Adsorption of Tetracycline by Magnetic Mesoporous Silica Derived from Bottom Ash—Biomass Power Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Magnetic Mesoporous Silica
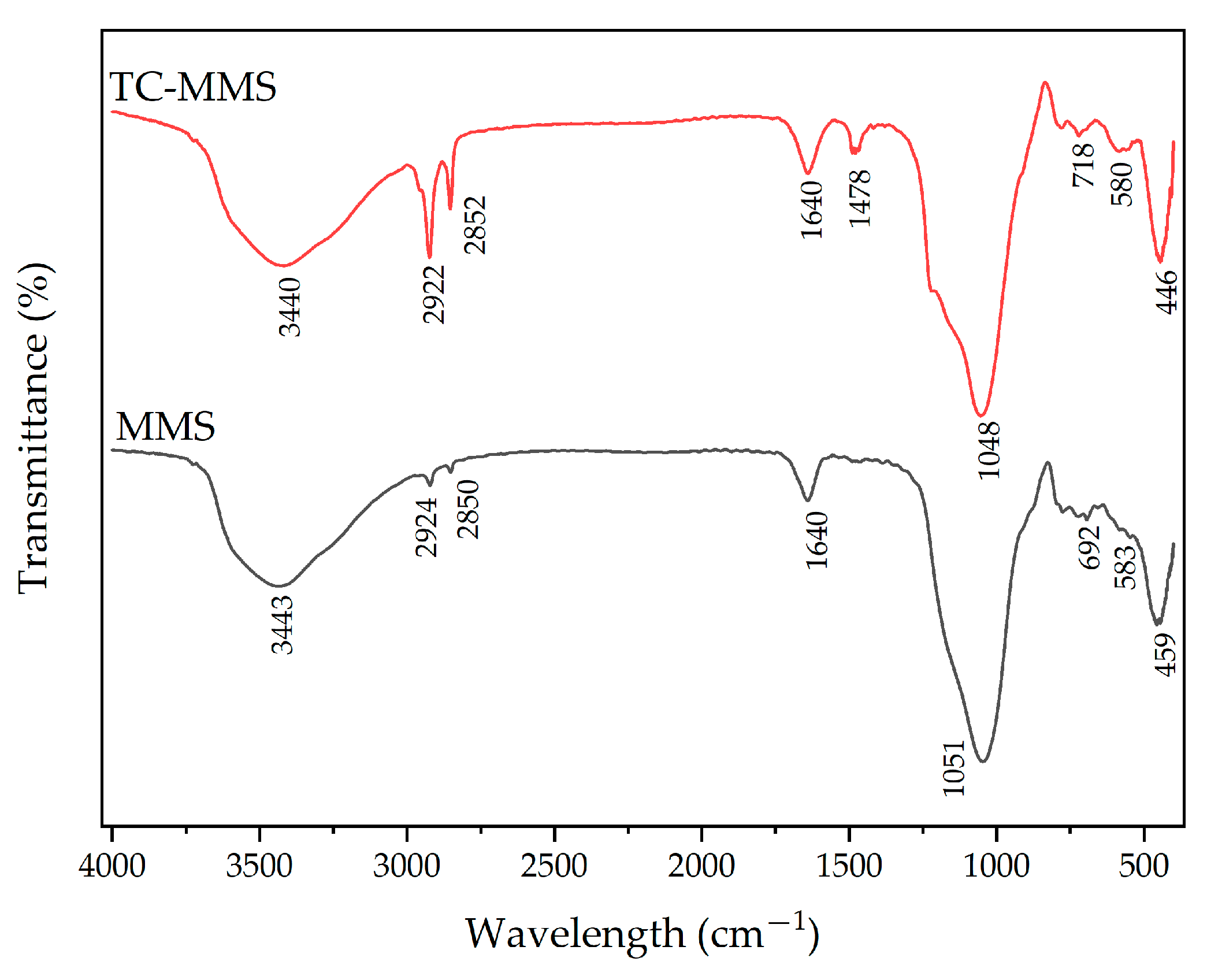
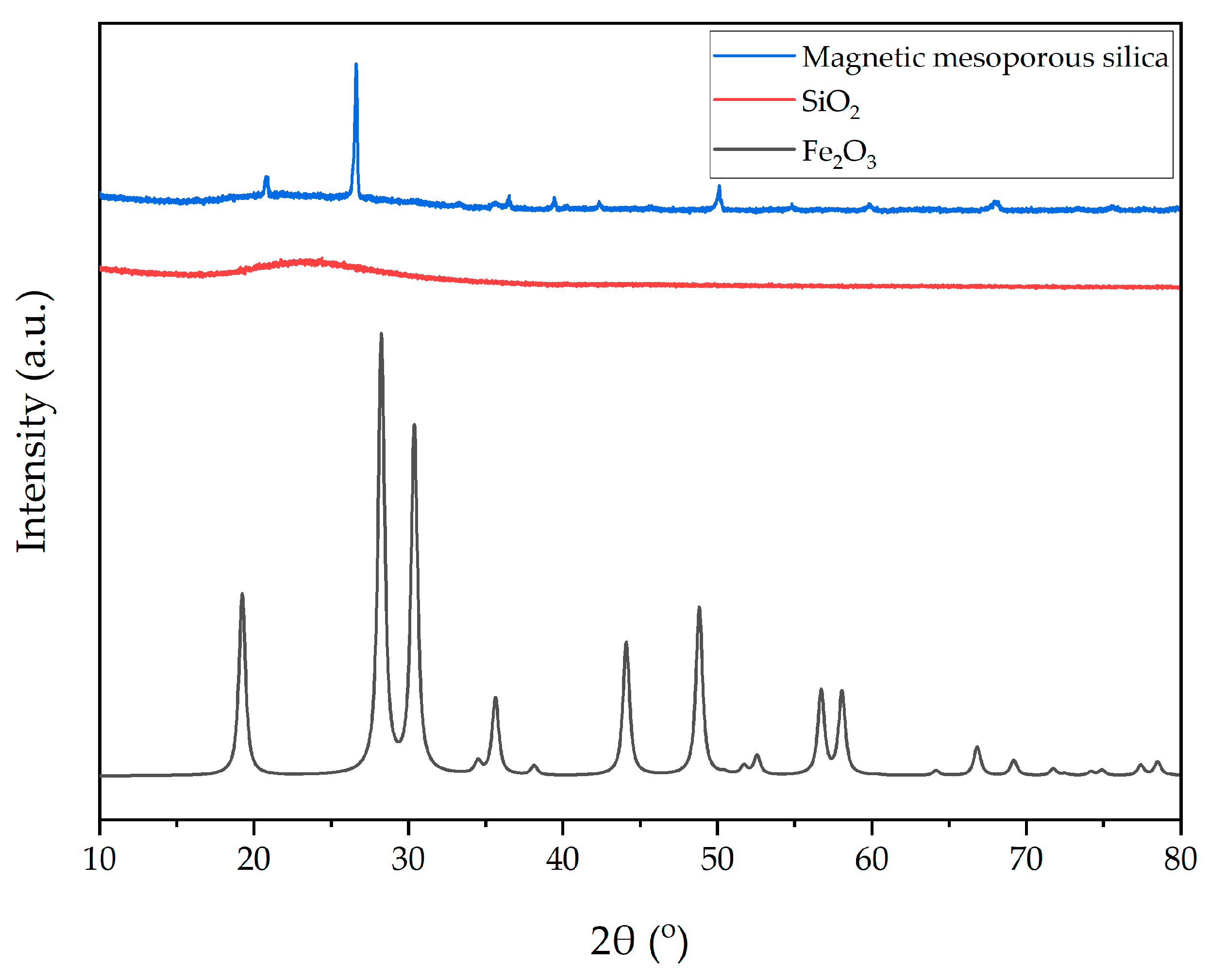
2.2.2. Characterization
2.2.3. Adsorption Experiments
- Qe is the amount of TC adsorption per unit weight of adsorbent (mg/g);
- Co is the initial concentration of TCs (mg/mL);
- Ce (mg/L) is the equilibrium concentration of TC;
- V is the solution volume (mL);
- m is the mass of adsorbent (g).
3. Results and Discussion
3.1. Characterization of MMS
3.2. Adsorption Isotherms of TC on MMS
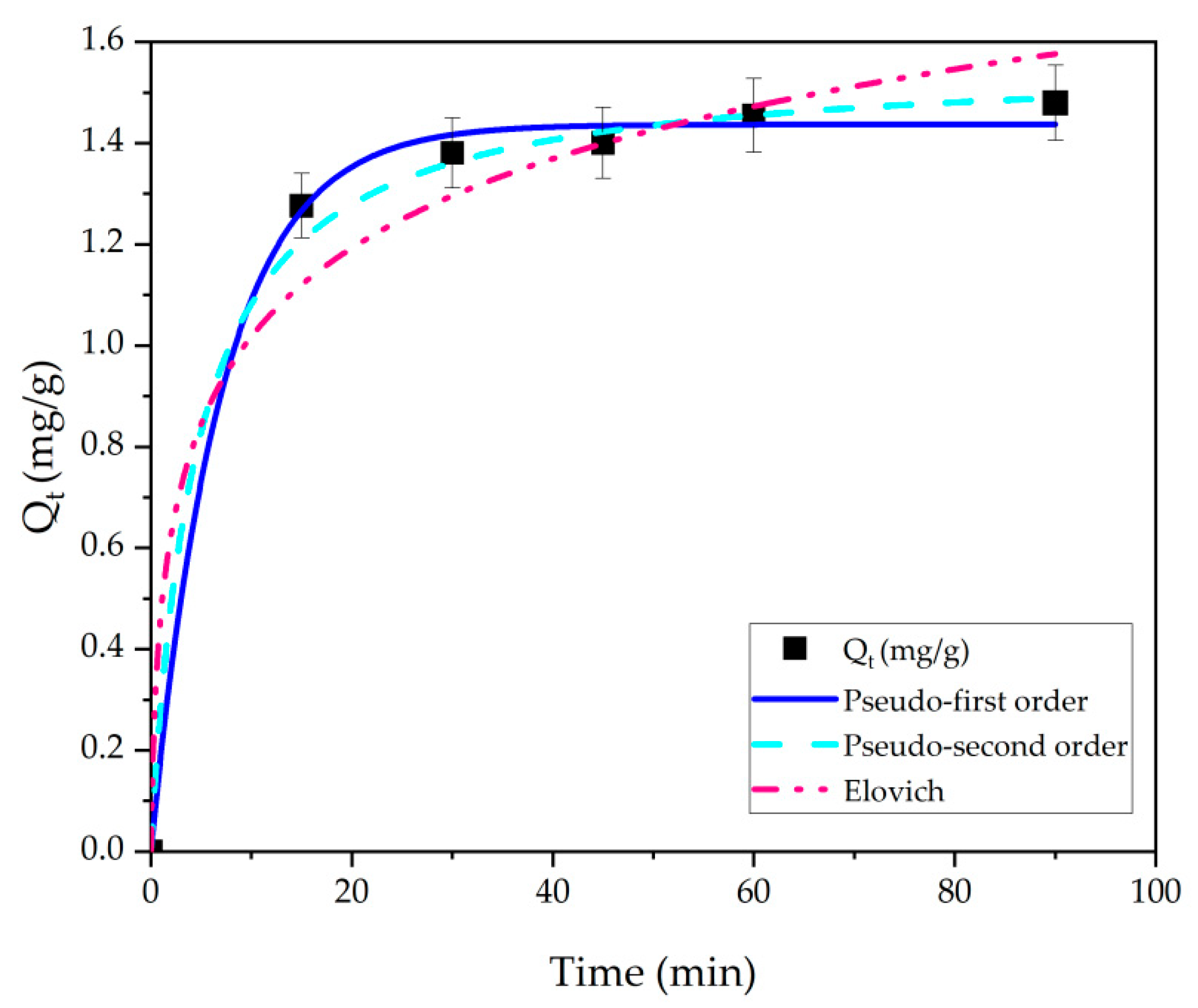
3.3. Adsorption Kinetics of TC on MMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [Green Version]
- Ötker, H.M.; Akmehmet-Balcıoğlu, I. Adsorption and degradation of enrofloxacin, a veterinary antibiotic on natural zeolite. J. Hazard. Mater. 2005, 122, 251–258. [Google Scholar] [CrossRef]
- Kim, S.; Shon, H.; Ngo, H. Adsorption characteristics of antibiotics trimethoprim on powdered and granular activated carbon. J. Ind. Eng. Chem. 2010, 16, 344–349. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Yi, X.; Ma, Y.; Yin, B.; Zhuo, H.; Li, J.; Huang, Y. Effects of administration mode of antibiotics on antibiotic resistance of Enterococcus faecalis in aquatic ecosystems. Chemosphere 2009, 76, 915–920. [Google Scholar] [CrossRef]
- Javid, A.; Mesdaghinia, A.; Nasseri, S.; Mahvi, A.H.; Alimohammadi, M.; Gharibi, H. Assessment of tetracycline contamination in surface and groundwater resources proximal to animal farming houses in Tehran, Iran. J. Environ. Health Sci. Eng. 2016, 14, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.-H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y.-H. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Tolls, J. Sorption of Veterinary Pharmaceuticals in Soils: A Review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Liu, H. Insight into the adsorption of tetracycline onto amino and amino–Fe3+ gunctionalized mesoporous silica: Effect of functionalized groups. J. Environ. Sci. 2018, 65, 171–178. [Google Scholar] [CrossRef]
- Lv, J.-M.; Ma, Y.-L.; Chang, X.; Fan, S.-B. Removal and removing mechanism of tetracycline residue from aqueous solution by using Cu-13X. Chem. Eng. J. 2015, 273, 247–253. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Sun, P.; Pei, J.; Li, D.; Huang, C.-H. Oxidation of tetracycline antibiotics induced by Fe(III) ions without light irradiation. Chemosphere 2015, 119, 1255–1261. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, H.; Li, Y.; Liu, Z. Photodegradation of oxytetracycline in aqueous by 5A and 13X loaded with TiO2 under UV irradiation. J. Hazard. Mater. 2010, 176, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hsu, F.; Liao, H. Biodegradation of three tetracyclines in swine wastewater. J. Environ. Sci. Health Part B 2014, 49, 449–455. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Susanto, H.; Nasseri, S.; Ulbricht, M. Influences of solution chemistry and polymeric natural organic matter on the removal of aquatic pharmaceutical residuals by nanofiltration. Water Res. 2009, 43, 3270–3280. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef]
- Hawker, D.W.; Chimpalee, D.; Vijuksangsith, P.; Boonsaner, M. The pH dependence of the cellulosic membrane permeation of tetracycline antibiotics. J. Environ. Chem. Eng. 2015, 3, 2408–2415. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: Review. Environ. Toxicol. Pharmacol. 2017, 50, 1–10. [Google Scholar] [CrossRef]
- Putra, E.K.; Pranowo, R.; Sunarso, J.; Indraswati, N.; Ismadji, S. Performance of activated carbon and bentonite for adsorption of amoxicillin from wastewater: Mechanisms, isotherms and kinetics. Water Res. 2009, 43, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-J.; Kim, S.-G.; Kim, S.-H. Removal of antibiotics by coagulation and granular activated carbon filtration. J. Hazard. Mater. 2008, 151, 38–43. [Google Scholar] [CrossRef]
- Li, Z.; Schulz, L.; Ackley, C.; Fenske, N. Adsorption of tetracycline on kaolinite with pH-dependent surface charges. J. Colloid Interface Sci. 2010, 351, 254–260. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Mesa, M.; Sierra, L.; Patarin, J.; Guth, J.-L. Morphology and porosity characteristics control of SBA-16 mesoporous silica. Effect of the triblock surfactant Pluronic F127 degradation during the synthesis. Solid State Sci. 2005, 7, 990–997. [Google Scholar] [CrossRef]
- Anbia, M.; Lashgari, M. Synthesis of amino-modified ordered mesoporous silica as a new nano sorbent for the removal of chlorophenols from aqueous media. Chem. Eng. J. 2009, 150, 555–560. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–713. [Google Scholar] [CrossRef]
- Wu, S.H.; Lin, H.P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Microscale scavenging of pentachlorophenol in water using amine and tripolyphosphate-grafted SBA-15 silica: Batch and modeling studies. J. Environ. Manag. 2014, 146, 42–49. [Google Scholar] [CrossRef] [Green Version]
- Sathe, T.R.; Agrawal, A.; Nie, S. Mesoporous Silica Beads Embedded with Semiconductor Quantum Dots and Iron Oxide Nanocrystals: Dual-Function Microcarriers for Optical Encoding and Magnetic Separation. Anal. Chem. 2006, 78, 5627–5632. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Li, W.; Wu, X.; Wang, S.; Deng, Q.; Huang, M. Recent Advances in the Synthesis, Surface Modifications and Applications of Core-Shell Magnetic Mesoporous Silica Nanospheres. Chem. Asian J. 2020, 15, 1248–1265. [Google Scholar] [CrossRef] [PubMed]
- Diagboya, P.N.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Yue, Q.; Zhang, Y.; Wang, C.; Wang, X.; Sun, Z.; Hou, X.-F.; Zhao, D.; Deng, Y. Magnetic yolk–shell mesoporous silica microspheres with supported Au nanoparticles as recyclable high-performance nanocatalysts. J. Mater. Chem. A 2015, 3, 4586–4594. [Google Scholar] [CrossRef]
- Faaliyan, K.; Abdoos, H.; Borhani, E.; Afghahi, S.S.S. Magnetite-silica nanoparticles with core-shell structure: Single-step synthesis, characterization and magnetic behavior. J. Sol-Gel Sci. Technol. 2018, 88, 609–617. [Google Scholar] [CrossRef]
- Cho, B.H.; Nam, B.H.; An, J.; Youn, H. Municipal Solid Waste Incineration (MSWI) Ashes as Construction Materials—A Review. Materials 2020, 13, 3143. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Stewart, D.I. Beneficial management of biomass combustion ashes. Renew. Sustain. Energy Rev. 2021, 151, 111555. [Google Scholar] [CrossRef]
- Yang, G.C.; Yang, T.-Y. Synthesis of zeolites from municipal incinerator fly ash. J. Hazard. Mater. 1998, 62, 75–89. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, F.-S.; Zhu, J.; Liu, Z. Effective utilization of waste ash from MSW and coal co-combustion power plant—Zeolite synthesis. J. Hazard. Mater. 2008, 153, 382–388. [Google Scholar] [CrossRef]
- Chandrasekar, G.; You, K.-S.; Ahn, J.-W.; Ahn, W.-S. Synthesis of hexagonal and cubic mesoporous silica using power plant bottom ash. Microporous Mesoporous Mater. 2008, 111, 455–462. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Xu, Y.; Nie, Q.; Yang, Z.; Sheng, W.; Qian, G. Municipal solid waste incineration residues recycled for typical construction materials—A review. RSC Adv. 2022, 12, 6279–6291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-S.; Li, W.-K.; Huang, C.-Y. Synthesis of mesoporous silica materials from municipal solid waste incinerator bottom ash. Waste Manag. 2014, 34, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Chen, Z.; Zhao, X.; Luo, X.; Wang, L.; Li, Y.; Teng, F. Preparation of magnetic mesoporous silica from rice husk for aflatoxin B1 removal: Optimum process and adsorption mechanism. PLoS ONE 2020, 15, e0238837. [Google Scholar] [CrossRef]
- Jabariyan, S.; Zanjanchi, M.A. A simple and fast sonication procedure to remove surfactant templates from mesoporous MCM-41. Ultrason. Sonochemistry 2012, 19, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 23. [Google Scholar] [CrossRef]
- Gu, C.; Karthikeyan, K.G. Interaction of Tetracycline with Aluminum and Iron Hydrous Oxides. Environ. Sci. Technol. 2005, 39, 2660–2667. [Google Scholar] [CrossRef]
- Prakasham, R.S.; Devi, G.S.; Laxmi, K.R.; Rao, C.S. Novel Synthesis of Ferric Impregnated Silica Nanoparticles and Their Evaluation as a Matrix for Enzyme Immobilization. J. Phys. Chem. C 2007, 111, 3842–3847. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, H.; Liu, H.; Li, H.; Qu, J. Iron-incorporated mesoporous silica for enhanced adsorption of tetracycline in aqueous solution. RSC Adv. 2015, 5, 42407–42413. [Google Scholar] [CrossRef]
- Suwunwong, T.; Patho, P.; Choto, P.; Phoungthong, K. Enhancement the rhodamine 6G adsorption property on Fe3O4-composited biochar derived from rice husk. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Bruckmann, F.D.S.; Schnorr, C.E.; Salles, T.d.R.; Nunes, F.B.; Baumann, L.; Müller, E.I.; Silva, L.F.O.; Dotto, G.L.; Rhoden, C.R.B. Highly Efficient Adsorption of Tetracycline Using Chitosan-Based Magnetic Adsorbent. Polymers 2022, 14, 4854. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2018, 276, 105–114. [Google Scholar] [CrossRef]
- Liu, Y. Is the Free Energy Change of Adsorption Correctly Calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar] [CrossRef]
- Dai, J.; Meng, X.; Zhang, Y.; Huang, Y. Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour. Technol. 2020, 311, 123455. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. A novel hay-derived biochar for removal of tetracyclines in water. Bioresour. Technol. 2018, 274, 162–172. [Google Scholar] [CrossRef]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Meng, Y.; Su, J.; Wang, X. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci. Total. Environ. 2017, 603–604, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, K.; Rajapaksha, A.U.; Adassoriya, N.; Sarkar, B.; Sirimuthu, N.M.; Cooray, A.; Ok, Y.S.; Vithanage, M. Clay-biochar composites for sorptive removal of tetracycline antibiotic in aqueous media. J. Environ. Manag. 2019, 238, 315–322. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Chen, C.-W.; Hung, C.-M.; Vo, T.-D.-H.; Chang, J.-H.; Dong, C.-D. Influence of pyrolysis temperature on polycyclic aromatic hydrocarbons production and tetracycline adsorption behavior of biochar derived from spent coffee ground. Bioresour. Technol. 2019, 284, 197–203. [Google Scholar] [CrossRef]
- Yu, C.; Chen, X.; Li, N.; Chen, J.; Yao, L.; Zhou, Y.; Lu, K.; Lai, Y.; Lai, X. Biomass ash pyrolyzed from municipal sludge and its adsorption performance toward tetracycline: Effect of pyrolysis temperature and KOH activation. Environ. Sci. Pollut. Res. 2022, 29, 81383–81395. [Google Scholar] [CrossRef]
- Chang, J.; Shen, Z.; Hu, X.; Schulman, E.; Cui, C.; Guo, Q.; Tian, H. Adsorption of Tetracycline by Shrimp Shell Waste from Aqueous Solutions: Adsorption Isotherm, Kinetics Modeling, and Mechanism. ACS Omega 2020, 5, 3467–3477. [Google Scholar] [CrossRef] [PubMed]


| Temp (°C) | 25 °C | 45 °C | 60 °C |
|---|---|---|---|
| Langmuir | |||
| Qm | 18.11 ± 2.65 | 43.92 ± 15.67 | 276.74 ± 159.62 |
| KL | 0.031 ± 0.009 | 0.009 ± 0.004 | 0.002 ± 0.001 |
| R2 | 0.9832 | 0.9876 | 0.9815 |
| SSE | 1.58 | 1.74 | 0.19 |
| χ2 | 0.53 | 0.53 | 0.53 |
| Freundlich | |||
| n | 0.54 ± 0.003 | 0.79 ± 0.001 | 0.96 ± 3.25 × 10−4 |
| KF | 1.31 ± 0.01 | 0.64 ± 0.003 | 0.56 ± 6.20 × 10−4 |
| R2 | 0.9867 | 0.9983 | 0.9999 |
| SSE | 138.27 | 32.07 | 1.66 |
| χ2 | 0.14 | 0.03 | 0.002 |
| Temkin | |||
| AT | 0.58 ± 0.01 | 0.58 ± 0.01 | 0.88 ± 0.03 |
| BT | 790.61 ± 7.53 | 790.62 ± 7.53 | 909.52 ± 10.28 |
| R2 | 0.9181 | 0.9181 | 0.8880 |
| SSE | 855.40 | 3806.77 | 8002.25 |
| χ2 | 0.86 | 3.89 | 8.03 |
| Sips | |||
| Qm | 12.92 ± 0.11 | 14.52 ± 0.17 | 17.07 ± 0.24 |
| KS | 0.18 ± 0.02 | 0.35 ± 0.08 | 0.47 ± 0.13 |
| A | 2.09 ± 0.18 | 3.79 ± 0.77 | 3.63 ± 0.87 |
| R2 | 0.9603 | 0.9415 | 0.9218 |
| SSE | 512.81 | 1055.65 | 2210.77 |
| χ2 | 0.51 | 1.06 | 2.22 |
| Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/(mol.K) |
|---|---|---|---|
| 298 | −23.61 | −7.62 | −15.94 |
| 318 | −21.92 | ||
| 333 | −18.79 |
| Model | Parameter | Value |
|---|---|---|
| pseudo-1st-order | K1 | 0.14 ± 0.02 |
| Qe | 1.44 ± 0.02 | |
| R2 | 0.9971 | |
| SSE | 0.005 | |
| χ2 | 0.00 | |
| pseudo-2nd-order | Qe | 1.56 ± 0.003 |
| K2 | 0.14 ± 0.002 | |
| R2 | 0.9646 | |
| SSE | 2.44 | |
| χ2 | 0.00 | |
| Elovich | β | 1.33 ± 0.05 |
| α | 3.90 ± 0.03 | |
| R2 | 0.9559 | |
| SSE | 4.20 | |
| χ2 | 4.20 |
| Adsorbent | Qm (mg g−1) | Refs. |
|---|---|---|
| Alkali modified magnetic biochar (MSBC) | 97.962 | [54] |
| Alkali-acid modified magnetic biochar (MSABC) | 98.334 | |
| The raw biochar (RBC) | 37.803 | |
| Alfalfa-derived biochar | 372 | [55] |
| Pinus taeda-derived activated biochar | 274.8 | [56] |
| Waste chicken-feather-derived multilayered graphene-phase biochar | 388.33 | [57] |
| Clay-biochar composites | 77.962 | [58] |
| Spent coffee-ground-derived biochar | 39.22 | [59] |
| Biomass ash pyrolyzed from municipal sludge | 50.75 | [60] |
| Shrimp Shell Waste | 229.98 | [61] |
| Magnetic mesoporous silica from BA-Biomass Power Plant | 276.74 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanh, P.T.H.; Phoungthong, K.; Chantrapromma, S.; Choto, P.; Thanomsilp, C.; Siriwat, P.; Wisittipanit, N.; Suwunwong, T. Adsorption of Tetracycline by Magnetic Mesoporous Silica Derived from Bottom Ash—Biomass Power Plant. Sustainability 2023, 15, 4727. https://doi.org/10.3390/su15064727
Hanh PTH, Phoungthong K, Chantrapromma S, Choto P, Thanomsilp C, Siriwat P, Wisittipanit N, Suwunwong T. Adsorption of Tetracycline by Magnetic Mesoporous Silica Derived from Bottom Ash—Biomass Power Plant. Sustainability. 2023; 15(6):4727. https://doi.org/10.3390/su15064727
Chicago/Turabian StyleHanh, Phan Thi Hong, Khamphe Phoungthong, Suchada Chantrapromma, Patcharanan Choto, Chuleeporn Thanomsilp, Piyanuch Siriwat, Nuttachat Wisittipanit, and Thitipone Suwunwong. 2023. "Adsorption of Tetracycline by Magnetic Mesoporous Silica Derived from Bottom Ash—Biomass Power Plant" Sustainability 15, no. 6: 4727. https://doi.org/10.3390/su15064727
APA StyleHanh, P. T. H., Phoungthong, K., Chantrapromma, S., Choto, P., Thanomsilp, C., Siriwat, P., Wisittipanit, N., & Suwunwong, T. (2023). Adsorption of Tetracycline by Magnetic Mesoporous Silica Derived from Bottom Ash—Biomass Power Plant. Sustainability, 15(6), 4727. https://doi.org/10.3390/su15064727









