Biodiesel from Recycled Sunflower and Palm Oil—A Sustainable Fuel for Microturbo-Engines Used in Airside Applications
Abstract
1. Introduction
- -
- Fossil fuel depletion
2. Materials and Methods
2.1. Determination of Physical-Chemical Properties for Fuel Blends
2.2. Fuel Blends Combustion
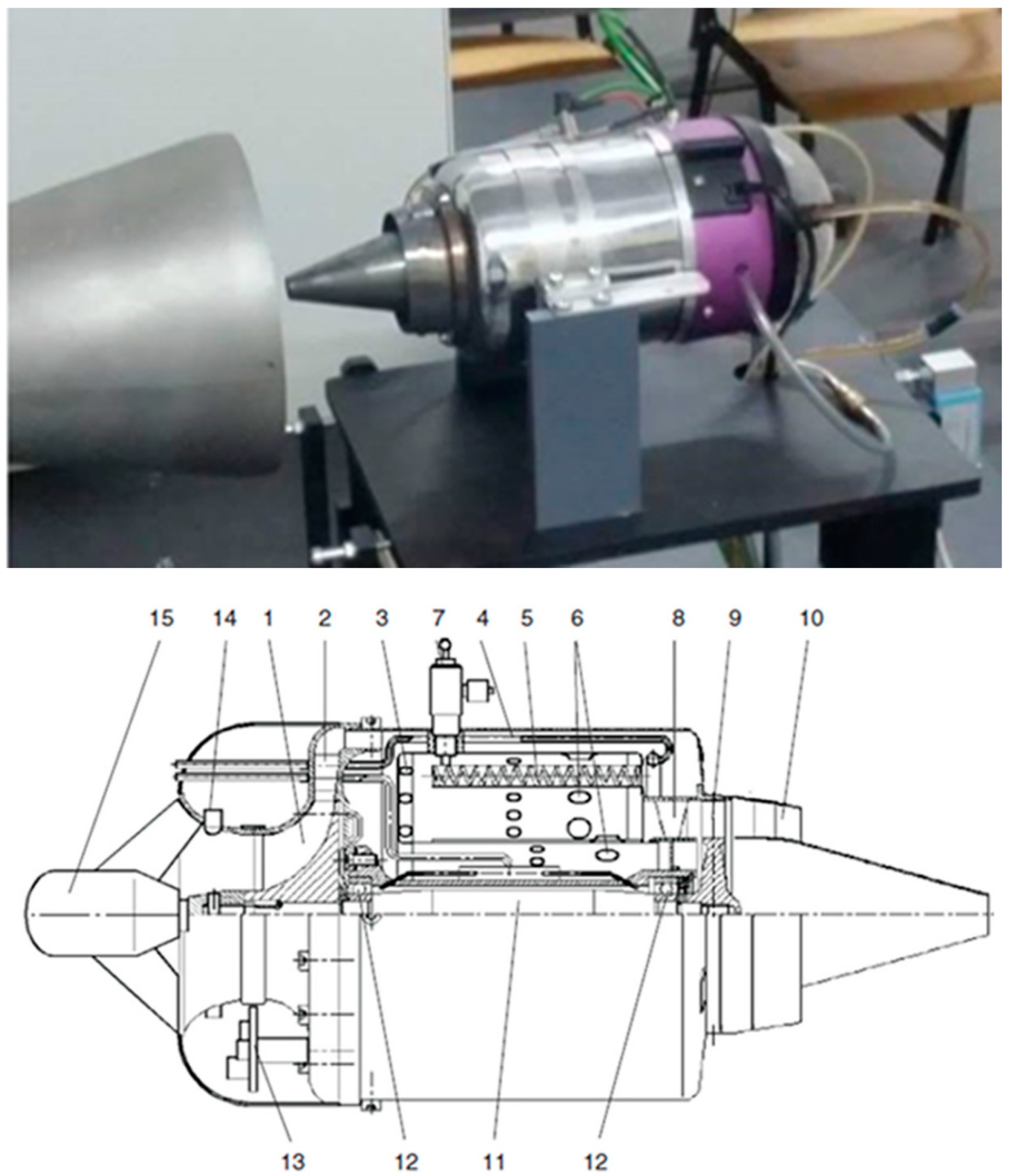
2.3. Micro Turb-Engine Experimental Procedure
3. Results and Discussion
3.1. Physical-Chemical Properties for Fuel Blends Experimental Results
- It can be observed that Flash point, Kinematic viscosity and density are increasing while biodiesel concentration is increasing too.
- Freezing point increases while the concentration of biodiesel increases, and at 100% SFP, the freezing point is −6 °C, thus making it unusable for aviation applications.
- Low calorific power decreases while biodiesel concentration increases making it a non-desirable property. As for elemental analysis, it can be observed that while the biodiesel concentration increases, carbon and hydrogen content decreases and oxygen concentration increases, leading to the conclusion that the resulting CO2 concentration resulting from combustion process will decrease.
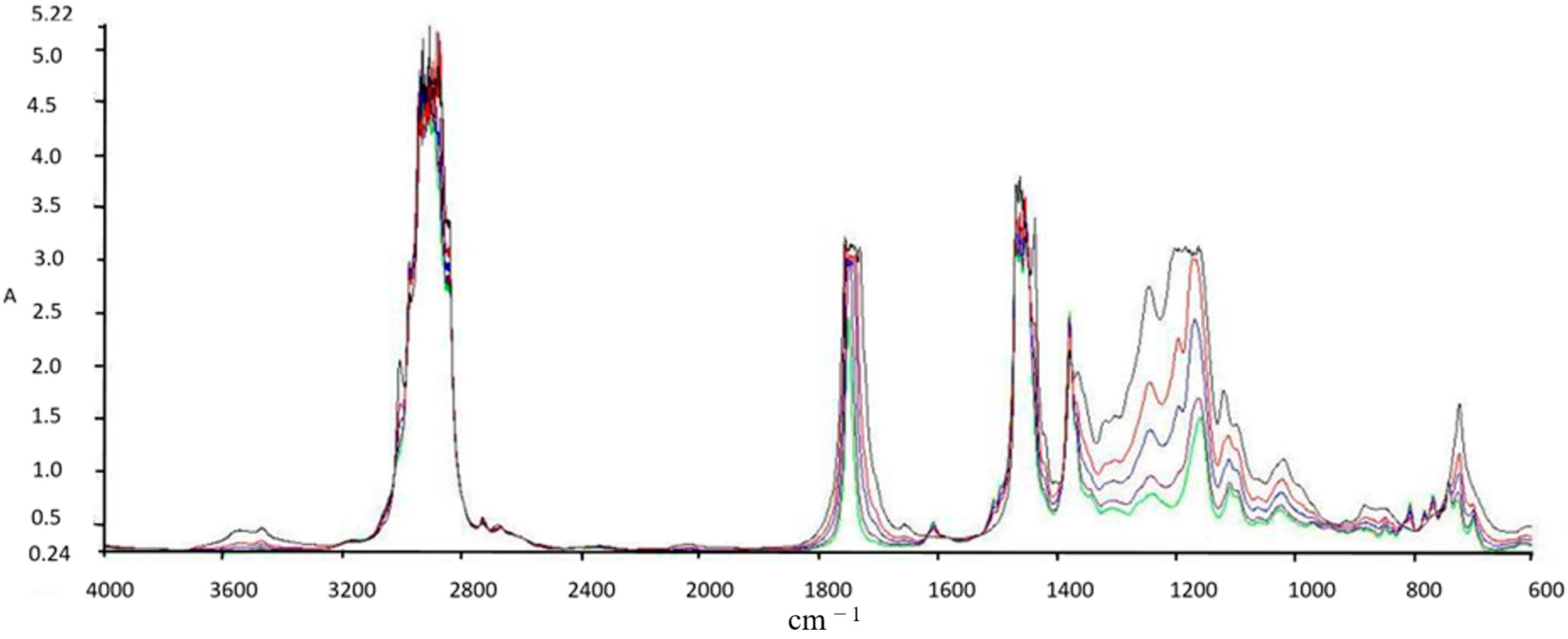
3.2. Combustion Reaction Analysis
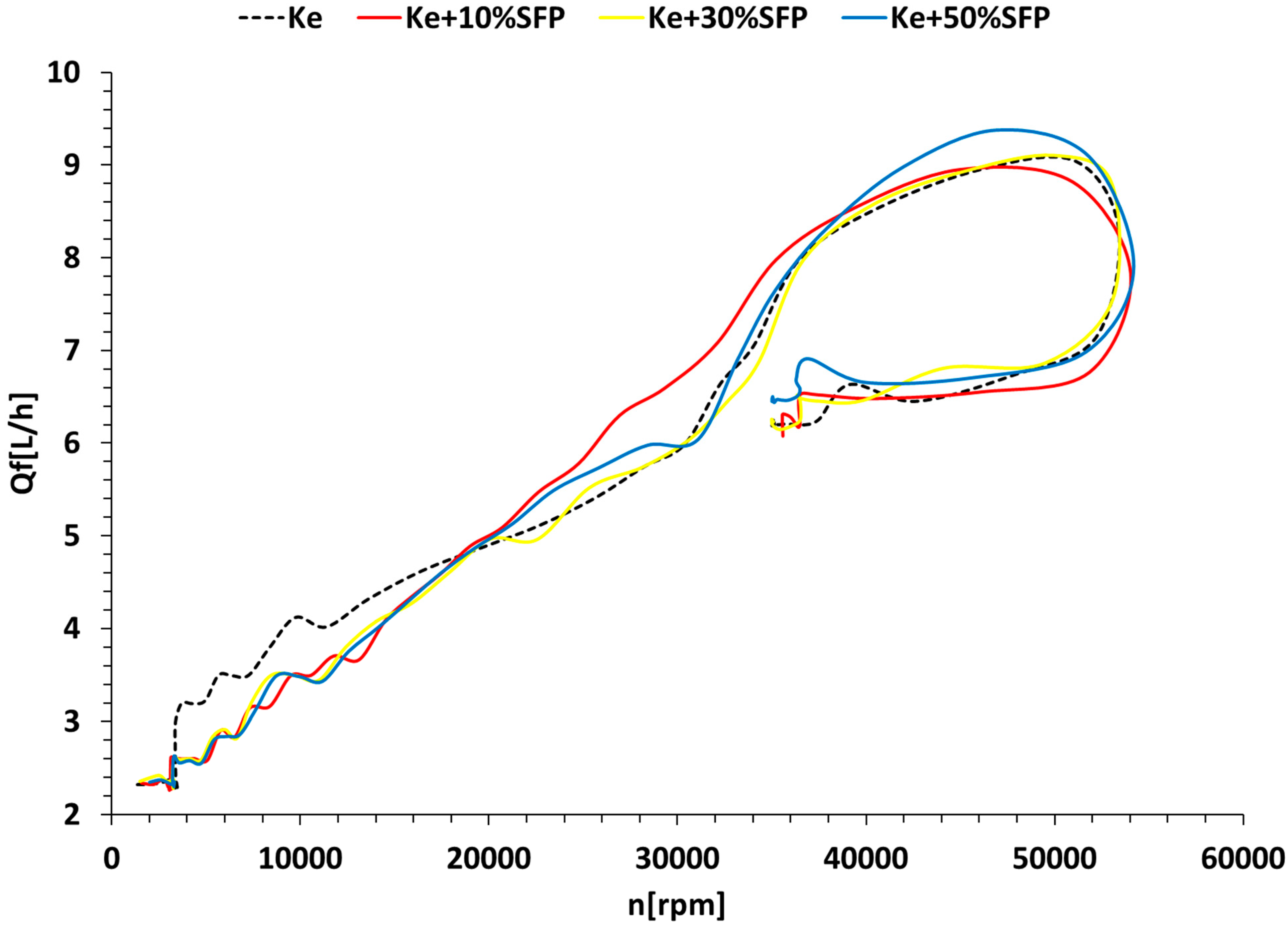
3.3. Micro Turbo-Engine Test Bench Experiments
Experimental Results
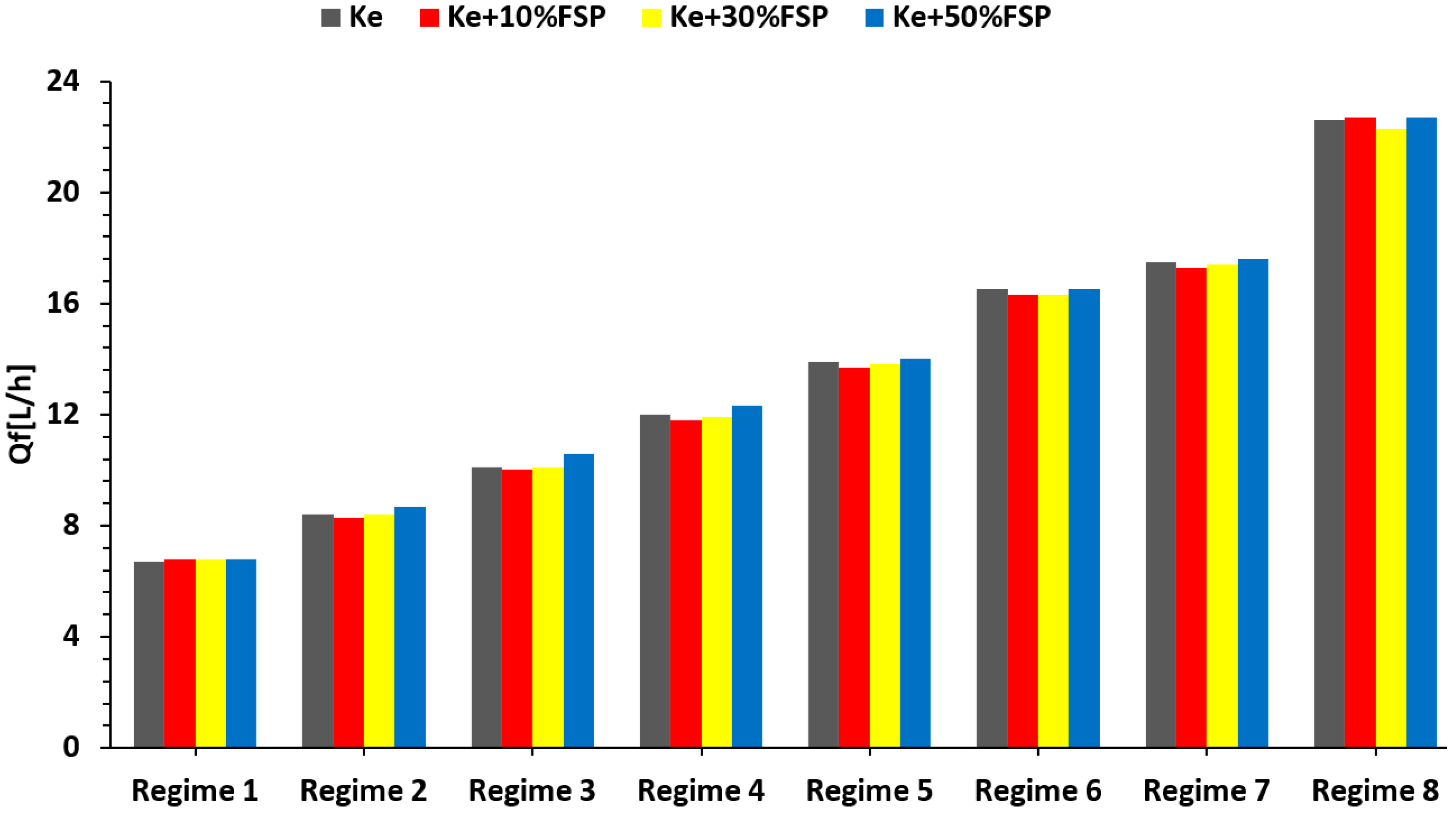
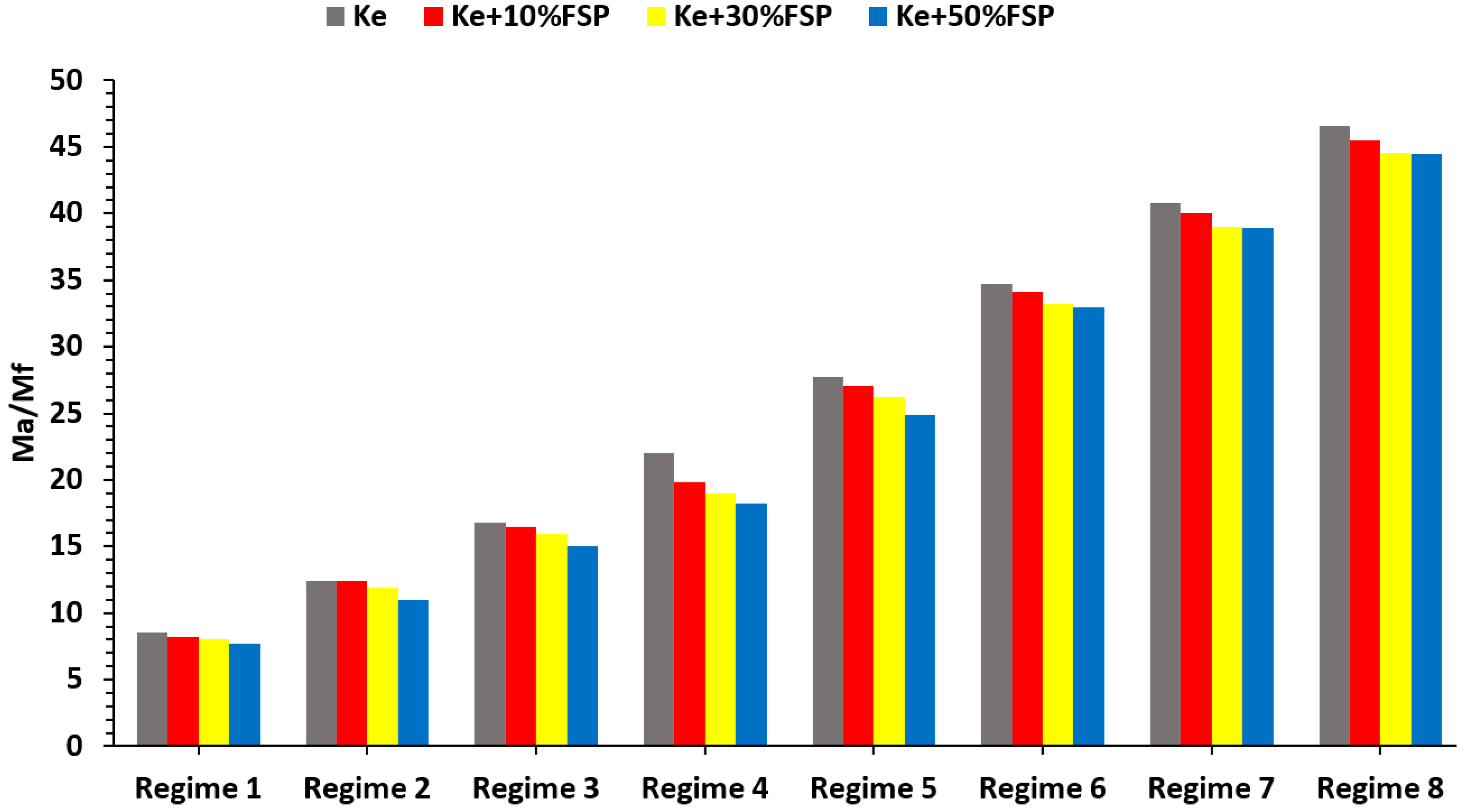
3.4. Jet Engine Performance Analysis
4. Conclusions
- The experiments performed on Jet CAT P80® micro-turbo engine highlights the possibility of using different percentages of biodiesel in fed fuel without putting in danger the engine’s integrity.
- Some of the properties of the used blends vary proportionally with the percentage of biodiesel within the blend. Thus, freezing point increases as the biodiesel concentration increases, making these blends unsuitable for high altitude flights.
- On contrast, some characteristics decrease as the biodiesel concentration increases. LCP decreases as the biodiesel concentration increases, leading to increased fuel consumption.
- Biodiesel has lower carbon content than usual Ke so the produced CO2 while burning biodiesel blends is lower, making it more environmentally friendly.
- Also, SPF has larger amount of O2 within its molecule, thus the requirements of O2 from air decreases, leading to lower CO2 generated during the burning process.
- Low LCP of the biodiesel is leading to increased fuel consumption as the percentages of biodiesel increases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://yearbook.enerdata.net/total-energy/world-consumption-statistics.html (accessed on 9 August 2022).
- Mahlia, T.M.I.; Ismail, N.; Hossain, N.; Silitonga, A.S.; Shamsuddin, A.H. Palm oil and its wastes as bioenergy sources: A comprehensive review. Environ. Sci. Pollut. Res. 2019, 26, 14849–14866. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.K.; Chowdhury, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Sangmesh, B.; Karuppasamy, K.S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B.K. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Yusoff, M.H.M.; Ayoub, M.; Jusoh, N.; Abdullah, A.Z. The Challenges of a Biodiesel Implementation Program in Malaysia. Processes 2020, 8, 1244. [Google Scholar]
- Noor, C.W.M.; Noor, M.M.; Mamat, R. Biodiesel as alternative fuel for marine diesel engine applications: A review. Renew. Sustain. Energy Rev. 2018, 94, 127–142. [Google Scholar] [CrossRef]
- Kirubakaran, M.; Selvan, V.A.M. A comprehensive review of low cost biodiesel production from waste chicken fat. Renew. Sustain. Energy Rev. 2018, 82, 390–401. [Google Scholar] [CrossRef]
- Global Monitoring Laboratory. Available online: https://gml.noaa.gov/ccgg/trends/gl_trend.html (accessed on 9 August 2022).
- Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 9 August 2022).
- de Oliveira Gonçalves, F.; Lopes, E.S.; Lopes, M.S.; Filho, R.M. Thorough evaluation of the available light-duty engine technologies to reduce greenhouse gases emissions in Brazil. J. Clean. Prod. 2022, 358, 132051. [Google Scholar] [CrossRef]
- Demirbas, A. Importance of biodiesel as transportation fuel. Energy Policy 2007, 35, 4661–4670. [Google Scholar] [CrossRef]
- Hou, S.; Chen, X.; Qiu, R. Sustainable biofuel consumption in air passenger transport driven by carbon-tax policy. Sustain. Prod. Consum. 2022, 31, 478–491. [Google Scholar] [CrossRef]
- Verger, T.; Azimov, U.; Adeniyi, O. Biomass-based fuel blends as an alternative for the future heavy-duty transport: A review. Renew. Sustain. Energy Rev. 2022, 161, 112391. [Google Scholar] [CrossRef]
- Ghosh, S.K. Biomass & Bio-waste Supply Chain Sustainability for Bio-energy and Bio-fuel Production. Procedia Environ. Sci. 2016, 31, 31–39. [Google Scholar]
- Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Sundaram, A.; Chang, S.W.; Kumar, G.; Shin, H.; Saratale, R.G.; Saratale, G.D. Transesterification and fuel characterization of rice bran oil: A biorefinery path. Fuel 2019, 253, 975–987. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Nguyen, D.D.; Shobana, S.; Saratale, G.D.; Arvindnarayan, S.; Atabani, A.E.; Chang, S.W.; Kumar, G. Engine performance, emission and bio characteristics of rice bran oil derived biodiesel blends. Fuel 2019, 239, 153–161. [Google Scholar] [CrossRef]
- Alam, F.; Mobin, S.; Chowdhury, H. Third generation biofuel from Algae. Procedia Eng. 2014, 105, 763–768. [Google Scholar] [CrossRef]
- Abbasi, M.; Pishvaee, M.S.; Mohseni, S. Third-generation biofuel supply chain: A comprehensive review and future research directions. J. Clean. Prod. 2021, 323, 129100. [Google Scholar] [CrossRef]
- Godbole, V.; Pal, M.K.; Gautam, P. A critical perspective on the scope of interdisciplinary approaches used in fourth-generation biofuel production. Algal Res. 2021, 58, 102436. [Google Scholar] [CrossRef]
- The Portal for Statistics (Statista). Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 9 August 2022).
- Font de Mora, E.; Torres, C.; Valero, A. Thermoeconomic Analysis of Biodiesel Production from Used Cooking Oils. Sustainability 2015, 7, 6321–6335. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Tournaki, S.; Gkouskos, Z.; Paraíba, O.; Giglio, F.; García, P.Q.; Braga, J.; Adrianos, H.; Filice, M. Quality Characteristics of Biodiesel Produced from Used Cooking Oil in Southern Europe. ChemEngineering 2019, 3, 19. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Belo, I. Microbial valorization of waste cooking oils for valuable compounds production—A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2583–2616. [Google Scholar] [CrossRef]
- da Silva Guabiroba, R.C.; da Silva, R.M.; da Silva, C.A.; da Silva, M.A.V. Value chain analysis of waste cooking oil for biodiesel production: Study case of one oil collection company in Rio de Janeiro-Brazil. J. Clean. Prod. 2017, 142, 3928–3937. [Google Scholar] [CrossRef]
- Urban Strategies for Waste Management in Tourist Cities. Available online: http://www.decisive2020.eu/wp-content/uploads/2019/07/D-4.1-%E2%80%9CURBANWASTE-prevention-and-management-strategies-and-guidelines-for-implementation.pdf (accessed on 9 August 2022).
- de Albuquerque Landi, F.F.; Fabiani, C.; Castellani, B.; Cotana, F.; Pisello, A.L. Environmental assessment of four waste cooking oil valorization pathways. Waste Manag. 2022, 138, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Foo, W.H.; Koay, S.S.N.; Chia, S.R.; Chia, W.Y.; Tang, D.Y.Y.; Nomanbhay, S.; Chew, K.W. Recent advances in the conversion of waste cooking oil into value-added products: A review. Fuel 2022, 324, 124539. [Google Scholar] [CrossRef]
- Çamur, H.; Alassi, E. Physicochemical Properties Enhancement of Biodiesel Synthesis from Various Feedstocks of Waste/Residential Vegetable Oils and Palm Oil. Energies 2021, 14, 4928. [Google Scholar] [CrossRef]
- Simbi, I.; Aigbe, U.O.; Oyekola, O.; Osibote, O.A. Optimization of biodiesel produced from waste sunflower cooking oil over bi-functional catalyst. Results Eng. 2022, 13, 100374. [Google Scholar] [CrossRef]
- Plata, V.; Ferreira-Beltrán, D.; Gauthier-Maradei, P. Effect of Cooking Conditions on Selected Properties of Biodiesel Produced from Palm-Based Waste Cooking Oils. Energies 2022, 15, 908. [Google Scholar] [CrossRef]
- Naik, B.D.; Meivelu, U.; Thangarasu, V.; Annamalai, S.; Sivasankaralingam, V. Experimental and empirical analysis of a diesel engine fuelled with ternary blends of diesel, waste cooking sunflower oil biodiesel and diethyl ether. Fuel 2022, 320, 123961. [Google Scholar] [CrossRef]
- Vergel-Ortega, M.; Valencia-Ochoa, G.; Duarte-Forero, J. Experimental study of emissions in single-cylinder diesel engine operating with diesel-biodiesel blends of palm oil-sunflower oil and ethanol. Case Stud. Therm. Eng. 2021, 26, 101190. [Google Scholar] [CrossRef]
- Elnajjar, E.; Al-Omari, S.; Selim, M.; Purayil, S. CI engine performance and emissions with waste cooking oil biodiesel boosted with hydrogen supplement under different load and engine parameters. Alex. Eng. J. 2022, 61, 4793–4805. [Google Scholar] [CrossRef]
- Agrisoma Biosciences Inc. Biojet Blend Fuels Transatlantic Flight. Biomass Magazine, 2018. Available online: http://biomassmagazine.com/articles/15596/biojet-blend-fuels-transatlantic-flight (accessed on 9 August 2022).
- Rucinski, T. United Airlines Targets 50 Percent Cut in Greenhouse Gas Emissions. 2018. Available online: https://www.reuters.com/article/us-ual-emissions-idUSKCN1LT32A (accessed on 9 August 2022).
- Chakraborty, D.; Kotoky, A. Oil from Seeds Helps Propel SpiceJet’s First. 2018, Bloomberg. Available online: https://www.bloomberg.com/news/articles/2018-08-27/india-s-spicejet-makes-maiden-flight-usingblended-bio-fuel (accessed on 9 August 2022).
- Willige, A. An Airbus Powered by Cooking Oil: Is Sustainable Aviation Fuel the Future of Aviation? 2022. Available online: https://climatechampions.unfccc.int/an-airbus-powered-by-cooking-oil-is-sustainable-aviation-fuel-the-future-of-aviation/?gclid=CjwKCAiAqt-dBhBcEiwATw-ggLj4hxSBtkp9Ys0LBXCBifrE-Iqyr4Rrq7SqUYKgCWshS-8sBKmBZhoCtnIQAvD_BwE (accessed on 21 December 2022).
- Sundararaj, R.H.; Kumar, R.D.; Raut, A.K.; Sekar, T.C.; Pandey, V.; Kushari, A.; Puri, S.K. Combustion and emission characteristics from biojet fuel blends in a gas turbine combustor. Energy 2019, 182, 689–705. [Google Scholar] [CrossRef]
- Suchocki, T.; Witanowski, Ł.; Lampart, P.; Kazimierski, P.; Januszewicz, K.; Gawron, B. Experimental investigation of performance and emission characteristics of a miniature gas turbine supplied by blends ofkerosene and waste tyre pyrolysis oil. Energy 2021, 215, 119125. [Google Scholar] [CrossRef]
- Cican, G.; Toma, A.; Puşcaşu, C.; Catana, R. Jet CAT P80 thermal analyses and performance assessment using different fuels types. J. Therm. Sci. 2018, 27, 389–393. [Google Scholar] [CrossRef]
- Cican, G.; Plesu, V.; Deaconu, M.; Toma, A.; Cretu, M. Performances and Emissions Evaluation of a Microturbojet Engine Running on Biodiesel Blends. ASME J. Energy Resour. Technol. 2019, 141, 072003. [Google Scholar] [CrossRef]
- Przysowa, R.; Gawron, B.; Białecki, T.; Łęgowik, A.; Merkisz, J.; Jasiński, R. Performance and Emissions of a Microturbine and Turbofan Powered by Alternative Fuels. Aerospace 2021, 8, 25. [Google Scholar] [CrossRef]
- Cican, G.; Deaconu, M.; Mirea, R.; Ceatra, L.C.; Cretu, M. An Experimental Investigation to Use the Biodiesel Resulting from Recycled Sunflower Oil, and Sunflower Oil with Palm Oil as Fuels for Aviation Turbo-Engines. Int. J. Environ. Res. Public Health 2021, 18, 5189. [Google Scholar] [CrossRef]
- Cican, G.; Deaconu, M.; Mirea, R.; Ceatra, L.; Cretu, M.; Dobre, T. Investigating the Use of Recycled Pork Fat-Based Biodiesel in Aviation Turbo Engines. Processes 2020, 8, 1196. [Google Scholar] [CrossRef]
- Ratkovská, K.; Hocko, M.; Čerňan, J.; Cúttová, M. The Influence of Biofuel on the Operational Characteristics of Small Experimental Jet Engine 1, Mechanics. Mater. Sci. Eng. 2017. [Google Scholar]
- Balogun, S.A.; Ghazali, I.; Mohammed, A.T.; Hermansyah, D.; Amanah, A.; Kurnia, M.T. Renewable Aviation Fuel: Review of Bio-jet Fuel for Aviation Industry. Eng. Sci. Lett. 2022, 01, 7–11. [Google Scholar] [CrossRef]
- SR EN ISO 3675/2003; European Committee for Standardization. Crude Petroleum and Liquid Petroleum Products—Laboratory Determination of Density—Hydrometer Method. ASRO: Bucharest, Romania, 2003.
- ASTM D92-05a; ASTM International. Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM International: West Conshohocken, PA, USA, 2009.
- SR EN ISO 3104/2002; European Committee for Standardization. Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. ASRO: Bucharest, Romania, 2002.
- ASTM D240-17; ASTM International. Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2017.
- SR 13552; Romanian Standards Association. Standardized Method for Determining the Pour Point of Petroleum Based Products. ASRO: Bucharest, Romania. Available online: https://magazin.asro.ro/ro/standard/200503 (accessed on 9 August 2022).
- ASTM D5291-16; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants. ASTM International: West Conshohocken, PA, USA, 2016.
- Jet Cat USA. Jet Cat Instruction Manual. U.S. Patent No. 6216440, 17 April 2001. [Google Scholar]
- Lois, A.L. Biodiesel and Biokerosenes: Production, Characterization, Soot & Pah Emissions. Ph.D. Thesis, Polytechnic University of Madrid, Madrid, Spain, 2015. [Google Scholar]
- Oyerinde, A.Y.; Bello, E.I. Use of Fourier Transformation Infrared (FTIR) Spectroscopy for Analysis ofFunctional Groups in Peanut Oil Biodiesel and Its Blends. Br. J. Appl. Sci. Technol. 2016, 13, 22178. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Industrial Hydrocarbon Processes; Gulf Professional Publishing: Houston, TX, USA, 2020; ISSN 978-0-12-809923-0. Available online: https://www.sciencedirect.com/book/9780750686327/handbook-of-industrial-hydrocarbon-processes (accessed on 9 August 2022).
- Mattingly, J. Elements of Propulsion: Gas Turbines and Rockets, 2nd ed.; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2006. [Google Scholar]






| Sample | Flash Point [°C] | Kinematic Viscosity at 40 °C [cSt] | Density at 22 °C [g/cm3] | Freezing Point [°C] | Low Calorific Power [kJ/kg] | Elemental Analysis % |
|---|---|---|---|---|---|---|
| Ke + 5% Aeroshell 500 Oil | 42.3 | 1.39 | 0.817 | <−35 °C | 42,399 | C: 85.17 H: 13.31 N: 0.07 O: 1.45 |
| Ke + 10% SFP | 45.6 | 1.75 | 0.832 | <−35 °C | 41,989 | C: 84.52 H: 13.24 N: 0.07 O: 2.17 |
| Ke + 30% SFP | 53.5 | 2.54 | 0.854 | −29 °C | 41,169 | C: 83.21 H: 13.1 N: 0.07 O: 3.62 |
| Ke + 50% SFP | 71 | 3.37 | 0.863 | −23 °C | 40,350 | C: 81.91 H: 12.96 N: 0.07 O: 5.06 |
| Blend | MO [kg] | Mair [kg] | CO2 [kg] | H2O [kg] |
|---|---|---|---|---|
| Ke | 3.32 | 14.45 | 3.12 | 1.20 |
| Ke + 10% SFP | 3.29 | 14.32 | 3.10 | 1.19 |
| Ke + 30% SFP | 3.23 | 14.05 | 3.05 | 1.18 |
| Ke + 50% SFP | 3.17 | 13.79 | 3.00 | 1.17 |
| SFP | 3.02 | 13.14 | 2.88 | 1.13 |
| Fuel | Ke | Ke + 10% SFP | Ke + 30% SFP | Ke + 50% SFP |
|---|---|---|---|---|
| [%] | 5.490 | 5.501 | 5.525 | 5.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cican, G.; Crunteanu, D.E.; Mirea, R.; Ceatra, L.C.; Leventiu, C. Biodiesel from Recycled Sunflower and Palm Oil—A Sustainable Fuel for Microturbo-Engines Used in Airside Applications. Sustainability 2023, 15, 2079. https://doi.org/10.3390/su15032079
Cican G, Crunteanu DE, Mirea R, Ceatra LC, Leventiu C. Biodiesel from Recycled Sunflower and Palm Oil—A Sustainable Fuel for Microturbo-Engines Used in Airside Applications. Sustainability. 2023; 15(3):2079. https://doi.org/10.3390/su15032079
Chicago/Turabian StyleCican, Grigore, Daniel Eugeniu Crunteanu, Radu Mirea, Laurentiu Constantin Ceatra, and Constantin Leventiu. 2023. "Biodiesel from Recycled Sunflower and Palm Oil—A Sustainable Fuel for Microturbo-Engines Used in Airside Applications" Sustainability 15, no. 3: 2079. https://doi.org/10.3390/su15032079
APA StyleCican, G., Crunteanu, D. E., Mirea, R., Ceatra, L. C., & Leventiu, C. (2023). Biodiesel from Recycled Sunflower and Palm Oil—A Sustainable Fuel for Microturbo-Engines Used in Airside Applications. Sustainability, 15(3), 2079. https://doi.org/10.3390/su15032079






