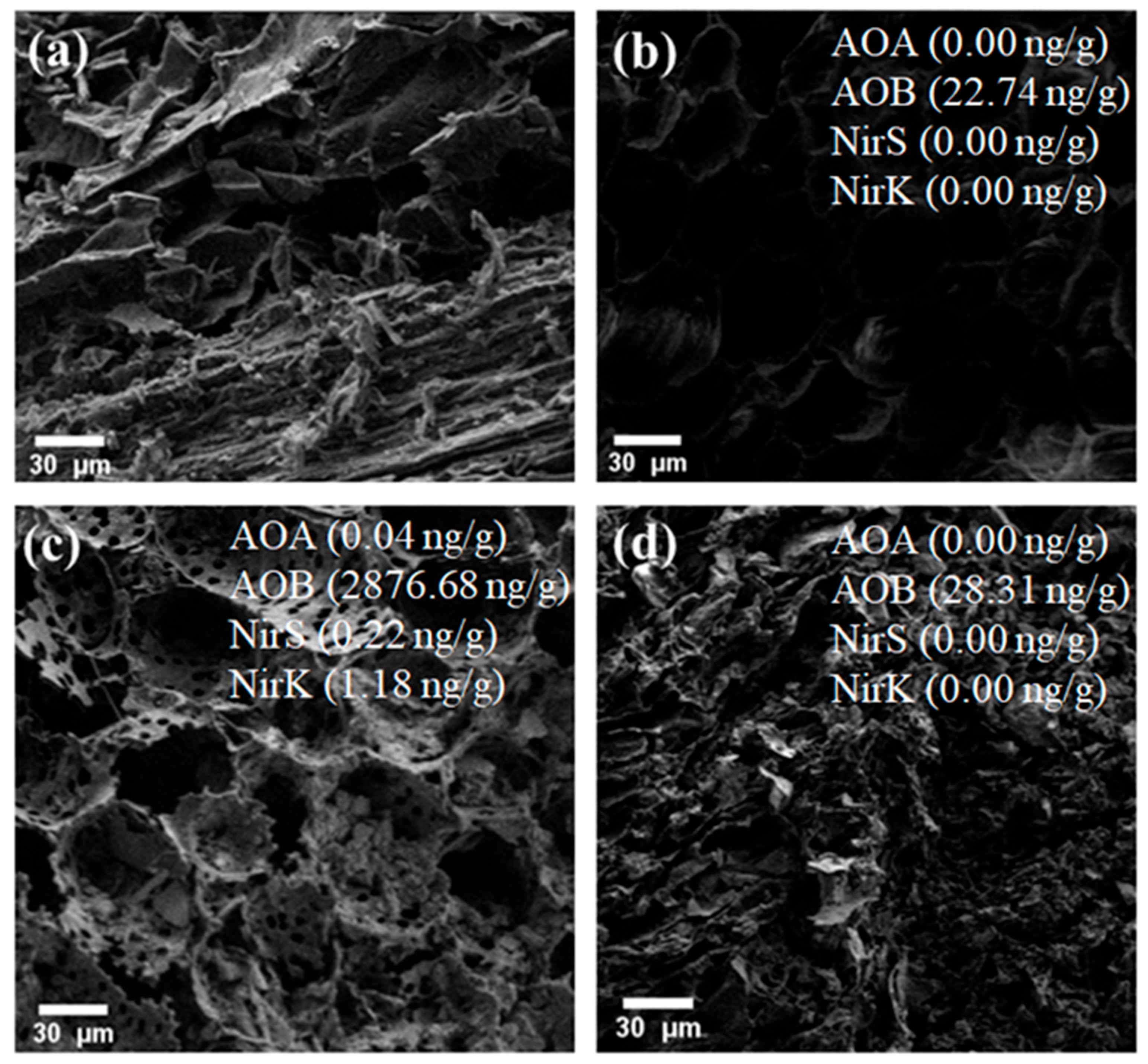
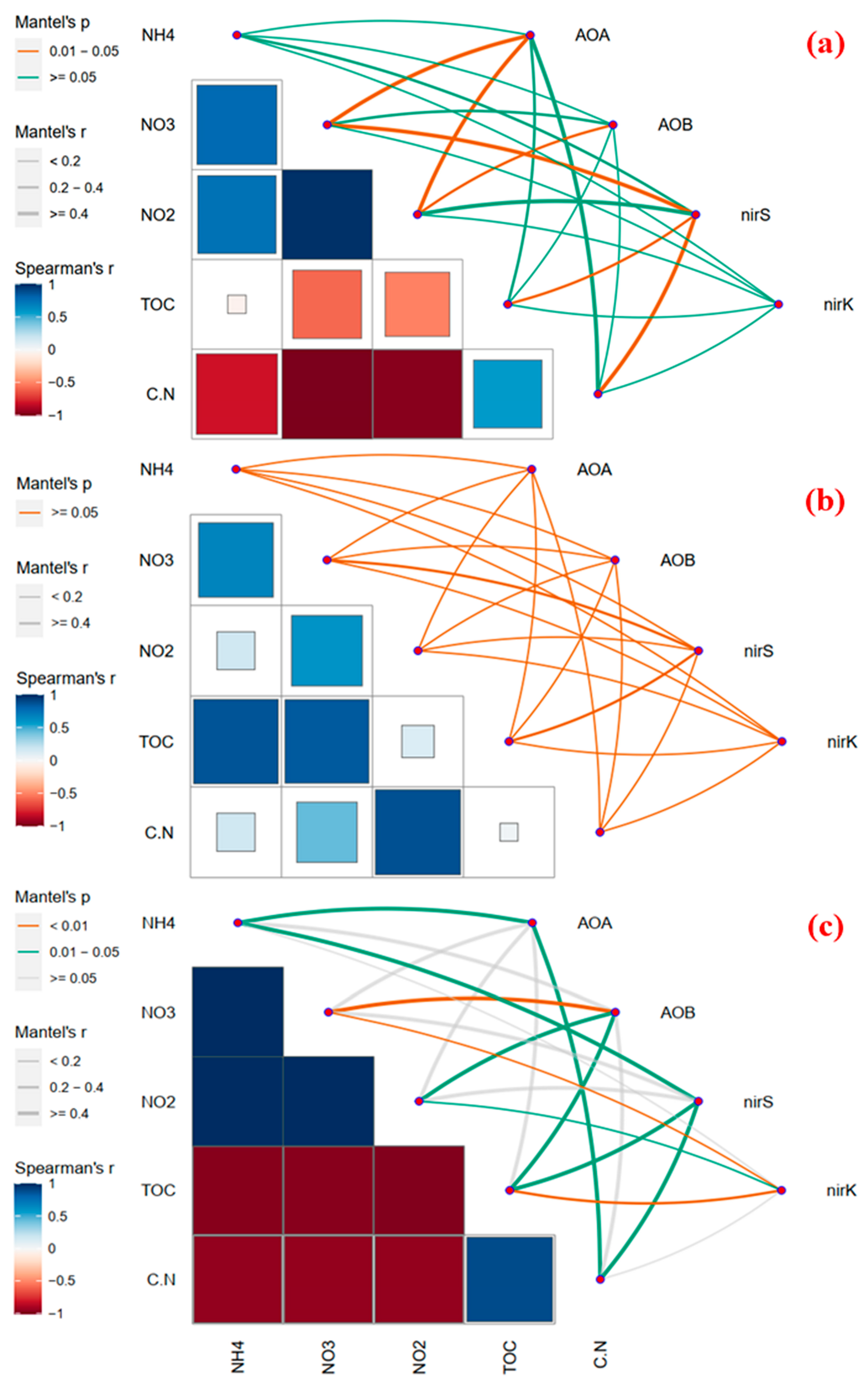
3.3. BC Provides an Inhabitation for AOA, AOB, nirK, and nirS Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, K.; Sang, C.; Guan, M.; Wu, Y.; Xia, H.; Chen, Y.; Nie, C. Performance and Stratified Microbial Community of Vermi-Filter Affected by Acorus calamus and Epipremnum aureum during Recycling of Concentrated Excess Sludge. Chemosphere 2021, 280, 130609. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.J. Sludge Treatment: Current Research Trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Huang, K.; Sang, C.; Guan, M.; Xia, H.; Li, Y. Performance and Mechanisms of Biochar for Promoting the Removal Efficiency of Organic Solids in the Vermi-Wetland during the Recycling of Excess Sludge. J. Clean. Prod. 2022, 360, 132172. [Google Scholar] [CrossRef]
- Singh, R.; Bhunia, P.; Dash, R.R. A Mechanistic Review on Vermifiltration of Wastewater: Design, Operation and Performance. J. Environ. Manag. 2017, 197, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Dash, R.R.; Bhunia, P. A Comparative Study of Macrophytes Influence on Performance of Hybrid Vermifilter for Dairy Wastewater Treatment. J. Environ. Chem. Eng. 2018, 6, 4714–4726. [Google Scholar] [CrossRef]
- Hu, S.; Zuo, X.; Lv, Z.; He, J.; Wu, Y.; Liu, H.; Chen, Z. Drained Water Quality in Sludge Treatment Wetlands: Effects of Earthworm Densities and Plant Species. J. Clean. Prod. 2020, 247, 119128. [Google Scholar] [CrossRef]
- Hu, S.; Lv, Z.; Zuo, X.; Liu, H.; Vymazal, J.; Chen, Z. Effects of Loading Rates and Plant Species on Sludge Characteristics in Earthworm Assistant Sludge Treatment Wetlands. Sci. Total Environ. 2020, 730, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xing, D.; Twagirayezu, G.; Lin, S.; Gu, S.; Tu, C.; Hill, P.W.; Jones, D.L. Effects of Field-Aging on the Impact of Biochar on Herbicide Fate and Microbial Community Structure in the Soil Environment. Chemosphere 2023. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Gong, X.; Cai, L.; Li, S.; Chang, S.X.; Sun, X.; An, Z. Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotoxicol. Environ. Saf. 2018, 156, 197–204. [Google Scholar] [CrossRef]
- Blenis, N.; Hue, N.; Maaz, T.M.C.; Kantar, M. Biochar Production, Modification, and Its Uses in Soil Remediation: A Review. Sustainability 2023, 15, 3442. [Google Scholar] [CrossRef]
- Deng, Z.; Gu, S.; Cheng, H.; Xing, D.; Twagirayezu, G.; Wang, X.; Ning, W.; Mao, M. Removal of Phosphate from Aqueous Solution by Zeolite-Biochar Composite: Adsorption Performance and Regulation Mechanism. Appl. Sci. 2022, 12, 5334. [Google Scholar] [CrossRef]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in Heavy Metal Bioavailability and Speciation from a Pb-Zn Mining Soil Amended with Biochars from Co-Pyrolysis of Rice Straw and Swine Manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Li, J.; Hu, B.; Chu, G. Ammonia-Oxidizing Bacteria Are Sensitive and Not Resilient to Organic Amendment and Nitrapyrin Disturbances, but Ammonia-Oxidizing Archaea Are Resistant. Geoderma 2021, 384, 114814. [Google Scholar] [CrossRef]
- Coca-Salazar, A.; Richaume, A.; Florio, A.; Carnol, M. Response of Ammonia-Oxidizing Bacteria and Archaea Abundance and Activity to Land Use Changes in Agricultural Systems of the Central Andes. Eur. J. Soil Biol. 2021, 102, 103263. [Google Scholar] [CrossRef]
- Koch, H.; van Kessel, M.A.H.J.; Lücker, S. Complete Nitrification: Insights into the Ecophysiology of Comammox Nitrospira. Appl. Microbiol. Biotechnol. 2019, 103, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Ginawi, A.; Wang, L.; Wang, H.; Yu, B.; Yunjun, Y. Effects of Environmental Variables on Abundance of Ammonia-Oxidizing Communities in Sediments of Luotian River, China. PeerJ 2020, 8, e8256. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Duff, A.M.; Smith, C.J. Community and Functional Shifts in Ammonia Oxidizers across Terrestrial and Marine (Soil/Sediment) Boundaries in Two Coastal Bay Ecosystems. Environ. Microbiol. 2018, 20, 2834–2853. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xia, H.; Cui, G.; Li, F. Effects of Earthworms on Nitrification and Ammonia Oxidizers in Vermicomposting Systems for Recycling of Fruit and Vegetable Wastes. Sci. Total Environ. 2017, 578, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhao, Y.; Zhang, J.; Bello, A.; Sun, Y.; Han, Y.; Wang, B.; Egbeagu, U.U.; Li, D.; Jong, C.; et al. Insight to Nitrification during Cattle Manure-Maize Straw and Biochar Composting in Terms of Multi-Variable Interaction. Bioresour. Technol. 2021, 323, 124572. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pang, Q.; Peng, F.; Zhang, A.; Zhou, Y.; Lian, J.; Zhang, Y.; Yang, F.; Zhu, Y.; Ding, C.; et al. Response Characteristics of Nitrifying Bacteria and Archaea Community Involved in Nitrogen Removal and Bioelectricity Generation in Integrated Tidal Flow Constructed Wetland-Microbial Fuel Cell. Front. Microbiol. 2020, 11, 1385. [Google Scholar] [CrossRef]
- Karmegam, N.; Jayakumar, M.; Govarthanan, M.; Kumar, P.; Ravindran, B.; Biruntha, M. Precomposting and Green Manure Amendment for Effective Vermitransformation of Hazardous Coir Industrial Waste into Enriched Vermicompost. Bioresour. Technol. 2021, 319, 124136. [Google Scholar] [CrossRef] [PubMed]
- CJ/T 221-2005; Determination Method for Municipal Sludge in Wastewater Treatment Plant. Chinese Standard: Beijing, China, 2005.
- Huang, K.; Li, F.; Wei, Y.; Chen, X.; Fu, X. Changes of Bacterial and Fungal Community Compositions during Vermicomposting of Vegetable Wastes by Eisenia Foetida. Bioresour. Technol. 2013, 150, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Golestan, H.A.; Razavi, H.; Golestan, N.; Fotouhi, F.; Khosravi, A. Development of a Robust TaqMan Probe-Based One-Step Multiplex RT-QPCR for Simultaneous Detection of SARS-CoV-2 and Inuenza A/B Viruses. BMC Microbiol. 2023, 23, 335. [Google Scholar]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Kõiv-Vainik, M.; Mander, Ü.; Truu, M. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Zhou, X.; Chu, J.; Li, Y.; Wang, M. Effects of Emergent Aquatic Plants on Abundance and Community Structure of Ammonia-Oxidising Microorganisms. Ecol. Eng. 2015, 81, 504–513. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Chinese Standard: Beijing, China, 2002.
- Xue, R.; Wang, C.; Liu, X.; Liu, M. Earthworm Regulation of Nitrogen Pools and Dynamics and Marker Genes of Nitrogen Cycling: A Meta-Analysis. Pedosphere 2022, 32, 131–139. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced Nitrogen Removal of Low C/N Domestic Wastewater Using a Biochar-Amended Aerated Vertical Flow Constructed Wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef]
- Verhamme, D.T.; Prosser, J.I.; Nicol, G.W. Ammonia Concentration Determines Differential Growth of Ammonia-Oxidising Archaea and Bacteria in Soil Microcosms. ISME J. 2011, 5, 1067–1071. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, J.; Li, H.; Li, T.; Hou, R.; Liu, D.; Ji, Y. Effects of Biochar Amendment on Nitrogen Mineralization in Black Soil with Different Moisture Contents under Freeze-Thaw Cycles. Geoderma 2019, 353, 459–467. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Zhang, J.; Hu, Z.; Liang, S. Preparation and Evaluation of Wetland Plant-Based Biochar for Nitrogen Removal Enhancement in Surface Flow Constructed Wetlands. Environ. Sci. Pollut. Res. 2018, 25, 13929–13937. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Yang, J.; Xing, M.; Li, X.; Yi, D.; Deng, D. Earthworm-Microorganism Interactions: A Strategy to Stabilize Domestic Wastewater Sludge. Water Res. 2010, 44, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of Biochar on Nitrogen Transformation and Heavy Metals in Sludge Composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Wu, Y.; Yin, X.A.; Zhang, G. Effects of Surface-Modified Biochars and Activated Carbon on the Transformation of Soil Inorganic Nitrogen and Growth of Maize under Chromium Stress. Chemosphere 2019, 227, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Knowles, O.A.; Robinson, B.H.; Contangelo, A.; Clucas, L. Biochar for the Mitigation of Nitrate Leaching from Soil Amended with Biosolids. Sci. Total Environ. 2011, 409, 3206–3210. [Google Scholar] [CrossRef]
- Cardelli, R.; Becagli, M.; Marchini, F.; Saviozzi, A. Effect of Biochar, Green Compost, and Vermicompost on the Quality of a Calcareous Soil: A 1-Year Laboratory Experiment. Soil Sci. 2017, 182, 248–255. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Zhang, Q.; Liu, C. Long-Term Effects of Biochar Addition and Straw Return on N2O Fluxes and the Related Functional Gene Abundances under Wheat-Maize Rotation System in the North China Plain. Appl. Soil Ecol. 2019, 135, 44–55. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Luo, J.; Zhang, L.; Wu, Z.; Lindsey, S. Effects of Biochar and 3,4-Dimethylpyrazole Phosphate (DMPP) on Soil Ammonia-Oxidizing Bacteria and NosZ-N2O Reducers in the Mitigation of N2O Emissions from Paddy Soils. J. Soils Sediments 2021, 21, 1089–1098. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Z.; Azari, M.; Kartal, B.; Du, H.; Cai, M.; Herbold, C.W.; Ding, X.; Denecke, M.; Li, X.; et al. Discovery of a New Genus of Anaerobic Ammonium Oxidizing Bacteria with a Mechanism for Oxygen Tolerance. Water Res. 2022, 226, 119165. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Yang, X.; Zhou, Y.; Zhang, L.; Yang, Y.; Luo, L.; Yan, Q. Responses of Ammonia-Oxidizing Microorganisms to Biochar and Compost Amendments of Heavy Metals-Polluted Soil. J. Environ. Sci. 2021, 102, 263–272. [Google Scholar] [CrossRef]
- Kumar, S.; Pratap, B.; Dubey, D.; Dutta, V. Microbial Communities in Constructed Wetland Microcosms and Their Role in Treatment of Domestic Wastewater. In Emerging Eco-Friendly Green Technologies for Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2020; pp. 311–327. [Google Scholar]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial Functional Genes Involved in Nitrogen Fixation, Nitrification and Denitrification in Forest Ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, L.; Xu, X.; Meng, Q.; Men, M.; Xu, B.; Deng, L. Correlation between Ammonia-Oxidizing Microorganisms and Environmental Factors during Cattle Manure Composting. Rev. Argent. Microbiol. 2019, 51, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Schleper, C.; Wagner, M. The Thaumarchaeota: An Emerging View of Their Phylogeny and Ecophysiology. Curr. Opin. Microbiol. 2011, 14, 300–306. [Google Scholar] [CrossRef] [PubMed]
| Layers | AOA/nirS (ng/g) | AOB × 103/nirK (ng/g) | ||
|---|---|---|---|---|
| Without BC | With BC | Without BC | With BC | |
| V | 0.017 ± 0.006 b/ 0.15 ± 0.03 b | 0.023 ± 0.004 a/ 0.33 ± 0.13 a | 0.56 ± 0.39 a/ 0.87 ± 0.35 b | 0.94 ± 0.84 a/ 1.79 ± 0.07 a |
| C | 0.010 ± 0.005 a/ 0.1 ± 0.09 b | 0.012 ± 0.005 a/ 0.17 ± 0.02 a | 0.45 ± 0.16 b/ 1.47 ± 0.82 a | 0.63 ± 0.19 a/ 1.74 ± 1.01 a |
| M | 0.010 ± 0.001 b/ 0.84 ± 0.07 b | 0.019 ± 0.001 a/ 1.71 ± 0.39 a | 1.40 ± 0.26 b/ 1.72 ± 0.76 b | 12.71 ± 1.76 a/ 3.86 ± 0.62 a |
| Total | 0.038 ± 0.011 b/ 1.09 ± 0.19 b | 0.054 ± 0.010 a/ 2.22 ± 1.16 a | 2.41 ± 0.81 b/ 4.05 ± 2.5 b | 14.33 ± 1.06 a/ 7.39 ± 2.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, T.; Twagirayezu, G.; Wang, Z.; Xia, H.; Sang, C.; Huang, K.; Cheng, H. Biochar Amendment in Vermi-Wetland for Enhancing Nitrification during Excess Sludge Recycling. Sustainability 2023, 15, 16551. https://doi.org/10.3390/su152416551
Bai T, Twagirayezu G, Wang Z, Xia H, Sang C, Huang K, Cheng H. Biochar Amendment in Vermi-Wetland for Enhancing Nitrification during Excess Sludge Recycling. Sustainability. 2023; 15(24):16551. https://doi.org/10.3390/su152416551
Chicago/Turabian StyleBai, Ting, Gratien Twagirayezu, Zhen Wang, Hui Xia, Chunlei Sang, Kui Huang, and Hongguang Cheng. 2023. "Biochar Amendment in Vermi-Wetland for Enhancing Nitrification during Excess Sludge Recycling" Sustainability 15, no. 24: 16551. https://doi.org/10.3390/su152416551






