Effects of Replacing Fishmeal with Algal Biomass (Pavlova sp. 459) on Membrane Lipid Composition of Atlantic Salmon (Salmo salar) Parr Muscle and Liver Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Fish and Set-Up
2.3. Tissue Sampling
2.4. Ethical Approval
2.5. Lipid Extraction
2.6. Fatty Acid Methyl Ester (FAME) Derivatization
2.7. Neutral Lipid/Polar Lipid Separation
2.8. Sterol Derivatization and Analysis
2.9. Quantitative Lipid Analysis
2.10. Compound-Specific Stable Isotope Analysis (CSIA)
2.11. Statistical Analysis
3. Results
3.1. Pavlova sp. 459 Oil and Experimental Diets Composition
3.2. Growth Performance
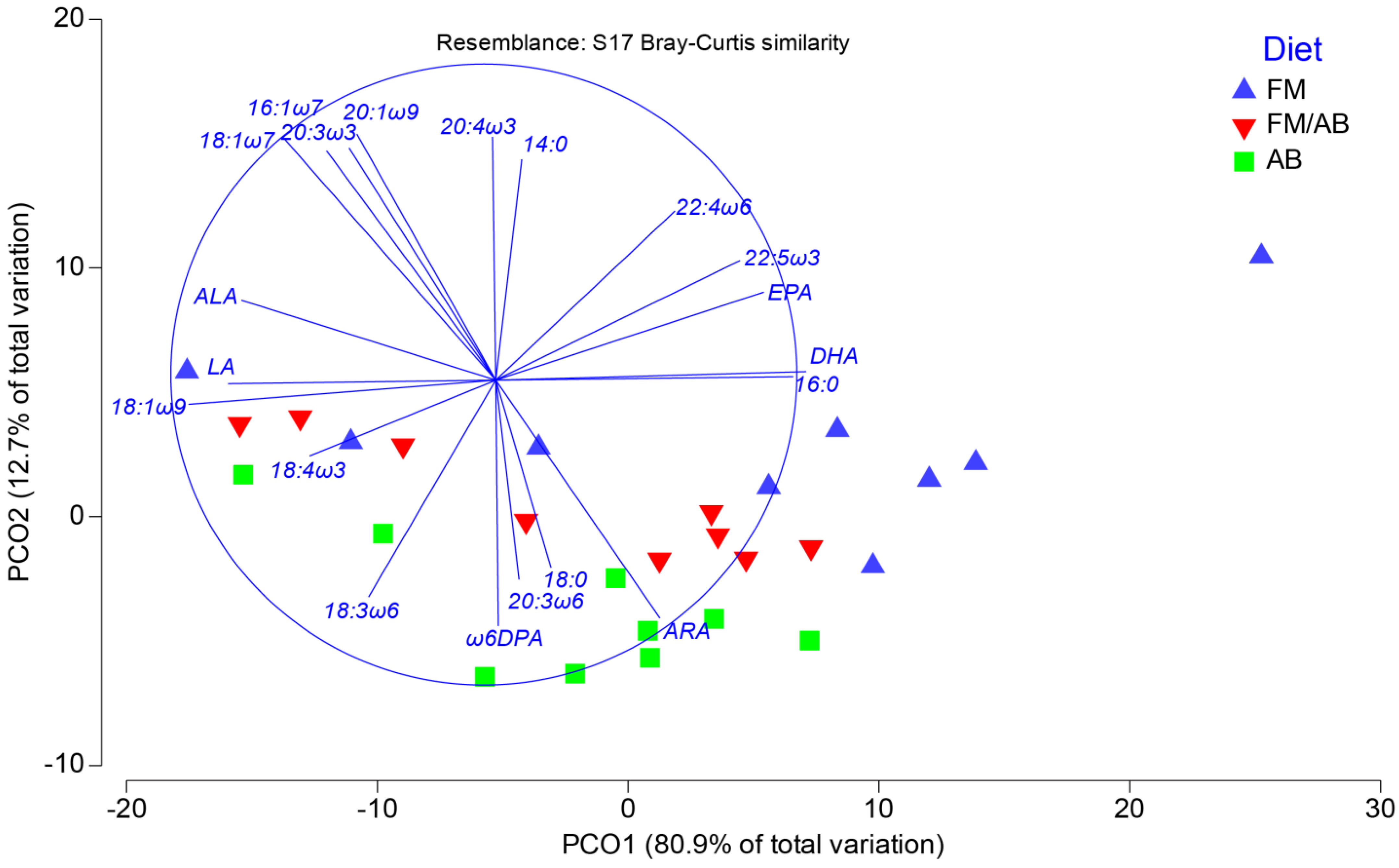
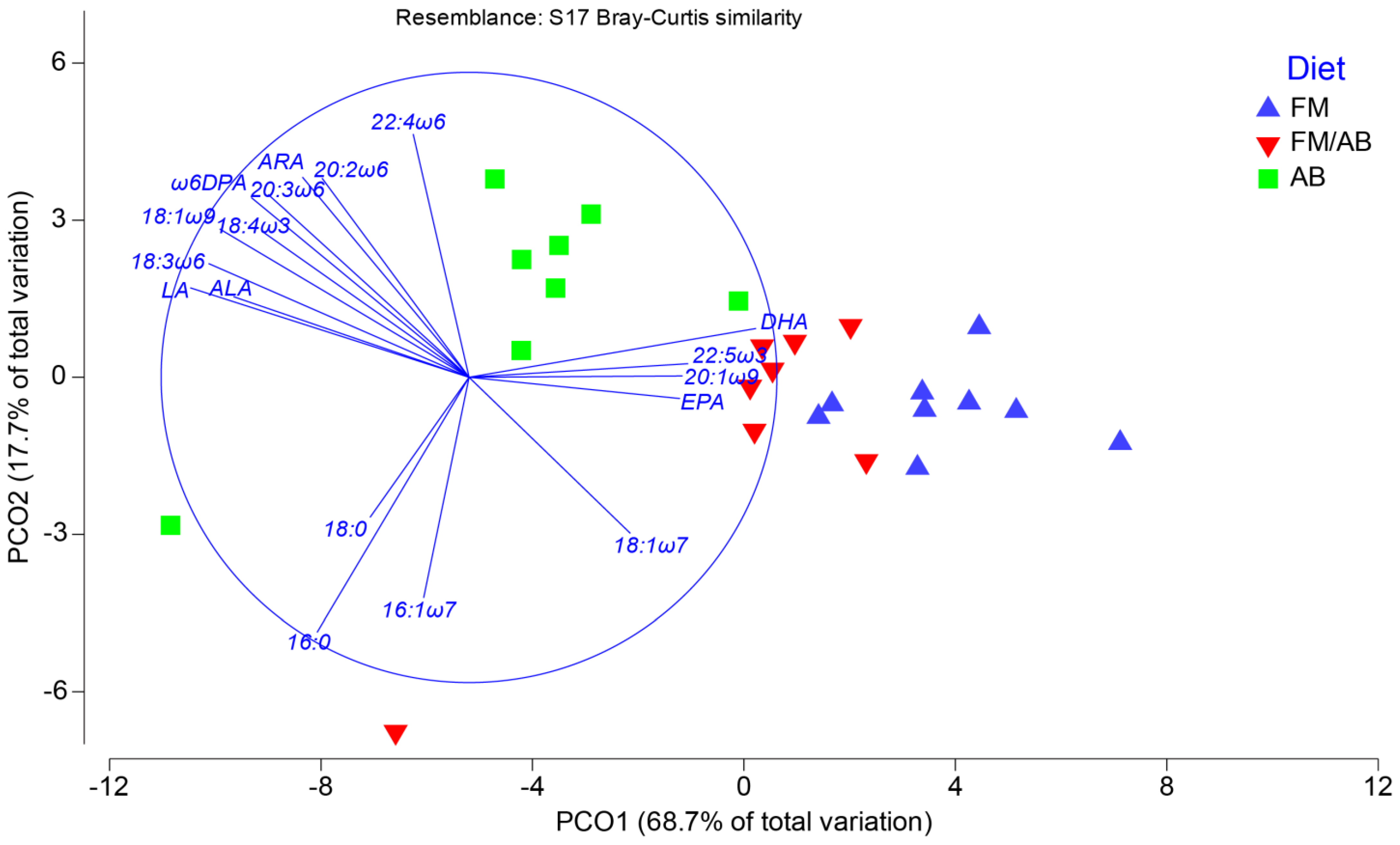
3.3. Liver Tissue Lipid Classes and Fatty Acid Composition
3.4. Liver Tissue Phospholipid Fatty Acid Composition
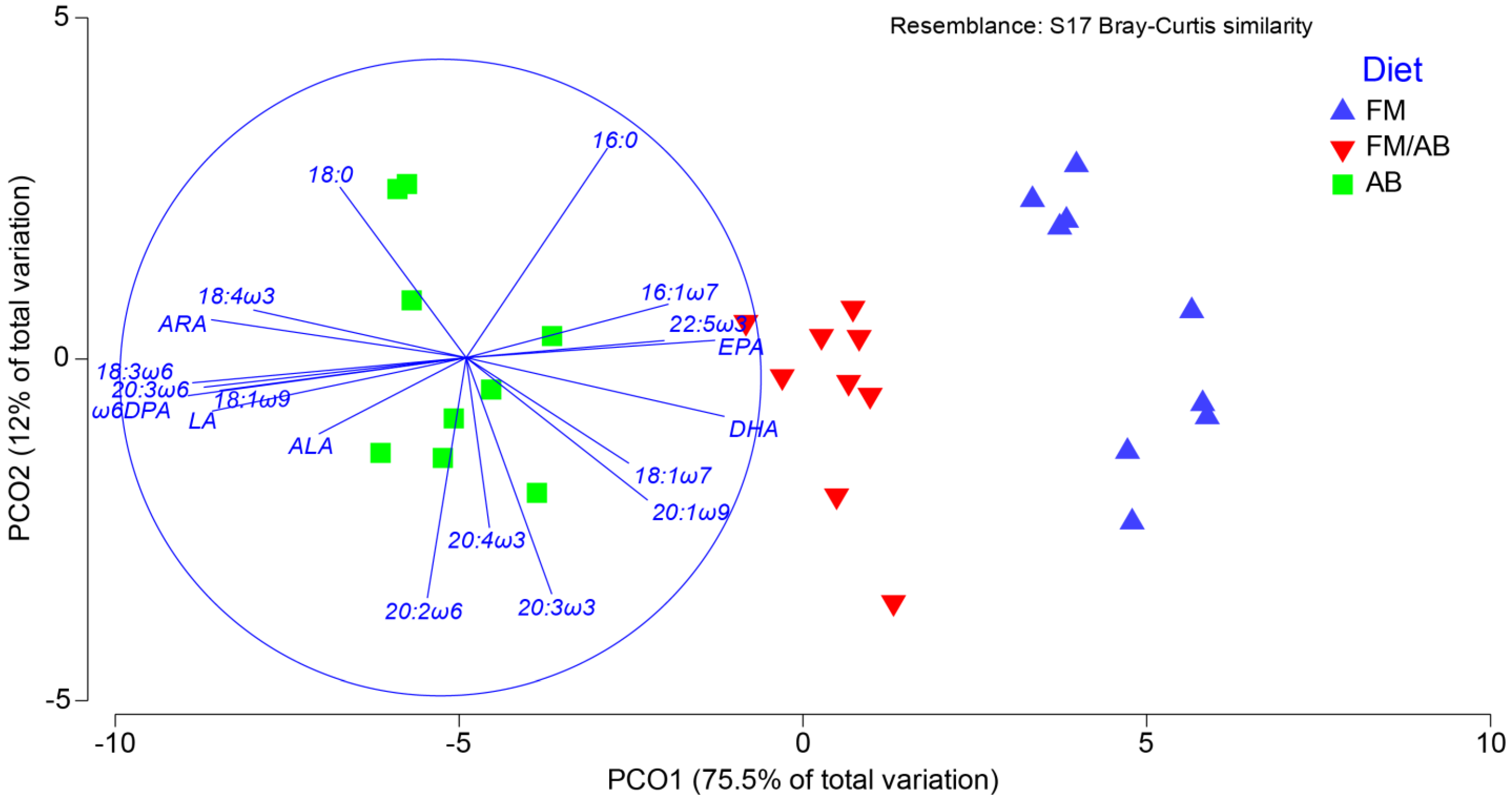
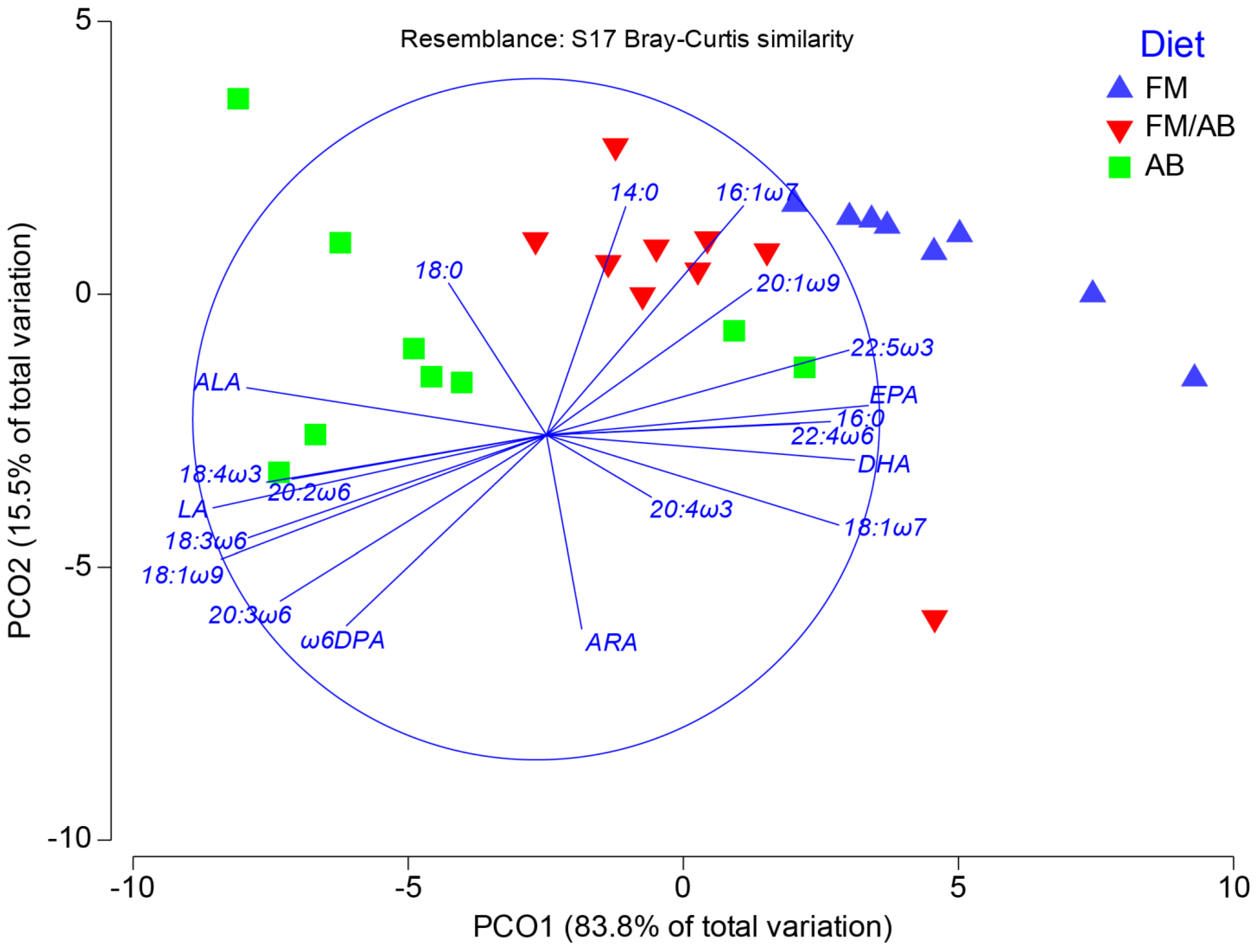
3.5. Muscle Tissue Lipid Class and Fatty Acid Composition
3.6. Muscle Tissue Phospholipid Fatty Acid Composition
3.7. Muscle Tissue Sterol Composition
3.8. Compound-Specific Stable Isotope Analysis
4. Discussion
4.1. Liver Tissue
4.2. Muscle Tissue
5. Compound-Specific Stable Isotope Analysis Reveals Pav459 Contribution to EPA and DHA in Atlantic Salmon Muscle and Liver tissues
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ingredient (g/kg) 1 | FM | FM/AB | AB |
|---|---|---|---|
| Pav459 algal biomass 2 | 0 | 100 | 200 |
| Fish meal | 200 | 100 | 0 |
| Fish (herring) oil | 70 | 45 | 17.5 |
| Ground wheat | 149 | 117.7 | 87.8 |
| Soy protein concentrate | 84 | 107 | 127.5 |
| Corn protein concentrate | 140 | 140 | 140 |
| Poultry fat | 55 | 67.5 | 78.75 |
| Canola oil | 55 | 67.5 | 78.75 |
| Poultry by-product meal | 150 | 150 | 150 |
| Blood meal | 40 | 40 | 40 |
| Vitamin/mineral mix 3 | 2 | 2 | 2 |
| Special pre-mix 4 | 20 | 20 | 20 |
| Dicalcium phosphate | 22 | 25 | 30 |
| L-lysine | 0.5 | 3 | 8.5 |
| L-methionine | 1 | 2.8 | 3.9 |
| Choline chloride | 10.5 | 10.5 | 10.5 |
| L-tryptophan | 1 | 2 | 3 |
| Threonine | 0 | 0 | 1.8 |
| TOTAL | 1000 | 1000 | 1000 |
| Proximate Composition (%) | |
|---|---|
| Dry matter | 95.23 |
| Ash | 9.80 |
| Crude protein, N 6.25 × (%) | 60.87 |
| Esterifiable lipid | 10.68 |
| Crude lipid | 12.25 |
| Minerals (%) | |
| Calcium | 0.36 |
| Magnesium | 0.41 |
| Phosphorous | 1.20 |
| Potassium | 1.40 |
| Sodium | 1.23 |
| Carotenoids (mg/100 g) | |
| Astaxanthin | 9.09 |
| Canthaxanthin | 40.61 |
| Chlorophyll a | 2259.69 |
| Chlorophyll b | 62.10 |
| α-carotene | 5.39 |
| β-carotene | 65.87 |
| Fucoxanthin | 630.07 |
| Lutein | 266.50 |
| Neoxanthin | 6.87 |
| Zeaxanthi | 106.25 |
| FM | FM/AB | AB | |
|---|---|---|---|
| Proximate Composition (%) | |||
| Dry matter | 94.4 ± 0.3 a | 93.7 ± 0.0 b | 91.5 ± 0.1 c |
| Crude protein | 48.0 ± 0.3 | 48.5 ± 0.4 | 49.0 ± 0.3 |
| Crude fat | 22.9 ± 0.4 | 22.9 ± 0.2 | 23.0 ± 0.1 |
| Ash | 8.2 ± 0.1 a | 7.6 ± 0.2 b | 6.7 ± 0.1 c |
| Minerals 1 | |||
| Calcium (%) | 2.1 ± 0.06 a | 1.7 ± 0.0 b | 1.3 ± 0.0 c |
| Potassium (%) | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| Magnesium (%) | 0.3 ± 0.0 b | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Phosphorus (%) | 1.6 ± 0.0 a | 1.5 ± 0.2 b | 1.3 ± 0.0 c |
| Sodium (%) | 0.4 ± 0.0 b | 0.4 ± 0.2 ab | 0.5 ± 0.0 a |
| Copper (ppm) | 11.7 ± 3. | 12.5 ± 0.5 | 18.0 ± 0.8 |
| Iron (ppm) | 489.5 ± 6.0 c | 880.5 ± 10.2 b | 1318.6 ± 0.2 a |
| Manganese (ppm) | 27.2 ± 2.0 c | 33.9 ± 0.5 b | 43.0 ± 0.0 a |
| Zinc (ppm) | 179.4 ± 8.4 a | 150.1 ± 1.3 b | 120.8 ± 3.4 c |
Appendix B
| FM | FM/AB | AB | |||
|---|---|---|---|---|---|
| Average Similarity: 82.6 | Average Similarity: 89.1 | Average Similarity: 90.4 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| DHA | 23.85 | 18:1ω9 | 28.95 | 18:1ω9 | 28.62 |
| 18:1ω9 | 21.94 | DHA | 20.58 | DHA | 20.09 |
| 16:0 | 14.33 | 16:0 | 11.75 | 16:0 | 11.93 |
| LA | 8.52 | LA | 10.08 | LA | 10.03 |
| EPA | 6.15 | - | - | - | - |
| FM & FM/AB | FM &AB | FM/AB &AB | |||
| Average dissimilarity = 15.4 | Average dissimilarity = 16.8 | Average dissimilarity = 10.7 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| 18:1ω9 | 31.30 | 18:1ω9 | 27.33 | 18:1ω9 | 26.28 |
| DHA | 26.68 | DHA | 24.57 | DHA | 21.92 |
| 16:0 | 8.47 | 16:0 | 7.20 | 16:0 | 8.48 |
| LA | 6.91 | LA | 6.99 | LA | 5.86 |
| - | - | EPA | 6.59 | ARA | 5.76 |
| - | - | - | - | EPA | 4.89 |
| FM | FM/AB | AB | |||
|---|---|---|---|---|---|
| Average Similarity: 96.1 | Average Similarity: 97.0 | Average Similarity: 96.6 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| DHA | 37.96 | DHA | 36.72 | DHA | 33.58 |
| 16:0 | 18.23 | 16:0 | 17.38 | 16:0 | 16.79 |
| 18:1ω9 | 12.01 | 18:1ω9 | 12.82 | 18:1ω9 | 13.41 |
| EPA | 7.28 | EPA | 5.71 | LA | 6.16 |
| - | - | - | - | 18:0 | 5.64 |
| FM & FM/AB | FM & AB | FM/AB & AB | |||
| Average dissimilarity = 5.5 | Average dissimilarity = 10.3 | Average dissimilarity = 6.4 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| EPA | 13.64 | DHA | 20.19 | DHA | 25.35 |
| DHA | 12.38 | EPA | 12.59 | ω6DPA | 10.89 |
| ω6DPA | 10.23 | ω6DPA | 12.18 | ARA | 10.34 |
| 16:0 | 8.94 | ARA | 10.30 | EPA | 9.31 |
| LA | 8.68 | LA | 7.45 | 16:0 | 7.59 |
| ARA | 8.58 | 18:1ω9 | 7.06 | 20:3ω6 | 6.95 |
| 18:1ω9 | 7.52 | 20:3ω6 | 6.75 | - | - |
| 18:0 | 7.38 | - | - | - | - |
| FM | FM/AB | AB | |||
|---|---|---|---|---|---|
| Average Similarity: 90.7 | Average Similarity: 95.3 | Average Similarity: 92.8 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| 18:1ω9 | 31.13 | 18:1ω9 | 35.00 | 18:1ω9 | 38.49 |
| 16:0 | 17.20 | 16:0 | 15.20 | LA | 17.12 |
| LA | 14.05 | LA | 14.83 | 16:0 | 11.40 |
| DHA | 9.08 | DHA | 9.04 | DHA | 7.31 |
| FM & FM/AB | FM & AB | FM/AB & AB | |||
| Average dissimilarity = 8.8 | Average dissimilarity = 13.6 | Average dissimilarity = 7.9 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| 18:1ω9 | 28.19 | 18:1ω9 | 25.86 | 18:1ω9 | 21.66 |
| DHA | 15.14 | 16:0 | 13.83 | DHA | 17.41 |
| LA | 9.78 | DHA | 11.84 | 16:0 | 16.11 |
| 16:0 | 8.35 | LA | 11.53 | LA | 10.94 |
| 14:0 | 7.18 | 16:1ω7 | 6.39 | 18:0 | 5.88 |
| 16:1ω7 | 6.53 | 14:0 | 4.91 | - | - |
| FM | FM/AB | AB | |||
|---|---|---|---|---|---|
| Average Similarity: 96.5 | Average Similarity: 87.0 | Average Similarity: 94.8 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| DHA | 40.08 | DHA | 34.69 | DHA | 34.82 |
| 16:0 | 20.50 | 16:0 | 23.54 | 16:0 | 21.25 |
| 18:1ω9 | 10.00 | 18:1ω9 | 11.79 | 18:1ω9 | 12.09 |
| - | - | - | - | EPA | 6.89 |
| FM & FM/AB | FM & AB | FM/AB & AB | |||
| Average similarity: 96.0 | Average dissimilarity = 9.9 | Average dissimilarity = 9.1 | |||
| FAs | Contribution | FAs | Contribution | FAs | Contribution |
| DHA | 31.05 | DHA | 28.61 | DHA | 35.42 |
| 16:0 | 20.33 | 18:1ω9 | 11.44 | 16:0 | 18.61 |
| EPA | 12.11 | ω6DPA | 11.28 | 18:1ω9 | 10.32 |
| 18:1ω9 | 9.17 | EPA | 11.15 | ω6DPA | 6.03 |
| - | - | LA | 8.31 | - | - |
Appendix C
| Sample ID | Amount (mg) | Mean δ13CVPDB/‰ of All Analyses 1 | St. Dev. of δ13CVPDB/‰ of All Analyses 2 |
|---|---|---|---|
| L-glutamic acid | 1.124 | −26.67 | 0.07 |
| L-glutamic acid | 1.201 | −26.67 | 0.07 |
| L-glutamic acid | 1.186 | −26.67 | 0.07 |
| L-glutamic acid | 1.143 | −26.67 | 0.07 |
| Blank capsule | 0.000 | ||
| Blank capsule | 0.000 | ||
| Blank capsule | 0.000 | ||
| EDTA #2 | 1.285 | −40.38 | 0.08 |
| EDTA #2 | 1.159 | −40.38 | 0.08 |
| D-Fructose | 1.197 | −10.53 | 0.08 |
| D-Fructose | 1.121 | −10.53 | 0.08 |
| L-glutamic acid | 1.697 | −26.67 | 0.07 |
| L-glutamic acid | 1.566 | −26.67 | 0.07 |
| L-glutamic acid | 0.958 | −26.67 | 0.07 |
| L-glutamic acid | 0.658 | −26.67 | 0.07 |
| L-glutamic acid | 0.415 | −26.67 | 0.07 |
| Pav 459-1 | 1.258 | −56.17 | |
| EDTA #2 | 1.280 | −40.38 | 0.08 |
| D-Fructose | 1.175 | −10.53 | 0.08 |
| Pav 459-2 | 1.215 | −55.60 | |
| Pav 459-3 | 1.273 | −55.30 | |
| L-glutamic acid | 1.152 | −26.67 | 0.07 |
| EDTA #2 | 1.246 | −40.38 | 0.08 |
| D-Fructose | 1.188 | −10.53 | 0.08 |
| L-glutamic acid | 1.159 | −26.67 | 0.07 |
References
- Fry, J.P.; Love, D.C.; MacDonald, G.K.; West, P.C.; Engstrom, P.M.; Nachman, K.E.; Lawrence, R.S. Environmental health impacts of feeding crops to farmed fish. Environ. Int. 2016, 91, 201–214. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Bandara, T. Alternative feed ingredients in aquaculture: Opportunities and challenges. J. Entomol. Zool. Stud. 2018, 6, 3087–3094. [Google Scholar]
- Stenberg, O.K.; Holen, E.; Piemontese, L.; Liland, N.S.; Lock, E.J.; Espe, M.; Belghit, I. Effect of dietary replacement of fish meal with insect meal on in vitro bacterial and viral induced gene response in Atlantic salmon (Salmo salar) head kidney leukocytes. Fish Shellfish Immunol. 2019, 91, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Burr, G.S.; Wolters, W.R.; Barrows, F.T.; Hardy, R.W. Replacing fishmeal with blends of alternative proteins on growth performance of rainbow trout (Oncorhynchus mykiss), and early or late stage juvenile Atlantic salmon (Salmo salar). Aquaculture 2012, 334, 110–116. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; Senadheera, S.P.S.D.; Thanuthong, T.; De Silva, S.S. Fish oil replacement with different vegetable oils in Murray cod: Evidence of an “omega-3 sparing effect” by other dietary fatty acids. Aquaculture 2011, 315, 250–259. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Fowler, L.G. Poultry by-product meal as a dietary protein source in fall chinook salmon diets. Aquaculture 1991, 99, 309–321. [Google Scholar] [CrossRef]
- Galkanda-Arachchige, H.S.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Kiron, V.; Sørensen, M.; Huntley, M.; Vasanth, G.K.; Gong, Y.; Dahle, D.; Palihawadana, A.M. Defatted biomass of the microalga, Desmodesmus sp., can replace fishmeal in the feeds for Atlantic salmon. Front. Mar. Sci. 2016, 3, 67. [Google Scholar] [CrossRef]
- Zatti, K.M.; Ceballos, M.J.; Vega, V.V.; Denstadli, V. Full replacement of fish oil with algae oil in farmed Atlantic salmon (Salmo salar)–Debottlenecking omega 3. Aquaculture 2023, 574, 739653. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Khanjani, M.H.; Mozanzadeh, M.T.; Sharifinia, M.; Emerenciano, M.G.C. Biofloc: A sustainable dietary supplement, nutritional value and functional properties. Aquaculture 2023, 562, 738757. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Patelakis, S.J.; Whitney-Lalonde, C.G.; Garrison, L.L.; Wall, C.L.; MacQuarrie, S.P. Nutrient composition and protein quality of microalgae meals produced from the marine prymnesiophyte Pavlova sp. 459 mass-cultivated in enclosed photobioreactors for potential use in salmonid aquafeeds. J. Appl. Phycol. 2020, 32, 299–318. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Patelakis, S.J.J. Apparent digestibility coefficients (ADCs) of intact-cell marine microalgae meal (Pavlova sp. 459) for juvenile Atlantic salmon (Salmo salar L.). Aquaculture 2022, 546, 737236. [Google Scholar] [CrossRef]
- Volkman, J.K. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 2003, 60, 495–506. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Wei, M.; Parrish, C.C.; Guerra, N.I.; Tibbetts, S.M.; Colombo, S.M. Dietary inclusion of a marine microalgae meal for Atlantic salmon (Salmo salar): Impact of Pavlova sp. 459 on growth performance and tissue lipid composition. Aquaculture 2022, 553, 738084. [Google Scholar] [CrossRef]
- Parrish, C.C. Determination of total lipid, lipid classes, and fatty acids in aquatic samples. In Lipids in Freshwater Ecosystems; Springer: New York, NY, USA, 1999; pp. 4–20. [Google Scholar]
- Hailat, I.; Helleur, R.J. Direct analysis of sterols by derivatization matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Leng, Y.; Lu, Q.; Zhou, W. The application of microalgae biomass and bio-products as aquafeed for aquaculture. Algal Res. 2021, 60, 102541. [Google Scholar] [CrossRef]
- Turchini, G.; Mailer, R.J. Rapeseed (canola) oil and other monounsaturated fatty acid-rich vegetable oils. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2011; pp. 161–208. [Google Scholar]
- Bureau, D.P.; Meeker, D.L. Terrestrial animal fats. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2010; pp. 245–266. [Google Scholar]
- Campos, I.; Matos, E.; Maia, M.R.; Marques, A.; Valente, L.M. Partial and total replacement of fish oil by poultry fat in diets for European seabass (Dicentrarchus labrax) juveniles: Effects on nutrient utilization, growth performance, tissue composition and lipid metabolism. Aquaculture 2019, 502, 107–120. [Google Scholar] [CrossRef]
- De Silva, S.; Francis, D.S.; Tacon, A.G. Fish oil in aquaculture: In retrospect. In Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds; CRC Press: Boca Raton, FL, USA, 2011; pp. 1–20. [Google Scholar]
- Peterson, B.C.; Burr, G.S.; Barrows, F.T.; Block, S.; Bowzer, J.; Buentello, A. Growth performance of Atlantic salmon smolts fed diets containing heterotrophic algal biomass as replacement of fish oil. N. Am. J. Aquac. 2019, 81, 364–371. [Google Scholar] [CrossRef]
- Sørensen, M.; Berge, G.M.; Reitan, K.I.; Ruyter, B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar)—Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 2016, 460, 116–123. [Google Scholar] [CrossRef]
- Perez-Velazquez, M.; Gatlin III, D.M.; González-Félix, M.L.; García-Ortega, A.; de Cruz, C.R.; Juárez-Gómez, M.L.; Chen, K. Effect of fishmeal and fish oil replacement by algal meals on biological performance and fatty acid profile of hybrid striped bass (Morone crhysops♀× M. saxatilis♂). Aquaculture 2019, 507, 83–90. [Google Scholar] [CrossRef]
- Haas, S.; Bauer, J.L.; Adakli, A.; Meyer, S.; Lippemeier, S.; Schwarz, K.; Schulz, C. Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J. Appl. Phycol. 2016, 28, 1011–1021. [Google Scholar] [CrossRef]
- Pakravan, S.; Akbarzadeh, A.; Sajjadi, M.M.; Hajimoradloo, A.; Noori, F. Partial and total replacement of fish meal by marine microalga Spirulina platensis in the diet of Pacific white shrimp Litopenaeus vannamei: Growth, digestive enzyme activities, fatty acid composition and responses to ammonia and hypoxia stress. Aquac. Res. 2017, 48, 5576–5586. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary supplementation with ω6 LC-PUFA-rich algae modulates zebrafish immune function and improves resistance to streptococcal infection. Front. Immunol. 2018, 9, 1960. [Google Scholar] [CrossRef]
- Nayak, S.; Al Ashhab, A.; Zilberg, D.; Khozin-Goldberg, I. Dietary supplementation with omega-6 LC-PUFA-rich microalgae regulates mucosal immune response and promotes microbial diversity in the zebrafish gut. Biology 2020, 9, 119. [Google Scholar] [CrossRef]
- Long, X.; Wu, X.; Zhao, L.; Liu, J.; Cheng, Y. Effects of dietary supplementation with Haematococcus pluvialis cell powder on coloration, ovarian development and antioxidation capacity of adult female Chinese mitten crab, Eriocheir sinensis. Aquaculture 2017, 473, 545–553. [Google Scholar] [CrossRef]
- Skalli, A.; Firmino, J.P.; Andree, K.B.; Salomón, R.; Estévez, A.; Puig, P.; Sabater-Martínez, M.; Hechavarria, T.; Gisbert, E. The inclusion of the microalga Scenedesmus sp. in diets for rainbow trout, Onchorhynchus mykiss, juveniles. Animals 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Betancor, M.B.; Howarth, F.J.; Glencross, B.D.; Tocher, D.R. Influence of dietary docosahexaenoic acid in combination with other long-chain polyunsaturated fatty acids on expression of biosynthesis genes and phospholipid fatty acid compositions in tissues of post-smolt Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 172, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Zhol, S.; Ackman, R.G.; Morrison, C. Storage of lipids in the myosepta of Atlantic salmon (Salmo salar). Fish Physiol. Biochem. 1995, 14, 171–178. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Lund, E.K.; Harvey, L.J.; Ladha, S.; Clark, D.C.; Johnson, I.T. Effects of dietary fish oil supplementation on the phospholipid composition and fluidity of cell membranes from human volunteers. Ann. Nutr. Metab. 1999, 43, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 181–257. [Google Scholar]
- Stubhaug, I.; Lie, Ø.; Torstensen, B.E. Fatty acid productive value and β-oxidation capacity in Atlantic salmon (Salmo salar L.) fed on different lipid sources along the whole growth period. Aquac. Nutr. 2007, 13, 145–155. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Calder, P.C. n−3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- Arts, M.T.; Kohler, C.C. Health and condition in fish: The influence of lipids on membrane competency and immune response. In Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009; pp. 237–256. [Google Scholar]
- Sargent, J.; McEvoy, L.; Estevez, A.; Bell, G.; Bell, M.; Henderson, J.; Tocher, D. Lipid nutrition of marine fish during early development: Current status and future directions. Aquaculture 1999, 179, 217–229. [Google Scholar] [CrossRef]
- Milke, L.M.; Bricelj, V.M.; Parrish, C.C. Biochemical characterization and nutritional value of three Pavlova spp. in unialgal and mixed diets with Chaetoceros muelleri for postlarval sea scallops, Placopecten magellanicus. Aquaculture 2008, 276, 130–142. [Google Scholar] [CrossRef]
- Parrish, C.C.; Milke, L.M.; Bricelj, V.M. Characterisation of 4α-methyl sterols in Pavlova spp. and postlarval sea scallops, Placopecten magellanicus. Aquaculture 2011, 311, 261–262. [Google Scholar] [CrossRef]
- Volkman, J.K.; Kearney, P.; Jeffrey, S.W. A new source of 4-methyl sterols and 5α (H)-stanols in sediments: Prymnesiophyte microalgae of the genus Pavlova. Org. Geochem. 1990, 15, 489–497. [Google Scholar] [CrossRef]
- Ahmed, F.; Zhou, W.; Schenk, P.M. Pavlova lutheri is a high-level producer of phytosterols. Algal Res. 2015, 10, 210–217. [Google Scholar] [CrossRef]
- Volkman, J.K.; Barrett, S.M.; Blackburn, S.I. Eustigmatophyte microalgae are potential sources of C29 sterols, C22–C28 n-alcohols and C28–C32 n-alkyl diols in freshwater environments. Org. Geochem. 1999, 30, 307–318. [Google Scholar] [CrossRef]
- Véron, B.; Dauguet, J.C.; Billard, C. Sterolic biomarkers in marine phytoplankton. I. Free and conjugated sterols of Pavlova lutheri (Haptophyta). Eur. J. Phycol. 1996, 31, 211–215. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Merkel, P. Sterols of freshwater microalgae: Potential implications for zooplankton nutrition. J. Plankton Res. 2016, 38, 865–877. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Ruxton, C.H.S.; Calder, P.C.; Reed, S.C.; Simpson, M.J.A. The impact of long-chain n-3 polyunsaturated fatty acids on human health. Nutr. Res. Rev. 2005, 18, 113–129. [Google Scholar] [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Banni, S.; Carta, G. Nutritional properties of dietary omega-3-enriched phospholipids. BioMed Res. Int. 2013, 2013, 965417. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Moradi, Y.; Bakar, J.; Motalebi, A.A.; Syed Muhamad, S.H.; Che Man, Y. A review on fish lipid: Composition and changes during cooking methods. J. Aquat. Food Prod. Technol. 2011, 20, 379–390. [Google Scholar] [CrossRef]
- Phillips, D.L.; Newsome, S.D.; Gregg, J.W. Combining sources in stable isotope mixing models: Alternative methods. Oecologia 2005, 144, 520–527. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon isotopes in photosynthesis. Bioscience 1988, 38, 328–336. [Google Scholar] [CrossRef]
| Lipid Class Composition (%) | |
|---|---|
| Total lipid (mg/g) | 109.6 ± 10.4 |
| TAG | 24.7 ± 0.4 |
| FFA | 18.5 ± 1.1 |
| ST | 10.9 ± 1.0 |
| AMPL | 22.9 ± 1.7 |
| PL | 16.6 ± 1.4 |
| Fatty acid composition (%) | |
| 14:0 | 15.6 ± 0.1 |
| 15:0 | 0.2 ± 0.0 |
| 16:0 | 7.3 ± 0.2 |
| Total SFA 2 | 23.0 ± 0.2 |
| 16:1ω7 | 6.4 ± 0.0 |
| 16:1ω5 | 3.7 ± 0.0 |
| 18:1ω9 | 0.2 ± 0.0 |
| 18:1ω7 | 0.1 ± 0.0 |
| Total MUFA 3 | 11.2 ± 0.2 |
| 16:2ω4 | 2.2 ± 0.0 |
| 18:2ω6 (LA) | 3.5 ± 0.1 |
| 18:3ω6 | 0.4 ± 0.0 |
| 20:4ω6 (ARA) | 0.5 ± 0.5 |
| 22:5ω6 (ω6DPA) | 7.2 ± 0.1 |
| 18:3ω3 (ALA) | 3.6 ± 0.1 |
| 18:4ω3 | 6.3 ± 0.1 |
| 20:5ω3 (EPA) | 26.8 ± 0.1 |
| 22:5ω3 | 0.0 ± 0.0 |
| 22:6ω3 (DHA) | 13.6 ± 0.2 |
| Total PUFA 4 | 64.5 ± 0.3 |
| Total ω3 | 50.3 ± 0.3 |
| Total ω6 | 11.7 ± 0.4 |
| ω3/ω6 ratio | 4.3 ± 0.2 |
| EPA+DHA | 40.4 ± 0.3 |
| Sterol | Common Name | Formula | MW + TMS | Sterol Content (µg mg−1) of Lipids |
|---|---|---|---|---|
| Cholest-5-en-3β-ol | Cholesterol | 27:1Δ5 | 458 | 8.7 |
| 5α-cholestan-3β-ol | Cholestanol | 27:0, 5αΔ0 | 460 | 0.5 |
| 24-methylcholesta-5,22E-dien-3β-ol | Brassicasterol | 28:2, Δ5,22Me24 | 470 | 0.7 |
| 24-methylcholest-5-en-3β-ol | Campesterol | 28:1Δ5Me24 | 472 | 3.8 |
| 24-ethylcholesta-5,22E-dien-3β-ol | Stigmasterol | 29:2, Δ5,22Et24 | 484 | 41.7 |
| 24-ethyl-5α-cholest-22E-en-3β-ol * | Stigmasta-22-en-3-β-ol | 29:1, 5αΔ22Et24 | 486 | 21.6 |
| 23,24-dimethylcholest-5-en-3β-ol | 29:1Δ5 Me23,24 | 486 | 6.4 | |
| 4α-methyl-24-ethyl-5α-cholest-22E-en-3β-ol | 4α-Methylporiferast-22-enol | 30:1, 5αΔ22Me4Et24 | 500 | 12.3 |
| 24-ethyl-5α-cholest-22E-en-3β,4β-diol | 4-Desmethyl-22-dehydropavlovol | 29:0, 5αΔ22Et24OH | 502 | 0.2 |
| 5α-cholestan-3β,4β-diol | 24-Methylpavlovol | 30:0, 5αΔ°Me4,24OH | 504 | 0.5 |
| 4α-methyl-24-ethyl-5α-cholestan-3β,4β-diol | 24-Ethylpavlovol | 30:0, 5αΔ°Me4Et24OH | 518 | 2.0 |
| Total | 9.83% |
| FM | FM/AB | AB | |
|---|---|---|---|
| Total lipid (mg/g) | 144.2 ± 23.6 | 200.0 ± 38.2 | 174.5 ± 34.8 |
| TAG | 74.1 ± 1.8 | 75.1 ± 1.1 | 72.7 ± 2.2 |
| FFA | 9.0 ± 1.1 | 9.1 ± 0.3 | 8.5 ± 0.9 |
| ST | 3.5 ± 0.4 b | 7.5 ± 0.7 a | 4.7 ± 1.7 b |
| AMPL | 5.7 ± 0.8 b | 5.0 ± 0.7 b | 8.0 ± 1.0 a |
| Phospholipid | 6.3 ± 1.7 | 3.1 ± 0.5 | 6.1 ± 2.2 |
| Fatty acid composition (%) | |||
| 14:0 | 2.3 ± 0.4 | 1.9 ± 0.5 | 2.2 ± 0.1 |
| 16:0 | 14.9 ± 0.7 | 13.9 ± 0.8 | 14.0 ± 0.1 |
| 18:0 | 4.0 ± 0.2 | 4.3 ± 0.6 | 3.7 ± 0.0 |
| Total SFA 2 | 22.3 ± 0.9 | 21.4 ± 0.6 | 20.8 ± 0.1 |
| 16:1ω7 | 4.7 ± 0.2 a | 3.9 ± 0.5 ab | 3.8 ± 0.0 b |
| 16:1ω5 | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.4 ± 0.0 a |
| 18:1ω9 | 31.6 ± 0.3 c | 36.2 ± 1.2 b | 38.7 ± 0.3 a |
| 18:1ω7 | 2.6 ± 0.0 a | 2.5 ± 0.0 b | 2.3 ± 0.0 c |
| 20:1ω9 | 2.0 ± 0.1 a | 1.5 ± 0.3 b | 0.6 ± 0.1 c |
| Total MUFA 3 | 44.8 ± 0.6 | 47.1 ± 1.6 | 46.8 ± 0.2 |
| 16:2ω4 | 0.4 ± 0.0 a | 0.3 ± 0.0 b | 0.3 ± 0.0 b |
| 18:2ω6 (LA) | 14.6 ± 0.2 c | 16.0 ± 0.7 b | 18.8 ± 0.0 a |
| 18:3ω6 | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.1 ± 0.0 b |
| 20:3ω6 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:4ω6 (ARA) | 0.4 ± 0.0 a | 0.4 ± 0.0 b | 0.3 ± 0.0 b |
| 22:5ω6 (ω6DPA) | 0.1 ± 0.0 c | 0.4 ± 0.0 b | 0.6 ± 0.0 a |
| 18:3ω3 (ALA) | 2.8 ± 0.1 b | 3.2 ± 0.3 b | 3.9 ± 0.0 a |
| 18:4ω3 | 0.9 ± 0.0 b | 0.9 ± 0.1 b | 1.2 ± 0.0 a |
| 20:4ω3 | 0.3 ± 0.0 a | 0.2 ± 0.0 b | 0.1 ± 0.0 c |
| 20:5ω3 (EPA) | 5.3 ± 0.2 a | 4.4 ± 0.3 b | 3.8 ± 0.0 c |
| 22:5ω3 | 0.7 ± 0.0 a | 0.4 ± 0.0 b | 0.2 ± 0.0 c |
| 22:6ω3 (DHA) | 4.4 ± 0.2 a | 3.2 ± 0.2 b | 1.9 ± 0.1 c |
| Total PUFA 4 | 32.3 ± 0.8 | 31.0 ± 1.3 | 32.0 ± 0.2 |
| Total ω3 | 14.8 ± 0.5 a | 12.4 ± 0.7 b | 11.1 ± 0.1 c |
| Total ω6 | 16.1 ± 0.2 c | 17.5 ± 0.7 b | 20.4 ± 0.1 a |
| ω3/ω6 ratio | 0.9 ± 0.0 a | 0.7 ± 0.0 b | 0.5 ± 0.0 c |
| EPA+DHA | 9.7 ± 0.4 a | 7.5 ± 0.4 b | 5.6 ± 0.1 c |
| DHA/EPA ratio | 0.8 ± 0.0 a | 0.7 ± 0.1 b | 0.5 ± 0.0 c |
| EPA/ARA ratio | 12.5 ± 0.4 | 12.3 ± 1.0 | 11.7 ± 0.9 |
| DHA/ARA ratio | 10.4 ± 0.2 a | 8.8 ± 0.1 b | 5.7 ± 0.4 c |
| Initial | FM | FM/AB | AB | |
|---|---|---|---|---|
| Total lipid (mg/g) | 18.3 ± 4.5 | 28.3 ± 4.9 | 24.4 ± 4.5 | 26.3 ± 8.3 |
| Neutral Lipid | 60.7 ± 4.6 | 65.1 ± 5.8 | 62.1 ± 6.5 | 62.1 ± 4.7 |
| Polar Lipid | 39.3 ± 4.6 | 34.9 ± 5.8 | 37.9 ± 6.5 | 37.9 ± 4.7 |
| Lipid class composition (%) | ||||
| TAG | 1.5 ± 1.2 | 12.4 ± 16.3 | 10.1 ± 10.6 | 6.8 ± 7.0 |
| FFA | 30.8 ± 2.5 | 30.2 ± 8.8 | 25.4 ± 6.3 | 28.7 ± 5.3 |
| ST | 26.9 ± 3.9 | 20.4 ± 4.1 | 23.9 ± 3.5 | 21.9 ± 3.6 |
| PL | 26.7 ± 3.2 | 23.4 ± 5.4 | 25.4 ± 5.3 | 24.1 ± 4.3 |
| PL/ST ratio 2 | 1.0 ± 0.2 | 1.2 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.1 |
| Fatty acid composition (%) | ||||
| 14:0 | 1.5 ± 0.1 | 1.2 ± 0.2 a | 1.1 ± 0.1 ab | 1.0 ± 0.1 b |
| 16:0 | 18.0 ± 1.3 | 12.6 ± 2.3 | 11.1 ± 1.8 | 11.2 ± 1.4 |
| 18:0 | 5.6 ± 0.5 | 4.2 ± 0.5 | 3.9 ± 0.4 | 4.4 ± 0.8 |
| Total SFA 3 | 25.7 ± 0.9 | 18.4 ± 2.3 | 16.6 ± 2.2 | 17.1 ± 1.9 |
| 16:1ω7 | 2.4 ± 0.2 | 2.5 ± 0.5 a | 2.4 ± 0.3 ab | 2.0 ± 0.2 b |
| 16:1ω5 | 0.1 ± 0.0 | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.3 ± 0.1 a |
| 18:1ω9 | 11.4 ± 1.6 | 23.0 ± 9.9 | 28.0 ± 5.6 | 27.5 ± 4.4 |
| 18:1ω7 | 2.5 ± 0.2 | 2.7 ± 0.3 | 2.6 ± 0.3 | 2.4 ± 0.4 |
| Total MUFA 4 | 17.6 ± 2.3 | 32.9 ± 11.2 | 37.7 ± 7.0 | 36.0 ± 5.5 |
| 18:2ω6 (LA) | 4.3 ± 0.3 | 7.7 ± 1.7 b | 9.2 ± 1.0 ab | 9.5 ± 1.2 a |
| 18:3ω6 | 0.2 ± 0.0 | 0.2 ± 0.0 c | 0.3 ± 0.0 b | 0.4 ± 0.1 a |
| 20:3ω6 | 0.9 ± 0.2 | 1.2 ± 0.3 b | 1.4 ± 0.1 b | 2.2 ± 0.3 a |
| 20:4ω6 (ARA) | 4.6 ± 0.6 | 2.5 ± 0.8 b | 2.7 ± 0.8 b | 3.7 ± 0.7 a |
| 22:5ω6 (ω6DPA) | 0.9 ± 0.1 | 0.4 ± 0.1 c | 1.0 ± 0.2 b | 1.8 ± 0.3 a |
| 18:3ω3 (ALA) | 0.5 ± 0.1 | 0.9 ± 0.3 | 1.1 ± 0.2 | 1.0 ± 0.2 |
| 18:4ω3 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.1 |
| 20:4ω3 | 0.5 ± 0.1 | 0.7 ± 0.2 a | 0.6 ± 0.1 ab | 0.5 ± 0.1 b |
| 20:5ω3 (EPA) | 7.4 ± 1.2 | 5.5 ± 1.2 a | 4.0 ± 0.9 b | 3.5 ± 0.8 b |
| 22:5ω3 | 2.7 ± 0.5 | 1.3 ± 0.2 a | 0.9 ± 0.1 b | 0.8 ± 0.2 b |
| 22:6ω3 (DHA) | 32.5 ± 1.8 | 23.7 ± 8.2 | 20.3 ± 4.5 | 19.6 ± 3.6 |
| Total PUFA 5 | 56.7 ± 1.7 | 48.3 ± 9.0 | 45.4 ± 5.1 | 46.7 ± 3.9 |
| Total ω3 | 43.9 ± 1.8 | 32.6 ± 9.2 | 27.6 ± 5.2 | 26.0 ± 4.2 |
| Total ω6 | 11.6 ± 0.9 | 14.1 ± 1.2 c | 16.5 ± 0.5 b | 19.7 ± 0.5 a |
| ω3/ω6 ratio | 3.8 ± 0.4 | 2.3 ± 0.7 a | 1.7 ± 0.3 b | 1.3 ± 0.2 b |
| EPA+DHA | 39.9 ± 1.7 | 29.2 ± 9.3 a | 24.3 ± 5.4 b | 23.1 ± 4.3 b |
| DHA/EPA ratio | 4.5 ± 0.9 | 4.2 ± 0.7 b | 5.1 ± 0.5 a | 5.7 ± 0.5 a |
| EPA/ARA ratio | 1.6 ± 0.2 | 2.3 ±0.5 a | 1.5 ± 0.3 b | 0.9 ± 0.2 c |
| DHA/ARA ratio | 7.2 ± 1.1 | 9.5 ± 1.2 a | 7.7 ± 1.0 b | 5.3 ± 0.5 c |
| FM | FM/AB | AB | |
|---|---|---|---|
| 14:0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| 16:0 | 17.4 ± 0.8 a | 16.8 ± 0.7 ab | 16.3 ± 0.8 b |
| 18:0 | 5.2 ± 0.9 | 5.0 ± 0.3 | 5.7 ± 0.6 |
| Total SFA 2 | 24.1 ± 1.3 | 23.2 ± 1.0 | 23.3 ± 1.1 |
| 16:1ω7 | 1.4 ± 0.2 a | 1.2 ± 0.1 b | 1.0 ± 0.1 c |
| 16:1ω5 | 0.1 ± 0.0 c | 0.1 ± 0.0 b | 0.2 ± 0.0 a |
| 18:1ω9 | 11.5 ± 0.4 c | 12.2 ± 0.3 b | 12.9 ± 0.5 a |
| 18:1ω7 | 1.5 ± 0.4 a | 12.2 ± 0.3 ab | 12.9 ± 0.5 b |
| 20:1ω9 | 1.6 ± 0.3 a | 1.4 ± 0.3 ab | 1.2 ± 0.2 b |
| Total MUFA 3 | 17.0 ± 0.6 | 17.2 ± 0.7 | 17.4 ± 0.5 |
| 18:2ω6 (LA) | 4.5 ± 0.2 c | 5.4 ± 0.3 b | 6.0 ± 0.3 a |
| 18:3ω6 | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.3 ± 0.0 a |
| 20:2ω6 | 1.5 ± 0.3 | 1.6 ± 0.2 | 1.6 ± 0.3 |
| 20:3ω6 | 1.3 ± 0.2 c | 1.8 ± 0.2 b | 2.7 ± 0.3 a |
| 20:4ω6 (ARA) | 3.4 ± 0.4 c | 4.2 ± 0.6 b | 5.5 ± 0.6 a |
| 22:4ω6 | 0.2 ± 0.1 a | 0.1 ± 0.0 b | 0.2 ± 0.1 a |
| 22:5ω6 (ω6DPA) | 0.6 ± 0.0 c | 1.7 ± 0.1 b | 3.1 ± 0.1 a |
| 18:3ω3 (ALA) | 0.4 ± 0.0 b | 0.5 ± 0.0 a | 0.5 ± 0.0 a |
| 18:4ω3 | 0.0 ± 0.0 b | 0.1 ± 0.0 b | 0.1 ± 0.0 a |
| 20:3ω3 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:4ω3 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| 20:5ω3 (EPA) | 7.2 ± 0.7 a | 5.7 ± 0.6 b | 4.6 ± 0.6 c |
| 22:5ω3 | 1.6 ± 0.2 a | 1.2 ± 0.1 b | 1.1 ± 0.2 b |
| 22:6ω3 (DHA) | 36.1 ± 1.1 a | 35.2 ± 1.0 a | 32.0 ± 0.7 b |
| Total PUFA 4 | 58.5 ± 1.2 | 59.3 ± 0.9 | 59.1 ± 1.0 |
| P/S ratio 5 | 2.4 ± 0.2 | 2.6 ± 0.1 | 2.5 ± 0.2 |
| Total ω3 | 45.9 ± 1.2 a | 43.3 ± 1.0 b | 39.0 ± 0.8 c |
| Total ω6 | 11.7 ± 0.3 c | 15.1 ± 0.5 b | 19.4 ± 0.5 a |
| ω3/ω6 ratio | 3.9 ± 0.2 a | 2.9 ± 0.1 b | 2.0 ± 0.1 c |
| EPA+DHA | 43.2 ± 1.0 a | 40.9 ± 1.0 b | 36.7 ± 0.8 c |
| DHA/EPA ratio | 5.1 ± 0.5 b | 6.2 ± 0.7 a | 7.0 ± 0.8 a |
| EPA/ARA ratio | 2.1 ± 0.4 a | 1.4 ± 0.3 b | 0.8 ± 0.1 c |
| DHA/ARA ratio | 10.7 ± 1.6 a | 8.5 ± 1.3 b | 5.9 ± 0.6 c |
| Initial | FM | FM/AB | AB | |
|---|---|---|---|---|
| Total lipid (mg/g) | 15.8 ± 6.7 | 70.4 ± 15.9 | 64.5 ± 12.7 | 63.4 ± 12.5 |
| Neutral lipid | 78.9 ± 7.1 | 61.4 ± 9.0 b | 75.5 ± 7.7 a | 62.8 ± 10.2 b |
| Polar lipid | 5.6 ± 5.5 | 38.6 ± 9.0 a | 24.5 ± 7.7 b | 37.2 ± 10.2 a |
| TAG | 56.0 ± 11.6 | 43.9 ± 10.3 | 54.8 ± 4.9 | 49.2 ± 8.6 |
| FFA | 5.4 ± 3.4 | 4.1 ± 1.0 b | 7.5 ± 2.2 a | 4.2 ± 0.7 b |
| ST | 16.2 ± 7.9 | 11.0 ± 2.2 a | 11.9 ± 3.8 a | 6.2 ± 4.3 b |
| PL | 15.6 ± 8.2 | 20.3 ± 5.1 a | 8.1 ± 5.2 b | 18.4 ± 7.4 a |
| PL/ST ratio 2 | 1.1 ± 0.6 | 2.0 ± 0.1 b | 0.8 ± 0.7 b | 4.5 ± 2.7 a |
| 14:0 | 3.6 ± 2.2 | 2.3 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.1 |
| 16:0 | 16.0 ± 2.5 | 14.7± 0.4 a | 14.0 ± 0.6 ab | 13.3 ± 0.5 b |
| 18:0 | 3.7 ± 0.2 | 3.3 ± 0.1 | 3.5 ± 0.3 | 3.5 ± 0.6 |
| Total SFA 3 | 24.2 ± 4.7 | 21.1 ± 0.4 a | 20.4 ± 0.5 ab | 19.5 ± 0.8 b |
| 16:1ω7 | 6.1 ± 2.0 | 4.6 ± 0.3 a | 4.4 ± 0.3 ab | 3.5 ± 0.1 b |
| 16:1ω5 | 0.1 ± 0.0 | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.3 ± 0.1 a |
| 18:1ω9 | 18.5 ± 2.1 | 30.1 ± 1.4 b | 33.2 ± 0.8 a | 35.0 ± 2.4 a |
| 18:1ω7 | 3.4 ± 0.6 | 2.2 ± 0.3 | 2.0 ± 0.3 | 1.7 ± 0.4 |
| Total MUFA 4 | 33.6 ± 2.8 | 42.1 ± 1.6 | 43.7 ± 0.6 | 43.4 ± 2.3 |
| 18:2ω6 (LA) | 8.1 ± 0.6 | 12.8 ± 0.6 a | 14.1 ± 0.4 b | 15.4 ± 0.7 c |
| 18:3ω6 | 0.2 ± 0.0 | 0.4 ± 0.1 c | 0.6 ± 0.1 b | 0.7 ± 0.1 a |
| 20:3ω6 | 0.3 ± 0.0 | 0.4 ± 0.0 c | 0.5 ± 0.1 b | 0.7 ± 0.1 a |
| 20:4ω6 (ARA) | 1.0 ± 0.2 | 0.6 ± 0.1 b | 0.6 ± 0.0 ab | 0.6 ± 0.1 a |
| 22:5ω6 (ω6DPA) | 0.5 ± 0.3 | 0.2 ± 0.1 c | 0.4 ± 0.0 b | 0.8 ± 0.1 a |
| 18:3ω3 (ALA) | 1.5 ± 0.1 | 2.3 ± 0.1 b | 2.4 ± 0.2 b | 2.7 ± 0.1 a |
| 18:4ω3 | 1.0 ± 0.2 | 0.9 ± 0.1 c | 1.1 ± 0.1 b | 1.2 ± 0.1 a |
| 20:4ω3 | 0.8 ± 0.1 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.1 |
| 20:5ω3 (EPA) | 4.8 ± 1.0 | 3.9 ± 0.4 a | 2.9 ± 0.1 ab | 2.7 ± 0.5 b |
| 22:5ω3 | 2.0 ± 0.4 | 1.3 ± 0.1 a | 1.0 ± 0.1 b | 0.9 ± 0.2 b |
| 22:6ω3 (DHA) | 17.4 ± 4.9 | 9.8 ± 1.6 | 8.3 ± 0.5 | 7.6 ± 2.1 |
| Total PUFA 5 | 41.3 ± 6.0 | 36.3 ± 1.6 | 35.4 ± 0.3 | 36.4 ± 2.3 |
| Total ω3 | 28.0 ± 6.2 | 19.2 ± 2.0 | 16.8 ± 0.5 | 15.9 ± 2.7 |
| Total ω6 | 10.8 ± 0.7 | 15.2 ± 0.5 c | 16.8 ± 0.8 b | 19.2 ± 0.7 a |
| ω3/ω6 ratio | 2.6 ± 0.6 | 1.3 ± 0.2 a | 1.0 ± 0.1 a | 0.8 ± 0.2 b |
| EPA+DHA | 22.2 ± 5.9 | 13.7 ± 2.0 a | 11.2 ± 0.5 b | 10.3 ± 2.6 b |
| EPA+DHA (mg/g) | 2.38 ± 0.96 | 6.00 ± 2.04 | 5.37 ± 0.94 | 4.63 ± 1.08 |
| DHA/EPA ratio | 3.6 ± 0.5 | 2.5 ± 0.3 b | 2.9 ± 0.1 a | 2.8 ± 0.3 a |
| EPA/ARA ratio | 4.8 ± 0.3 | 7.0 ± 0.7 a | 5.2 ± 0.3 b | 4.2 ± 0.4 c |
| DHA/ARA ratio | 17.1 ± 2.1 | 17.3 ± 1.1 a | 14.9 ± 0.7 a | 11.6 ± 1.6 b |
| DHA + EPA/112 g | 266.6 | 672.0 | 601.4 | 518.6 |
| FM | FM/AB | AB | |
|---|---|---|---|
| 14:0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| 16:0 | 19.7 ± 0.6 | 20.4 ± 2.3 | 20.0 ± 1.2 |
| 18:0 | 3.3 ± 0.2 | 3.4 ± 0.7 | 3.3 ± 0.3 |
| Total SFA 2 | 24.2 ± 1.3 | 25.1 ± 2.8 | 24.2 ± 0.9 |
| 16:1ω7 | 1.5 ± 0.2 ab | 1.4 ± 0.3 a | 1.3 ± 0.1 b |
| 16:1ω5 | 0.1 ± 0.0 c | 0.1 ± 0.0 b | 0.2 ± 0.0 a |
| 18:1ω9 | 9.6 ± 0.6 | 10.1 ± 0.7 | 11.2 ± 0.5 |
| 18:1ω7 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.5 ± 0.1 |
| 20:1ω9 | 0.4 ± 0.0 a | 0.3 ± 0.1 ab | 0.3 ± 0.0 b |
| Total MUFA 3 | 14.0 ± 0.9 | 14.2 ± 1.0 | 15.0 ± 0.7 |
| 18:2ω6 (LA) | 4.1 ± 0.3 c | 4.7 ± 0.3 b | 5.6 ± 0.4 a |
| 18:3ω6 | 0.1 ± 0.0 c | 0.2 ± 0.0 b | 0.2 ± 0.0 a |
| 20:2ω6 | 0.5 ± 0.1 b | 0.5 ± 0.1 ab | 0.6 ± 0.0 a |
| 20:3ω6 | 0.5 ± 0.1 c | 0.8 ± 0.1 b | 1.1 ± 0.2 a |
| 20:4ω6 (ARA) | 1.3 ± 0.1 b | 1.5 ± 0.2 b | 1.9 ± 0.1 a |
| 22:4ω6 | 0.2 ± 0.0 ab | 0.2 ± 0.0 b | 0.2 ± 0.0 a |
| 22:5ω6 (ω6DPA) | 0.7 ± 0.1 c | 1.6 ± 0.2 b | 2.7 ± 0.4 a |
| 18:3ω3 (ALA) | 1.1 ± 0.1 b | 1.3 ± 0.1 ab | 1.4 ± 0.1 a |
| 18:4ω3 | 0.3 ± 0.0 b | 0.4 ± 0.1 b | 0.5 ± 0.1 a |
| 20:3ω3 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 20:4ω3 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| 20:5ω3 (EPA) | 8.7 ± 0.5 a | 7.0 ± 0.2 b | 6.7 ± 0.7 b |
| 22:5ω3 | 2.3 ± 0.2 a | 1.8 ± 0.2 b | 1.7 ± 0.2 b |
| 22:6ω3 (DHA) | 38.2 ± 1.9 | 36.9 ± 3.4 | 33.3 ± 3.1 |
| Total PUFA 4 | 60.4 ± 1.5 | 59.2 ± 3.6 | 59.3 ± 1.0 |
| P/S ratio 5 | 2.4 ± 0.1 | 2.4 ± 0.3 | 2.5 ± 0.1 |
| Total ω3 | 51.7 ± 1.6 c | 48.6 ± 3.4 b | 45.9 ± 1.4 a |
| Total ω6 | 7.4 ± 0.4 | 9.4 ± 0.5 | 12.3 ± 0.7 |
| ω3/ω6 ratio | 7.0 ± 0.5 a | 5.2 ± 0.4 b | 3.7 ± 0.3 c |
| DHA/EPA ratio | 4.4 ± 0.4 b | 5.3 ± 0.5 a | 5.0 ± 0.7 ab |
| EPA/ARA ratio | 6.5 ± 0.8 a | 4.6 ± 0.5 b | 3.5 ± 0.4 b |
| DHA/ARA ratio | 28.5 ± 2.3 a | 24.4 ± 1.8 b | 17.5 ± 1.4 c |
| Sterol | Common Name | Formula | MW | RT (min) | Sterol Content (µg mg−1) of Lipids |
|---|---|---|---|---|---|
| Cholest-5-en-3β-ol | Cholesterol | 27:1Δ5 | 458 | 47.043 | 43.9 |
| 5α-cholestan-3β-ol * | Cholestanol | 27:0, 5αΔ° | 460 | 47.265 | tr |
| 5α-cholest-7-en-3β-ol | Lathosterol | 27:1Δ7 | 458 | 48.256 | 0.1 |
| 24-methylcholest-5-en-3β-ol * | Campesterol | 28:1Δ5Me24 | 472 | 49.125 | 0.2 |
| 5α-cholest-8(14)-en-3β-ol | 27:1Δ8(14) | 458 | 49.592 | 0.2 | |
| 23,24-dimethylcholest-5-en-3β-ol * | 29:1Δ5 Me23,24 | 486 | 50.902 | 0.1 | |
| Total | 4.44% |
| Fatty Acids (‰) | FM 1 | AB 1 | p-Value | Liver TFA (FM) 1 | Liver TFA (AB) 1 | p-Value | RC (%) 2 |
|---|---|---|---|---|---|---|---|
| LA | −26.1 ± 0.4 | −25.8 ± 0.0 | 0.322 | −25.6 ± 0.1 | −25.9 ± 0.2 | 0.127 | |
| ALA | −31.8 ± 0.2 | −30.9 ± 0.1 | <0.05 | ND | ND | ||
| ARA | ND | ND | −23.5 ± 0.5 | −28.4 ± 0.4 | <0.05 | ||
| EPA | −24.8 ± 0.7 | −39.6 ± 0.1 | <0.05 | −24.8 ± 0.3 | −38.0 ± 1.2 | <0.05 | 42.7% |
| DHA | −24.4 ± 0.1 | −36.0 ± 0.2 | <0.05 | −24.4 ± 0.2 | −35.6 ± 0.6 | <0.05 | 56.9% |
| Fatty Acids (‰) | FM 1 | AB 1 | p-Value | Liver PL (FM) 2 | Liver PL (AB) 2 | p-Value | RC (%) 3 |
|---|---|---|---|---|---|---|---|
| LA | −26.1 ± 0.4 | −25.8 ± 0.0 | 0.322 | −25.1 ± 0.4 | −26.0 ± 0.4 | <0.05 | |
| ALA | −31.8 ± 0.2 | −30.9 ± 0.1 | <0.05 | ND | ND | ||
| ARA | ND | ND | −24.1 ± 0.7 | −28.3 ± 1.0 | <0.05 | ||
| EPA | −24.8 ± 0.7 | −39.6 ± 0.1 | <0.05 | −24.8 ± 1.1 | −37.6 ± 0.2 | <0.05 | 41.3% |
| DHA | −24.4 ± 0.1 | −36.0 ± 0.2 | <0.05 | −24.2 ± 1.0 | −35.0 ± 1.5 | <0.05 | 54.0% |
| Fatty Acids (‰) | FM 1 | AB 1 | p-Value | Muscle TFA (FM) 2 | Muscle TFA (AB) 2 | p-Value | RC (%) 3 |
|---|---|---|---|---|---|---|---|
| LA | −26.1 ± 0.4 | −25.8 ± 0.0 | 0.322 | −26.7 ± 0.4 | −27.0 ± 0.5 | 0.128 | |
| ALA | −31.8 ± 0.2 | −30.9 ± 0.1 | <0.05 | −30.6 ± 0.4 | −31.4 ± 0.2 | <0.05 | |
| ARA | ND | ND | −24.5 ± 0.5 | −28.0 ± 0.4 | <0.05 | ||
| EPA | −24.8 ± 0.7 | −39.6 ± 0.1 | <0.05 | −25.2 ± 0.4 | −36.1 ± 1.3 | <0.05 | 36.6% |
| DHA | −24.4 ± 0.1 | −36.0 ± 0.2 | <0.05 | −25.3 ± 0.4 | −32.9 ± 1.5 | <0.05 | 43.1% |
| Fatty Acids (‰) | FM 1 | AB 1 | p-Value | Muscle PL (FM) 1 | Muscle PL (AB) 1 | p-Value | RC (%) 2 |
|---|---|---|---|---|---|---|---|
| LA | −26.1 ± 0.4 | −25.8 ± 0.0 | 0.322 | −25.6 ± 0.4 | −26.3 ± 0.2 | 0.073 | |
| ALA | −31.8 ± 0.2 | −30.9 ± 0.1 | <0.05 | −30.7 ± 1.2 | −30.6 ± 0.3 | 0.836 | |
| ARA | ND | ND | −23.8 ± 0.4 | −27.5 ± 0.2 | <0.05 | ||
| EPA | −24.8 ± 0.7 | −39.6 ± 0.1 | <0.05 | −25.2 ± 0.1 | −36.6 ± 0.7 | <0.05 | 38.1% |
| DHA | −24.4 ± 0.1 | −36.0 ± 0.2 | <0.05 | −25.5 ± 0.4 | −32.7 ± 1.0 | <0.05 | 42.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra, N.; Parrish, C.C.; Wei, M.; Perry, J.; Del Ángel-Rodríguez, J.A.; Tibbetts, S.M.; Emam, M.; Colombo, S.M. Effects of Replacing Fishmeal with Algal Biomass (Pavlova sp. 459) on Membrane Lipid Composition of Atlantic Salmon (Salmo salar) Parr Muscle and Liver Tissues. Sustainability 2023, 15, 16599. https://doi.org/10.3390/su152416599
Guerra N, Parrish CC, Wei M, Perry J, Del Ángel-Rodríguez JA, Tibbetts SM, Emam M, Colombo SM. Effects of Replacing Fishmeal with Algal Biomass (Pavlova sp. 459) on Membrane Lipid Composition of Atlantic Salmon (Salmo salar) Parr Muscle and Liver Tissues. Sustainability. 2023; 15(24):16599. https://doi.org/10.3390/su152416599
Chicago/Turabian StyleGuerra, Nigel, Christopher C. Parrish, Minmin Wei, Judy Perry, Jorge A. Del Ángel-Rodríguez, Sean M. Tibbetts, Mohamed Emam, and Stefanie M. Colombo. 2023. "Effects of Replacing Fishmeal with Algal Biomass (Pavlova sp. 459) on Membrane Lipid Composition of Atlantic Salmon (Salmo salar) Parr Muscle and Liver Tissues" Sustainability 15, no. 24: 16599. https://doi.org/10.3390/su152416599
APA StyleGuerra, N., Parrish, C. C., Wei, M., Perry, J., Del Ángel-Rodríguez, J. A., Tibbetts, S. M., Emam, M., & Colombo, S. M. (2023). Effects of Replacing Fishmeal with Algal Biomass (Pavlova sp. 459) on Membrane Lipid Composition of Atlantic Salmon (Salmo salar) Parr Muscle and Liver Tissues. Sustainability, 15(24), 16599. https://doi.org/10.3390/su152416599








