Comparative Study of Effective Pretreatments on the Structural Disruption and Hydrodepolymerization of Rice Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Pretreatment of Rice Straw
2.2.1. Hydrothermal Pretreatment
2.2.2. Microwave Pretreatment
2.2.3. Cryocrushing Pretreatment
2.2.4. Hydrodepolymerization of Pretreated RS Residues
2.2.5. Analysis and Characterization of RS Hydrolyzates
2.2.6. Characterization of the Straw Residue
3. Results and Discussion
3.1. Effects of Hydrothermal Pretreatment on RS
3.2. Effects of Microwave Pretreatment on RS
3.2.1. Effects of Microwave Power on Microwave Pretreatment of RS
3.2.2. Effects of Time on Microwave Pretreatment of RS
3.3. Effects of Cryocrushing Pretreatment on RS
3.4. Comparison of RS and Pretreated RS Residues and Solutions Parameters
3.5. Characterization and Comparison of RS and Pretreated RS Residues
3.5.1. SEM-EDS Results
3.5.2. FTIR Results
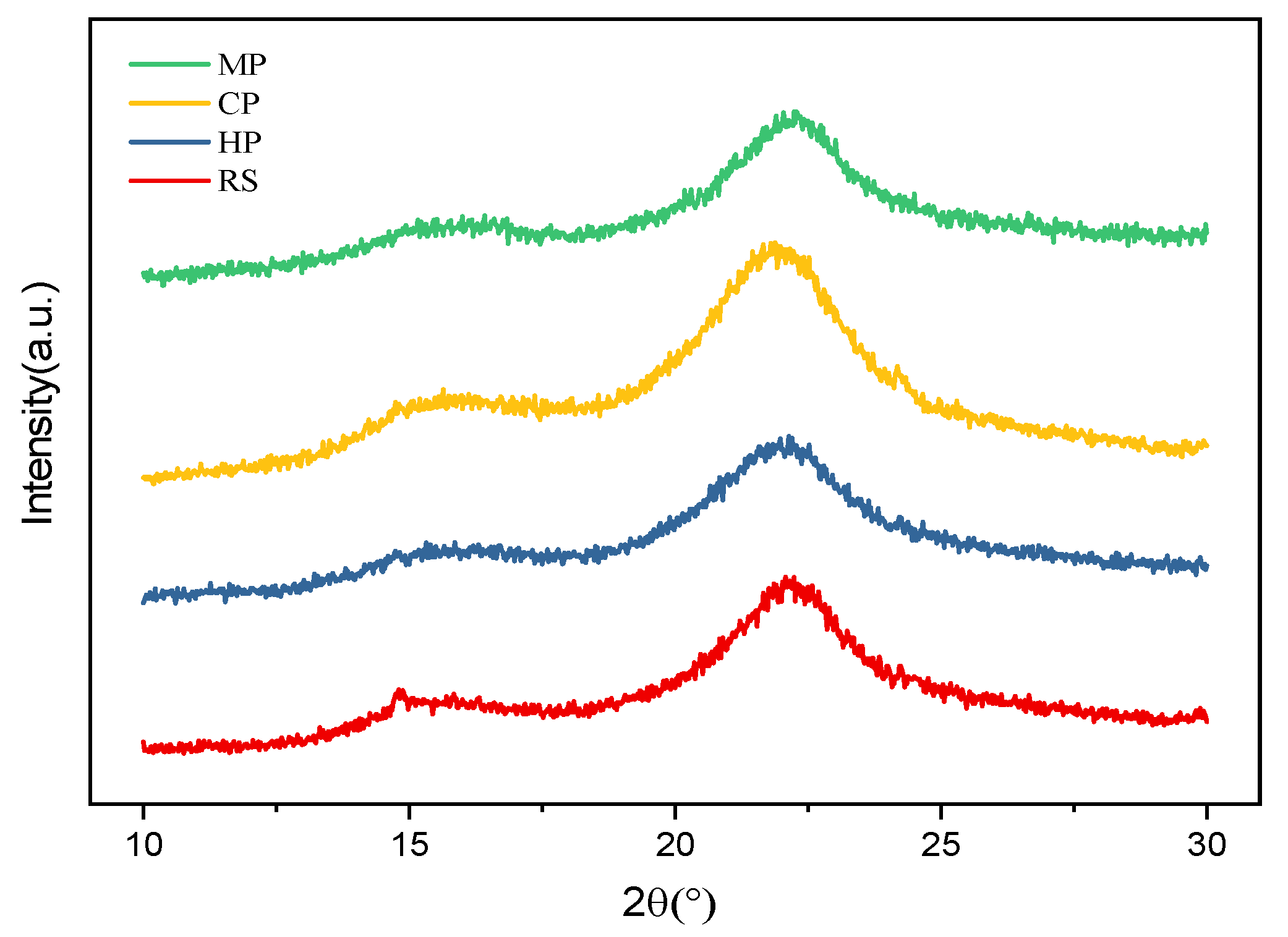
3.5.3. XRD Results
3.5.4. N2 Physical Adsorption–Desorption Results
3.6. Effects on Hydrodepolymerization of RS Residues after Various Pretreatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, H.; Wang, Y.; Feng, X.; Li, S.; Leong, Y.K.; Chang, J.S. Renewable biohydrogen production from straw biomass-Recent advances in pretreatment/hydrolysis technologies and future development. Int. J. Hydrogen Energy 2022, 47, 37359–37373. [Google Scholar] [CrossRef]
- Rajput, A.A.; Hassan, M. Enhancing biogas production through co-digestion and thermal pretreatment of wheat straw and sunflower meal. Renew. Energy 2021, 168, 1–10. [Google Scholar] [CrossRef]
- Valles, A.; Capilla, M.; Álvarez-Hornos, F.J.; García-Puchol, M.; San-Valero, P.; Gabaldón, C. Optimization of alkali pretreatment to enhance rice straw conversion to butanol. Biomass Bioenerg. 2021, 150, 106131. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, Q.; Zhou, Z.; Ding, Y.; Zubair, M. Effect of substrate load on anaerobic fermentation of rice straw with rumen liquid as inoculum: Hydrolysis and acidogenesis efficiency, enzymatic activities and rumen bacterial community structure. Waste Manag. 2021, 124, 235–243. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, D.; Yadav, S.K.; Krishania, M. Process scale-up of an efficient acid-catalyzed steam pretreatment of rice straw for xylitol production by C. Tropicalis MTCC 6192. Bioresour. Technol. 2021, 320, 124422. [Google Scholar] [CrossRef]
- Huang, Z.J.; Feng, G.J.; Lin, K.P.; Pu, F.L.; Tan, Y.M.; Tu, W.C.; Han, Y.L.; Hou, X.D.; Zhang, H.M.; Zhang, Y. Significant boost in xylose yield and enhanced economic value with one-pot process using deep eutectic solvent for the pretreatment and saccharification of rice straw. Ind. Crop. Prod. 2020, 152, 112515. [Google Scholar] [CrossRef]
- Doan, H.T.; Nguyen, P.T.M.; Tran, T.T.; Nguyen, T.K.; Tran, M.D.; Nguyen, D.B. Optimizing lime pretreat- ment of rice straw for biolipid production using oleaginous microorganisms. Chemosphere 2021, 269, 129390. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathit-sukanoh, N. Effects of polyol-based deep eutectic solvents on the efficiency of rice straw enzymatic hydrolysis. Ind. Crop. Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Li, W.; Chen, C.; Liu, G. Enhanced methane production and energy potential from rice straw by employing microaerobic pretreatment via anaerobic digestion. J. Clean. Prod. 2021, 296, 126434. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Liu, J.; Zhang, X.; Li, F. Pretreatment enhanced structural disruption, enzymatic hydrolysis, fermentative hydrogen production from rice straw. Int. J. Hydrogen Energy 2022, 47, 11778–11786. [Google Scholar] [CrossRef]
- Maleki, M.; Ariaeenejad, S.; Salekdeh, G.H. Efficient saccharification of ionic liquid-pretreated rice straw in a one-pot system using novel metagenomics derived cellulases. Bioresour. Technol. 2022, 345, 126536. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xia, T.; Zhang, Y.; Wei, Z.; Qu, F.; Zheng, G.; Song, C.; Zhao, Y.; Kang, K.; Yang, H. Identifying driving factors of humic acid formation during rice straw composting based on Fenton pretreatment with bacterial inoculation. Bioresour. Technol. 2021, 33, 125403. [Google Scholar] [CrossRef]
- Wang, W.; Tan, X.; Imtiaz, M.; Wang, Q.; Miao, C.; Yuan, Z.; Zhuang, X. Rice straw pretreatment with KOH/urea for enhancing sugar yield and ethanol production at low temperature. Ind. Crop. Prod. 2021, 170, 113776. [Google Scholar]
- Abdolmaleki, A.; Nabavizadeh, S.S.; Badbedast, M. 1-(Carboxymethyl)pyridinium chloride as an acidic ionic liquid for rice straw effective pretreatment. Renew. Energy 2021, 177, 544–553. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Organosolv pretreatments of rice straw followed by microbial hydroly- sis for efficient biofuel production. Renew. Energy 2020, 148, 923–934. [Google Scholar] [CrossRef]
- Sabeeh, M.; Liaquat, R.; Maryam, A. Effect of alkaline and alkaline-photocatalytic pretreatment on characteristics and biogas production of rice straw. Bioresour. Technol. 2020, 309, 123449. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Process development for sodium carbonate pretreatment and enzymatic saccharification of rice straw for bioethanol production. Biomass Bioenerg. 2020, 138, 105574. [Google Scholar]
- Yang, X.; Wang, B.; Lu, T.; Zhou, L. Depolymerization of wheat straw to produce glucose by self-catalyzed hydrolysis. Energy Fuel. 2020, 34, 5990–5996. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Ye, J.; Wu, Y.; Liu, J.; Fang, W.; Xu, D.; Wang, B.; Yan, L.; Zeng, G. Comparison of various pretreatments for ethanol production enhancement from solid residue after rumen fluid digestion of rice straw. Bioresour. Technol. 2018, 247, 147–156. [Google Scholar] [CrossRef]
- Hartati, I.; Sulistyo, H.; Sediawan, W.B.; Azis, M.M.; Fahrurrozi, M. Microwave-assisted urea-based- hydrotropic pretreatment of rice straw: Experimental data and mechanistic kinetic models. ACS Omega 2021, 6, 13225–13239. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Ultrasonic assisted petha waste water pretreatment of rice straw for optimum production of methane and ethanol using mixed microbial culture. Renew. Energy 2020, 145, 682–690. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-oligosaccharides, fermentable sugars, and bioenergy production from sugarcane straw using steam explosion pretreatment at pilot-scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, B.; Yang, T.; Kai, X.; Zhang, W. Hydrogenation of rice stalk in situ in supercritical ethanol-water co-solvent via catalytic ethanol steam reforming. J. Supercrit. Fluid. 2018, 133, 309–317. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Lu, J.; Du, J.; Tao, Y.; Cheng, Y.; Li, Q.; Wang, H. Combinatorial pretreatments of reed straw using liquid hot water and lactic acid for fermentable sugar production. Fuel 2023, 331, 125916. [Google Scholar] [CrossRef]
- Kim, D.H.; Jo, I.S.; Kang, B.J.; Lee, B.D.; Kumar, S.; Kim, S.H.; Yoon, J.J. Evaluation of bio-hydrogen production using rice straw hydrolysate extracted by acid and alkali hydrolysis. Int. J. Hydrogen Energy 2022, 47, 37385–37393. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, C.; Xu, M.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Alkali-catalyzed organosolv pretreatment of lignocellulose enhances enzymatic hydrolysis and results in highly antioxidative lignin. Energ. Fuel. 2021, 35, 5039–5048. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Muhammad, N.; Hallett, J.P. Production of food-grade glucose from rice and wheat residues using a biocompatible ionic liquid. ACS Sustain. Chem. Eng. 2021, 9, 8080–8089. [Google Scholar] [CrossRef]
- Aggarwal, N.; Pal, P.; Sharma, N.; Saravanamurugan, S. Consecutive Organosolv and alkaline pretreatment: An efficient approach toward the production of cellulose from rice Straw. ACS Omega 2021, 6, 27247–27258. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Y.; Tong, Z.; Meng, J.; Shi, X.; Cao, K.; Xiao, I.; Yu, H. Molecular dynamics mechanism of metal salt hydrate-based deep eutectic solvent to dissolve cellulose at room temperature. J. For. Eng. 2022, 7, 64–71. [Google Scholar]
- Tang, S.; Dong, Q.; Fang, Z.; Miao, Z. Complete recovery of cellulose from rice straw pretreated with ethylene glycol and aluminum chloride for enzymatic hydrolysis. Bioresour. Technol. 2019, 284, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kaur, B.; Raheja, Y.; Agrawal, D.; Basotra, N.; Falco, D.M.; Tsang, A.; Chadha, S.B. Lignocellulolytic enzymes from Aspergillus allahabadii for efficient bioconversion of rice straw into fermentable sugars and biogas. Bioresour. Technol. 2022, 360, 127507. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Bhadana, B.; Singh, C.P.; Adsul, M.; Kumar, R.; Gupta, R.P.; Satlewal, A. Understanding the effects of low enzyme dosage and high solid loading on the enzyme inhibition and strategies to improve hydrolysis yields of pilot scale pretreated rice straw. Fuel 2022, 327, 125114. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Wang, W.; Yu, Q.; Chen, X.; Miao, C.; Guo, Y.; Zhang, Y.; Zhuang, X.; Sun, Y.; et al. A novel recyclable alkaline biphasic 2-phenoxyethanol/water system for rice straw biorefinery under mild conditions. ACS Sustain. Chem. Eng. 2020, 8, 7649–7655. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Ling, Z.; Lai, C.; Yong, Q. Revealing migration discipline of lignin during producing fermentable sugars from wheat straw through autohydrolysis. Ind. Crops Prod. 2021, 171, 113849. [Google Scholar] [CrossRef]
- Moirangthem, K.; Zaky, A.S.; Tucker, G.A. Microwave subcritical water pre-treatment and enzymatic hydrolysis of geographical identification (GI) tag Indian black rice (Chakhao Poireiton) straw for fermentable sugar production. Biofuels 2022, 13, 815–822. [Google Scholar] [CrossRef]
- Castoldi, R.; Correa, V.G.; de Morais, G.R. Liquid nitrogen pretreatment of eucalyptus sawdust and rice hull for enhanced enzymatic saccharification. Bioresour. Technol. 2017, 224, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Teramura, H.; Oshima, T.; Matsuda, F.; Sasaki, K.; Ogino, C.; Yamasaki, M.; Kondo, A. Glucose content in the liquid hydrolysate after dilute acid pretreatment is affected by the starch content in rice straw. Bioresour. Technol. 2013, 149, 520–524. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, Q.; Li, Y. Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour. Technol. 2016, 216, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhao, J.; Liang, J.; Zhu, J. Efficient and selective catalytic conversion of hemicellulose in rice straw by metal catalyst under mild conditions. Sustainability 2020, 12, 106011. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Templeton, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1671, 1–16. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic aid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chang, K.; Chen, X.; Han, Y.; Wang, X.; Potprommanee, L.; Ning, X.; Liu, J.; Sun, J.; Peng, Y.; Sun, S. Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Bioresour. Technol. 2016, 214, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Imman, S.; Arnthong, J.; Burapatana, V.; Champreda, V.; Laosiripojana, N. Effects of acid and alkali promoters on compressed liquid hot water pretreatment of rice straw. Bioresour. Technol. 2014, 171, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Y.; Li, Y. Liquid hot water and alkaline pretreatment of soybean straw for improving cellulose digestibility. Bioresour. Technol. 2011, 102, 6254–62259. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Qi, W.; Wang, W.; Wang, Q.; Tan, X. Pretreatment of sugarcane bagasse with liquid hot water and aqueous ammonia. Bioresour. Technol. 2013, 144, 210–215. [Google Scholar] [CrossRef]
- Santucci, B.S.; Maziero, P.; Rabelo, S.C.; Curvelo, A.A.S.; Pimenta, M.T.B. Autohydrolysis of hemicelluloses from sugarcane bagasse during hydrothermalb pretreatment: A kinetic assessment. BioEnergy Res. 2015, 8, 1778–1787. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Sun, J.; Li, D.; Feng, Y. Effects of liquid hot water pretreatment process parameters on components and cellulose digestibility of corn stover. CIESC J. 2015, 66, 1529–1536. [Google Scholar]
- Jiang, W.; Chang, S.; Li, H.; Oleskowicz-Popiel, P.; Xu, J. Liquid hot water pretreatment on different parts of cotton stalk to facilitate ethanol production. Bioresour. Technol. 2015, 176, 175–180. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Q.; Liao, Q.; Xia, A.; Huang, Y.; Zhu, X.; Zhu, X. Study on degradation kinetics of hemicellulose in wheat straw hydrothermal pretreatment. CIESC J. 2020, 71, 3098–3105. [Google Scholar]
- Norazlina, I.; Dhinashini, R.S.; Nurhafizah, I.; Norakma, M.N.; Noor Fazreen, D. Extraction of xylose from rice straw and lemongrass via microwave assisted. Mater. Today: Proc. 2022, 48, 784–789. [Google Scholar] [CrossRef]
- Sindhu, R.; Kuttiraj, M.; Prabisha, T.P.; Binod, P.; Sukumaran, R.K.; Pandey, A. Development of a combined pretreatment and hydrolysis strategy of rice straw for the production of bioethanol and biopolymer. Bioresour. Technol. 2016, 215, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ji, Q.; Yu, X.; Li, M.; Abiola, F.O.; Yagoub, A.E.A.; Chen, L.; Zhou, C. Multimode-ultrasound and microwave assisted natural ternary deep eutectic solvent sequential pretreatments for corn straw biomass deconstruction under mild conditions. Ultrason. Sonochem. 2021, 72, 105414. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; Xue, S.; Yu, S.; Chao, Z.; Bing, L.; Kang, Y. Effects of microwave power and microwave irradiation time on pretreatment efficiency and characteristics of corn stover using combination of steam explosion and microwave irradiation (SE–MI) pretreatment. Bioresour. Technol. 2012, 118, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, Z.; Dai, S.; Tian, G.; Wang, Z. Production of hemicelluloses sugars, cellulose pulp, and lignosulf- onate surfactant using corn stalk by prehydrolysis and alkaline sulfite cooking. Ind. Crop. Prod. 2023, 192, 115880. [Google Scholar]
- Tian, F.; Guan, M.; Du, K.; Yong, C.; Zhao, C. Fiber properties of straw pretreated by fermentation and its degradable composites. J. For. Eng. 2022, 7, 128–133. [Google Scholar]
- Zhang, Y.; Li, L.; Dai, T.; Li, W.; Ma, Z. Effects of pretreatment on degaradation of corn stalk. J. Cellulose Sci. Technol. 2009, 17, 35–38. [Google Scholar]
- Patil, R.; Cimon, C.; Eskicioglu, C.; Goud, V. Effect of ozonolysis and thermal pre-treatment on rice straw hydrolysis for the enhancement of biomethane production. Renew. Energy 2021, 179, 467–474. [Google Scholar] [CrossRef]
- Mariani, M.; Zaccheria, F.; Psaro, R.; Zaccheria, F. Some insight into the role of different copper species as acids in cellulose deconstruction. Catal. Commun. 2014, 44, 19–23. [Google Scholar] [CrossRef]
- Cao, G.; Xia, X.; Zhao, L.; Wang, Z.; Li, X.; Yang, Q. Development of AFEX-based consolidated bioprocessing on wheat straw for biohydrogen production using anaerobic microflora. Int. J. Hydrogen Energy 2013, 38, 15653–15659. [Google Scholar] [CrossRef]
- Rajput, A.A.; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef]
- Boonmanumsin, P.; Treeboobpha, S.; Jeamjumnunja, K.; Luengnaruemitchai, A.; Chaisuwan, T.; Wongkasemjit, S. Release of monomeric sugars from miscanthus sinensis by microwave-assisted ammonia and phosphoric acid treatments. Bioresour. Technol. 2012, 103, 425–431. [Google Scholar] [CrossRef]
- Sorn, V.; Chang, K.; Phitsuwan, P.; Ratanakhanokchai, K.; Dong, C. Effect of microwave-assisted ionic liquid/ acidic ionic liquid pretreatment on the morphology, structure, and enhanced delignification of rice straw. Bioresour. Technol. 2019, 293, 121929. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Pu, Y.; Yang, B.; Ragauskas, A.; Wyman, C.E. Comparison of microwaves to fluidized sand baths for heating 2tubular reactors for hydrothermal and dilute acid batch pretreatment of corn stover. Bioresour. Technol. 2011, 102, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Kamiya, N. Powerful peracetic acid-ionic liquid pretreatment process for the efficient chemical hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2016, 214, 487–495. [Google Scholar]
- Ling, Z.; Lai, C.; Huang, C.; Xu, F.; Yong, Q. Research progress in variations of cellulose supramolecular structures via biomass pretreatment. J. For. Eng. 2021, 6, 24–34. [Google Scholar]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from loktak lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–257. [Google Scholar] [CrossRef]
- Li, Y.; Liu, P.; Huang, J.; Zhang, R.; Hu, Z.; Feng, S.; Wang, Y.; Wang, L.; Xia, T.; Peng, L. Mild chemical pretreatments are n sufficient for bioethanol production in transgenic rice straws overproducing glucosidase. Green Chem. 2018, 20, 2047–2056. [Google Scholar] [CrossRef]



| Pretreat Condition (°C, min) | logR0 | RS Recovery (%) | Cellulose Recovery (%) | Hemicellulose Recovery (%) | Lignin Removal (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Reduction Sugar Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| RS | _ | _ | _ | _ | _ | 37.8 ± 0.3 | 20.5 ± 0.3 | 19.0 ± 0.2 | _ |
| 100, 5 | 0.7 | 87.2 | 90.9 | 86.8 | 9.6 | 39.4 ± 0.3 | 20.4 ± 0.7 | 19.7 ± 0.4 | 0.6 |
| 100, 10 | 1.0 | 86.9 | 89.0 | 83.9 | 11.7 | 38.7 ± 0.2 | 19.8 ± 0.5 | 19.3 ± 0.3 | 0.8 |
| 100, 30 | 1.5 | 86.2 | 88.3 | 79.9 | 13.8 | 38.7 ± 0.2 | 19.0 ± 0.4 | 19.0 ± 0.3 | 0.9 |
| 120, 5 | 2.6 | 86.0 | 88.0 | 79.3 | 14.4 | 38.7 ± 0.3 | 18.9 ± 0.5 | 18.9 ± 0.1 | 0.9 |
| 120, 10 | 2.9 | 85.5 | 87.8 | 78.8 | 15.9 | 38.8 ± 0.5 | 18.9 ± 0.2 | 18.7 ± 0.3 | 1.0 |
| 120, 30 | 3.4 | 84.9 | 87.6 | 78.7 | 19.1 | 39.0 ± 0.6 | 19.0 ± 0.5 | 18.1 ± 0.3 | 1.0 |
| 140, 5 | 4.5 | 84.7 | 86.5 | 74.0 | 23.8 | 38.6 ± 0.9 | 17.9 ± 0.7 | 17.1 ± 0.1 | 1.8 |
| 140, 10 | 4.8 | 84.0 | 87.6 | 69.2 | 25.3 | 39.4 ± 1.0 | 16.9 ± 0.5 | 16.9 ± 0.8 | 2.0 |
| 140, 30 | 5.3 | 81.8 | 85.5 | 69.8 | 25.9 | 39.5 ± 0.2 | 17.5 ± 0.3 | 17.2 ± 0.4 | 1.8 |
| 160, 5 | 6.4 | 80.0 | 87.4 | 63.6 | 27.2 | 41.3 ± 1.0 | 16.3 ± 0.8 | 17.3 ± 0.5 | 5.1 |
| 160, 10 | 6.7 | 78.2 | 86.1 | 61.4 | 25.5 | 41.6 ± 0.8 | 16.1 ± 0.7 | 18.1 ± 0.2 | 5.6 |
| 160, 30 | 7.0 | 73.5 | 84.6 | 58.1 | 26.1 | 43.5 ± 0.5 | 16.2 ± 0.4 | 19.1 ± 0.8 | 5.2 |
| 180, 5 | 8.3 | 62.2 | 79.5 | 22.5 | 36.2 | 48.3 ± 0.4 | 7.4 ± 0.1 | 19.5 ± 1.0 | 8.4 |
| 180, 10 | 8.6 | 59.0 | 74.6 | 14.1 | 41.3 | 47.8 ± 0.8 | 4.9 ± 0.0 | 18.9 ± 0.7 | 14.9 |
| 180, 30 | 9.0 | 58.7 | 75.9 | 9.5 | 40.1 | 48.9 ± 1.0 | 3.3 ± 0.0 | 19.4 ± 0.5 | 8.8 |
| 200, 5 | 10.1 | 58.3 | 75.6 | 9.1 | 50.0 | 49.0 ± 0.2 | 3.2 ± 0.1 | 16.3 ± 0.3 | 9.0 |
| 200, 10 | 10.4 | 54.8 | 71.5 | 4.8 | 53.3 | 49.3 ± 0.5 | 1.8 ± 0.0 | 16.2 ± 0.4 | 14.6 |
| 200, 30 | 10.9 | 54.1 | 72.6 | 4.0 | 54.2 ± | 50.7 ± 1.1 | 1.5 ± 0.0 | 16.1 ± 0.6 | 5.8 |
| Power (W) | RS Recovery (%) | Cellulose Recovery (%) | Hemicellulose Recovery (%) | Lignin Removal (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Reduction Sugar Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 65 | 88.1 | 83.4 | 78.2 | 15.1 | 35.8 ± 0.8 | 18.2 ± 0.0 | 18.3 ± 0.2 | 1.0 |
| 195 | 87.9 | 83.0 | 73.8 | 19.1 | 35.7 ± 0.4 | 17.2 ± 0.3 | 17.6 ± 0.5 | 1.1 |
| 325 | 87.8 | 82.5 | 72.4 | 19.6 | 35.5 ± 0.5 | 16.9 ± 0.1 | 17.4 ± 0.2 | 1.2 |
| 455 | 87.7 | 82.8 | 71.4 | 20.6 | 35.7 ± 0.5 | 16.7 ± 0.1 | 17.2 ± 0.3 | 1.3 |
| 585 | 87.6 | 81.8 | 71.4 | 22.2 | 35.3 ± 0.5 | 16.7 ± 0.0 | 16.9 ± 0.4 | 1.4 |
| 650 | 87.6 | 81.1 | 70.5 | 22.5 | 35.0 ± 0.8 | 16.5 ± 0.1 | 16.8 ± 0.9 | 1.9 |
| Time (min) | RS Recovery (%) | Cellulose Recovery (%) | Hemicellulose Recovery (%) | Lignin Removal (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Reduction Sugar Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 5 | 88.9 | 84.2 | 80.2 | 16.2 | 35.8 ± 0.3 | 18.5 ± 0.1 | 17.9 ± 0.3 | 0.4 |
| 10 | 88.7 | 83.8 | 78.7 | 15.0 | 35.7 ± 0.5 | 18.2 ± 0.5 | 18.2 ± 0.6 | 0.6 |
| 15 | 88.3 | 82.9 | 77.1 | 17.7 | 35.5 ± 0.9 | 17.9 ± 0.4 | 17.7 ± 0.5 | 0.7 |
| 20 | 87.9 | 82.8 | 76.3 | 17.2 | 35.6 ± 0.4 | 17.8 ± 0.1 | 17.9 ± 0.9 | 1.5 |
| 25 | 87.8 | 82.2 | 75.0 | 22.4 | 35.4 ± 0.6 | 17.5 ± 0.1 | 16.8 ± 0.3 | 1.7 |
| 30 | 87.6 | 81.1 | 74.5 | 22.5 | 35.0 ± 1.0 | 17.4 ± 0.0 | 16.8 ± 0.3 | 1.9 |
| Soaking Time (h) | RS Recovery (%) | Cellulose Recovery (%) | Hemicellulose Recovery (%) | Lignin Removal (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|---|---|---|---|
| 0 | 92.9 | 90.4 | 88.8 | 9.1 | 36.8 ± 0.3 | 19.6 ± 0.3 | 18.6 ± 1.0 |
| 0.16 | 91.8 | 91.8 | 87.3 | 9.6 | 37.8 ± 0.8 | 19.5 ± 0.7 | 18.7 ± 0.9 |
| 0.5 | 90.3 | 92.5 | 87.2 | 12.0 | 38.7 ± 0.0 | 19.8 ± 0.2 | 18.3 ± 0.4 |
| 1 | 89.9 | 91.3 | 85.1 | 12.3 | 38.4 ± 0.6 | 19.4 ± 0.5 | 18.2 ± 0.8 |
| 5 | 89.6 | 89.8 | 84.4 | 12.9 | 37.9 ± 0.2 | 19.3 ± 0.8 | 18.2 ± 0.4 |
| 10 | 89.5 | 89.0 | 84.3 | 13.3 | 37.6 ± 0.9 | 19.3 ± 0.7 | 18.4 ± 0.3 |
| 20 | 89.4 | 89.2 | 83.7 | 14.8 | 37.7 ± 0.2 | 19.2 ± 0.4 | 18.1 ± 0.9 |
| a | |||||||
|---|---|---|---|---|---|---|---|
| Samples | RS Recovery (%) | Cellulose Recovery (%) | Hemicellulose Recovery (%) | Lignin Removal (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
| RS | - | - | - | - | 37.8 | 20.5 | 19.0 |
| HP | 59.0 | 74.6 | 14.1 | 41.3 | 47.8 | 4.9 | 18.9 |
| MP | 87.6 | 81.1 | 74.5 | 22.5 | 35.0 | 16.5 | 16.8 |
| CP | 92.9 | 90.4 | 88.8 | 9.1 | 36.8 | 19.6 | 18.6 |
| b | |||||||
| Liquid Samples | pH | Conductivity (µs/cm) | TDS (mg/L) | Salinity (%) | Reduction Sugar Yield (%) | ||
| HP | 3.9 | 2250.0 | 1126.0 | 0.1 | 14.9 | ||
| MP | 6.4 | 613.0 | 305.0 | 0.0 | 1.9 | ||
| CP | - | - | - | - | - | ||
| Samples | C (%) | O (%) | Si (%) | Cl (%) | K (%) |
|---|---|---|---|---|---|
| RS | 72.2 | 24.4 | 2.1 | 0.4 | 1.0 |
| HP | 59.7 | 38.5 | 1.6 | 0.0 | 0.3 |
| MP | 59.6 | 35.9 | 4.4 | 0.1 | 0.0 |
| CP | 58.7 | 39.6 | 1.6 | 0.1 | 0.0 |
| Samples | Specific Surface Area (m2/g) | Aperture (nm) |
|---|---|---|
| RS | 2.5 | 13.0 |
| HP | 9.3 | 15.8 |
| MP | 6.3 | 23.1 |
| CP | 3.0 | 28.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, X.; Liang, J.; Zhu, J. Comparative Study of Effective Pretreatments on the Structural Disruption and Hydrodepolymerization of Rice Straw. Sustainability 2023, 15, 4728. https://doi.org/10.3390/su15064728
Yang X, Li X, Liang J, Zhu J. Comparative Study of Effective Pretreatments on the Structural Disruption and Hydrodepolymerization of Rice Straw. Sustainability. 2023; 15(6):4728. https://doi.org/10.3390/su15064728
Chicago/Turabian StyleYang, Xiaorui, Xiaotong Li, Jinhua Liang, and Jianliang Zhu. 2023. "Comparative Study of Effective Pretreatments on the Structural Disruption and Hydrodepolymerization of Rice Straw" Sustainability 15, no. 6: 4728. https://doi.org/10.3390/su15064728




