The Synergic Effect of Erythrosine and Gold Nanoparticles in Photodynamic Inactivation
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinagreiro, C.S.; Zangirolami, A.; Schaberle, F.A.; Nunes, S.C.; Blanco, K.C.; Inada, N.M.; da Silva, G.J.; Pais, A.A.; Bagnato, V.S.; Arnaut, L.G. Antibacterial photodynamic inactivation of antibiotic-resistant bacteria and biofilms with nanomolar photosensitizer concentrations. ACS Infect. Dis. 2020, 6, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Huang, W. Evaluation of photodynamic inactivation efficiency using conventional and decorative light-emitting diode lamps. Sens. Mater 2017, 29, 1569–1577. [Google Scholar] [CrossRef] [Green Version]
- Penha, C.B.; Bonin, E.; da Silva, A.F.; Hioka, N.; Zanqueta, É.B.; Nakamura, T.U.; de Abreu Filho, B.A.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Photodynamic inactivation of foodborne and food spoilage bacteria by curcumin. LWT Food Sci. Technol. 2017, 76, 198–202. [Google Scholar] [CrossRef]
- Al-Asmari, F.; Mereddy, R.; Sultanbawa, Y. A novel photosensitization treatment for the inactivation of fungal spores and cells mediated by curcumin. J. Photochem. Photobiol., B 2017, 173, 301–306. [Google Scholar] [CrossRef]
- Ghate, V.S.; Zhou, W.; Yuk, H.G. Perspectives and trends in the application of photodynamic inactivation for microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhen, D.; Li, C.; Jiang, N.; Geng, H.; Qiao, Y.; Cai, Q. One-step self-assembly of ZnPc/KMnF3: Yb, Er upconversion photodynamic therapy system for antibacterial applications. Nano 2020, 15, 2050075. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.; Faustino, M.A.; Tome, J.P.; Neves, M.G.; Tome, A.C.; Cavaleiro, J.A.; Cunha, A.; Almeida, A. Involvement of type I and type II mechanisms on the photoinactivation of non-enveloped DNA and RNA bacteriophages. J. Photochem. Photobiol. B 2013, 120, 10–16. [Google Scholar] [CrossRef]
- Dong, L.; Qin, J.; Tai, L.; Mou, K.; Liao, X.; Chen, F.; Hu, X. Inactivation of bacillus subtilis by curcumin-mediated photodynamic technology through inducing oxidative stress response. Microorganisms 2022, 10, 802. [Google Scholar] [CrossRef]
- Hasenleitner, M.; Plaetzer, K. In the right light: Photodynamic inactivation of microorganisms using a LED-based illumination device tailored for the antimicrobial application. Antibiotics 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Tan, L.; Li, H.; Chen, B.; Huang, J.; Zhao, Y.; Wang, J.; Ou, J. Photodynamic inactivation of planktonic Staphylococcus aureus by sodium magnesium chlorophyllin and its effect on the storage quality of lettuce. Photochem. Photobiol. Sci. 2021, 20, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Y.; Chang, K.-C.; Chen, L.-Y.; Wang, P.-C.; Chou, C.-C.; Wu, Z.-B.; Hu, A. Blue light irradiation triggers the antimicrobial potential of ZnO nanoparticles on drug-resistant Acinetobacter baumannii. J. Photochem. Photobiol. B 2018, 180, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Pei, J.; Xue, F.; Cui, X.; Xiong, X.; Li, C. The application of photodynamic inactivation to microorganisms in food. Food Chem. X 2021, 12, 100150. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.H.; Sarmento-Neto, J.F.; Souza, S.O.; Raposo, B.L.; Silva, B.P.; Borges, C.P.; Santos, B.S.; Cabral Filho, P.E.; Reboucas, J.S.; Fontes, A. Advances on antimicrobial photodynamic inactivation mediated by Zn(II) porphyrins. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100454. [Google Scholar] [CrossRef]
- Duguay, B.A.; Herod, A.; Pringle, E.S.; Monro, S.M.; Hetu, M.; Cameron, C.G.; McFarland, S.A.; McCormick, C. Photodynamic inactivation of human coronaviruses. Viruses 2022, 14, 110. [Google Scholar] [CrossRef]
- Su, L.; Huang, J.; Li, H.; Pan, Y.; Zhu, B.; Zhao, Y.; Liu, H. Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int. J. Biol. Macromol. 2021, 172, 231–240. [Google Scholar] [CrossRef]
- Cossu, M.; Ledda, L.; Cossu, A. Emerging trends in the photodynamic inactivation (PDI) applied to the food decontamination. Food Res. Int. 2021, 144, 110358. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.; Wang, L.; Sun, J. Photodynamic inactivation and its application in food preservation. Crit. Rev. Food Sci. Nutr. 2021, 63, 1–15. [Google Scholar] [CrossRef]
- Amin, R.M.; Mohamed, M.B.; Ramadan, M.A.; Verwanger, T.; Krammer, B. Rapid and sensitive microplate assay for screening the effect of silver and gold nanoparticles on bacteria. Nanomed. Nanotechnol. Biol. Med. 2009, 4, 637–643. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Aparna, R.; Prasad, R. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 2014, 137, 75–78. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Kim, Y.; Park, H.; Lee, J.; Kim, H.; Lee, S. Effect of potassium iodide on erythrosine-mediated photodynamic therapy on Streptococcus mutans biofilms. J. Korean Acad. Pediatr. Dent. 2022, 49, 321–328. [Google Scholar] [CrossRef]
- Yassunaka, N.N.; de Freitas, C.F.; Rabello, B.R.; Santos, P.R.; Caetano, W.; Hioka, N.; Nakamura, T.U.; de Abreu Filho, B.A.; Mikcha, J.M.G. Photodynamic inactivation mediated by erythrosine and its derivatives on foodborne pathogens and spoilage bacteria. Curr. Microbiol. 2015, 71, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Seo, H.; Lee, S.; Park, H.; Lee, J. Susceptibility of Mutans streptococci in the planktonic and biofilm state to erythrosine. J. Korean Acad. Pediatr. Dent. 2019, 46, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Park, H.-W.; Lee, J.-H.; Seo, H.-W.; Lee, S.-Y. The photodynamic therapy on Streptococcus mutans biofilms using erythrosine and dental halogen curing unit. Int. J. Oral Sci. 2012, 4, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Christopher, S. Type II mechanisms of photodynamic action. Light-Act. Pestic. Am. Chem. Soc. 1987, 22–38. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Akhtar, F.; Khan, A.U.; Misba, L.; Akhtar, K.; Ali, A. Antimicrobial and antibiofilm photodynamic therapy against vancomycin resistant Staphylococcus aureus (VRSA) induced infection in vitro and in vivo. Eur. J. Pharm. Biopharm. 2021, 160, 65–76. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P.; Pratten, J.; Parkin, I.P.; Wilson, M. Nanoparticles: Their potential use in antibacterial photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 712–720. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Zhou, L.; Ye, Z.; Wang, J.; Ying, Y.; Ruan, C.; Wang, R.; Li, Y. Label-free capacitive immunosensor based on quartz crystal Au electrode for rapid and sensitive detection of Escherichia coli O157: H7. Anal. Chim. Acta 2011, 687, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, H.; Sato, M.; Kezuka, K.; Sato, I.; Feng, X.; Okumura, S.; Fujita, T.; Yokoyama, U.; Eguchi, H.; Ishikawa, Y. Effect of ascorbic acid on reactive oxygen species production in chemotherapy and hyperthermia in prostate cancer cells. J. Physiol. Sci. 2012, 62, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bodannes, R.S.; Chan, P.C. Ascorbic acid as a scavenger of singlet oxygen. FEBS Lett. 1979, 105, 195–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dong, W.; Shen, S.; Meng, F.; Wang, J.; Yang, K.; Lin, D. Enhancement of E. coli inactivation by photosensitized erythrosine-based solar disinfection under weakly acidic conditions. Water Res. 2022, 212, 118125. [Google Scholar] [CrossRef]
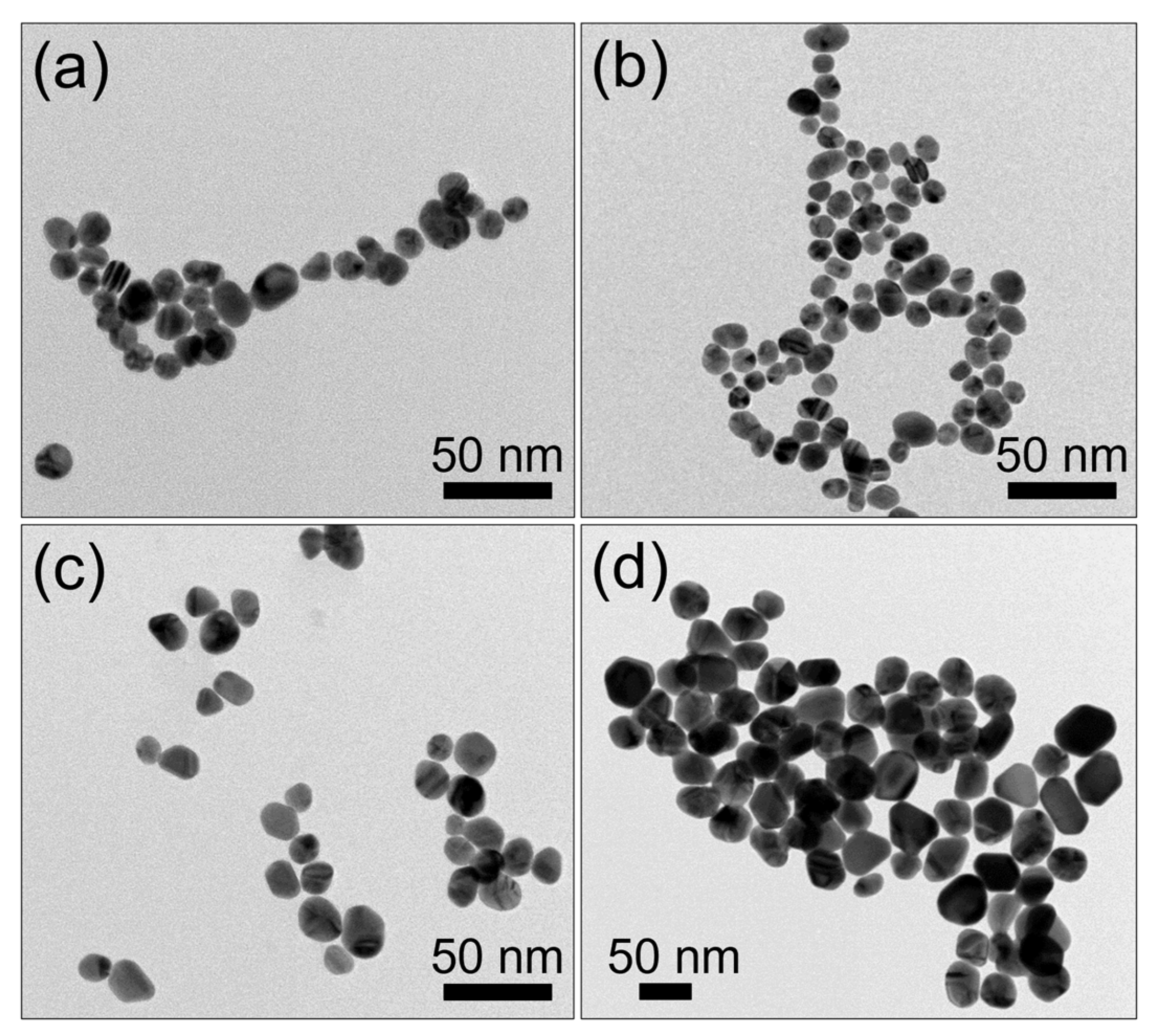

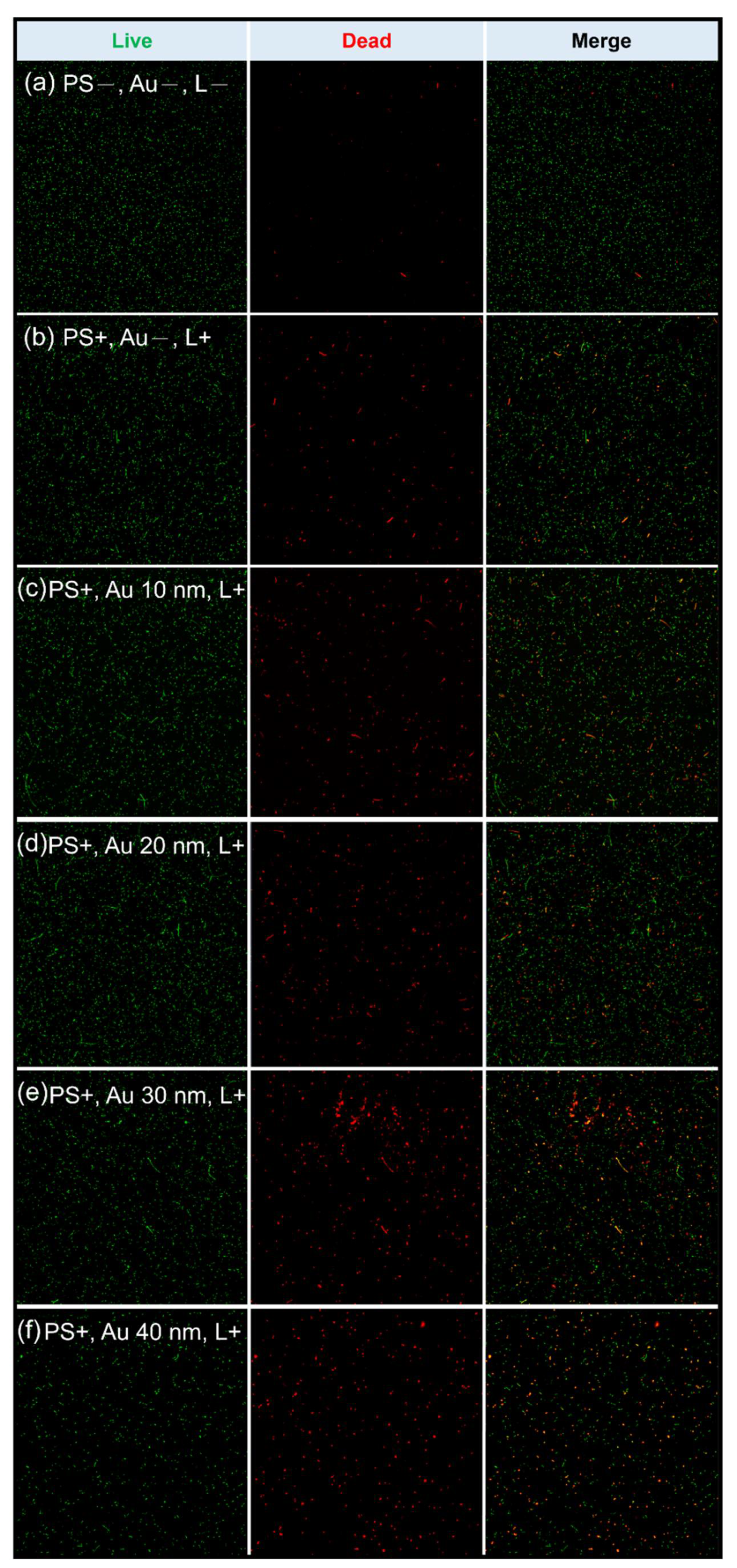
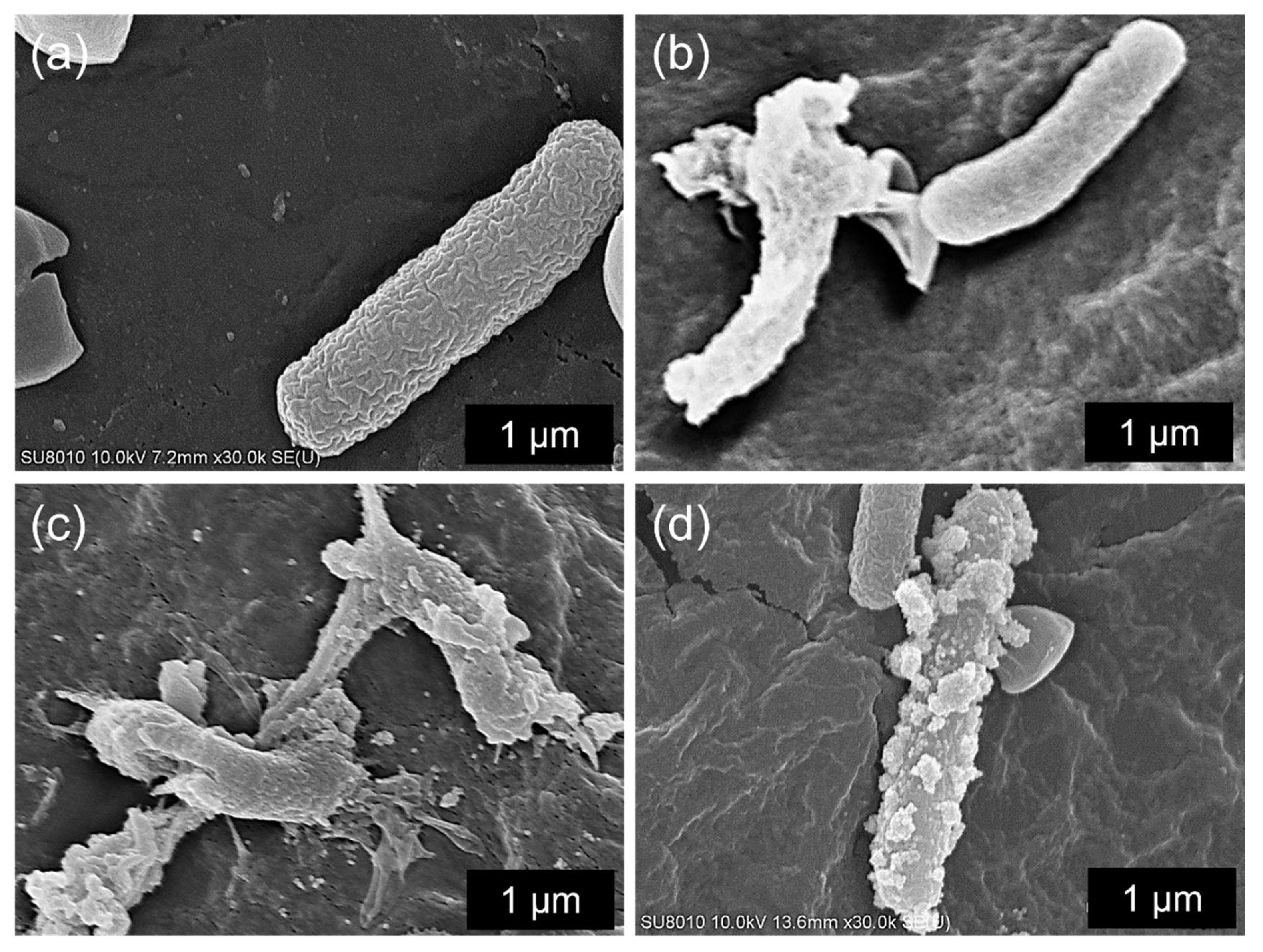




| PS−, Au−, L− | No treatment, allowing natural bacterial growth |
| PS−, Au−, L+ | Influence of only green light irradiation on bacteria |
| PS−, Au+, L− | Influence of only Au NPs on bacteria |
| PS−, Au+, L+ | Influence of Au NPs and green light irradiation on bacteria |
| PS+, Au+, L− | Influence of erythrosine and Au NPs on bacteria |
| PS+, Au−, L+ | Influence of erythrosine and green light irradiation on bacteria |
| PS+, Au+, L+ | Influence of erythrosine, Au NPs, and green light irradiation on bacteria |
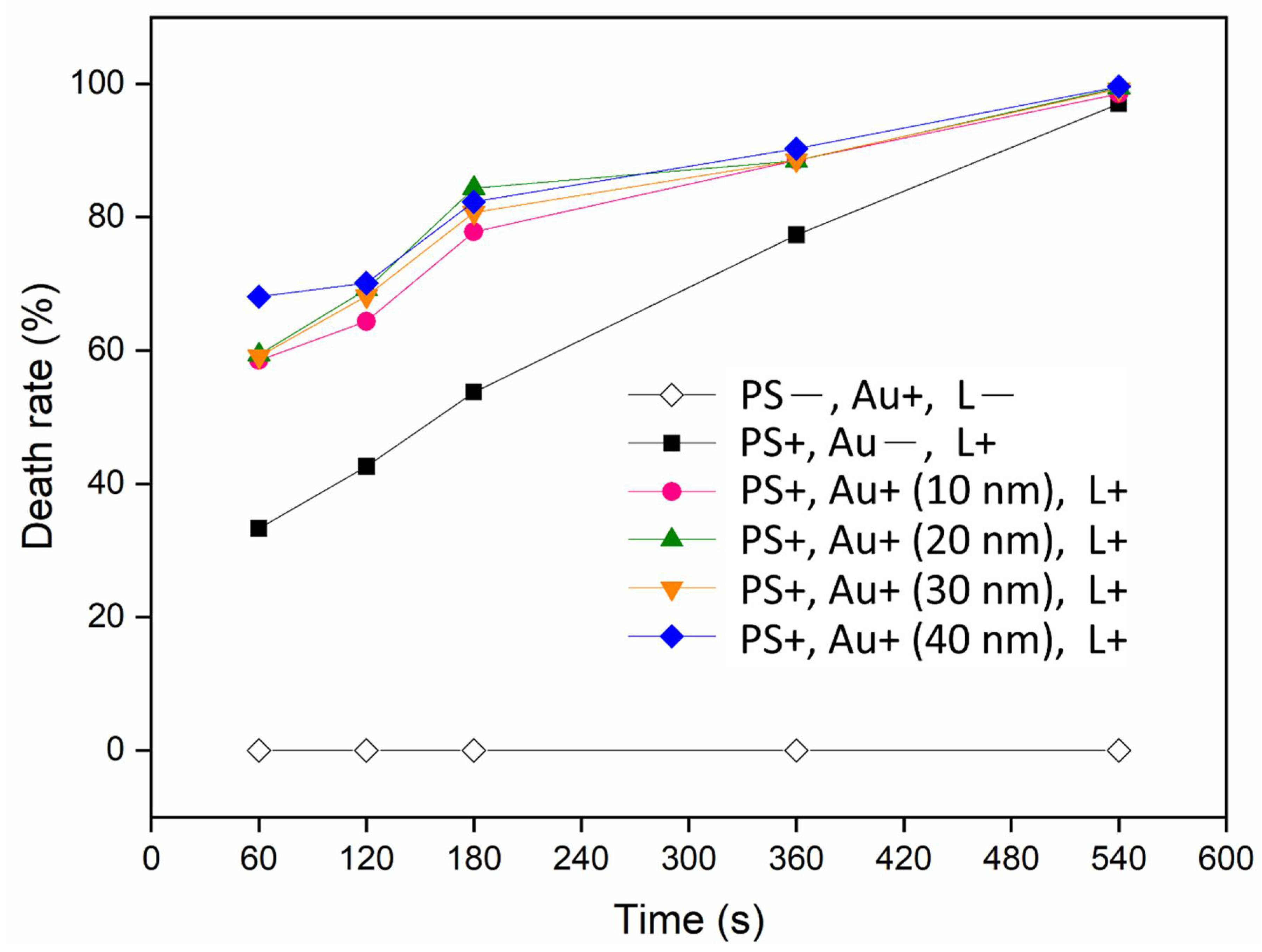
| Time (s) | Condition | CFU | AVG | SD | Death Rate (%) | p-Value (× 10−3) | ||
|---|---|---|---|---|---|---|---|---|
| 0 | PS−, Au−, L− | 436 | 413 | 416 | 422 | 10.20 | 0 | - |
| 0 | PS−, Au+, L− | 428 | 419 | 430 | 426 | 5.86 | 0 | |
| 60 | PS+, Au−, L+ | 303 | 286 | 255 | 281 | 19.87 | 33.28 | 0.88718 |
| PS+, Au+ (10 nm), L+ | 168 | 191 | 166 | 175 | 11.34 | 58.50 | 0.02169 | |
| PS+, Au+ (20 nm), L+ | 170 | 178 | 166 | 171 | 4.988 | 59.37 | 0.00632 | |
| PS+, Au+ (30 nm), L+ | 191 | 152 | 184 | 172 | 14.42 | 59.13 | 0.03726 | |
| PS+, Au+ (40 nm), L+ | 134 | 120 | 150 | 135 | 12.25 | 68.06 | 0.01416 | |
| 120 | PS+, Au−, L+ | 247 | 243 | 236 | 242 | 4.54 | 42.61 | 0.02216 |
| PS+, Au+ (10 nm), L+ | 140 | 167 | 144 | 150 | 11.897 | 64.35 | 0.01653 | |
| PS+, Au+ (20 nm), L+ | 149 | 118 | 123 | 130 | 13.589 | 69.17 | 0.01710 | |
| PS+, Au+ (30 nm), L+ | 138 | 132 | 133 | 134 | 2.624 | 68.14 | 0.00270 | |
| PS+, Au+ (40 nm), L+ | 135 | 113 | 130 | 126 | 9.416 | 70.12 | 0.00724 | |
| 180 | PS+, Au−, L+ | 187 | 217 | 181 | 195 | 15.748 | 53.75 | 0.06891 |
| PS+, Au+ (10 nm), L+ | 108 | 86 | 87 | 94 | 10.143 | 77.79 | 0.00552 | |
| PS+, Au+ (20 nm), L+ | 60 | 72 | 66 | 66 | 4.898 | 84.35 | 0.00153 | |
| PS+, Au+ (30 nm), L+ | 87 | 62 | 95 | 81 | 14.055 | 80.71 | 0.01009 | |
| PS+, Au+ (40 nm), L+ | 66 | 81 | 77 | 75 | 6.342 | 82.29 | 0.00215 | |
| 360 | PS+, Au−, L+ | 95 | 95 | 97 | 96 | 0.942 | 77.31 | 0.00146 |
| PS+, Au+ (10 nm), L+ | 54 | 34 | 57 | 48 | 10.208 | 88.84 | 0.00333 | |
| PS+, Au+ (20 nm), L+ | 54 | 51 | 41 | 49 | 5.557 | 88.46 | 0.00141 | |
| PS+, Au+ (30 nm), L+ | 36 | 49 | 60 | 48 | 9.809 | 88.54 | 0.00308 | |
| PS+, Au+ (40 nm), L+ | 41 | 45 | 37 | 41 | 3.265 | 90.28 | 0.00094 | |
| 540 | PS+, Au−, L+ | 10 | 10 | 18 | 13 | 3.771 | 97.00 | 0.00075 |
| PS+, Au+ (10 nm), L+ | 3 | 9 | 7 | 6 | 2.494 | 98.50 | 0.00061 | |
| PS+, Au+ (20 nm), L+ | 1 | 2 | 4 | 2 | 1.247 | 99.45 | 0.00054 | |
| PS+, Au+ (30 nm), L+ | 2 | 3 | 5 | 3 | 1.247 | 99.21 | 0.00054 | |
| PS+, Au+ (40 nm), L+ | 0 | 2 | 3 | 2 | 1.247 | 99.60 | 0.00053 | |
| Conditions | Green | SD | Red | SD | Death Rate: Red/Green × 100 (%) |
|---|---|---|---|---|---|
| PS−, Au−, L− | 361.6 | 29.85 | 9.8 | 2.86 | 2.71 |
| PS+, Au−, L− | 341.4 | 21.69 | 3.8 | 2.04 | 1.11 |
| PS−, Au−, L+ | 205.4 | 14.58 | 2.4 | 1.51 | 1.16 |
| PS−, Au+ (10 nm), L− | 254.2 | 30.56 | 6.0 | 2.23 | 2.36 |
| PS−, Au+ (20 nm), L− | 273.4 | 12.48 | 4.2 | 1.92 | 1.53 |
| PS−, Au+ (30 nm), L− | 346.0 | 10.58 | 6.8 | 3.27 | 1.96 |
| PS−, Au+ (40 nm), L− | 296.0 | 6.44 | 5.2 | 3.27 | 1.75 |
| PS−, Au+ (10 nm), L+ | 273.6 | 20.62 | 7.2 | 3.56 | 2.63 |
| PS−, Au+ (20 nm), L+ | 539.4 | 25.11 | 7.6 | 4.39 | 1.40 |
| PS−, Au+ (30 nm), L+ | 307.4 | 20.98 | 3.8 | 1.92 | 1.23 |
| PS−, Au+ (40 nm), L+ | 324.0 | 25.94 | 6.2 | 1.92 | 1.91 |
| PS+, Au−, L+ | 260.2 | 12.15 | 23.8 | 3.11 | 9.14 |
| PS+, Au+ (10 nm), L+ | 248.4 | 20.03 | 42.2 | 7.98 | 16.98 |
| PS+, Au+ (20 nm), L+ | 270.4 | 6.76 | 48.8 | 7.91 | 18.04 |
| PS+, Au+ (30 nm), L+ | 222.0 | 29.29 | 43.6 | 9.12 | 19.63 |
| PS+, Au+ (40 nm), L+ | 127.8 | 10.63 | 33.8 | 7.75 | 26.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.-C.; Yang, S.-W.; Xu, Y.-C.; Lu, F.-I. The Synergic Effect of Erythrosine and Gold Nanoparticles in Photodynamic Inactivation. Sustainability 2023, 15, 3621. https://doi.org/10.3390/su15043621
Shi S-C, Yang S-W, Xu Y-C, Lu F-I. The Synergic Effect of Erythrosine and Gold Nanoparticles in Photodynamic Inactivation. Sustainability. 2023; 15(4):3621. https://doi.org/10.3390/su15043621
Chicago/Turabian StyleShi, Shih-Chen, Shu-Wen Yang, Yu-Chen Xu, and Fu-I Lu. 2023. "The Synergic Effect of Erythrosine and Gold Nanoparticles in Photodynamic Inactivation" Sustainability 15, no. 4: 3621. https://doi.org/10.3390/su15043621






