Optimization of Xanthan Gum Production by Demerara Sugar Using Response Surface Methodology
Abstract
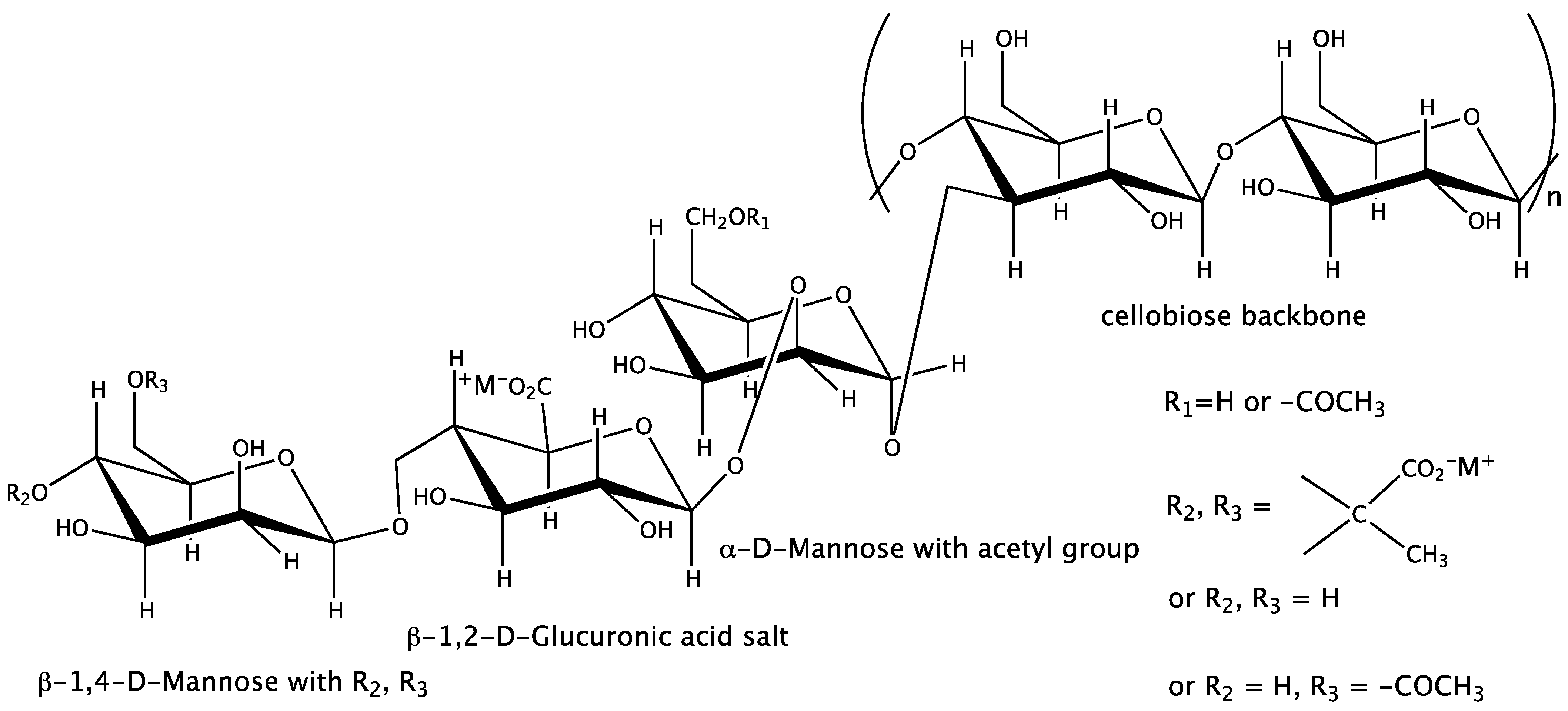
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Xanthan Gum Production
2.3. Xanthan Gum Recovery
2.4. Experimental Design and Data Analysis
3. Results and Discussion
3.1. Analysis of Variance (ANOVA)
3.2. Response Surface Methodology (RSM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; Gautam, S.; Wadhawan, S. Xanthomonas. Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 811–817. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of xanthan gum for whole cell immobilization and its impact in bioremediation—A review. Bioresour. Technol. 2022, 351, 126918. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B.; De Vos, W.H.; Depypere, F.; Van der Meeren, P.; Dewettinck, K. Guar and xanthan gum differentially affect shear induced breakdown of native waxy maize starch. Food Hydrocoll. 2014, 35, 546–556. [Google Scholar] [CrossRef]
- García-Ochoa, F.; Santos, V.E.; Casas, J.A.; Gomez, E. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef] [PubMed]
- Abu Elella, M.H.; Goda, E.S.; Gab-Allah, M.A.; Hong, S.E.; Pandit, B.; Lee, S.; Gamal, H.; Rehman, A.U.; Yoon, K.R. Xanthan gum-derived materials for applications in environment and eco-friendly materials: A review. J. Environ. Chem. Eng. 2020, 9, 104702. [Google Scholar] [CrossRef]
- Furtado, I.F.S.P.C.; Sydney, E.B.; Rodrigues, S.A.; Sydney, A.C.N. Xanthan gum: Applications, challenges, and advantages of this asset of biotechnological origin. Biotechnol. Res. Innov. 2022, 6, e202204. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Al-Muhanna, M.K.; Anwar, N.; Hasnain, S.; Nayak, A.K. Chapter 1–Synthesis of tailor-made polysaccharides: An overview. In Tailor-Made Polysaccharides in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–27. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Şen, E.; Demirci, A.S.; Palabiyik, I. Xanthan gum characterization and production kinetics from pomace of Vitis vinifera. J. Food Process. Preserv. 2022, 46, e17098. [Google Scholar] [CrossRef]
- Xanthan Gum Market Will Be Worth USD 730.39 Million by 2030. Available online: https://www.marketresearchfuture.com/reports/xanthan-gum-market-748 (accessed on 13 February 2023).
- Maurya, D.K.; Kumar, A.; Chaurasiya, U.; Hussain, T.; Singh, S.K. Modern era of microbial biotechnology: Opportunities and future prospects. Microbiomes Plant Health Panoply Appl. 2020, 1, 317–343. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, preparation method, and application in food industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef]
- Chaitali, M.; Kapadi, M.; Suraishkumar, G.K.; Gudi, R.D. Productivity Improvement in Xanthan Gum Fermentation Using Multiple Substrate Optimization. Biotechnol. Prog. 2008, 19, 1190–1198. [Google Scholar] [CrossRef]
- Habibi, H.; Khosravi-Darani, K. Effective variables on production and structure of xanthan gum and its food applications: A review. Biocatal. Agric. Biotechnol. 2017, 10, 130–140. [Google Scholar] [CrossRef]
- Silva, M.F.; Fornari, R.C.; Mazutti, M.A.; de Oliveira, D.; Padilha, F.F.; Cichoski, A.J.; Cansian, R.L.; Di Luccio, M.; Treichel, H. Production and characterization of xantham gum by Xanthomonas campestris using cheese whey as sole carbon source. J. Food Eng. 2009, 90, 119–123. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; González López, Ó. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef]
- Rottava, I.; Batesini, G.; Silva, M.F.; Lerin, L.A.; de Oliveira, D.; Padilha, F.F.; Toniazzo, G.; Mossi, A.; Cansian, R.L.; Di Luccio, M.; et al. Xanthan gum production and rheological behavior using different strains of Xanthomonas sp. Carbohydr. Polym. 2009, 77, 65–71. [Google Scholar] [CrossRef]
- Jesus, M.; Mata, F.; Batista, R.A.; Ruzene, D.S.; Albuquerque-Júnior, R.; Cardoso, J.C.; Vaz-Velho, M.; Pires, P.; Padilha, F.F.; Silva, D.P. Corncob as Carbon Source in the Production of Xanthan Gum in Different Strains Xanthomonas sp. Sustainability 2023, 15, 2287. [Google Scholar] [CrossRef]
- da Silva, J.A.; Cardoso, L.G.; de Jesus Assis, D.; Gomes, G.V.P.; Oliveira, M.B.P.P.; de Souza, C.O.; Druzian, J.I. Xanthan Gum Production by Xanthomonas campestris pv campestris IBSBF 1866 and 1867 from Lignocellulosic Agroindustrial Wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef]
- Demirci, A.S.; Palabiyik, I.; Apaydın, D.; Mirik, M.; Gumus, T. Xanthan gum biosynthesis using Xanthomonas isolates from waste bread: Process optimization and fermentation kinetics. LWT 2018, 101, 40–47. [Google Scholar] [CrossRef]
- Cuadrado-Osorio, P.D.; Ramírez-Mejía, J.M.; Mejía-Avellaneda, L.F.; Mesa, L.; Bautista, E.J. Agro-industrial residues for microbial bioproducts: A key booster for bioeconomy. Bioresour. Technol. Rep. 2022, 20, 101232. [Google Scholar] [CrossRef]
- Bernardo, R.; Lourenzani, W.L.; Satolo, E.G.; Caldas, M.M. Analysis of the agricultural productivity of the sugarcane crop in regions of new agricultural expansions of sugarcane. Gestão Produção 2019, 26. [Google Scholar] [CrossRef]
- Faria, S.; Petkowicz, C.L.D.O.; de Morais, S.A.L.; Terrones, M.G.H.; de Resende, M.M.; de França, F.P.; Cardoso, V.L. Characterization of xanthan gum produced from sugar cane broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, C.; Li, W.; Hang, F.; Li, K.; Shi, C.; Doherty, W.O. Nutritional and antioxidant properties of non-centrifugal cane sugar derived from membrane clarified juice. LWT 2020, 131, 109717. [Google Scholar] [CrossRef]
- Baikow, V. Manufacture and Refining of Raw Cane Sugar; Elsevier: New York, NY, USA, 2013; pp. 1–588. [Google Scholar]
- Jaffé, W.R. Nutritional and functional components of non centrifugal cane sugar: A compilation of the data from the analytical literature. J. Food Compos. Anal. 2015, 43, 194–202. [Google Scholar] [CrossRef]
- D’Adamo, I.; Gastaldi, M.; Morone, P.; Rosa, P.; Sassanelli, C.; Settembre-Blundo, D.; Shen, Y. Bioeconomy of Sustainability: Drivers, Opportunities and Policy Implications. Sustainability 2021, 14, 200. [Google Scholar] [CrossRef]
- Letisse, F.; Lindley, N.; Roux, G. Development of a Phenomenological Modeling Approach for Prediction of Growth and Xanthan Gum Production Using Xanthomonas campestris. Biotechnol. Prog. 2003, 19, 822–827. [Google Scholar] [CrossRef]
- Soleymanpour, Z.; Nikzad, M.; Talebnia, F.; Niknezhad, V. Xanthan gum production from acid hydrolyzed broomcorn stem as a sole carbon source by Xanthomonas campestris. 3 Biotech 2018, 8, 296. [Google Scholar] [CrossRef]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. LIBRO: Statistics Experimenters; Wiley: New York, NY, USA, 2009; pp. 1–655. [Google Scholar]
- Vaishnav, A.; Upadhyay, K.; Koradiya, M.; Tipre, D.; Dave, S. Valorisation of fruit waste for enhanced exopolysaccharide production by Xanthomonas campestries using statistical optimisation of medium and process. Food Biosci. 2022, 46, 101608. [Google Scholar] [CrossRef]
- Mohsin, A.; Akyliyaevna, K.A.; Zaman, W.Q.; Hussain, M.H.; Mohsin, M.Z.; Al-Rashed, S.; Tan, X.; Tian, X.; Aida, K.; Tariq, M.; et al. Kinetically modelled approach of xanthan production using different carbon sources: A study on molecular weight and rheological properties of xanthan. Int. J. Biol. Macromol. 2021, 193, 1226–1236. [Google Scholar] [CrossRef]
- Gunasekar, V.; Reshma, K.; Treesa, G.; Gowdhaman, D.; Ponnusami, V. Xanthan from sulphuric acid treated tapioca pulp: Influence of acid concentration on xanthan fermentation. Carbohydr. Polym. 2014, 102, 669–673. [Google Scholar] [CrossRef]
- Prajapati, J.; Panchal, R.; Patel, D.; Goswami, D. Production and characterization of xanthan gum by Xanthomonas campestris using sugarcane bagasse as sole carbon source. In Biotechnology and Biological Sciences: Proceedings of the 3rd International Conference of Biotechnology and Biological Sciences (BIOSPECTRUM 2019), Kolkata, India, 8–10 August 2019; Taylor and Francis: London, UK, 2019; pp. 363–367. [Google Scholar] [CrossRef]
- Mohsin, A.; Zhang, K.; Hu, J.; Rehman, S.U.; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: A kinetic model approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef]
- Li, P.; Zeng, Y.; Xie, Y.; Li, X.; Kang, Y.; Wang, Y.; Xie, T.; Zhang, Y. Effect of pretreatment on the enzymatic hydrolysis of kitchen waste for xanthan production. Bioresour. Technol. 2017, 223, 84–90. [Google Scholar] [CrossRef]
- Demirci, A.S. Xanthan Gum Production from Hydrolyzed Rice Bran as a Carbon Source by Xanthomonas spp. Microbiol. Biotechnol. Lett. 2012, 40, 356–363. [Google Scholar] [CrossRef]
- Ozdal, M.; Kurbanoglu, E.B. Valorisation of chicken feathers for xanthan gum production using Xanthomonas campestris MO-03. J. Genet. Eng. Biotechnol. 2018, 16, 259–263. [Google Scholar] [CrossRef]
- Soltaninejad, A.; Jazini, M.; Karimi, K. Biorefinery for efficient xanthan gum, ethanol, and biogas production from potato crop residues. Biomass Bioenergy 2022, 158, 106354. [Google Scholar] [CrossRef]
- Rončević, Z.; Grahovac, J.; Dodić, S.; Vučurović, D.; Dodić, J. Utilisation of winery wastewater for xanthan production in stirred tank bioreactor: Bioprocess modelling and optimisation. Food Bioprod. Process. 2019, 117, 113–125. [Google Scholar] [CrossRef]
- Brandão, L.V.; Assis, D.; Lopez, J.A.; Espiridião, M.C.A.; Echevarria, E.M.; Druzian, J.I. Bioconversion from crude glycerin by Xanthomonas campestris 2103: Xanthan production and characterization. Braz. J. Chem. Eng. 2013, 30, 737–746. [Google Scholar] [CrossRef]
- Mishra, A.; Mohite, A.M.; Sharma, N. Influence of particle size on physical, mechanical, thermal, and morphological properties of tamarind- fenugreek mucilage biodegradable films. Polym. Bull. 2022, 80, 3119–3133. [Google Scholar] [CrossRef]
- Haas, I.C.D.S.; Toaldo, I.M.; Burin, V.M.; Bordignon-Luiz, M.T. Extraction optimization for polyphenolic profiling and bioactive enrichment of extractives of non-pomace residue from grape processing. Ind. Crop. Prod. 2018, 112, 593–601. [Google Scholar] [CrossRef]
- Miranda, A.L.; Costa, S.S.; Assis, D.D.J.; Jesus, C.S.; Gil Guimarães, A.; Druzian, J.I. Influence of strain and fermentation time on the production, composition, and properties of xanthan gum. J. Appl. Polym. Sci. 2019, 137, 48557. [Google Scholar] [CrossRef]
- Trindade, R.A.; Munhoz, A.P.; Burkert, C.A. Impact of a carbon source and stress conditions on some properties of xanthan gum produced by Xanthomonas campestris pv. mangiferaeindicae. Biocatal. Agric. Biotechnol. 2018, 15, 167–172. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Asadollahi, M.A.; Zamani, A.; Biria, D. Production of xanthan gum by free and immobilized cells of Xanthomonas campestris and Xanthomonas pelargonii. Int. J. Biol. Macromol. 2016, 82, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, H.; Annadurai, G.; Chellapandian, M.; Krishnan, M.R.V. Influence of Nutrients on Cell Growth and Xanthan Production by Xanthomonas campestris. Bioprocess Eng. 1996, 14, 307–309. [Google Scholar] [CrossRef]
- Rabiya, R.; Sen, R. Artificial intelligence driven advanced optimization strategy vis-à-vis response surface optimization of production medium: Bacterial exopolysaccharide production as a case-study. Biochem. Eng. J. 2021, 178, 108271. [Google Scholar] [CrossRef]
- Kalogiannis, S.; Iakovidou, G.; Liakopoulou-Kyriakides, M.; Kyriakidis, D.A.; Skaracis, G.N. Optimization of xanthan gum production by Xanthomonas campestris grown in molasses. Process. Biochem. 2003, 39, 249–256. [Google Scholar] [CrossRef]
- Faria, S.; Vieira, P.A.; Resende, M.M.; Ribeiro, E.J.; Cardoso, V.L. Application of a model using the phenomenological approach for prediction of growth and xanthan gum production with sugar cane broth in a batch process. LWT 2010, 43, 498–506. [Google Scholar] [CrossRef]
- Li, R.; Feke, D.L. Rheological and kinetic study of the ultrasonic degradation of xanthan gum in aqueous solution: Effects of pyruvate group. Carbohydr. Polym. 2015, 124, 216–221. [Google Scholar] [CrossRef]
- Mesomo, M.; Silva, M.F.; Boni, G.; Padilha, F.F.; Mazutti, M.; Mossi, A.; de Oliveira, D.; Cansian, R.L.; Di Luccio, M.; Treichel, H. Xanthan gum produced by Xanthomonas campestris from cheese whey: Production optimisation and rheological characterisation. J. Sci. Food Agric. 2009, 89, 2440–2445. [Google Scholar] [CrossRef]
- Xu, L.; Dong, M.; Gong, H.; Sun, M.; Li, Y. Effects of inorganic cations on the rheology of aqueous welan, xanthan, gellan solutions and their mixtures. Carbohydr. Polym. 2015, 121, 147–154. [Google Scholar] [CrossRef]
- Katherine, R.F.; Muthukumaran, C.; Sharmila, G.; Kumar, N.M.; Tamilarasan, K.; Jaiganesh, R. Xanthan gum production using jackfruit-seed-powder-based medium: Optimization and characterization. 3 Biotech 2017, 7, 248. [Google Scholar] [CrossRef]





| Independent Variable (Concentration on g∙L−1) | Symbol | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Sucrose or demerara sugar | X1 | 30 | 50 | 70 |
| MgSO4∙7H2O | X2 | 0.2 | 0.6 | 1.0 |
| K2HPO4 | X3 | 0.01 | 0.50 | 1.00 |
| Runs | X1 | X2 | X3 | Production of Xanthan Gum (g∙L−1) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Strain 290 | Strain 472 | Strain S6 | |||||||
| Sucrose | Demerara Sugar | Sucrose | Demerara Sugar | Sucrose | Demerara Sugar | ||||
| 1 | −1 | −1 | −1 | 0.2993 | 0.3192 | 0.2857 | 0.3931 | 0.1528 | 0.4533 |
| 2 | −1 | −1 | +1 | 0.5166 | 0.2465 | 0.7171 | 1.2739 | 0.0200 | 0.0600 |
| 3 | −1 | +1 | +1 | 0.5992 | 0.2070 | 0.8308 | 1.3075 | 0.9382 | 1.3839 |
| 4 | −1 | +1 | −1 | 0.2847 | 0.2317 | 0.2585 | 0.3619 | 0.5968 | 0.4644 |
| 5 | +1 | +1 | +1 | 0.2784 | 0.3924 | 0.5254 | 0.3750 | 0.0712 | 0.3219 |
| 6 | +1 | −1 | +1 | 0.6840 | 0.2741 | 1.0034 | 0.6109 | 0.0920 | 0.3996 |
| 7 | +1 | −1 | −1 | 0.1127 | 0.2263 | 0.3713 | 0.1373 | 0.0619 | 0.4166 |
| 8 | +1 | +1 | −1 | 0.1454 | 0.1811 | 0.4683 | 0.8674 | 0.9255 | 0.3644 |
| 9 | 0 | 0 | 0 | 0.4926 | 0.3761 | 0.3480 | 0.7854 | 0.4634 | 0.7212 |
| 10 | 0 | 0 | 0 | 0.2859 | 0.4738 | 0.3436 | 0.6200 | 0.5775 | 0.7488 |
| 11 | 0 | 0 | 0 | 0.4182 | 0.4273 | 0.2495 | 0.4562 | 0.6876 | 0.6121 |
| 12 | 0 | 0 | 0 | 0.2750 | 0.3885 | 0.2326 | 0.6213 | 0.4677 | 0.6193 |
| 13 | 0 | 0 | 0 | 0.3010 | 0.4708 | 0.2875 | 0.6315 | 0.7182 | 0.6533 |
| SS | dF | MS | F | p-Value | |
|---|---|---|---|---|---|
| Strain 290 | |||||
| (1) sucrose (L) | 0.028716 | 1 | 0.028716 | 1.97056 | 0.219346 |
| sucrose (Q) | 0.000339 | 1 | 0.000339 | 0.02327 | 0.884727 |
| (2) MgSO4∙7H2O (L) | 0.011621 | 1 | 0.011621 | 0.79742 | 0.412776 |
| (3) K2HPO4 (L) | 0.190993 | 1 | 0.190993 | 13.10634 | 0.015215 |
| 1L by 2L | 0.024299 | 1 | 0.024299 | 1.66746 | 0.253072 |
| 1L by 3L | 0.003720 | 1 | 0.003720 | 0.25524 | 0.634884 |
| 2L by 3L | 0.014544 | 1 | 0.014544 | 0.99802 | 0.363654 |
| Error | 0.072863 | 5 | 0.014573 | ||
| Total SS | 0.347094 | 12 | |||
| Strain 472 | |||||
| (1) sucrose (L) | 0.009543 | 1 | 0.009543 | 0.63434 | 0.461891 |
| sucrose (Q) | 0.216603 | 1 | 0.216603 | 14.39851 | 0.012699 |
| (2) MgSO4∙7H2O (L) | 0.010841 | 1 | 0.010841 | 0.72066 | 0.434676 |
| (3) K2HPO4 (L) | 0.358239 | 1 | 0.358239 | 23.81361 | 0.004553 |
| 1L by 2L | 0.027320 | 1 | 0.027320 | 1.81604 | 0.235625 |
| 1L by 3L | 0.012364 | 1 | 0.012364 | 0.82187 | 0.406201 |
| 2L by 3L | 0.023555 | 1 | 0.023555 | 1.56582 | 0.266171 |
| Error | 0.075217 | 5 | 0.015043 | ||
| Total SS | 0.733682 | 12 | |||
| Strain S6 | |||||
| (1) sucrose (L) | 0.038809 | 1 | 0.038809 | 0.67477 | 0.448775 |
| sucrose (Q) | 0.156573 | 1 | 0.156573 | 2.72232 | 0.159867 |
| (2) MgSO4∙7H2O (L) | 0.607753 | 1 | 0.607753 | 10.56694 | 0.022679 |
| (3) K2HPO4 (L) | 0.047370 | 1 | 0.047370 | 0.82362 | 0.405737 |
| 1L by 2L | 0.033722 | 1 | 0.033722 | 0.58632 | 0.478413 |
| 1L by 3L | 0.133334 | 1 | 0.133334 | 2.31827 | 0.188357 |
| 2L by 3L | 0.021033 | 1 | 0.021033 | 0.36570 | 0.571750 |
| Error | 0.287573 | 5 | 0.057515 | ||
| Total SS | 1.326168 | 12 |
| SS | dF | MS | F | p-Value | |
|---|---|---|---|---|---|
| Strain 290 | |||||
| (1) Demerara sugar (L) | 0.000604 | 1 | 0.000604 | 0.30652 | 0.604 |
| Demerara sugar (Q) | 0.086340 | 1 | 0.086340 | 43.83216 | 0.001 |
| (2) MgSO4∙7H2O (L) | 0.000363 | 1 | 0.000363 | 0.18436 | 0.685 |
| (3) K2HPO4 (L) | 0.003268 | 1 | 0.003268 | 1.65925 | 0.254 |
| 1L by 2L | 0.005005 | 1 | 0.005005 | 2.54089 | 0.172 |
| 1L by 3L | 0.015887 | 1 | 0.015887 | 8.06512 | 0.036 |
| 2L by 3L | 0.005592 | 1 | 0.005592 | 2.83865 | 0.153 |
| Error | 0.009849 | 5 | 0.001970 | ||
| Total SS | 0.126907 | 12 | |||
| Strain 472 | |||||
| (1) Demerara sugar (L) | 0.226397 | 1 | 0.226397 | 6.05021 | 0.057 |
| Demerara sugar (Q) | 0.005688 | 1 | 0.005688 | 0.15200 | 0.713 |
| (2) MgSO4∙7H2O (L) | 0.030826 | 1 | 0.030826 | 0.82380 | 0.406 |
| (3) K2HPO4 (L) | 0.408427 | 1 | 0.408427 | 10.91476 | 0.021 |
| 1L by 2L | 0.030233 | 1 | 0.030233 | 0.80795 | 0.410 |
| 1L by 3L | 0.425595 | 1 | 0.425595 | 11.37356 | 0.020 |
| 2L by 3L | 0.101520 | 1 | 0.101520 | 2.71301 | 0.160 |
| Error | 0.187099 | 5 | 0.037420 | ||
| Total SS | 1.415786 | 12 | |||
| Strain S6 | |||||
| (1) Demerara sugar (L) | 0.092257 | 1 | 0.092257 | 1.930789 | 0.223 |
| Demerara sugar (Q) | 0.108667 | 1 | 0.108667 | 2.274231 | 0.192 |
| (2) MgSO4∙7H2O (L) | 0.181533 | 1 | 0.181533 | 3.799212 | 0.109 |
| (3) K2HPO4 (L) | 0.027226 | 1 | 0.027226 | 0.569801 | 0.484 |
| 1L by 2L | 0.268242 | 1 | 0.268242 | 5.613882 | 0.064 |
| 1L by 3L | 0.042881 | 1 | 0.042881 | 0.897424 | 0.387 |
| 2L by 3L | 0.207143 | 1 | 0.207143 | 4.335177 | 0.092 |
| Error | 0.238909 | 5 | 0.047782 | ||
| Total SS | 1.166857 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, L.C.; Jesus, M.S.; Pires, P.; Fontes-Junior, A.S.; Nunes, E.S.; Santos, K.S.; Teixeira, J.A.; Padilha, F.F.; Ruzene, D.S.; Silva, D.P. Optimization of Xanthan Gum Production by Demerara Sugar Using Response Surface Methodology. Sustainability 2023, 15, 5080. https://doi.org/10.3390/su15065080
Ramos LC, Jesus MS, Pires P, Fontes-Junior AS, Nunes ES, Santos KS, Teixeira JA, Padilha FF, Ruzene DS, Silva DP. Optimization of Xanthan Gum Production by Demerara Sugar Using Response Surface Methodology. Sustainability. 2023; 15(6):5080. https://doi.org/10.3390/su15065080
Chicago/Turabian StyleRamos, Larissa Castor, Meirielly Santos Jesus, Preciosa Pires, Alberto S. Fontes-Junior, Erica S. Nunes, Klebson S. Santos, José António Teixeira, Francine Ferreira Padilha, Denise Santos Ruzene, and Daniel Pereira Silva. 2023. "Optimization of Xanthan Gum Production by Demerara Sugar Using Response Surface Methodology" Sustainability 15, no. 6: 5080. https://doi.org/10.3390/su15065080
APA StyleRamos, L. C., Jesus, M. S., Pires, P., Fontes-Junior, A. S., Nunes, E. S., Santos, K. S., Teixeira, J. A., Padilha, F. F., Ruzene, D. S., & Silva, D. P. (2023). Optimization of Xanthan Gum Production by Demerara Sugar Using Response Surface Methodology. Sustainability, 15(6), 5080. https://doi.org/10.3390/su15065080










