Selenium Mediated Alterations in Physiology of Wheat under Different Soil Moisture Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup andPlant Material
2.2. Physiological Traits
2.3. Statistical Analysis
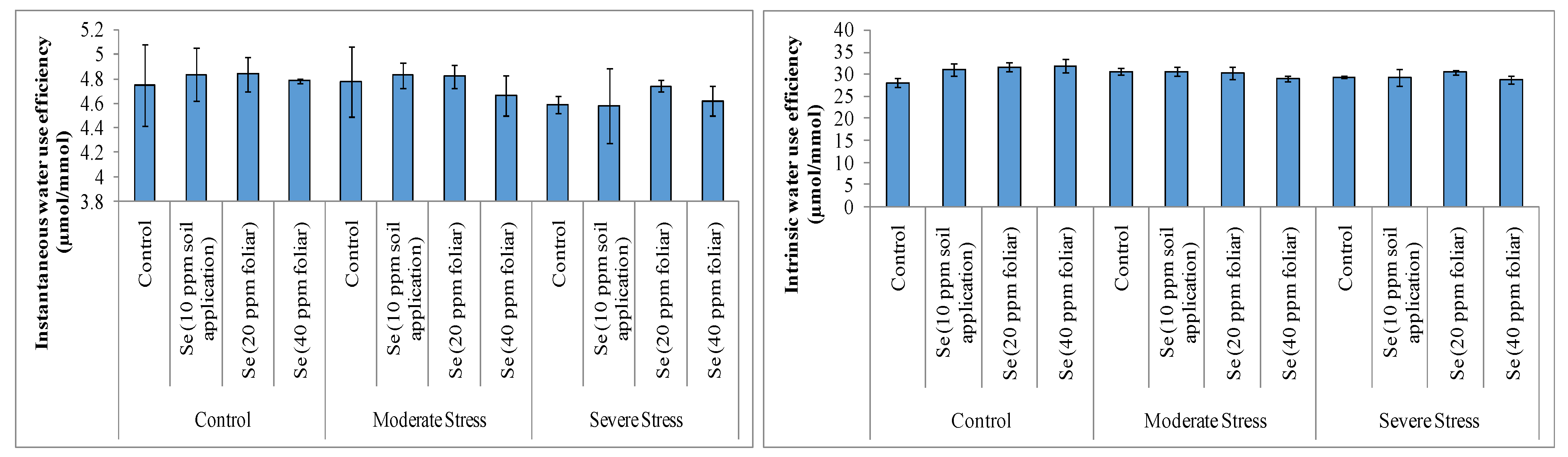
3. Results
3.1. Plant Water Relations
3.2. Gas ExchangeAttribute
3.3. Grain Yield and Biological Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, B.; Jatttan, M.; Dhansu, P.; Madan, S.; Kumari, N.; Dutt, K. Mycorrhizal symbiosis improved drought resistance in wheat using physiological traits. Cereal Res. Commun. 2022. [Google Scholar] [CrossRef]
- Nandwal, A.S.; Chand, M.; Kumari, A.; Rani, B.; Goel, V.; Singh, S. Genotypic differences in growth behavior and quality parameters of sugarcane (Saccharum officinarum) varieties under moisture stress conditions. Ind. J. Agric. Sci. 2019, 89, 65–72. [Google Scholar]
- Nandwal, A.S.; Chand, M.; Singh, K.; Mishra, A.K.; Kumar, A.; Kumari, A.; Rani, B. Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Ind. J. Exp. Biol. 2019, 57, 721–732. [Google Scholar]
- Nandwal, A.S.; Chand, M.; Pal, A.; Kumari, A.; Rani, B.; Goel, V.; Kulshreshtha, N. Soil moisture deficit induced changes in antioxidative defense mechanism of sugarcane (Saccharum officinarum) varieties differing in maturity. Ind. J. Agric. Sci. 2020, 90, 507–512. [Google Scholar]
- Farooq, M.; Hussain, M.; Siddique, K.H. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349. [Google Scholar] [CrossRef]
- Sharma, P.C.; Kumar, A.; Mann, A. Physiology of salt tolerance in crops. Sustain. Agric. 2021, 2021, 199. [Google Scholar]
- Kumar, A.; Singh, J.; Kumar, A.; Krishnamurthy, S.L.; Mann, A. Relative performance of wheat genotypes under individual and combined water deficit and salinity stress. Ind. J. Expt. Biol. 2022, 60, 49–58. [Google Scholar]
- Soni, S.; Kumar, A.; Sehrawat, N.; Kumar, A.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Biol. Sci. 2021, 28, 2510. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. FAO Statistical Databases; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Nuccio, M.L.; Paul, M.; Bate, N.J.; Cohn, J.; Cutler, S.R. Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Sci. 2018, 273, 110–119. [Google Scholar] [CrossRef]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019, 5, 2406. [Google Scholar] [CrossRef] [Green Version]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N.; Bukhari, M.A. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem. 2015, 175, 350–357. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Element. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Wang, Y.; Wang, S.H.; Yin, L.P.; Xu, G.J.; Zheng, C.; Zhang, M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lyciumchinense leaves. J. Sci. Food Agric. 2013, 93, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P.; Nasini, L.; Del Buono, D.; D‘Amato, R.; Tedeschini, E.; Businelli, D. Selenium protects olive (Olea europaea L.) from drought stress. Sci. Hort. 2013, 164, 165–171. [Google Scholar] [CrossRef]
- Abul-Soud, M.A.; Abd-Elrahman, S.H. Foliar selenium application to improve the tolerance of eggplant grown under salt stress conditions. Int. J. Plant Soil Sci. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, J. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F.; Mattson, N.S. The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol. Plant. 2016, 38, 1–14. [Google Scholar] [CrossRef]
- Ducsay, L.; Zapletalová, A.; Slepčan, M.; Vicianová, M.; Hozlár, P.; Bušo, R. Selenium effect on wheat grain yield and quality applied in different growth stages. Plant Soil Environ. 2021, 67, 147–153. [Google Scholar]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Expt. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Chilimba, A.D.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water—Culture Method for Growing Plants without Soil; California Agricultural Experiment Station Circular: Berkeley, CA, USA, 1950; Volume 347, pp. 1–32. [Google Scholar]
- Weatherley, P. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, A.K.; Singh, K.; Lata, C.; Kumar, A.; Krishnamurthy, S.L.; Kumar, P. Diurnal changes and effect of elevated CO2 on gas exchange under individual and interactive salt and water stress in wheat (Triticum aestivum). Ind. J. Agric. Sci. 2019, 89, 763. [Google Scholar] [CrossRef]
- Vesala, T.; Sevanto, S.; Grönholm, T.; Salmon, Y.; Nikinmaa, E.; Hari, P.; Hölttä, T. Effect of leaf water potential on internal humidity and CO2 dissolution: Reverse transpiration and improved water use efficiency under negative pressure. Front. Plant Sci. 2017, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci. 2017, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecot. Environ. Saf. 2015, 1, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.L.; Ampim, P.A.; Gao, M. Crop protection under drought stress. In Crop Protection under Changing Climate; Springer: Cham, Switzerland, 2020; pp. 145–170. [Google Scholar]
- Aissa, N.; Malagoli, M.; Radhouane, L. An approach to alleviate the impact of drought stress with selenium amendment. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 283–288. [Google Scholar] [CrossRef]
- Sattar, A.; Cheema, M.A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Nawaz, A.; Abbas, T.; Ali, Q. Physiological and biochemical attributes of bread wheat (Triticum aestivum L.) seedlings are influenced by foliar application of silicon and selenium under water deficit. Acta Physiol. Plant 2019, 41, 146. [Google Scholar] [CrossRef]
- Bashir, T.; Naz, S. Plant growth promoting rhizobacteria in combination with plant growth regulators attenuate the effect of drought stress. Pak. J. Bot. 2020, 52, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant 2018, 40, 1–3. [Google Scholar] [CrossRef]
- Hajiboland, R.; Sadeghzadeh, N.; Ebrahimi, N.; Sadeghzadeh, B.; Mohammadi, S. Influence of selenium in drought-stressed wheat plants under greenhouse and field conditions. Acta Agric. Slov. 2015, 105, 175–191. [Google Scholar] [CrossRef]
- Beltrano, J.; Ronco, M.G.; Arango, M.C. Soil drying and rewatering applied at three grain developmental stages affect differentially growth and grain protein deposition in wheat (Triticum aestivum L.). Braz. J. Plant Physiol. 2006, 18, 341–350. [Google Scholar] [CrossRef]
- Sharma, P.C.; Singh, D.; Sehgal, D.; Singh, G.; Hash, C.T.; Yadav, R.S. Further evidence that a terminal drought tolerance QTL of pearl millet is associated with reduced salt uptake. Environ. Expt. Bot. 2014, 1, 48–57. [Google Scholar] [CrossRef]
- Stiller, W.N.; Read, J.J.; Constable, G.A.; Reid, P.E. Selection for water use efficiency traits in cotton breeding program: Cultivar differences. Crop Sci. 2005, 45, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Rachaputi, R.C.; Chauhan, Y.; Douglas, C.; Martin, W.; Krosch, S.; Agius, P.; King, K. Physiological basis of yield variation in response to row spacing and plant density of mungbean grown in subtropical environments. Field Crops Res. 2015, 1, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, Y.S.; Williams, R. Physiological and agronomic strategies to increase mungbean yield in climatically variable environments of Northern Australia. Agronomy 2018, 26, 83. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Liu, D.; Cheng, J.; Zhao, G.; Fahima, T.; Yan, J. Effects of different selenium application methods on wheat (Triticum aestivum L.) biofortification and nutritional quality. Phyton 2020, 89, 423. [Google Scholar] [CrossRef]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.; Liu, F.; Graham, R.D. Selenium increases seed production in brassica. Plant Soil. 2009, 318, 73–80. [Google Scholar] [CrossRef]
| SMC | Texture | Saturation Capacity | pH | ECe | Ca | N | P | K |
|---|---|---|---|---|---|---|---|---|
| 14% | sandy | 25% | 8.72 | 0.35 dS m−1 | 4.5 ppm | 10.3 ppm | 2.5 ppm | 18.0 ppm |
| Environment (E)/Soil Moisture Level | Water Potential (−MPa) at 70 DAS | Water Potential (−MPa) at 90 DAS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T)/Application of Sodium Selenite (Na2SeO3) in ppm | ||||||||||
| Soil | Foliar | Soil | Foliar | |||||||
| 0 | 10 | 20 | 40 | Mean | 0 | 10 | 20 | 40 | Mean | |
| Control | 0.87 | 0.79 | 0.74 | 0.68 | 0.77C | 0.86 | 0.81 | 0.76 | 0.73 | 0.79C |
| Moderate stress | 0.95 | 0.88 | 0.91 | 0.88 | 0.91B | 1.00 | 0.97 | 0.94 | 0.91 | 0.96B |
| Severe stress | 1.12 | 1.02 | 1.09 | 1.06 | 1.07A | 1.21 | 1.10 | 1.13 | 1.06 | 1.13A |
| Mean | 0.98A | 0.90BC | 0.91B | 0.88C | 0.96AB | 0.94B | 0.90C | 0.98A | ||
| LSD (p ≤ 0.05) | E = 0.021, T = 0.024, E*T = NS | E = 0.026, T = 0.030, E*T = 0.042 | ||||||||
| Osmotic potential (−MPa) at 70 DAS | Osmotic potential (−MPa) at 90 DAS | |||||||||
| Control | 0.13 | 0.14 | 0.17 | 0.18 | 0.16C | 0.20 | 0.22 | 0.23 | 0.24 | 0.22C |
| Moderate stress | 0.17 | 0.20 | 0.18 | 0.21 | 0.19B | 0.25 | 0.25 | 0.26 | 0.28 | 0.26B |
| Severe stress | 0.22 | 0.26 | 0.23 | 0.26 | 0.24A | 0.31 | 0.34 | 0.33 | 0.36 | 0.34A |
| Mean | 0.17C | 0.20B | 0.19BC | 0.22A | 0.27B | 0.27B | 0.29A | 0.17C | ||
| LSD (p ≤ 0.05) | E = 0.015, T = 0.014, E*T = NS | E = 0.011, T = 0.008, E*T = 0.013 | ||||||||
| Relative Water Content (%) at 70 DAS | Relative Water Content (%) at 90 DAS | |||||||||
| Control | 82.5 | 85.0 | 86.3 | 87.0 | 85.2A | 82.0 | 82.6 | 83.9 | 84.0 | 83.1A |
| Moderate stress | 73.1 | 76.0 | 73.5 | 76.6 | 74.8B | 70.0 | 72.1 | 73.0 | 73.9 | 72.3B |
| Severe stress | 59.7 | 65.9 | 61.5 | 65.2 | 63.1C | 55.0 | 59.0 | 56.1 | 59.4 | 57.4C |
| Mean | 71.7C | 74.8B | 74.6B | 76.3A | 71.2AB | 71.0AB | 72.4A | 71.7AB | ||
| LSD (p ≤ 0.05) | E= 0.95, T = 1.09, E*T = 1.89 | E= 1.48, T = 1.71, E*T = 2.11 | ||||||||
| Environment (E)/Soil Moisture Level | A (mM m−2s−1) at 70 DAS | A (mM m−2s−1) at 90 DAS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T)/Application of Sodium Selenite (Na2SeO3) in ppm | ||||||||||
| Soil | Foliar | Soil | Foliar | |||||||
| 0 | 10 | 20 | 40 | Mean | 0 | 10 | 20 | 40 | Mean | |
| Control | 10.0 | 10.3 | 10.9 | 11.5 | 10.7A | 11.5 | 12.8 | 13.2 | 13.4 | 12.7A |
| Moderate stress | 8.70 | 9.40 | 9.12 | 9.85 | 9.27B | 9.73 | 10.4 | 10.8 | 11.0 | 10.5B |
| Severe stress | 6.74 | 8.54 | 7.85 | 8.20 | 7.83C | 7.34 | 8.50 | 8.30 | 8.92 | 8.27C |
| Mean | 8.48D | 9.43B | 9.28BC | 9.85A | 9.52C | 10.6B | 10.7B | 11.1A | ||
| LSD (p ≤ 0.05) | E = 0.19, T = 0.23, E*T = 0.39 | E = 0.24, T = 0.28, E*T = 0.48 | ||||||||
| E (mM m−2s−1) at 70 DAS | E (mM m−2s−1) at 90 DAS | |||||||||
| Control | 1.80 | 1.85 | 1.96 | 2.07 | 1.92A | 2.43 | 2.65 | 2.72 | 2.80 | 2.65A |
| Moderate stress | 1.51 | 1.65 | 1.57 | 1.72 | 1.61B | 2.04 | 2.16 | 2.24 | 2.31 | 2.19B |
| Severe stress | 1.20 | 1.28 | 1.35 | 1.44 | 1.32C | 1.60 | 1.86 | 1.75 | 1.93 | 1.79C |
| Mean | 1.50D | 1.59C | 1.63B | 1.74A | 2.02D | 2.22C | 2.24B | 2.35A | ||
| LSD (p ≤ 0.05) | E = 0.030, T = 0.035, E*T = 0.061 | E = 0.050, T = 0.057, E*T = 0.103 | ||||||||
| gS (mM m−2s−1) at 70 DAS | gS (mM m−2s−1) at 90 DAS | |||||||||
| Control | 0.320 | 0.340 | 0.360 | 0.381 | 0.350A | 0.409 | 0.412 | 0.415 | 0.420 | 0.414A |
| Moderate stress | 0.266 | 0.295 | 0.280 | 0.315 | 0.289B | 0.317 | 0.340 | 0.357 | 0.380 | 0.349B |
| Severe stress | 0.197 | 0.244 | 0.214 | 0.250 | 0.226C | 0.250 | 0.291 | 0.272 | 0.300 | 0.278C |
| Mean | 0.261D | 0.293B | 0.285C | 0.315A | 0.325C | 0.348B | 0.348B | 0.367A | ||
| LSD (p ≤ 0.05) | E = 0.026, T = 0.017, E*T = NS | E = 0.011, T = 0.010, E*T = 0.016 | ||||||||
| Fv/Fm at 70 DAS | Fv/Fm at 90 DAS | |||||||||
| Control | 0.642 | 0.650 | 0.670 | 0.720 | 0.671A | 0.690 | 0.720 | 0.737 | 0.810 | 0.739A |
| Moderate stress | 0.580 | 0.610 | 0.600 | 0.617 | 0.602B | 0.610 | 0.640 | 0.650 | 0.680 | 0.645B |
| Severe stress | 0.500 | 0.554 | 0.520 | 0.540 | 0.529C | 0.510 | 0.580 | 0.550 | 0.600 | 0.560C |
| Mean | 0.574C | 0.605B | 0.597B | 0.626A | 0.603C | 0.647B | 0.646B | 0.697A | ||
| LSD (p ≤ 0.05) | E = 0.012, T = 0.014, E*T = 0.024 | E = 0.025, T = 0.032, E*T = NS | ||||||||
| Environment (E)/Soil Moisture Level | Biological Yield per Plant (g) | Grain Yield per Plant (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T)/Application of Sodium Selenite (Na2SeO3) in ppm | ||||||||||
| Soil | Foliar | Soil | Foliar | |||||||
| 0 | 10 | 20 | 40 | Mean | 0 | 10 | 20 | 40 | Mean | |
| Control | 14.0 | 15.6 | 16.4 | 18.0 | 16.0A | 5.16 | 6.23 | 5.70 | 5.90 | 5.64A |
| Moderate stress | 10.2 | 11.4 | 12.7 | 13.4 | 11.9B | 4.50 | 4.96 | 5.07 | 5.21 | 4.94B |
| Severe stress | 8.01 | 8.90 | 8.5 | 9.17 | 8.71C | 3.17 | 3.51 | 3.39 | 3.82 | 3.47C |
| Mean | 10.8D | 12.1C | 12.4B | 13.5A | 4.28C | 4.60B | 4.66B | 4.98A | ||
| LSD (p ≤0.05) | E = 0.27, T = 0.30, E*T = 0.53 | E = 0.13, T = 0.15, E*T = 0.26 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Sharma, S.; Sharma, K.D.; Dhansu, P.; Devi, S.; Preet, K.; Ahlawat, P.; Kamboj, P.; Rani, P.; Rani, B.; et al. Selenium Mediated Alterations in Physiology of Wheat under Different Soil Moisture Levels. Sustainability 2023, 15, 1771. https://doi.org/10.3390/su15031771
Yadav S, Sharma S, Sharma KD, Dhansu P, Devi S, Preet K, Ahlawat P, Kamboj P, Rani P, Rani B, et al. Selenium Mediated Alterations in Physiology of Wheat under Different Soil Moisture Levels. Sustainability. 2023; 15(3):1771. https://doi.org/10.3390/su15031771
Chicago/Turabian StyleYadav, Sapna, Sinky Sharma, Kamal Dutt Sharma, Pooja Dhansu, Suman Devi, Kumar Preet, Pooja Ahlawat, Paras Kamboj, Preety Rani, Babita Rani, and et al. 2023. "Selenium Mediated Alterations in Physiology of Wheat under Different Soil Moisture Levels" Sustainability 15, no. 3: 1771. https://doi.org/10.3390/su15031771






