Cost and Heat Integration Analysis for CO2 Removal Using Imidazolium-Based Ionic Liquid-ASPEN PLUS Modelling Study
Abstract
:1. Introduction
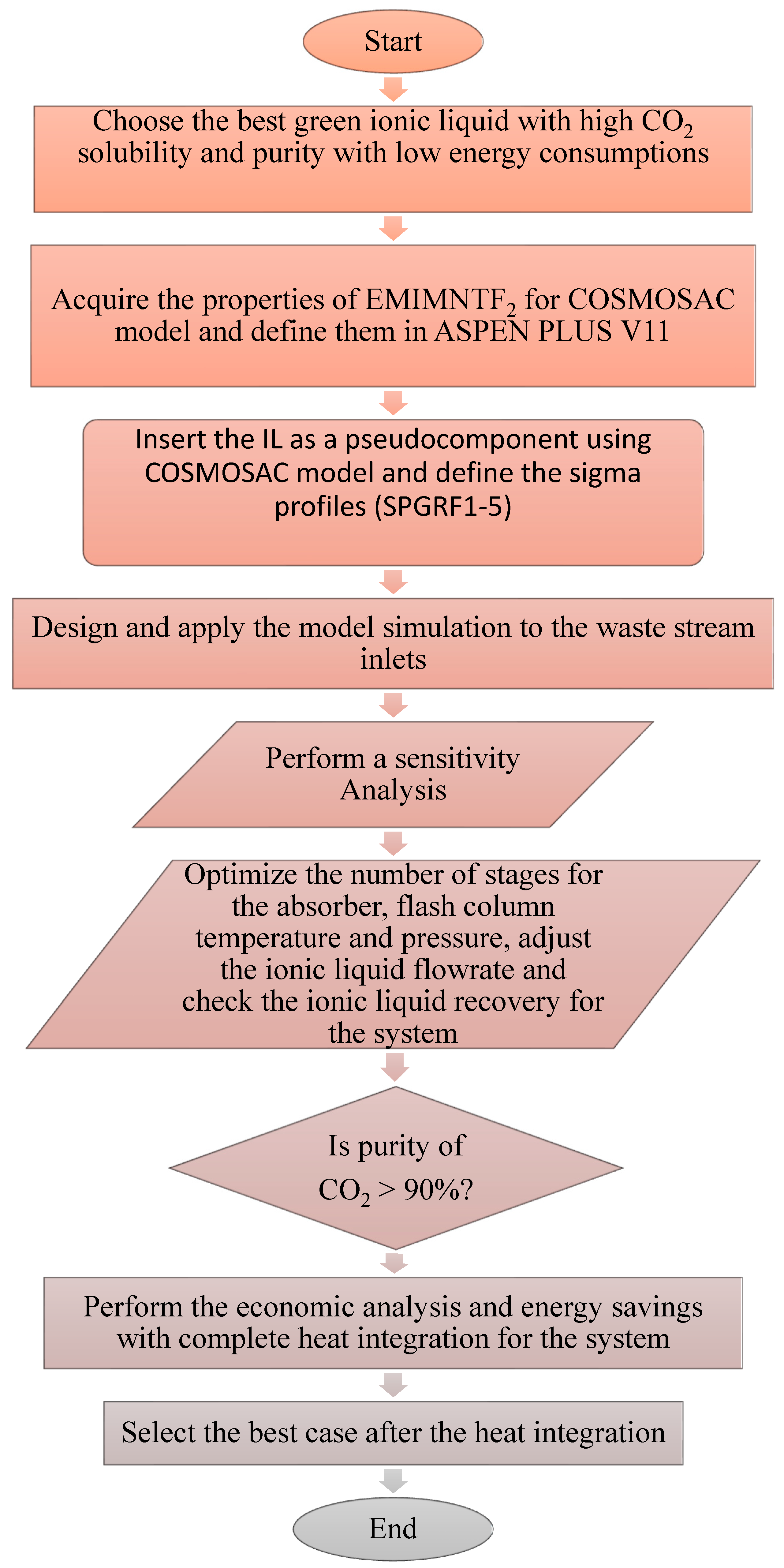
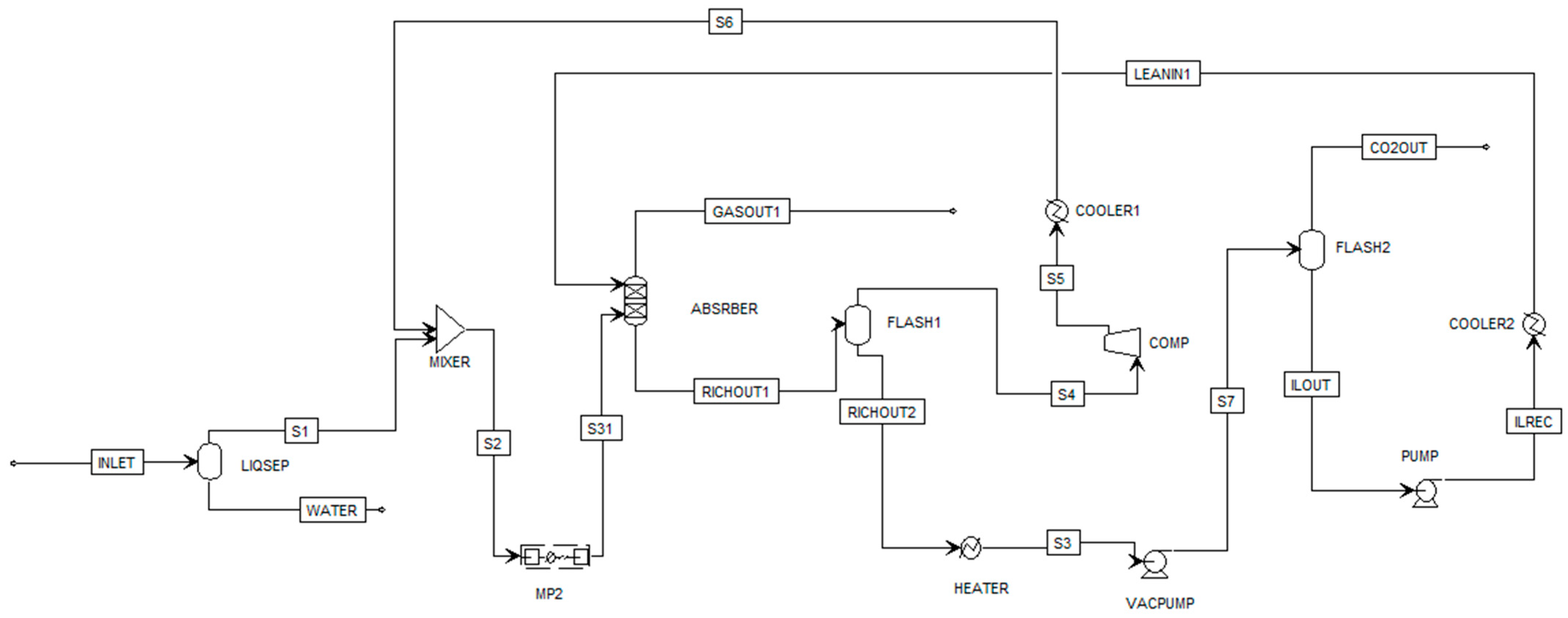
2. Modelling and Validation of CO2 Capture on Aspen Plus
3. Results and Discussion
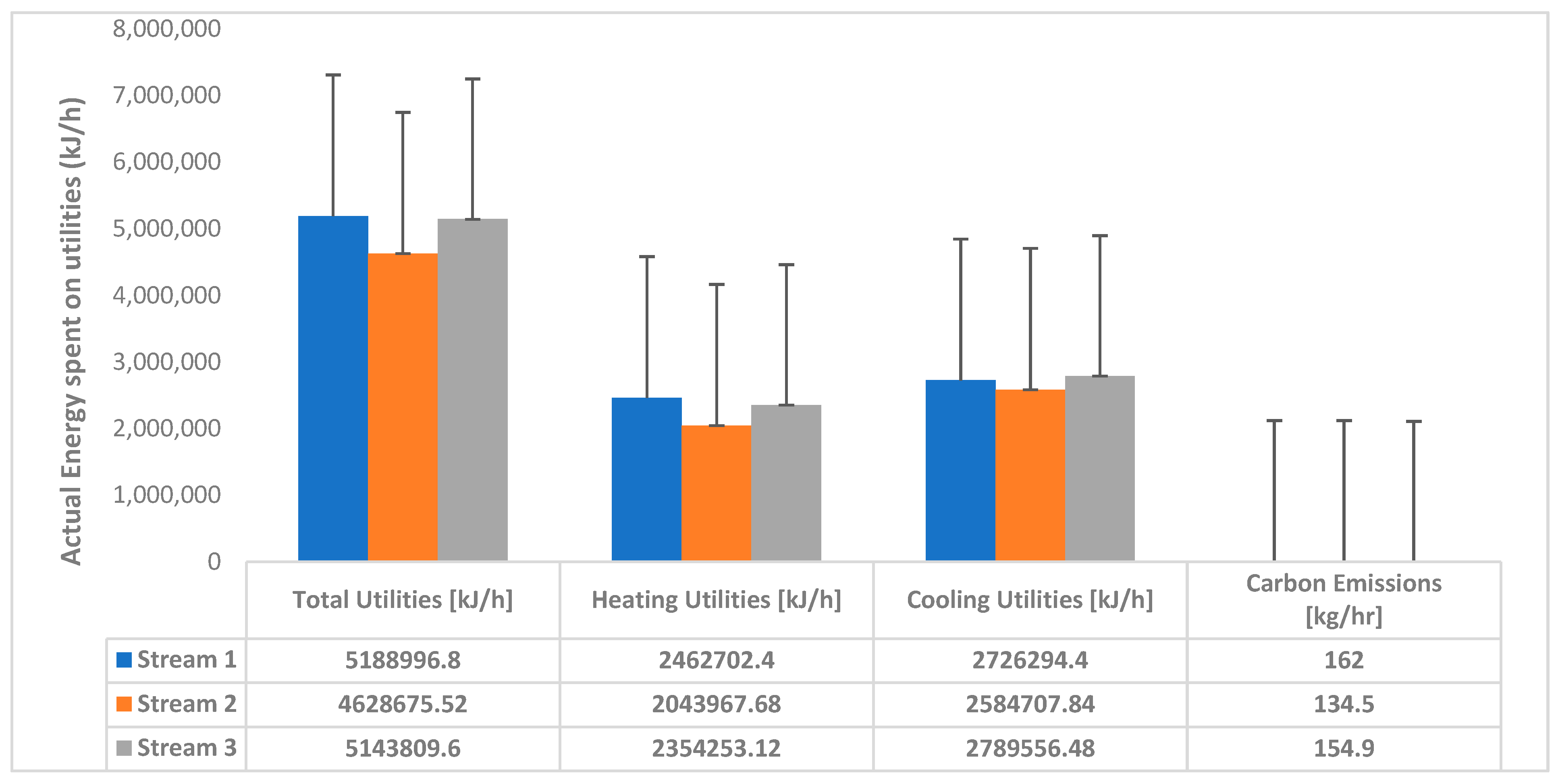
3.1. CO2 Purity Results
3.2. Cost Evaluation
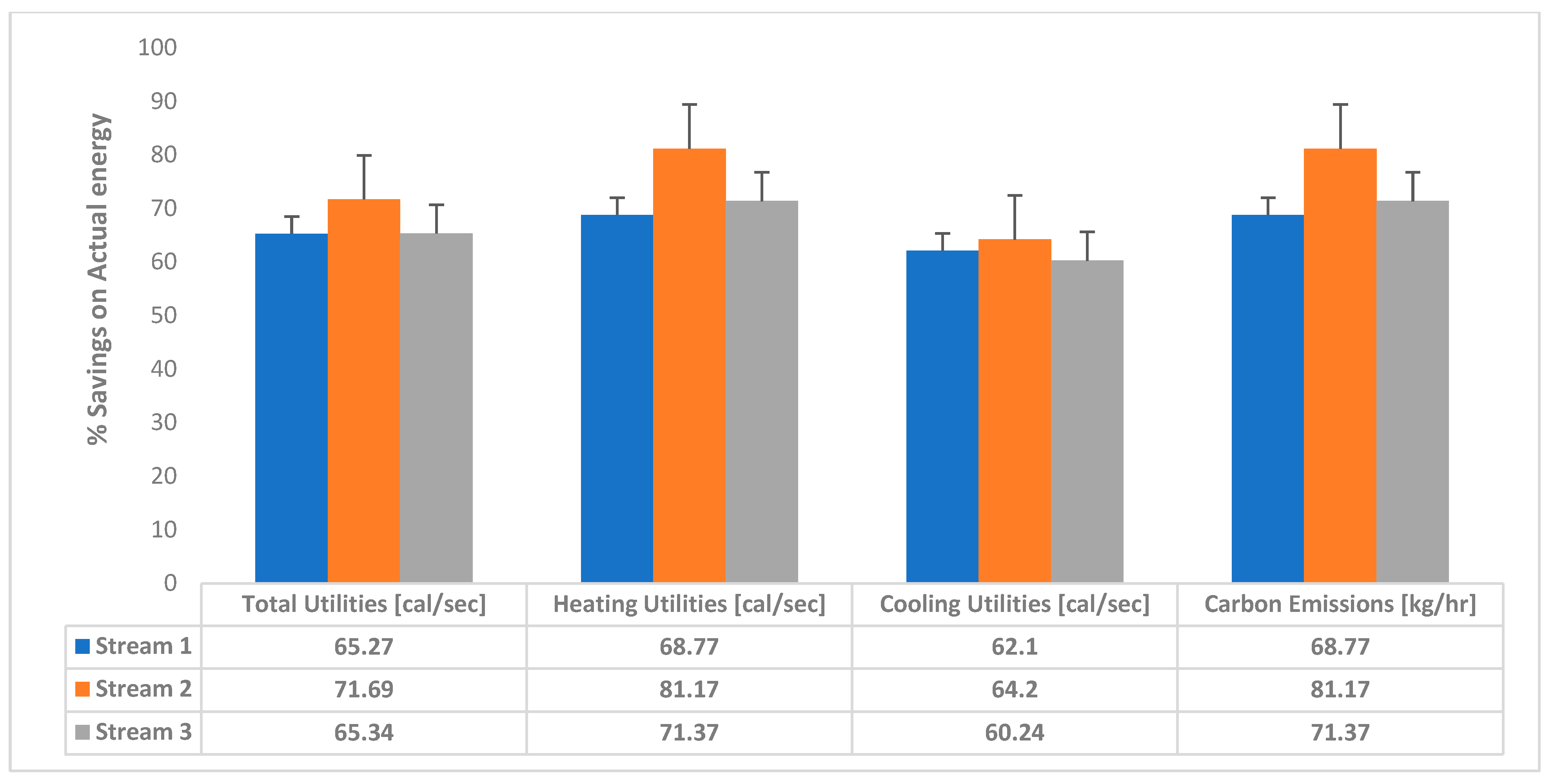
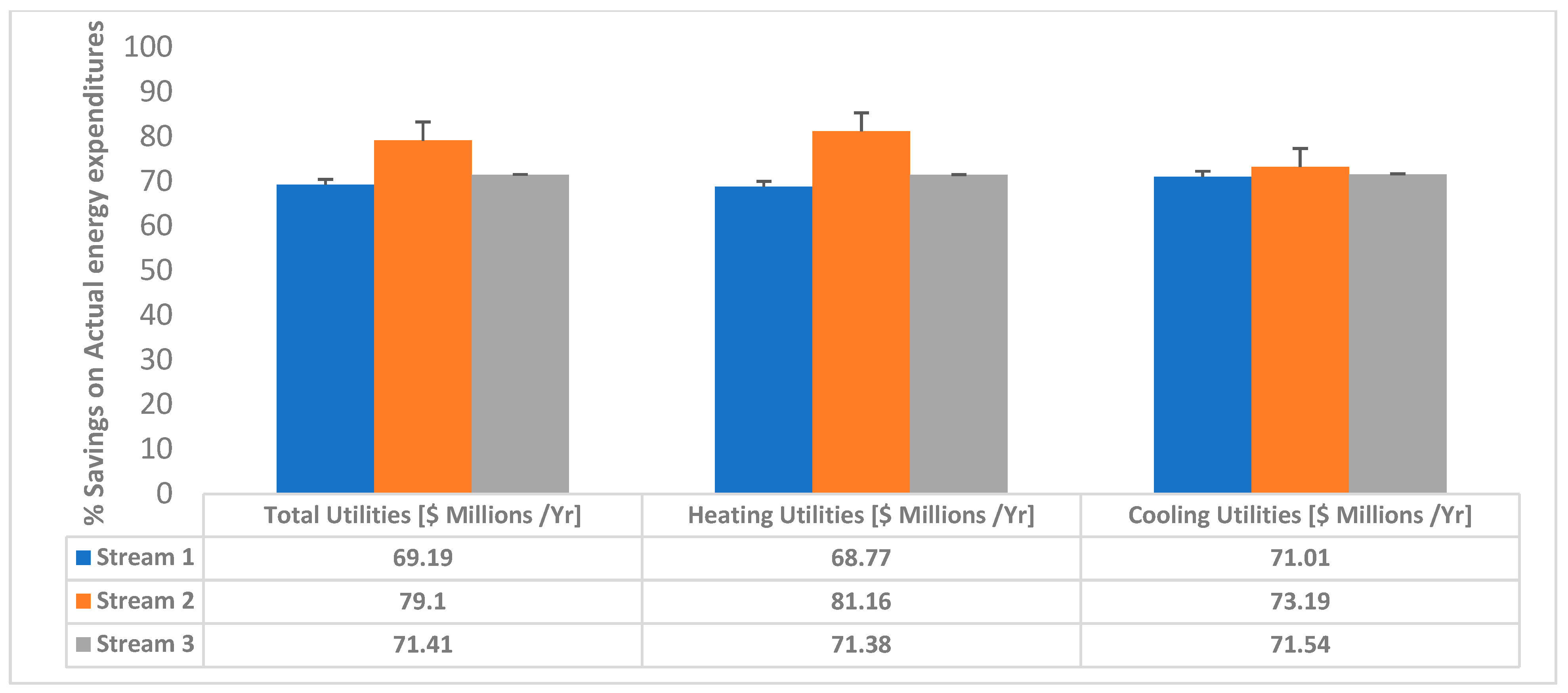
3.3. Energy Savings Analysis
3.4. Process Integration of the Proposed Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aghel, B.; Behaein, S.; Wongwises, S.; Shadloo, M.S. A review of recent progress in biogas upgrading: With emphasis on carbon capture. Biomass Bioenergy 2022, 160, 106422. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Wongwises, S.; Shadloo, M.S. Review on CO2 capture by blended amine solutions. Int. J. Greenh. Gas Control. 2022, 119, 103715. [Google Scholar] [CrossRef]
- Safarov, J.; El-Awady, W.A.; Shahverdiyev, A.; Hassel, E. Thermodynamic Properties of 1-Ethyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 2011, 56, 106–112. [Google Scholar] [CrossRef]
- Wang, S.; Li, G.; Fang, C. Urbanization, economic growth, energy consumption, and CO2 emissions: Empirical evidence from countries with different income levels. Renew. Sustain. Energy Rev. 2018, 81, 2144–2159. [Google Scholar] [CrossRef]
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Majid, M.Z.A. A global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten CO2 emitting countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- Li, R.; Jiang, R. Investigating effect of R&D investment on decoupling environmental pressure from economic growth in the global top six carbon dioxide emitters. Sci. Total Environ. 2020, 740, 140053. [Google Scholar]
- Salvia, M.; Reckien, D.; Pietrapertosa, F.; Eckersley, P.; Spyridaki, N.-A.; Krook-Riekkola, A.; Olazabal, M.; Hurtado, S.D.G.; Simoes, S.G.; Geneletti, D. Will climate mitigation ambitions lead to carbon neutrality? An analysis of the local-level plans of 327 cities in the EU. Renew. Sustain. Energy Rev. 2021, 135, 110253. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, X.; Chen, B.; Shang, Y.; Song, M. Challenges toward carbon neutrality in China: Strategies and countermeasures. Resour. Conserv. Recycl. 2022, 176, 105959. [Google Scholar] [CrossRef]
- Karami, B.; Ghaemi, A.; Shahhosseini, S. Eco-Friendly Deep Eutectic Solvents Blended with Diethanolamine Solution for Postcombustion CO2 Capture. Energy Fuels 2022, 36, 945–957. [Google Scholar] [CrossRef]
- Abdi-Khanghah, M.; Bemani, A.; Naserzadeh, Z.; Zhang, Z. Prediction of solubility of N-alkanes in supercritical CO2 using RBF-ANN and MLP-ANN. J. CO2 Util. 2018, 25, 108–119. [Google Scholar] [CrossRef]
- Muthuraj, R.; Mekonnen, T. Recent progress in carbon dioxide (CO2) as feedstock for sustainable materials development: Co-polymers and polymer blends. Polymer 2018, 145, 348–373. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Last, G.V.; Schmick, M.T. Identification and Selection of Major Carbon Dioxide Stream Compositions; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2011.
- Ghanbari, T.; Abnisa, F.; Daud, W.M.A.W. A review on production of metal organic frameworks (MOF) for CO2 adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, M.; Xu, J.; Zhao, S.; Wang, J.; Wang, Z. Recent Advances of Gas Transport Channels Constructed with Different Dimensional Nanomaterials in Mixed-Matrix Membranes for CO2 Separation. Small Methods 2020, 4, 1900749. [Google Scholar] [CrossRef]
- Dalane, K.; Dai, Z.; Mogseth, G.; Hillestad, M.; Deng, L. Potential applications of membrane separation for subsea natural gas processing: A review. J. Nat. Gas Sci. Eng. 2017, 39, 101–117. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.; Matsuura, T. Effect of operating conditions on the physical and chemical CO2 absorption through the PVDF hollow fiber membrane contactor. J. Membr. Sci. 2010, 353, 192–200. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, Z.; Zhang, W.; Zhang, L.; Baena-Moreno, F.M.; Lichtfouse, E. CO2 capture from coalbed methane using membranes: A review. Environ. Chem. Lett. 2020, 18, 79–96. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Okabe, H.; Yamada, H.; Onoda, M.; Fujioka, Y. Synthesis and selection of hindered new amine absorbents for CO2 capture. Energy Procedia 2011, 4, 201–208. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.; Graham, B.; Boxall, J.; Da Costa, J.D.; May, E. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94, 123–154. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A. State-of-the-art adsorption and membrane separation processes for carbon dioxide production from carbon dioxide emitting industries. Sep. Sci. Technol. 2009, 44, 1273–1421. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Tang, D.; Sholl, D.S. Selecting adsorbents to separate diverse near-azeotropic chemicals. J. Phys. Chem. C 2020, 124, 3664–3670. [Google Scholar] [CrossRef]
- Jiang, N.; Shang, R.; Heijman, S.G.; Rietveld, L.C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water Res. 2018, 144, 145–161. [Google Scholar] [CrossRef]
- Awual, M.R.; Khraisheh, M.; Alharthi, N.H.; Luqman, M.; Islam, A.; Karim, M.R.; Rahman, M.M.; Khaleque, M.A. Efficient detection and adsorption of cadmium (II) ions using innovative nano-composite materials. Chem. Eng. J. 2018, 343, 118–127. [Google Scholar] [CrossRef]
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent membrane developments for CO2 separation and capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic effect of adding graphene oxide and ZIF-301 to polysulfone to develop high performance mixed matrix membranes for selective carbon dioxide separation from post combustion flue gas. J. Membr. Sci. 2016, 514, 35–43. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A review of post-combustion CO2 capture technologies from coal-fired power plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Rafiq, S.; Maulud, A.; Man, Z.; Mutalib, M.I.A.; Ahmad, F.; Khan, A.U.; Khan, A.L.; Ghauri, M.; Muhammad, N. Modelling in mixed matrix membranes for gas separation. Can. J. Chem. Eng. 2015, 93, 88–95. [Google Scholar] [CrossRef]
- Tierling, S.; Jindal, S.; Abascal, R. Considerations for the use of carbon dioxide removal membranes in an offshore environment. In Proceedings of the OTC Brasil, Rio de Janeiro, Brazil, 4–6 October 2011. [Google Scholar]
- Jahn, J.; Van Den Bos, P.; Van Den Broeke, L.J. Evaluation of membrane processes for acid gas treatment. In Proceedings of the SPE International Production and Operations Conference & Exhibition, Doha, Qatar, 14–16 May 2012. [Google Scholar]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Martins, F.; Alvim-Ferraz, M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Zhou, X.; Rong, Y.; Fang, S.; Wang, K.; Zhi, X.; Qiu, L.; Chi, X. Thermodynamic analysis of an organic Rankine–vapor compression cycle (ORVC) assisted air compression system for cryogenic air separation units. Appl. Therm. Eng. 2021, 189, 116678. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An Improved CO2 Separation and Purification System Based on Cryogenic Separation and Distillation Theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef]
- Song, C.F.; Kitamura, Y.; Li, S.H. Evaluation of Stirling cooler system for cryogenic CO2 capture. Appl. Energy 2012, 98, 491–501. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, Z.; Zhang, Z.; Liu, P.; Shang, L.; Li, B. Effect of porous media and sodium dodecyl sulphate complex system on methane hydrate formation. Energy Fuels 2018, 32, 5736–5749. [Google Scholar] [CrossRef]
- Di Profio, P.; Canale, V.; D’Alessandro, N.; Germani, R.; Di Crescenzo, A.; Fontana, A. Separation of CO2 and CH4 from biogas by formation of clathrate hydrates: Importance of the driving force and kinetic promoters. ACS Sustain. Chem. Eng. 2017, 5, 1990–1997. [Google Scholar] [CrossRef]
- Prah, B.; Yun, R. CO2 hydrate slurry transportation in carbon capture and storage. Appl. Therm. Eng. 2018, 128, 653–661. [Google Scholar] [CrossRef]
- Smirnov, V.G.; Manakov, A.Y.; Dyrdin, V.V.; Ismagilov, Z.R.; Mikhailova, E.S.; Rodionova, T.V.; Villevald, G.V.; Malysheva, V.Y. The formation of carbon dioxide hydrate from water sorbed by coals. Fuel 2018, 228, 123–131. [Google Scholar] [CrossRef]
- Tajima, H.; Yamasaki, A.; Kiyono, F. Energy consumption estimation for greenhouse gas separation processes by clathrate hydrate formation. Energy 2004, 29, 1713–1729. [Google Scholar] [CrossRef]
- Li, A.; Wang, J.; Bao, B. High-efficiency CO2 capture and separation based on hydrate technology: A review. Greenh. Gases Sci. Technol. 2019, 9, 175–193. [Google Scholar] [CrossRef]
- Nath, D.; Henni, A. Solubility of carbon dioxide (CO2) in four bis (trifluoromethyl-sulfonyl) imide ([Tf2N]) based ionic liquids. Fluid Phase Equilibria 2020, 524, 112757. [Google Scholar] [CrossRef]
- Hasib-ur-Rahman, M.; Siaj, M.; Larachi, F. CO2 capture in alkanolamine/room-temperature ionic liquid emulsions: A viable approach with carbamate crystallization and curbed corrosion behavior. Int. J. Greenh. Gas Control. 2012, 6, 246–252. [Google Scholar] [CrossRef]
- Zheng, S.; Zeng, S.; Li, Y.; Bai, L.; Bai, Y.; Zhang, X.; Liang, X.; Zhang, S. State of the art of ionic liquid—Modified adsorbents for CO2 capture and separation. AIChE J. 2022, 68, e17500. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Huang, X.; Yang, D.; Wu, C.; Chen, J. Deep eutectic solvents composed of bio-phenol-derived superbase ionic liquids and ethylene glycol for CO2 capture. Chem. Commun. 2022, 58, 2160–2163. [Google Scholar] [CrossRef]
- Isaacs-Páez, E.; García-Pérez, A.; Nieto-Delgado, C.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J. Enhanced CO2 capture kinetics by using macroporous carbonized natural fibers impregnated with an ionic liquid. J. Mol. Liq. 2022, 350, 118602. [Google Scholar] [CrossRef]
- Lian, S.; Song, C.; Liu, Q.; Duan, E.; Ren, H.; Kitamura, Y. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization. J. Environ. Sci. 2021, 99, 281–295. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, W.; Zhang, R.; Luo, X.; Liang, Z. Study of Equilibrium Solubility, NMR Analysis, and Reaction Kinetics of CO2 Absorption into Aqueous N-1, N-2-Dimethylethane-1, 2-diamine Solutions. Energy Fuels 2020, 34, 672–682. [Google Scholar] [CrossRef]
- Golzar, K.; Amjad-Iranagh, S.; Modarress, H. Prediction of density, surface tension, and viscosity of quaternary ammonium-based ionic liquids ([N222 (n)] Tf2N) by means of artificial intelligence techniques. J. Dispers. Sci. Technol. 2014, 35, 1809–1829. [Google Scholar] [CrossRef]
- Izgorodina, E.I.; Seeger, Z.L.; Scarborough, D.L.; Tan, S.Y. Quantum chemical methods for the prediction of energetic, physical, and spectroscopic properties of ionic liquids. Chem. Rev. 2017, 117, 6696–6754. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale studies on ionic liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Guo, Y.; Zeng, Q.; Lin, W. Experimental Studies and Rate-Based Process Simulations of CO2 Absorption with Aqueous Ammonia Solutions. Ind. Eng. Chem. Res. 2012, 51, 5309–5319. [Google Scholar] [CrossRef]
- Shiflett, M.B.; Drew, D.W.; Cantini, R.A.; Yokozeki, A. Carbon dioxide capture using ionic liquid 1-butyl-3-methylimidazolium acetate. Energy Fuels 2010, 24, 5781–5789. [Google Scholar] [CrossRef]
- Khonkaen, K.; Siemanond, K.; Henni, A. Simulation of carbon dioxide capture using ionic liquid 1-Ethyl-3-methylimidazolium acetate. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2014; Volume 33, pp. 1045–1050. [Google Scholar]
- Amiri, N.; Lounis, Z.; Benyounes, H. CO2 Capture from Natural Gas with Ionic Liquid. In ICREEC 2019; Springer: Berlin/Heidelberg, Germany, 2020; pp. 647–652. [Google Scholar]
- Xie, Y.; Björkmalm, J.; Ma, C.; Willquist, K.; Yngvesson, J.; Wallberg, O.; Ji, X. Techno-economic evaluation of biogas upgrading using ionic liquids in comparison with industrially used technology in Scandinavian anaerobic digestion plants. Appl. Energy 2018, 227, 742–750. [Google Scholar] [CrossRef]
- Ma, C.; Liu, C.; Lu, X.; Ji, X. Techno-economic analysis and performance comparison of aqueous deep eutectic solvent and other physical absorbents for biogas upgrading. Appl. Energy 2018, 225, 437–447. [Google Scholar] [CrossRef]
- Haider, J.; Qyyum, M.A.; Riaz, A.; Naquash, A.; Kazmi, B.; Yasin, M.; Nizami, A.-S.; Byun, M.; Lee, M.; Lim, H. State-of-the-art process simulations and techno-economic assessments of ionic liquid-based biogas upgrading techniques: Challenges and prospects. Fuel 2022, 314, 123064. [Google Scholar] [CrossRef]
- Shaahmadi, F.; Shahraki, B.H.; Farhadi, A. The CO2/CH4 gas mixture solubility in ionic liquids [Bmim][Ac],[Bmim][BF4] and their binary mixtures. J. Chem. Thermodyn. 2020, 141, 105922. [Google Scholar] [CrossRef]
- Ramdin, M.; de Loos, T.W.; Vlugt, T.J. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Kortenbruck, K.; Pohrer, B.; Schluecker, E.; Friedel, F.; Ivanovic-Burmazovic, I. Determination of the diffusion coefficient of CO2 in the ionic liquid EMIM NTf2 using online FTIR measurements. J. Chem. Thermodyn. 2012, 47, 76–80. [Google Scholar] [CrossRef]
- Giammanco, C.H.; Kramer, P.L.; Yamada, S.A.; Nishida, J.; Tamimi, A.; Fayer, M.D. Carbon dioxide in an ionic liquid: Structural and rotational dynamics. J. Chem. Phys. 2016, 144, 104506. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.B.; Elliott, B.A.; Yokozeki, A. Phase behavior of vinyl fluoride in room-temperature ionic liquids [emim][Tf2N],[bmim][N (CN) 2],[bmpy][BF4],[bmim][HFPS] and [omim][TFES]. Fluid Phase Equilibria 2012, 316, 147–155. [Google Scholar] [CrossRef]
- Li, L.; Huang, X.; Jiang, Q.; Xia, L.; Wang, J.; Ai, N. New process development and process evaluation for capturing CO2 in flue gas from power plants using ionic liquid [emim][Tf2N]. Chin. J. Chem. Eng. 2020, 28, 721–732. [Google Scholar] [CrossRef]
- Sultan, H.; Bhatti, U.H.; Muhammad, H.A.; Nam, S.C.; Baek, I.H. Modification of postcombustion CO2 capture process: A techno-economic analysis. Greenh. Gases Sci. Technol. 2021, 11, 165–182. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Guan, H.; Jia, Z.; Zhang, Y.; Zhao, G.; Gao, M. Optimization study of CO2 capture unit for subcritical coal-fired power generation unit based on Ebsilon and Aspen plus. Energy Convers. Manag. 2022, 269, 116111. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lin, S.-T. Screening of ionic liquids for CO2 capture using the COSMO-SAC model. Chem. Eng. Sci. 2015, 121, 157–168. [Google Scholar] [CrossRef]
- Palomar, J.; Gonzalez-Miquel, M.; Polo, A.; Rodriguez, F. Understanding the physical absorption of CO2 in ionic liquids using the COSMO-RS method. Ind. Eng. Chem. Res. 2011, 50, 3452–3463. [Google Scholar] [CrossRef]
- García-Gutiérrez, P.; Jacquemin, J.; McCrellis, C.; Dimitriou, I.; Taylor, S.R.; Hardacre, C.; Allen, R.W. Techno-economic feasibility of selective CO2 capture processes from biogas streams using ionic liquids as physical absorbents. Energy Fuels 2016, 30, 5052–5064. [Google Scholar] [CrossRef]
- Micari, M.; Dakhchoune, M.; Agrawal, K. Techno-economic assessment of postcombustion carbon capture using high-performance nanoporous single-layer graphene membranes. J. Membr. Sci. 2021, 624, 119103. [Google Scholar] [CrossRef]
- Miladi, R.; Frikha, N.; Gabsi, S. Modeling and energy analysis of a solar thermal vacuum membrane distillation coupled with a liquid ring vacuum pump. Renew. Energy 2021, 164, 1395–1407. [Google Scholar] [CrossRef]
- Ottiger, S.; Pini, R.; Storti, G.; Mazzotti, M. Competitive adsorption equilibria of CO2 and CH4 on a dry coal. Adsorption 2008, 14, 539–556. [Google Scholar] [CrossRef]
- Von Storch, H.; Becker-Hardt, S.; Sattler, C. (Solar) Mixed Reforming of Methane: Potential and Limits in Utilizing CO2 as Feedstock for Syngas Production—A Thermodynamic Analysis. Energies 2018, 11, 2537. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Gani, R.; Zhou, T. Comparative economic analysis of physical, chemical, and hybrid absorption processes for carbon capture. Ind. Eng. Chem. Res. 2020, 59, 2005–2012. [Google Scholar] [CrossRef]
- Rashid, T.U. Ionic liquids: Innovative fluids for sustainable gas separation from industrial waste stream. J. Mol. Liq. 2021, 321, 114916. [Google Scholar] [CrossRef]
- Kazmi, B.; Raza, F.; Taqvi, S.A.A.; Ali, S.I.; Suleman, H. Energy, exergy and economic (3E) evaluation of CO2 capture from natural gas using pyridinium functionalized ionic liquids: A simulation study. J. Nat. Gas Sci. Eng. 2021, 90, 103951. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Paramio, C.; Palomar, J. Aspen plus supported design of pre-combustion CO2 capture processes based on ionic liquids. Sep. Purif. Technol. 2022, 290, 120841. [Google Scholar] [CrossRef]
- Haider, J.; Qyyum, M.A.; Kazmi, B.; Ali, I.; Nizami, A.-S.; Lee, M. Simulation study of deep eutectic solvent-based biogas upgrading process integrated with single mixed refrigerant biomethane liquefaction. Biofuel Res. J. 2020, 7, 1245. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Du, Z.; Abdeltawab, A.A.; Al-Enizi, A.M.; Chen, X.; Yu, G. A process simulation study of CO2 capture by ionic liquids. Int. J. Greenh. Gas Control. 2017, 58, 223–231. [Google Scholar] [CrossRef]
- Leonzio, G.; Zondervan, E. Surface-Response Analysis for the Optimization of a Carbon Dioxide Absorption Process Using [hmim][Tf2N]. Processes 2020, 8, 1063. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Wang, J.; Song, Z.; Cheng, H.; Chen, L.; Deng, L.; Qi, Z. Multilevel screening of ionic liquid absorbents for simultaneous removal of CO2 and H2S from natural gas. Sep. Purif. Technol. 2020, 248, 117053. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Wang, Y.; Hu, J.; Cui, P. Ionic liquid-based CO2 capture in power plants for low carbon emissions. Int. J. Greenh. Gas Control. 2018, 75, 134–139. [Google Scholar] [CrossRef]
- Pilorgé, H.; McQueen, N.; Maynard, D.; Psarras, P.; He, J.; Rufael, T.; Wilcox, J. Cost Analysis of Carbon Capture and Sequestration of Process Emissions from the U.S. Industrial Sector. Environ. Sci. Technol. 2020, 54, 7524–7532. [Google Scholar] [CrossRef] [PubMed]
- AlNouss, A.; McKay, G.; Al-Ansari, T. A techno-economic-environmental study evaluating the potential of oxygen-steam biomass gasification for the generation of value-added products. Energy Convers. Manag. 2019, 196, 664–676. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Ferro, V.; Palomar, J. Techno-economic feasibility of ionic liquids-based CO2 chemical capture processes. Chem. Eng. J. 2021, 407, 127196. [Google Scholar] [CrossRef]
- Hu, H.; Westover, T.L.; Cherry, R.; Aston, J.E.; Lacey, J.A.; Thompson, D.N. Process simulation and cost analysis for removing inorganics from wood chips using combined mechanical and chemical preprocessing. BioEnergy Res. 2017, 10, 237–247. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, D.; Liu, X.; Zhang, Z.; Wang, Y. Separation of acetonitrile/methanol/benzene ternary azeotrope via triple column pressure-swing distillation. Sep. Purif. Technol. 2016, 169, 66–77. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Hallett, J.P.; Mac Dowell, N. Solvent selection and design for CO2 capture–how we might have been missing the point. Sustain. Energy Fuels 2017, 1, 2078–2090. [Google Scholar] [CrossRef]
- Mota-Martinez, M.T.; Brandl, P.; Hallett, J.P.; Mac Dowell, N. Challenges and opportunities for the utilisation of ionic liquids as solvents for CO2 capture. Mol. Syst. Des. Eng. 2018, 3, 560–571. [Google Scholar] [CrossRef]
- Akinola, T.E.; Oko, E.; Wang, M. Study of CO2 removal in natural gas process using mixture of ionic liquid and MEA through process simulation. Fuel 2019, 236, 135–146. [Google Scholar] [CrossRef]
- Seo, K.; Tsay, C.; Edgar, T.F.; Stadtherr, M.A.; Baldea, M. Economic Optimization of Carbon Capture Processes Using Ionic Liquids: Toward Flexibility in Capture Rate and Feed Composition. ACS Sustain. Chem. Eng. 2021, 9, 4823–4839. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, K.; Yu, M.; Dai, Y.; Yuan, R.; Zhu, Z.; Gao, J. An improvement scheme for pressure-swing distillation with and without heat integration through an intermediate connection to achieve energy savings. Comput. Chem. Eng. 2018, 119, 439–449. [Google Scholar] [CrossRef]
- Haider, J.; Qyyum, M.A.; Kazmi, B.; Zahoor, M.; Lee, M. Simulation study of biomethane liquefaction followed by biogas upgrading using an imidazolium-based cationic ionic liquid. J. Clean. Prod. 2019, 231, 953–962. [Google Scholar] [CrossRef]
- De Riva, J.; Suarez-Reyes, J.; Moreno, D.; Díaz, I.; Ferro, V.; Palomar, J. Ionic liquids for post-combustion CO2 capture by physical absorption: Thermodynamic, kinetic and process analysis. Int. J. Greenh. Gas Control. 2017, 61, 61–70. [Google Scholar] [CrossRef]
- Li, K.; Wu, W.; Wu, J.; Liang, H.; Zhang, H. Experiments on vapour-liquid equilibrium of CO2-ionic liquid under flow conditions and influence on its refrigeration cycle. Appl. Therm. Eng. 2020, 180, 115865. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Palomar, J. Improvement of CO2 capture processes by tailoring the reaction enthalpy of Aprotic N-Heterocyclic anion-based ionic liquids. Chem. Eng. J. Adv. 2022, 10, 100291. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Kumar, S.; Cho, J.H.; Moon, I. Ionic liquid-amine blends and CO2BOLs: Prospective solvents for natural gas sweetening and CO2 capture technology—A review. Int. J. Greenh. Gas Control. 2014, 20, 87–116. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Lu, X.; Ji, X. Energy consumption analysis for CO2 separation using imidazolium-based ionic liquids. Appl. Energy 2014, 136, 325–335. [Google Scholar] [CrossRef]
- Shankar, N.; Sivasubramanian, V.; Arunachalam, K. Steady state optimization and characterization of crude oil using Aspen HYSYS. Pet. Sci. Technol. 2016, 34, 1187–1194. [Google Scholar] [CrossRef]
- Lei, Y.; Zhou, Y.; Wei, Z.; Chen, Y.; Guo, F.; Yan, W. Optimal Design of an Ionic Liquid (IL)-Based Aromatic Extractive Distillation Process Involving Energy and Economic Evaluation. Ind. Eng. Chem. Res. 2021, 60, 3605–3616. [Google Scholar] [CrossRef]
- Linnhoff, B.; Mason, D.R.; Wardle, I. Understanding heat exchanger networks. Comput. Chem. Eng. 1979, 3, 295–302. [Google Scholar] [CrossRef]
- Zoughaib, A. Energy Integration of Continuous Processes: From Pinch Analysis to Hybrid Exergy/Pinch Analysis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–53. [Google Scholar] [CrossRef]
- Smith, R. State of the art in process integration. Appl. Therm. Eng. 2000, 20, 1337–1345. [Google Scholar] [CrossRef]
- Dagde, K.K.; Piagbo, B.K. Using Simulation Modelling to Improve the Performance of Chemical Engineering Processes: Part 1-Industrial Heat Exchanger Network Analysis. Int. J. Pure Appl. Sci. Technol. 2012, 12, 39. [Google Scholar]
- El-Halwagi, M.M. Pollution Prevention through Process Integration: Systematic Design Tools; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Furman, K.C.; Sahinidis, N.V. A critical review and annotated bibliography for heat exchanger network synthesis in the 20th century. Ind. Eng. Chem. Res. 2002, 41, 2335–2370. [Google Scholar]
- Jezowski, J. Heat Exchanger Network Grass Root and Retrofit Design. The Review of The State-of-The Art: Part I. Heat Exchanger Network Targeting and Insight Based Methods of Synthesis. Hung. J. Ind. Chem. 1994, 22, 279–294. [Google Scholar]
- Mardini, N.; Bicer, Y. Formic acid synthesis and utilization for solar energy storage through solar-driven chloralkali process and fuel cells. Energy Storage 2021, 3, e235. [Google Scholar] [CrossRef]
- Gatti, M.; Martelli, E.; Marechal, F.; Consonni, S. Review, modeling, Heat Integration, and improved schemes of Rectisol®-based processes for CO2 capture. Appl. Therm. Eng. 2014, 70, 1123–1140. [Google Scholar] [CrossRef]
- Harkin, T.; Hoadley, A.; Hooper, B. Reducing the energy penalty of CO2 capture and compression using pinch analysis. J. Clean. Prod. 2010, 18, 857–866. [Google Scholar] [CrossRef]
- Perevertaylenko, O.Y.; Gariev, A.O.; Damartzis, T.; Tovazhnyanskyy, L.L.; Kapustenko, P.O.; Arsenyeva, O.P. Searches of cost effective ways for amine absorption unit design in CO2 post-combustion capture process. Energy 2015, 90, 105–112. [Google Scholar] [CrossRef]
- Momeni, M.; Soltani, M.; Hosseinpour, M.; Nathwani, J. A comprehensive analysis of a power-to-gas energy storage unit utilizing captured carbon dioxide as a raw material in a large-scale power plant. Energy Convers. Manag. 2021, 227, 113613. [Google Scholar] [CrossRef]
- Brunner, C.; Slawitsch, B.; Giannakopoulou, K.; Schnitzer, H. Industrial Process Indicators and Heat Integration in Industries; Booklet IEA SHC Task; Joanneum Research: Graz, Austria, 2008. [Google Scholar]
- Shemfe, M.B.; Fidalgo, B.; Gu, S. Heat integration for bio-oil hydroprocessing coupled with aqueous phase steam reforming. Chem. Eng. Res. Des. 2016, 107, 73–80. [Google Scholar] [CrossRef]






| CO2 Separation Techniques | Description | Examples | Benefits | Drawbacks | CO2 Recovery | Energy Requirement | Ref. |
|---|---|---|---|---|---|---|---|
| Absorption | A process in which a gas is separated by an absorbent in which it is soluble; hence, solubility is the main factor defining absorption |
| High purity Low costs for MEA solvents are reported Simple design Less toxic Easy regeneration Chemical absorption holds good results in terms of removal efficiency | Methods used to get the equilibrium data for absorption through experiments are typically time-consuming Amine absorbents are corrosive MEA absorbent requires high energy during high-temperature absorbent regeneration Requires waste treatment Solvent degradation | 90–98% | 4–6 MJ/kgCO2 | [20,21,22,23] |
| Adsorption | Using adsorbents to separate the gas mixtures under variable pressures of temperatures |
| Design flexibility Easy operation | The available adsorbent for CO2 has low selectivity and capacity. Lower removal efficiency compared to other technologies (absorption and cryogenic) Temperature Swing Adsorption requires more energy because of additional heat demand compared to other processes. | 80–95% | 2–3 MJ/kgCO2 | [20,23,24,25,26,27] |
| Membrane separation | Membranes split gases by allowing a mixture of gases to pass through a membrane from one side of the membrane to the other. The concentration gradient created by a high partial pressure on one side of the membrane and a low partial pressure on the other induces this transportation. |
| Environmentally friendly technology Requires less energy Low cost for operation | Extreme temperatures or harsh chemicals may destroy the membrane Low removal efficiency and low CO2 purity Membranes are delicate to residues of sulfur compounds, such as H2S | 80–90% | 0.5–6 MJ/kgCO2 | [1,23,28,29,30,31,32,33,34,35,36] |
| Distillation (cryogenic) | A separation technique that liquefies the gases and then uses boiling points to achieve high-purity separation |
| Feasible operation Design that is environmentally friendly | Due to the low temperature of the gases during liquefaction, the frosting of gases can occur, which can damage the equipment Corrosion and fouling can occur due to acidic gases High maintenance costs | >95% | 6–10 MJ/kgCO2 | [20,23,37,38,39] |
| Hydrates separation | Utilizes the differential pressure in between gas components to generate hydrates and accomplish segregation in the mixture |
| Clean process Free of impurities Easy to transport | Not good for industrial use in terms of costs Slow process Very low temperatures are required to conduct the process Requires high pressure to operate | 99% | - | [40,41,42,43,44,45] |
| Equipment | Operating Input Parameters |
|---|---|
| LIQSEP (Flash Separator) | Temperature: 20 °C, Pressure:15 bar |
| Mixer | - |
| MP2 (Multistage Compressor) | Isentropic, 3 Stages, Discharge Pressure 18 bar |
| Absorber | 20 Stages, Column Pressure:15 bar, Packing Type: Flexiring, Material: Metal, Size: 0.625 In, Vendor: KOCH |
| Flash1 (Flash Column) | Temperature: 38.85 °C, Pressure: 2 bar |
| COMP (Compressor) | Isentropic, Discharge Pressure: 30 bar |
| Cooler1 | Temperature: 37.5 °C, Pressure: 30 bar |
| Heater | Temperature: 126.85 °C, Pressure: 2 bar |
| Flash2 (Flash Column) | Temperature: 131.85 °C, Pressure: 0.04 bar |
| Pump | Discharge Pressure: 20 bar |
| Vaccum Pump | Discharge Pressure: 0.04 bar |
| Cooler2 | Temperature: 30 °C, Pressure: 20 bar |
| Total Electric Power Demand | 13.15 kW |
| Net Duty | 364,744.59 kJ/h |
| Inlet Stream No. | CH4 Composition (%) | CO2 Composition (%) | H2S Composition (%) | H2O Composition (%) | N2 Composition (%) | H2 Composition (%) | CO Composition (%) |
|---|---|---|---|---|---|---|---|
| Stream 1 | 56 | 42 | 1 | 1 | - | - | - |
| Stream 2 | 83 | 14 | 1 | 2 | - | - | - |
| Stream 3 | 58 | 35 | 1 | 2 | 1 | 1 | 2 |
| Stream Variable | Value |
|---|---|
| Pressure, bar | 15 |
| Temperature, K | 326 |
| Total Mass Flow kg/h | 4000 |
| Type of Industry | Composition of Feed (%) | IL Used | CO2 Purity (%) | CH4 Purity (%) | Ref. |
|---|---|---|---|---|---|
| Coal-fired Power Plant | N2→78 CO2→13 H2O→1 | 1-butyl-3-methylimidazolium acetate ([Bmim][Ac]) | 98.7 | - | [57,79] |
| Natural-Gas (Sweetening Process) | CH4→80 CO2→20 | 3MEPYNTF2 | ≥99 | ≥99 | [80] |
| Biomethane Liquefaction | CH4→60 CO2→39 H2S→1 | [Bmim][PF6] | 98.5 | 99 | [82] |
| Model Flue gas | O2→3.81 CO2→13.30 N2→71.64 H2O→11.25 | [bmim][PF6] [bmim][BF4] | 90 90 | - | [83] |
| Coal Industry | N2→84 CO2→12 H2O (vapor)→4 | ([emim][Ac]) | 99.26 | - | [58] |
| Flue Gas | Ar→0.48 CH4→0.24 H2→37.5 N2→0.33 CO→6.27 CO2→23.87 H2O→30.68 NH3→0.16 H2S→0.47 | [hmim][Tf2N] | 93.7%, | - | [84] |
| Natural Gas | CH4→- CO2→- H2S→- | [DePYO] [H2PO4] [BeMPYO][H2PO4] [PMMIM] [H2PO4] [EMIM] [H2PO4] [BMIM] [MeSO4] [BMIM] [PF6] [BMIM] [TCM] | 90.73 89.96 88.26 91.42 84.11 85.18 86.64 | 97.47 97.36 97.21 97.52 97.01 96.86 97.26 | [86] |
| Biogas from sewage sludge | CH4→40–75 vol% CO2→15–60 vol% H2O (vapor)→5–10 vol% Trace→H2S | [C2MIm][Tf2N] [C6MIm][Tf2N] [P66614][Tf2N] | Complete removal of CO2 | 95% CH4 mol frac rich stream | [73] |
| Power Plant | N2→78 CO2→12.5 H2O→5 | [bmim][Tf2N] | 94.5 | - | [87] |
| Mixed Waste Industrial Stream (Stream 1) | CH4→56 CO2→42 H2O→1 H2S→1 | [EMIM][NTF2] | 99.4 | 98.36 | This Work |
| Mixed Waste Industrial Stream (Stream 2) | CH4→83 CO2→14 H2O→2 H2S→1 | [EMIM][NTF2] | 97.9 | 98.8 | This Work |
| Mixed Waste Industrial Stream (Stream 3) | CH4→58 CO2→35 H2O→1 H2S→1 N2→1 H2→1 CO→2 | [EMIM][NTF2] | 99.2 | 92.1 | This Work |
| Utility Type | Costing Rate | Inlet Temperature (°C) | Outlet Temperature (°C) | Pressure (Bar) |
|---|---|---|---|---|
| Electricity | 0.0775 $/kWh | - | - | - |
| HP Steam | 2.5·10−6 $/kJ | 250 | 249 | 39.7 |
| Cooling Water | 2.12·10−7 $/kJ | 20 | 25 | 1.01 |
| Design | Base Case | Target | New Area Cost Index (Cost) | Payback (Years) | Operating Savings (Cost/s) | DTmin (°C) | Pinch Temperatures (°C) | ||
|---|---|---|---|---|---|---|---|---|---|
| Heating Cost Index (Cost/s) | 0.04267 | 0.04747 | 0.001654 | 19,950 | 58,670 | 0.01078 | 10 | HOT | COLD |
| Heating Load (kJ/h) | 36,150,000 | 44,010,000 | 1,404,000 | 403.2 | 393.2 | ||||
| Cooling Cost Index (Cost/s) | 0.02766 | 0.03364 | 0.001215 | 113.29 | 103.29 | ||||
| Cooling Load (kJ/h) | 36,350,000 | 44,200,000 | 1,596,000 | ||||||
| Area (m2) | 516.8 | 585.2 | 3734 | ||||||
| New Area (m2) | 23.34 | ||||||||
| Shell | 8 | 7 | 38 | ||||||
| New Shell | 1 | ||||||||
| HEN Design Cross Pinch | 403.2/393.2 °C | 113.3/103.3 °C |
|---|---|---|
| Flash2_Heat_Exchanger (kJ/h) | 34,556,431.6 | 0 |
| B-10_Heat_Exchanger (kJ/h) | 0 | 0 |
| Heater (kJ/h) | 0 | 0 |
| Flash1_Heatexchanger (kJ/h) | 0 | 0 |
| Cooler2 (kJ/h) | 0 | 30,488,804.7 |
| Cooler1 (kJ/h) | 0 | 70,389.4058 |
| E-100(kJ/h) | 0 | 4,190,871.3 |
| Total Network Cross Pinch Load (kJ/h) | 34,556,431.6 | 34,750,065.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, T.; Khraisheh, M.; Almomani, F. Cost and Heat Integration Analysis for CO2 Removal Using Imidazolium-Based Ionic Liquid-ASPEN PLUS Modelling Study. Sustainability 2023, 15, 3370. https://doi.org/10.3390/su15043370
Qureshi T, Khraisheh M, Almomani F. Cost and Heat Integration Analysis for CO2 Removal Using Imidazolium-Based Ionic Liquid-ASPEN PLUS Modelling Study. Sustainability. 2023; 15(4):3370. https://doi.org/10.3390/su15043370
Chicago/Turabian StyleQureshi, Tooba, Majeda Khraisheh, and Fares Almomani. 2023. "Cost and Heat Integration Analysis for CO2 Removal Using Imidazolium-Based Ionic Liquid-ASPEN PLUS Modelling Study" Sustainability 15, no. 4: 3370. https://doi.org/10.3390/su15043370
APA StyleQureshi, T., Khraisheh, M., & Almomani, F. (2023). Cost and Heat Integration Analysis for CO2 Removal Using Imidazolium-Based Ionic Liquid-ASPEN PLUS Modelling Study. Sustainability, 15(4), 3370. https://doi.org/10.3390/su15043370








