Composting Waste from the White Wine Industry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
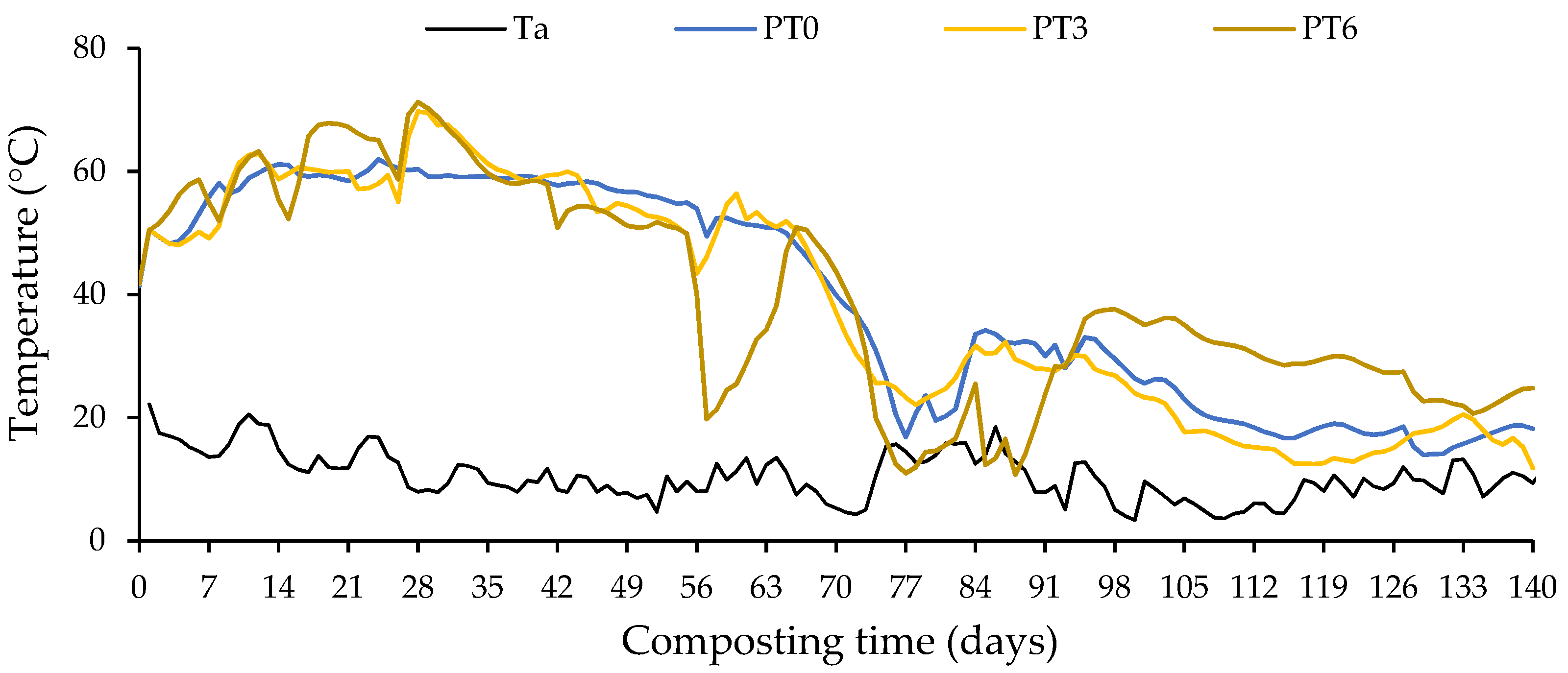
3.1. Temperature
3.2. Moisture Content
3.3. pH and Electrical Conductivity
3.4. Organic Matter Decomposition
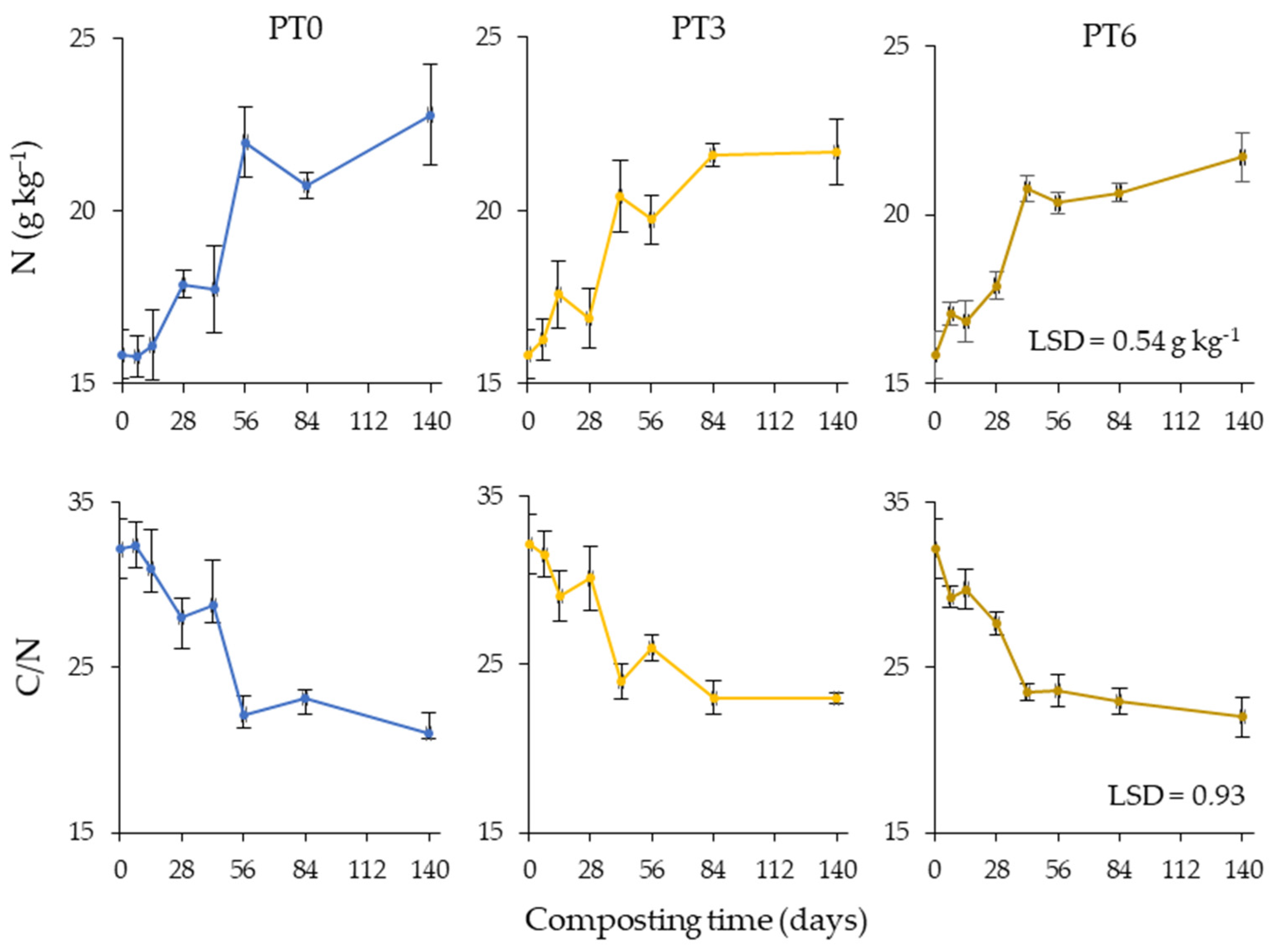
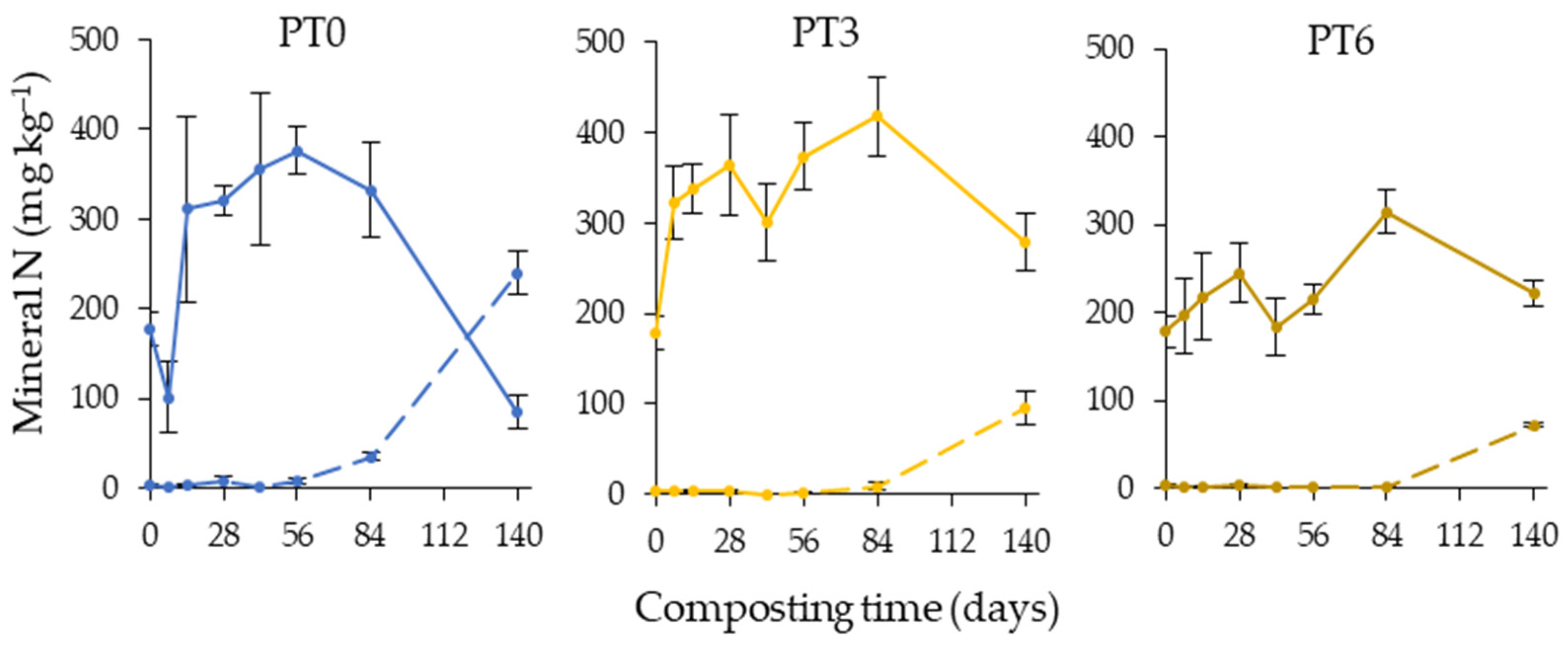
3.5. Nitrogen Transformations
3.6. Compost Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cataldo, E.; Salvi, L.; Sbraci, S.; Storchi, P.; Mattii, G.B. Sustainable viticulture: Effects of soil management in Vitis vinifera. Agronomy 2020, 10, 2–15. [Google Scholar] [CrossRef]
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Coll, P.; Cortet, J.; Le Cadre-Barthélemy, E.; D’errico, G.; et al. Vineyard Management and its impacts on soil biodiversity, functions, and ecosystems services. Front. Ecol. Evol. 2022, 10, 1–21. [Google Scholar] [CrossRef]
- Karimi, B.; Cahurel, J.; Gontier, L.; Chovelon, M.; Mahé, H.; Ranjard, L. A meta-analysis of the ecotoxicological impact of viticultural practices on soil biodiversity. Environ. Chem. Lett. 2020, 18, 1947–1966. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Climate change and winegrape quality in Australia. Clim. Res. 2008, 36, 99–111. [Google Scholar] [CrossRef]
- Ramos, M.C. Effects of compost amendment on the available soil water and grape yield in vineyards planted after land levelling. Agric. Water Manag. 2017, 191, 67–76. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of wine production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Lazcano, C.; Charlotte, D.; Wilson, S.G. Defining and managing for healthy vineyard soils, interactions with the concept of terroir. Front. Environ. Sci. 2020, 8, 68. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Alatzas, A.; Theocharis, S.; Miliords, D.; Leontaridou, K.; Kanellis, A.; Kotseridis, Y.; Hatzopoulos, P.; Koundouras, S. The effect of water deficit on two Greek Vitis vinifera L. cultivars: Physiology, grape composition and gene expression during berry development. Plants 2021, 10, 1947. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.; Oliveira, L.F.S.; Ferrari, V.; Taffarel, S.R.; Feijoo, G.; Moreira, M.T. Environment assessement of viticulture waste valorization through composting as a biofertilisation strategie for cerealand fruit crops. Environ. Pollut. 2020, 264, 114794. [Google Scholar] [CrossRef]
- Badalikova, B.; Burg, P.; Masán, V.; Prudil, J.; Jobbágy, J.; Cizková, A.; Kristof, K.; Vasinka, M. Deep placement of compost into vineyard soil affecting physical properties of soil, yield and quality of grapes. Sustainability 2022, 14, 7823. [Google Scholar] [CrossRef]
- Benbi, D.K.; Biswas, C.R.; Bawa, S.S.; Kumar, K. Influence of farmyard manure, inorganic fertilizers and weed control practices on some soil physical properties in a long-term experiment. Soil Use Manag. 1998, 14, 52–54. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, M.; Vallini, G.; Pera, A. The biology of composting: A review. Waste Manag. Res. 1983, 1, 157–176. [Google Scholar] [CrossRef]
- Liang, Y.; Leonard, J.J.; Feddes, J.J.R.; McGill, W.B. Influence of carbon and buffer amendments on ammonia volatilization in composting. Biorsource Technol. 2006, 97, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Brito, L.M.; Mourão, I.; Coutinho, J.; Smith, S.R. Simple technologies for on-farm composting of cattle slurry solid fraction. Waste Manag. 2012, 32, 1332–1340. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Brito, L.M.; Mourão, I.; Coutinho, J.; Smith, S.R. Co-composting of invasive Acacia longifolia with pine bark for horticultural use. Environ. Technol. 2015, 36, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the cost of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- INE-Instituto Nacional de Estatística 2021. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0004498&contexto=bd&selTab=tab2&xlang=pt (accessed on 1 February 2023).
- Moldes, A.B.; Vázquez, M.; Dominguez, J.M.; Díaz-Fierros, F.; Barral, M. Evaluation of mesophilic biodegraded grape marc as soil fertilizer. Appl. Biochem. Biotechnol. 2007, 14, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, E.; Sort, X.; Soliva, M.; Trillas, I. Composting winery waste: Sludges and grape stalks. Bioresour. Technol. 2004, 95, 203–208. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Diaz, M.J.; Madejon, E.; López, F.; López, R.; Cabrera, F. Optimization of the rate vinasse/grape marc for co-composting process. Process Biochem. 2002, 37, 1143–1150. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 2013, 116, 18–26. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterization of the solid by-product and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef]
- Dwier, K.; Hosseinian, F.; Rod, M. The market potential of grapes waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Marhuenda-Egea, F.C.; Perez-Espinosa, A.; Bernal, M.P.; Moral, R. Co-composting of distillery wastes with animal manures: Carbon and nitrogen transformations in the evolution of compost stability. Chemosphere 2008, 72, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.J.; Sánchez-Arias, V.; Villasenor, J.; Rodríguez, L. Evaluation of carbon degradation during co-composting of exhausted grape marc with different biowastes. Chemosphere 2008, 73, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Pergula, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The way for a sustainable agriculture. Appl. Soil Ecol. 2018, 123, 744–750. [Google Scholar] [CrossRef]
- CEN European Standards: 13037 (pH), 13038 (EC), 13040 (DMC); Soil Improvers and Growing Media. European Committee for Standardization: Brussels, Belgium, 1999.
- Zucconi, F.; Pera, A.; Forte, M.; De Bertoldi, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57.41. [Google Scholar]
- Mooijman, K.A. The new ISO 6579-1: A real horizontal standard for detection of Salmonella, at last! Food Microbiol. 2018, 71, 2–7. [Google Scholar] [CrossRef]
- Yoruk, N.G. Most probable number technique in Escherichia coli count using ISO 16649-3, ISO 7251, and rapid test enumeration device (tempo EC) methods in milk and dairy products. J. Food Saf. 2018, 38, e12502. [Google Scholar] [CrossRef]
- Paredes, C.; Roig, A.; Bernal, M.P.; Sánchez-Monedero, M.A.; Cegarra, J. Evolution of organic matter and nitrogen during co-composting of olive mill wastewater with solid organic wastes. Biol. Fertil. Soils 2000, 20, 222–227. [Google Scholar] [CrossRef]
- Tang, J.C.; Shibata, A.; Zhou, Q.; Katayama, A. Effect of temperature on reaction rate and microbial community in composting of cattle manure with rice straw. J. Biosci. Bioeng. 2007, 104, 321–328. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Richard, T.L.; Honeyman, M.S. Carbon, nutrient, and mass loss during composting. Nutr. Cycl. Agroecosys. 2002, 62, 15–42. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Vargas-Garcia, M.C.; Suárez-Estela, F.; Moreno, J. Evolution of the pathogen content during co-composting of winery and distillery wastes. Bioresour. Technol. 2008, 99, 7299–7306. [Google Scholar] [CrossRef]
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovás, J. Composting of wine industry wastes and their use as a substrate for growing soilless ornamental plants. Span. J. Agric. Res. 2012, 10, 482–491. [Google Scholar] [CrossRef]
- Portuguese Decree-Law 103/2015. Decreto-Lei nº 103/2015. In Diário da República; 1ª série; nº 114 de 15 de Junho; Ministério da Economia: Lisbon, Portugal, 2015; pp. 3756–3788. Available online: https://www.valorpneu.pt/wp-content/uploads/2020/01/2015_Decreto-Lei-103_alt-DL-178_2006.pdf (accessed on 1 January 2023).
- Brito, L.M.; Mourão, I.; Coutinho, J.; Smith, S. Composting for management and resource recovery of invasive Acacia species. Waste Manag. Res. 2013, 31, 1125–1132. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Kazmi, A.A. Effects of turning frequency on compost stability and some chemical characteristics in a rotary drum compost. Chemosphere 2009, 74, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Xue, J.; Cheng, D.; Li, Z. Effects of turning frequency on ammonia emission during the composting of chicken manure and soybean straw. Molecules 2022, 27, 472. [Google Scholar] [CrossRef]
- Brito, L.M.; Amaro, A.L.; Fernandes, A.S. Efeito do arejamento no processo de compostagem da fracção sólida do chorume de pecuária leiteira. Revista de Ciências Agrárias 2009, 33, 298–311. [Google Scholar] [CrossRef]
- El Kader, N.A.; Robin, P.; Paillat, J.; Leterme, P. Turning, compacting, and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour. Technol. 2007, 98, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Peigné, J.; Girardin, P. Environmental impacts of farm scale composting practices. Water Air Soil Pollut. 2004, 153, 45–68. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effect on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Raviv, M.; Medina, S.; Krasnovsky, A.; Ziadna, H. Organic matter and nitrogen conservation in manure compost for organic agriculture. Compost Sci. Util. 2004, 12, 6–10. [Google Scholar] [CrossRef]
- Brito, L.M.; Coutinho, J.; Smith, S.R. Methods to improve the composting process of the solid fraction of dairy cattle slurry. Bioresour. Technol. 2008, 99, 8955–8960. [Google Scholar] [CrossRef]
- Barros, E.S.C.; de Amorin, M.C.C.; Olszevski, N.; Silva, T.S. Composting of winery waste and characteristics of the final compost according to Brazilian legislation. J. Environ. Sci. Health Part B 2021, 5, 447–457. [Google Scholar] [CrossRef]
- Patti, A.F.; Issa, G.; Smernik, R.; Wilkinson, K. Chemical composition of composted grape marc. Water Sci. Technol. 2009, 60, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Soumaré, M.; Demeyer, A.; Tack, F.M.G.; Verloo, M.G. Chemical characteristics of Malian and Belgian solid waste composts. Bioresour. Technol. 2002, 81, 97–101. [Google Scholar] [CrossRef]
- Barrington, S.; Choinière, D.; Trigui, M.; Knight, W. Effect of carbon source on compost nitrogen and carbon losses. Bioresour. Technol. 2002, 83, 189–194. [Google Scholar] [CrossRef]
- Chinakwe, E.C.; Nwogwugwu, U.N.; Ibekwe, V.L.; Nwachukwu, I.N.; Ihejirika, C.E.; Ofoegbu, C.J.; Chinakwe, P.O.; Mejeha, O.K. Changes in microbial population numbers during composting of some organic wastes in greenhouse. J. Adv. Microbiol. 2019, 17, 1–10. [Google Scholar] [CrossRef]
- Brito, L.M.; Mourão, I.; Coutinho, J. Physicochemical Dynamics of composting screw pressed cattle slurry amended with Italian Reygrass straw or gorse bulking agent. Compost. Sci. Util. 2010, 18, 119–126. [Google Scholar] [CrossRef]
- Stentiford, E.T. Composting control, principles and practice. In The Science of Composting; DeBertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Chapman and Hall: London, UK, 1996; pp. 49–59. [Google Scholar]
- Chang, R.; Li, Y.; Li, J.; Chen, Q.; Zhao, H. Influences of the thermophilic period on biodegradation and nitrogen loss in stimulated vegetables waste composting. Global Ecol. Conserv. 2019, 18, 623. [Google Scholar] [CrossRef]
- Bueno, P.; Tapias, R.; López, F.; Díaz, M.J. Optimizing composting parameters for nitrogen conservation in composting. Bioresour. Technol. 2008, 99, 5069–5077. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Morales, J.; Mayoral, A.M.; Moral, R. Study of composting process of winery distillery wastes using multivariate techniques. Bioresour. Technol. 2009, 100, 4766–4772. [Google Scholar] [CrossRef]
- Raj, D.; Antil, R.S. Evaluation of maturity and stability parameters of composts prepared from agro-industrial wastes. Bioresour. Technol. 2011, 102, 2868–2873. [Google Scholar] [CrossRef]
- Antonic, B.; Janciková, S.; Dordevic, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G. Effect of composting on nutrient loss and nitrogen availability of cattle deep litter. Eur. J. Agron. 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Cáceres, R.; Malinska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, B.; Zelles, L.; Palojarvi, A.; Bai, Q. Emission of climate-relevant trace gases and succession of microbial communities during open-windrow composting. Appl. Environ. Microb. 1997, 63, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Sáez, J.A.; Clemente, R.; Bustamante, M.A.; Yañez, D.; Bernal, M.P. Evaluation of slurry management strategy and the integration of the composting technology in a pig farm. Agronomical and environmental implications. J. Environ. Manag. 2017, 192, 57–67. [Google Scholar] [CrossRef]
- Hwang, S.; Hanaki, K. Effects of oxygen concentration and moisture content of refuse on nitrification, denitrification and nitrous oxide production. Bioresour. Technol. 2000, 71, 159–165. [Google Scholar] [CrossRef]
- Wu, L.; Ma, L.Q.; Martinez, G.A. Comparison of methods for evaluating stability and maturity of biosolids compost. J. Environ. Qual. 2000, 29, 424–429. [Google Scholar] [CrossRef]
- Zucconi, F.; De Bertoldi, M. Composts specifications for the production and characterization of composts from municipal solid waste. In Compost: Quality and Use; de Bertoldi, M., Ferranti, M.P., L’Hermite, P., Zucconi, F., Eds.; Elsevier Applied Science: London, UK, 1987; pp. 30–50. [Google Scholar]
- Antil, R.S.; Bar-Tal, A.; Fine, P.; Hadas, A. Predicting nitrogen and carbon mineralization of composted manure and sewage sludge in soil. Compost Sci. Util. 2011, 19, 33–43. [Google Scholar] [CrossRef]
- Buchanan, M.; Brinton, W.; Shields, F.; West, J.; Thompson, W. Compost Maturity Index; CCQC—California Compost Quality Council: Nevada City, CA, USA, 2001. [Google Scholar]
- Tiquia, S. Reduction of compost phytotoxicity during the process of decomposition. Chemosphere 2010, 79, 506–512. [Google Scholar] [CrossRef]
- Komilis, D.P.; Tziouvaras, I.S. A statistical analysis to assess the maturity and stability of six composts. Waste Manag. 2009, 29, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Dominguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Commission of European Communities. Commission Staff Working Document on the Management of Biowaste in the European Union; Commission of European Communities: Brussels, Belgium, 2008; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52010SC0577&from=EL (accessed on 1 January 2023).
- Evanylo, G.; Sherony, C.; Spargo, J.; Starner, D.; Brosius, M.; Hearing, K. Soil and water environmental effects of fertilizer, manure, and compost-based fertility practices in an organic vegetable cropping system. Agric. Ecosyst. Environ. 2008, 127, 50–58. [Google Scholar] [CrossRef]

| Characteristics (Unit) | Pile | -------------------------------- Sample Day ------------------------------ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 28 | 42 | 56 | 84 | 140 | ||
| MC (g kg−1) | PT0 | 621 | 581 | 609 | 563 | 611 | 487 | 611 | 567 |
| PT3 | 621 | 638 | 577 | 585 | 481 | 417 | 440 | 422 | |
| PT6 | 621 | 606 | 552 | 530 | 439 | 335 | 402 | 408 | |
| LSD | 0 | 17 | 57 | 27 | 66 | 61 | 43 | 13 | |
| pH | PT0 | 3.9 | 5.3 | 4.6 | 5.0 | 4.4 | 7.8 | 8.0 | 7.7 |
| PT3 | 3.9 | 4.2 | 4.4 | 4.4 | 6.2 | 6.8 | 7.8 | 7.8 | |
| PT6 | 3.9 | 5.0 | 5.5 | 5.6 | 7.3 | 7.7 | 8.1 | 8.1 | |
| LSD | 0.0 | 0.7 | 1.3 | 0.7 | 0.8 | 0.1 | 0.2 | 0.1 | |
| EC (dS m−1) | PT0 | 2.2 | 1.8 | 2.0 | 2.0 | 2.7 | 1.6 | 1.1 | 1.5 |
| PT3 | 2.2 | 2,0 | 1.9 | 2.1 | 2.3 | 1.7 | 1.6 | 1.6 | |
| PT6 | 2.2 | 2.2 | 2.2 | 2.3 | 2.0 | 1.7 | 1.6 | 1.3 | |
| LSD | 0.0 | 0.2 | 0.5 | 0.2 | 0.4 | 0.2 | 0.2 | 0.1 | |
| RSG (%) | RRG (%) | GI (%) | ||
|---|---|---|---|---|
| Cress | TP0 | 101 | 86 | 87 |
| TP3 | 94 | 87 | 82 | |
| TP6 | 102 | 78 | 82 | |
| LSD | 11 | 24 | 24 | |
| Radish | TP0 | 96 | 118 | 113 |
| TP3 | 102 | 113 | 115 | |
| TP6 | 106 | 118 | 125 | |
| LSD | 7 | 17 | 20 |
| Pile | OM | N | C/N | NH4+-N | NO3−-N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | ---------- (g kg−1) ------------ | |||||
| PT0 | 860 | 22.8 | 21 | 84.1 | 239.7 | 4.3 | 24.5 | 6.7 | 2.3 |
| PT3 | 884 | 21.7 | 23 | 279.7 | 96.2 | 3.5 | 20.9 | 6.6 | 2.1 |
| PT6 | 869 | 21.7 | 22 | 221.8 | 71.4 | 3.5 | 24.9 | 5.3 | 2.2 |
| Pile | B | Cu | Zn | Pb | Cd | Cr | Ni | Hg |
|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | |
| PT0 | 24.0 | 46.9 | 21.8 | 0.27 | 0.11 | 1.85 | 0.66 | 0.003 |
| PT3 | 22.9 | 45.7 | 20.7 | 0.28 | 0.15 | 1.88 | 0.64 | 0.003 |
| PT6 | 25.2 | 38.7 | 23.3 | 0.31 | 0.12 | 4.93 | 1.52 | 0.003 |
| *LVR | - | 100 | 200 | 100 | 0.7 | 100 | 50 | 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, R.; Correia, C.; Mourão, I.; Moura, L.; Brito, L.M. Composting Waste from the White Wine Industry. Sustainability 2023, 15, 3454. https://doi.org/10.3390/su15043454
Pinto R, Correia C, Mourão I, Moura L, Brito LM. Composting Waste from the White Wine Industry. Sustainability. 2023; 15(4):3454. https://doi.org/10.3390/su15043454
Chicago/Turabian StylePinto, Rui, Cláudia Correia, Isabel Mourão, Luísa Moura, and Luis Miguel Brito. 2023. "Composting Waste from the White Wine Industry" Sustainability 15, no. 4: 3454. https://doi.org/10.3390/su15043454
APA StylePinto, R., Correia, C., Mourão, I., Moura, L., & Brito, L. M. (2023). Composting Waste from the White Wine Industry. Sustainability, 15(4), 3454. https://doi.org/10.3390/su15043454








