Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector
Abstract
1. Introduction
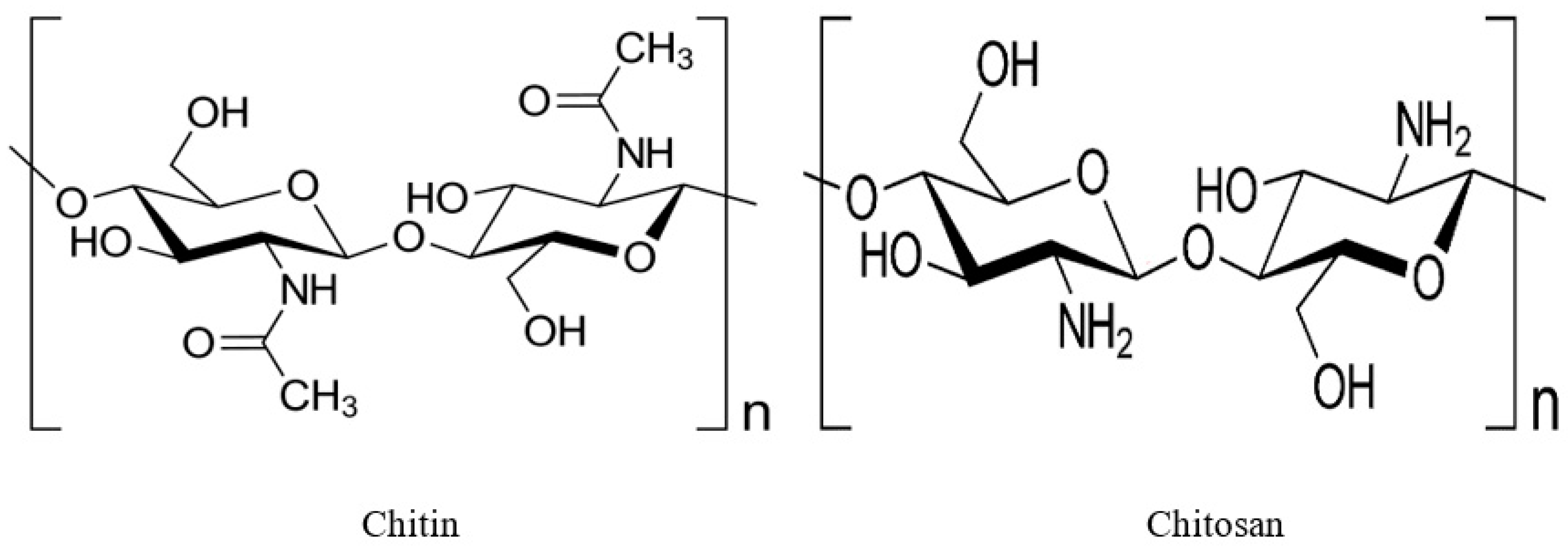
1.1. Chitin
1.2. Chitosan
2. Chitin and Chitosan Sources
3. Methods for Chitin and Chitosan Extraction
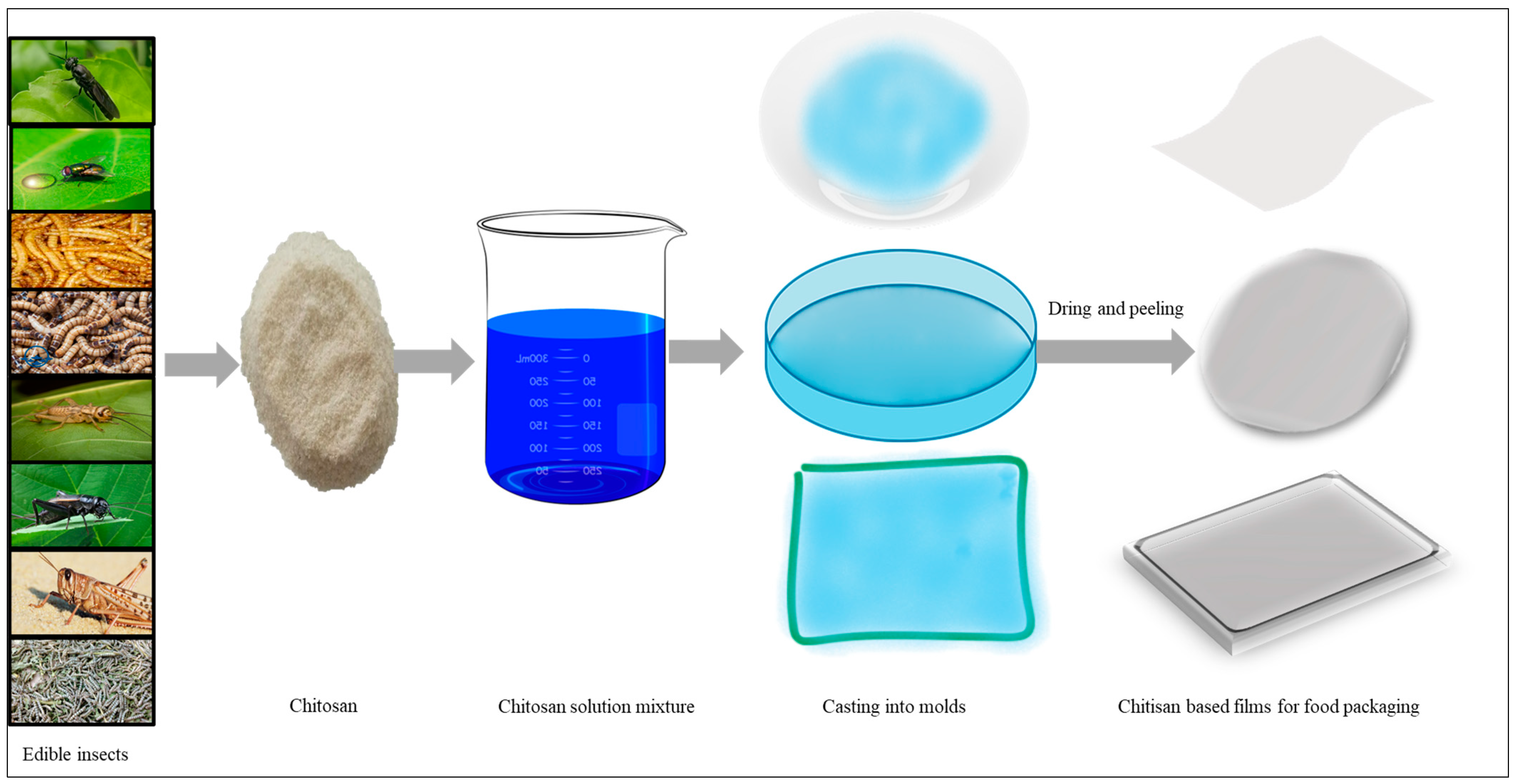
4. Insects as an Alternative Chitin and Chitosan Source
4.1. Black Soldier Fly
4.2. Housefly
4.3. Yellow Mealworm
4.4. Superworm
4.5. House Cricket
4.6. Field Cricket
4.7. Desert Locust
4.8. Silkworm
5. Applications of Chitin and Chitosan
5.1. Chitin and Chitosan Applications as Biomaterials
5.2. Chitin and Chitosan Applications in Food and Nutrition
5.3. Chitin and Chitosan Applications in Biomedicine
5.4. Chitin and Chitosan Applications in Agriculture
5.5. Chitin and Chitosan Applications in Cosmetics
5.6. Chitin and Chitosan Applications in Water Purification
5.7. Chitin and Chitosan-Based Food Packaging Bioplastics Production
5.8. Antibacterial Activities of Chitin and Chitosan
6. Conclusions
Funding
Institutional Review Board Statement
Informal Consent of Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Crini, G. Historical Landmarks in the Discovery of Chitin. In Sustainable Agriculture Reviews; Springer: Berlin, Germany, 2019; pp. 1–47. [Google Scholar]
- Peter, S.; Lyczko, N.; Gopakumar, D.; Maria, H.J.; Nzihou, A.; Thomas, S. Chitin and Chitosan Based Composites for Energy and Environmental Applications: A Review. Waste Biomass Valorization 2021, 12, 4777–4804. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res 2016, 4, 411–427. [Google Scholar]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of Chitin and Chitosan and Their Isolation. In Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 1–34. [Google Scholar]
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Hong, Y.; Ying, T. Characterization of a Chitin-Glucan Complex from the Fruiting Body of Termitomyces albuminosus (Berk.) Heim. Int. J. Biol. Macromol. 2019, 134, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- John Kasongo, K.; Tubadi, D.J.; Bampole, L.D.; Kaniki, T.A.; Kanda, N.J.M.; Lukumu, M.E. Extraction and Characterization of Chitin and Chitosan from Termitomyces titanicus. SN Appl. Sci. 2020, 2, 406. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-Based Biosorbents: Modification and Application for Biosorption of Heavy Metals and Radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef]
- Zemskova, L.; Egorin, A.; Tokar, E.; Ivanov, V. Chitosan-Based Biosorbents: Immobilization of Metal Hexacyanoferrates and Application for Removal of Cesium radionuclide from Aqueous Solutions. J. Solgel. Sci. Technol. 2019, 92, 459–466. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, Physicochemical Characterization, and Morphological Properties of Chitin and Chitosan from Cuticles of Edible Insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. A Fungal Chitin Derived from Hericium erinaceus Residue: Dissolution, Gelation and Characterization. Int. J. Biol. Macromol. 2020, 152, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Murakami, M.A.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. ichi Weak Gel of Chitin with Ionic Liquid, 1-Allyl-3-Methylimidazolium Bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Takada, A.; Kadokawa, J.-i. Preparation of Cellulosic Soft and Composite Materials Using Ionic Liquid Media and Ion Gels. Cellulose 2021, 29, 2745–2754. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Choline Chloride-Thiourea, a Deep Eutectic Solvent for the Production of Chitin Nanofibers. Carbohydr. Polym. 2014, 103, 466–471. [Google Scholar] [CrossRef]
- Hu, X.; Tang, Y.; Wang, Q.; Li, Y.; Yang, J.; Du, Y.; Kennedy, J.F. Rheological Behaviour of Chitin in NaOH/Urea Aqueous Solution. Carbohydr. Polym. 2011, 83, 1128–1133. [Google Scholar] [CrossRef]
- Kadokawa, J.-i. Dissolution, Derivatization, and Functionalization of Chitin in Ionic Liquid. Int. J. Biol. Macromol. 2019, 123, 732–737. [Google Scholar] [CrossRef]
- Huang, L.; Bi, S.; Pang, J.; Sun, M.; Feng, C.; Chen, X. Preparation and Characterization of Chitosan from Crab Shell (Portunus trituberculatus) by NaOH/Urea Solution Freeze-Thaw Pretreatment Procedure. Int. J. Biol. Macromol. 2020, 147, 931–936. [Google Scholar] [CrossRef]
- Silvestre, J.; Delattre, C.; Michaud, P.; de Baynast, H. Optimization of Chitosan Properties with the Aim of a Water Resistant Adhesive Development. Polymers 2021, 13, 4031. [Google Scholar] [CrossRef]
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.V.N. A Review on Cleaner Strategies for Extraction of Chitosan and Its Application in Toxic Pollutant Removal. Environ. Res. 2021, 196, 110996. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, Structural and Antimicrobial Properties of Poly Vinyl Alcohol-Chitosan Biodegradable Films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Niederhofer, A.; Müller, B.W. A Method for Direct Preparation of Chitosan with Low Molecular Weight from Fungi. Eur. J. Pharm. Biopharm. 2004, 57, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, E.L.; Wu, Z.M.; Li, H.; Zhu, Z.; Zhu, X.S.; Dong, Y. Synthesis of Chitosan Molecularly Imprinted Polymers for Solid-Phase Extraction of Methandrostenolone. Carbohydr. Polym. 2014, 101, 517–523. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A Potential Biopolymer for Wound Management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-Based Nanoparticles: An Overview of Biomedical Applications and Its Preparation. J. Drug Deliv Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-Based Nanoparticles against Bacterial Infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on Recent Progress in Chitosan-Based Hydrogels for Wastewater Treatment Application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-Based Hydrogels: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent Insights into the Extraction, Characterization, and Bioactivities of Chitin and Chitosan from Insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Chitin and Chitosan Derivatives: Global Strategic Business Report. Available online: https://www.researchandmarkets.com/reports/338576/chitin_and_chitosan_derivatives_global_strategic (accessed on 17 January 2023).
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Tacon, A.G.J. Global Trends in Aquaculture and Compound Aquafeed Production; World Aquaculture Society: Stavanger, Norway, 2018. [Google Scholar]
- Joseph, S.M.; Krishnamoorthy, S.; Paranthaman, R.; Moses, J.A.; Anandharamakrishnan, C. A Review on Source-Specific Chemistry, Functionality, and Applications of Chitin and Chitosan. Carbohydr. Polym. Technol. Appl. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Sheikh, I.; Dhiman, A.; Yadav, N.; Yadav, A.N.; Rastegari, A.A.; Singh, K.; Saxena, A.K. Endophytic Fungi: Biodiversity, Ecological Significance, and Potential Industrial Applications. In Recent Advancement in White Biotechnology through Fungi, Volume 1: Diversity and Enzymes Perspectives; Springer: Berlin, Germany, 2019. [Google Scholar]
- Fernando, L.D.; Dickwella Widanage, M.C.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.P.; Wang, P.; Zhang, L.; Wang, T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls from Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021, 8, 814. [Google Scholar] [CrossRef]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal Evolution: Diversity, Taxonomy and Phylogeny of the Fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan:: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Hazmi, A.T.; Ahmad, F.B.; Maziati Akmal, M.H.; Md Ralib, A.A.; Binti Ali, F. Fungal Chitosan for Potential Application in Piezoelectric Energy Harvesting: Review on Experimental Procedure of Chitosan Extraction. Alex. Eng. J. 2022, 67, 105–116. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’annibale, A. Chitosan Production by Fungi: Current State of Knowledge, Future Opportunities and Constraints. Fermentation 2022, 8, 76. [Google Scholar] [CrossRef]
- Shahbaz, U. Chitin, Characteristic, Sources, and Biomedical Application. Curr. Pharm. Biotechnol. 2020, 21, 1433–1443. [Google Scholar] [CrossRef]
- el Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and Efficient Extraction of Chitin and Chitosan for Scale-up Production: Effect of Process Parameters on Deacetylation Degree and Molecular Weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Yeul, V.S.; Rayalu, S.S. Unprecedented Chitin and Chitosan: A Chemical Overview. J. Polym. Environ. 2013, 21, 606–614. [Google Scholar] [CrossRef]
- Mathew, G.M.; Sukumaran, R.K.; Sindhu, R.; Binod, P.; Pandey, A. Green Remediation of the Potential Hazardous Shellfish Wastes Generated from the Processing Industries and Their Bioprospecting. Environ. Technol. Innov 2021, 24, 101979. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.L. Chitin Extraction from Shrimp Waste by Liquid Fermentation Using an Alkaline Protease-Producing Strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Gonil, P.; Sajomsang, W. Applications of Magnetic Resonance Spectroscopy to Chitin from Insect Cuticles. Int. J. Biol. Macromol. 2012, 51, 514–522. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- No, H.K.; Hur, E.Y. Control of Foam Formation by Antifoam during Demineralization of Crustacean Shell in Preparation of Chitin. J. Agric. Food Chem. 1998, 46, 3844–3846. [Google Scholar] [CrossRef]
- Bradić, B.; Novak, U.; Likozar, B. Crustacean Shell Bio-Refining to Chitin by Natural Deep Eutectic Solvents. Green Process Synth. 2020, 9, 13–25. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Franco, L.O.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of Chitosan and Its Oligomers from Shrimp Shell Waste, Their Characterization and Antimicrobial Effect. Vet. World 2017, 10, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F. Preparation of Chitin and Chitosan. In Chitin Chemistry; Palgrave: London, UK, 1992; pp. 54–84. [Google Scholar]
- Kostag, M.; el Seoud, O.A. Sustainable Biomaterials Based on Cellulose, Chitin and Chitosan Composites—A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Kou, S. Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2019; ISBN 9781119450467. [Google Scholar]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Hussain, R.; Maji, T.K.; Maji, T.K. Determination of Degree of Deacetylation of Chitosan and Their Effect on the Release Behavior of Essential Oil from Chitosan and Chitosan-Gelatin Complex Microcapsules. Int. J. Adv. Eng. Appl. 2013, 2, 4–12. [Google Scholar]
- Mukarram, M.; Naeem, M.; Aftab, T.; Khan, M.M.A. Chitin, Chitosan, and Chitooligosaccharides: Recent Advances and Future Perspectives. In Radiation-Processed Polysaccharides; Academic Press: Cambridge, MA, USA, 2022; pp. 339–353. [Google Scholar] [CrossRef]
- Philibert, T.; Lee, B.H.; Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef]
- Anand, M.; Kalaivani, R.; Maruthupandy, M.; Kumaraguru, A.K.; Suresh, S. Extraction and Characterization of Chitosan from Marine Crab and Squilla Collected from the Gulf of Mannar Region, South India. J. Chitin Chitosan Sci. 2014, 2, 280–287. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Suwalka, S.; Yogi, M.R.; Ali, W.; Das, A.; Alagirusamy, R. Green Synthesis of Chitosan-Cinnamaldehyde Cross-Linked Nanoparticles: Characterization and Antibacterial Activity. Carbohydr. Polym. 2019, 226, 115298. [Google Scholar] [CrossRef]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Mari, S.; Oh, J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Guerrero, I.; Huerta, S.; Saucedo, G.; Castillo, A.; Obdulia Gonzalez, R.; Hall, G.M. Effect of Initial Glucose Concentration and Inoculation Level of Lactic Acid Bacteria in Shrimp Waste Ensilation. Enzym. Microb. Technol. 2001, 28, 446–452. [Google Scholar] [CrossRef] [PubMed]
- De Holanda, H.D.; Netto, F.M. Recovery of Components from Shrimp (Xiphopenaeus kroyeri) Processing Waste by Enzymatic Hydrolysis. J. Food Sci. 2006, 71, C298–C303. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Hai, P.D.; My My, V.T.; Men, D.T.; Trung, L.D.; Bavor, H.J. Improving Product Added-Value from Shrimp (Litopenaeus vannamei) Waste by Using Enzymatic Hydrolysis and Response Surface Methodology. J. Aquat. Food Prod. Technol. 2021, 30, 880–892. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial Extraction of Chitin from Seafood Waste Using Sugars Derived from Fruit Waste-Stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- Rao, M.S.; Muñoz, J.; Stevens, W.F. Critical Factors in Chitin Production by Fermentation of Shrimp Biowaste. Appl. Microbiol. Biotechnol. 2000, 54, 808–813. [Google Scholar] [CrossRef]
- Yang, J.K.; Shih, I.L.; Tzeng, Y.M.; Wang, S.L. Production and Purification of Protease from a Bacillus Subtilis That Can Deproteinize Crustacean Wastes. Enzym. Microb. Technol. 2000, 26, 406–413. [Google Scholar] [CrossRef]
- Gartner, C.; Peláez, C.A.; López, B.L. Characterization of Chitin and Chitosan Extracted from Shrimp Shells by Two Methods. E-Polymers 2010, 10, 69. [Google Scholar] [CrossRef]
- Trung, T.S.; Tram, L.H.; van Tan, N.; van Hoa, N.; Minh, N.C.; Loc, P.T.; Stevens, W.F. Improved Method for Production of Chitin and Chitosan from Shrimp Shells. Carbohydr. Res 2020, 489, 107913. [Google Scholar] [CrossRef]
- Hongkulsup, C.; Khutoryanskiy, V.-v.; Niranjan, K. Enzyme Assisted Extraction of Chitin from Shrimp Shells (Litopenaeus Vannamei). J. Chem. Technol. Biotechnol. 2016, 91, 1250–1256. [Google Scholar] [CrossRef]
- Valdez-Peña, A.U.; Espinoza-Perez, J.D.; Sandoval-Fabian, G.C.; Balagurusamy, N.; Hernandez-Rivera, A.; De-la-Garza-Rodriguez, I.M.; Contreras-Esquivel, J.C. Screening of Industrial Enzymes for Deproteinization of Shrimp Head for Chitin Recovery. Food Sci. Biotechnol. 2010, 19, 553–557. [Google Scholar] [CrossRef]
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable Ecofriendly Phytoextract Mediated One Pot Green Recovery of Chitosan. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of Lignin and Chitin in Ionic Liquids and Their Biomedical Applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.S.; da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef] [PubMed]
- Idenoue, S.; Yamamoto, K.; Kadokawa, J.I. Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea. Chem. Eng. 2019, 3, 90. [Google Scholar] [CrossRef]
- Ramón, D.J.; Guillena, G. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley: Hoboken, NJ, USA, 2019; ISBN 9783527818471. [Google Scholar]
- Huang, W.C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and Facile Production of Chitin from Crustacean Shells Using a Natural Deep Eutectic Solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile Acid Base Sustainable Solvent for Fast Extraction of Various Molecular Weight Chitin from Lobster Shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and Physicochemical Properties of Chitin Polymer from Insect Farm Side Stream as a New Source of Renewable Biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Ji-bin, Z.; Jia, Z.; Jia-hui, L.I.; Tomerlin, J.K.; Xiao-peng, X.; Rehman, K. Black Soldier Fly: A New Vista for Livestock and Poultry Manure Management. J. Integr. Agric. 2020, 19, 2–14. [Google Scholar] [CrossRef]
- Rehman, K.U.; Hollah, C.; Wiesotzki, K.; Rehman, R.U.; Rehman, A.U.; Zhang, J.; Zheng, L.; Nienaber, T.; Heinz, V.; Aganovic, K. Black Soldier Fly, Hermetia illucens as a Potential Innovative and Environmentally Friendly Tool for Organic Waste Management: A Mini-Review. Waste Manag. Res. J. A Sustain. Circ. Econ. 2023, 41, 734242X2211054. [Google Scholar] [CrossRef]
- Lourenço, F.; Calado, R.; Medina, I.; Ameixa, O.M.C.C. The Potential Impacts by the Invasion of Insects Reared to Feed and Pet Animals in Europe and Other Regions: A Critical Review. Sustainability 2022, 14, 6361. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Li, X.; Qin, L. The Origin and Dispersal of the Domesticated Chinese Oak Silkworm, Antheraea pernyi, in China: A Reconstruction Based on Ancient Texts. J. Insect Sci. 2010, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Edible Insects Market Worth $9.60 Billion by 2030. Available online: https://www.meticulousresearch.com/pressrelease/184/edible-insects-market-2030 (accessed on 18 January 2023).
- Delgado, L.; Garino, C.; Moreno, F.J.; Zagon, J.; Broll, H. Sustainable Food Systems: EU Regulatory Framework and Contribution of Insects to the Farm-To-Fork Strategy. Food Rev. Int. 2022, 8, 1–22. [Google Scholar] [CrossRef]
- Petrescu, C.; Malina Petrescu-Mag, R.; Rizov, M.; Burny, P.; Markham Kim, H.; Joo, K.; Hwang, J. Are Customers Willing to Pay More for Eco-Friendly Edible Insect Restaurants? Focusing on the Internal Environmental Locus of Control. Sustainability 2022, 14, 10075. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of Chitin and Chitosan Derived from Hermetia illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Wang, H.; Kashif ur Rehman; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect Biorefinery: A Green Approach for Conversion of Crop Residues into Biodiesel and Protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef]
- Rehman, K.U.; Cai, M.; Xiao, X.; Zheng, L.; Wang, H.; Soomro, A.A.; Zhou, Y.; Li, W.; Yu, Z.; Zhang, J. Cellulose Decomposition and Larval Biomass Production from the Co-Digestion of Dairy Manure and Chicken Manure by Mini-Livestock (Hermetia illucens L.). J. Environ. Manag. 2017, 196, 458–465. [Google Scholar] [CrossRef]
- Somroo, A.A.; ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus Buchneri on Soybean Curd Residue Co-Conversion by Black Soldier Fly Larvae (Hermetia illucens) for Food and Feedstock Production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Feng, W.; Yang, D.; Rehman, R.U.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical Structure of Chitin in the Developing Stages of Black Soldier Fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef]
- Valdés, F.; Villanueva, V.; Durán, E.; Campos, F.; Avendaño, C.; Sánchez, M.; Domingoz-araujo, C.; Valenzuela, C. Insects as Feed for Companion and Exotic Pets: A Current Trend. Animals 2022, 12, 1450. [Google Scholar] [CrossRef]
- Greven, H.; Kaya, M.; Sargin, I.; Baran, T.; Møbjerg Kristensen, R.; Vinther Sørensen, M. Characterisation of Chitin in the Cuticle of a Velvet Worm (Onychophora). Turk. J. Zool. 2019, 43, 416–424. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current State of Chitin Purification and Chitosan Production from Insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Ji, R.; Schmitt, E.; Zibek, S. Chitosan Production with Larval Exoskeletons Derived from the Insect Protein Production. J. Biotechnol. 2020, 310, 62–67. [Google Scholar] [CrossRef]
- Kim, M.W.; Song, Y.S.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Park, Y.K.; Kang, S.H.; Kim, S.A.; Choi, C.; Jung, W.J. Production of Chitin and Chitosan from the Exoskeleton of Adult Two-Spotted Field Crickets (Gryllus bimaculatus). Entomol. Res. 2017, 47, 279–285. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of Chitin Extracted from Black Soldier Fly in Different Life Stages. Int. J. Biol. Macromol. 2020, 165, 3205–3214. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of Black Soldier Fly Prepupae and Systematic Approaches for Extraction and Fractionation of Proteins, Lipids and Chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- D’Hondt, E.; Soetemans, L.; Bastiaens, L.; Maesen, M.; Jespers, V.; Van den Bosch, B.; Voorspoels, S.; Elst, K. Simplified Determination of the Content and Average Degree of Acetylation of Chitin in Crude Black Soldier Fly Larvae Samples. Carbohydr. Res. 2020, 488, 107899. [Google Scholar] [CrossRef]
- Nafisah, A.; Nahrowi; Mutia, R.; Jayanegara, A. Chemical Composition, Chitin and Cell Wall Nitrogen Content of Black Soldier Fly (Hermetia illucens) Larvae after Physical and Biological Treatment. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Wuhan, China, 1 June 2019; IOP Publishing: Bristol, UK, 2019; Volume 546, p. 42028. [Google Scholar]
- Khayrova, A.A.; Lopatin, S.A.; Sinitsyna, O.A.; Sinitsyn, A.P.; Varlamov, V.P. Obtaining chitin from the black soldier fly Hermetia illucens by direct extraction. Proc. RAS Ufa Sci. Cent. 2018, 3, 84–88. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Black Soldier Fly Hermetia illucens as a Novel Source of Chitin and Chitosan. Int. J. Sci. 2019, 8, 81–86. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia illucens). J. Polym Environ. 2020, 28, 445–457. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V.; Antonov, A.; Ivanov, G.; Pastukhova, N.; Bovykina, G. Production of Chitin from Dead Hermetia illucens. IOP Conf. Ser. Earth Environ. Sci. 2019, 315, 042003. [Google Scholar] [CrossRef]
- Kim, M.W.; Han, Y.S.; Jo, Y.H.; Choi, M.H.; Kang, S.H.; Kim, S.A.; Jung, W.J. Extraction of Chitin and Chitosan from Housefly, Musca domestica, Pupa Shells. Entomol. Res. 2016, 46, 324–328. [Google Scholar] [CrossRef]
- Zhang, A.J.; Qin, Q.L.; Zhang, H.; Wang, H.T.; Li, X.; Miao, L.; Wu, Y.J. Preparation and Characterisation of Food-Grade Chitosan from Housefly Larvae. Czech J. Food Sci. 2011, 29, 616–623. [Google Scholar] [CrossRef]
- Ai, H.; Wang, F.; Yang, Q.; Zhu, F.; Lei, C. Preparation and Biological Activities of Chitosan from the Larvae of Housefly, Musca Domestica. Carbohydr. Polym. 2008, 72, 419–423. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, M.W.; Moon, C.; Seo, D.J.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.K.; Kim, S.A.; Kim, Y.W.; et al. Extraction of Chitin and Chitosan from Larval Exuvium and Whole Body of Edible Mealworm, Tenebrio Molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- Son, Y.J.; Hwang, I.K.; Nho, C.W.; Kim, S.M.; Kim, S.H. Determination of Carbohydrate Composition in Mealworm (Tenebrio molitor L.) Larvae and Characterization of Mealworm Chitin and Chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef]
- Song, Y.S.; Jo, Y.H.; Han, Y.S.; Jung, W.J. Production of Chitin- and Chitosan-Oligosaccharide Using the Edible Insect, Tenebrio molitor. Entomol. Res. 2022, 52, 207–213. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of Chitosan Extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and Their Antibacterial Activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and Physicochemical Characterization of Chitin and Chitosan from Zophobas morio Larvae in Varying Sodium Hydroxide Concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and Physicochemical Characterization of Chitin and Chitosan Isolated from House Cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef]
- Psarianos, M.; Dimopoulos, G.; Ojha, S.; Cavini, A.C.M.; Bußler, S.; Taoukis, P.; Schlüter, O.K. Effect of Pulsed Electric Fields on Cricket (Acheta Domesticus) Flour: Extraction Yield (Protein, Fat and Chitin) and Techno-Functional Properties. Innov. Food Sci. Emerg. Technol. 2022, 76, 102908. [Google Scholar] [CrossRef]
- Marei, N.; Elwahy, A.H.M.; Salah, T.A.; el Sherif, Y.; El-Samie, E.A. Enhanced Antibacterial Activity of Egyptian Local Insects’ Chitosan-Based Nanoparticles Loaded with Ciprofloxacin-HCl. Int. J. Biol. Macromol. 2019, 126, 262–272. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Samie, E.A.; Salah, T.; Saad, G.R.; Elwahy, A.H.M. Isolation and Characterization of Chitosan from Different Local Insects in Egypt. Int. J. Biol. Macromol. 2016, 82, 871–877. [Google Scholar] [CrossRef]
- Milusheva, R.Y.; Rashidova, S.S. Bombyx Mori Chitin and Chitosan: Synthesis, Properties, and Use, Tashkent; FAN: Tashkent, Uzbekistan, 2009; pp. 220–246. [Google Scholar]
- Zhang, M.; Haga, A.; Sekiguchi, H.; Hirano, S. Structure of Insect Chitin Isolated from Beetle Larva Cuticle and Silkworm (Bombyx mori) Pupa Exuvia. Int. J. Biol. Macromol. 2000, 27, 99–105. [Google Scholar] [CrossRef]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of Chitosan and Chitin Produced from Silkworm Crysalides. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Battampara, P.; Nimisha Sathish, T.; Reddy, R.; Guna, V.; Nagananda, G.S.; Reddy, N.; Ramesha, B.S.; Maharaddi, V.H.; Rao, A.P.; Ravikumar, H.N.; et al. Properties of Chitin and Chitosan Extracted from Silkworm Pupae and Egg Shells. Int. J. Biol. Macromol. 2020, 161, 1296–1304. [Google Scholar] [CrossRef]
- Pazmiño, M.F.; Del Hierro, A.G.; Flores, F.J. Genetic Diversity and Organic Waste Degrading Capacity of Hermetia illucens from the Evergreen Forest of the Equatorial Choco Lowland. PeerJ 2023, 11, e14798. [Google Scholar] [CrossRef]
- Salam, M.; Alam, F.; Dezhi, S.; Nabi, G.; Shahzadi, A.; Hassan, S.U.; Ali, M.; Saeed, M.A.; Hassan, J.; Ali, N.; et al. Exploring the Role of Black Soldier Fly Larva Technology for Sustainable Management of Municipal Solid Waste in Developing Countries. Environ. Technol. Innov 2021, 24, 101934. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the Physicochemical, Rheological, and Morphologic Properties of Chitosan from Four Insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic Changes of Nutrient Composition throughout the Entire Life Cycle of Black Soldier Fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.U.; Abdul; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of Mixtures of Dairy Manure and Soybean Curd Residue by Black Soldier Fly Larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Mazza, L.; Xiao, X.; ur Rehman, K.; Cai, M.; Zhang, D.; Fasulo, S.; Tomberlin, J.K.; Zheng, L.; Soomro, A.A.; Yu, Z.; et al. Management of Chicken Manure Using Black Soldier Fly (Diptera: Stratiomyidae) Larvae Assisted by Companion Bacteria. Waste Manag. 2020, 102, 312–318. [Google Scholar] [CrossRef]
- Zhu, Z.; Rehman, K.U.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.H.; Cai, M.; Zhang, J.; Yu, Z.; et al. De Novo Transcriptome Sequencing and Analysis Revealed the Molecular Basis of Rapid Fat Accumulation by Black Soldier Fly (Hermetia illucens, L.) for Development of Insectival Biodiesel. Biotechnol. Biofuels 2019, 12, 194. [Google Scholar] [CrossRef]
- Rehman, K.U.; Liu, X.; Wang, H.; Zheng, L.; Rehman, R.U.; Cheng, X.; Li, Q.; Li, W.; Cai, M.; Zhang, J.; et al. Effects of Black Soldier Fly Biodiesel Blended with Diesel Fuel on Combustion, Performance and Emission Characteristics of Diesel Engine. Energy Convers. Manag. 2018, 173, 489–498. [Google Scholar] [CrossRef]
- Kashif, R.U.; Clemens, H.; Heinz, V. Insects: Alternative Source of Protein and Fat in Livestock, Pets and Aquaculture Feed FEEDPLANET. 2021; pp. 28–35. Available online: https://feedplanetmagazine.com/blog/insects-alternative-source-of-protein-and-fat-in-livestock-pets-and-aquaculture-feed-1415 (accessed on 9 January 2023).
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The First Report of the Physicochemical Structure of Chitin Isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin/Chitosan-Melanin Complexes from Black Soldier Fly Hermetia illucens. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Ufa, Russia, 5–9 October 2020; Volume 809. [Google Scholar]
- Kurchenko, V.P.; Kukulyanskaya, T.A.; Azarko, I.I.; Zueva, O.Y.; Khizmatullin, R.G.; Varlamov, V.P. Physicochemical Properties of Chitin-Melanin and Melanoprotein Complexes from Bee Corpses. Appl. Biochem. Microbiol. 2006, 42, 331–334. [Google Scholar] [CrossRef]
- Scott, J.G. Evolution of Resistance to Pyrethroid Insecticides in Musca Domestica. Pest. Manag. Sci. 2017, 73, 716–722. [Google Scholar] [CrossRef]
- Cheng, Z.; Yu, L.; Li, H.; Xu, X.; Yang, Z. Use of Housefly (Musca domestica L.) Larvae to Bioconversion Food Waste for Animal Nutrition and Organic Fertilizer. Environ. Sci. Pollut. Res. 2021, 28, 48921–48928. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of Various Commodities for the Development of the Yellow Mealworm, Tenebrio Molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef] [PubMed]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a Source of Interesting Natural Compounds, Their Recovery Processes, Biological Effects, and Safety Aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef] [PubMed]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Nascimento Filho, M.A.; Pereira, R.T.; Oliveira, A.B.S.; Suckeveris, D.; Burin Junior, A.M.; Soares, C.A.P.; Menten, J.F.M. Nutritional Value of Tenebrio Molitor Larvae Meal for Broiler Chickens: Metabolizable Energy and Standardized Ileal Amino Acid Digestibility. J. Appl. Poult. Res. 2021, 30, 100102. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. The Superworm, Zophobas Morio (Coleoptera:Tenebrionidae): A ‘Sleeping Giant’ in Nutrient Sources. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Dragojlović, D.; Ðuragić, O.; Pezo, L.; Popović, L.; Rakita, S.; Tomičić, Z.; Spasevski, N. Comparison of Nutritional Profiles of Super Worm (Zophobas Morio) and Yellow Mealworm (Tenebrio Molitor) as Alternative Feeds Used in Animal Husbandry: Is Super Worm Superior? Animals 2022, 12, 1277. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska–Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio Molitor and Zophobas Morio Full-Fat Meals as Functional Feed Additives Affect Broiler Chickens’ Growth Performance and Immune System Traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef]
- Abd, M.D.; Jabir, R.; Razak, S.A.; Vikineswary, S. Nutritive Potential and Utilization of Super Worm (Zophobas Morio) Meal in the Diet of Nile Tilapia (Oreochromis Niloticus) Juvenile. Afr. J. Biotechnol. 2014, 11, 6592–6598. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The Potential Role of Insects as Feed: A Multi-Perspective Review. Animals 2019, 9, 119. [Google Scholar] [CrossRef]
- Soares Araújo, R.R.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Moreira Santos, E. Nutritional Composition of Insects Gryllus assimilis and Zophobas morio: Potential Foods Harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Finke, M. Complete Nutrient Composition of Commercially Raised Invertebrates Used as Food for Insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Adámková, A.; Mlček, J.; Kouřimská, L.; Borkovcová, M.; Bušina, T.; Adámek, M.; Bednářová, M.; Krajsa, J. Nutritional Potential of Selected Insect Species Reared on the Island of Sumatra. Int. J. Environ. Res Public Health 2017, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Kulma, M.; Kouřimská, L.; Homolková, D.; Božik, M.; Plachý, V.; Vrabec, V. Effect of Developmental Stage on the Nutritional Value of Edible Insects. A Case Study with Blaberus craniifer and Zophobas morio. J. Food Compos. Anal. 2020, 92, 103570. [Google Scholar] [CrossRef]
- Kaya, M.; Erdogan, S.; Mol, A.; Baran, T. Comparison of Chitin Structures Isolated from Seven Orthoptera Species. Int. J. Biol. Macromol. 2015, 72, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Seyyar, O.; Baran, T.; Turkes, T. Bat Guano as New and Attractive Chitin and Chitosan Source. Front. Zool. 2014, 11, 59. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread Enriched with Cricket Powder (Acheta domesticus): A Technological, Microbiological and Nutritional Evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Kulma, M.; Kouřimská, L.; Plachý, V.; Božik, M.; Adámková, A.; Vrabec, V. Effect of Sex on the Nutritional Value of House Cricket, Acheta domestica L. Food Chem. 2019, 272, 267–272. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Ploydee, E.; Chaiyanan, S. Production of High Viscosity Chitosan from Biologically Purified Chitin Isolated by Microbial Fermentation and Deproteinization. Int. J. Polym. Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Psarianos, M.; Ojha, S.; Schneider, R.; Schlüter, O.K. Chitin Isolation and Chitosan Production from House Crickets (Acheta Domesticus) by Environmentally Friendly Methods. Molecules 2022, 27, 5005. [Google Scholar] [CrossRef]
- Taylor, G.K.; Thomas, A.L.R. Dynamic Flight Stability in the Desert Locust Schistocerca gregaria. J. Exp. Biol. 2003, 206, 2803–2829. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.; Charnley, K. Mutualism between the Desert Locust Schistocerca gregaria and Its Gut Microbiota. Res. Microbiol. 2002, 153, 503–509. [Google Scholar] [CrossRef]
- Suresh, H.N.; Mahalingam, C.A.; Pallavi. Amount of Chitin, Chitosan and Chitosan Based on Chitin Weight in Pure Races of Multivoltine and Bivoltine Silkworm Pupae Bombyx mori L. Int. J. Sci. Nat. 2012, 3, 214–216. [Google Scholar]
- Mahesh, D.; Subbarayappa, C.T.; Shruthi, R. A Review—Bionutritional Science of Silkworm Pupal Residue to Mine New Ways for Utilization. Int. J. Adv. Res. Biol. Sci. 2015, 2, 135–140. [Google Scholar]
- Sánchez-Pérez, L.dC.; Barranco-Florido, J.E.; Rodríguez-Navarro, S.; Cervantes-Mayagoitia, J.F.; Ramos-López, M.Á. Enzymes of Entomopathogenic Fungi, Advances and Insights. Adv. Enzyme Res. 2014, 2, 65–76. [Google Scholar] [CrossRef]
- Milusheva, R.Y.; Rashidova, S.S. Bombyx Mori Chitosan Nanoparticles: Synthesis and Properties. Open J. Org. Polym. Mater. 2019, 9, 63–73. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Giraldo, J.D.; Garrido-Miranda, K.A.; Schoebitz, M. Chitin and Its Derivatives: Functional Biopolymers for Developing Bioproducts for Sustainable Agriculture—A Reality? Carbohydr. Polym. 2023, 299, 120196. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef]
- Ali, G.; Sharma, M.; Salama, E.S.; Ling, Z.; Li, X. Applications of Chitin and Chitosan as Natural Biopolymer: Potential Sources, Pretreatments, and Degradation Pathways. Biomass Convers. Biorefin. 2022 2022, 1, 1–15. [Google Scholar] [CrossRef]
- Rizvi, S.; Goswami, L.; Gupta, S.K. A Holistic Approach for Melanoidin Removal via Fe-Impregnated Activated Carbon Prepared from Mangifera indica Leaves Biomass. Bioresour. Technol. Rep. 2020, 12, 100591. [Google Scholar] [CrossRef]
- Ghourbanpour, J.; Sabzi, M.; Shafagh, N. Effective Dye Adsorption Behavior of Poly (Vinyl alcohol)/ Chitin Nanofiber/Fe(III) Complex. Int. J. Biol. Macromol. 2019, 137, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Lv, X.; Ma, M.; Oh, D.-H.; Jiang, Z.; Fu, X. Chitin and Chitin-Based Biomaterials: A Review of Advances in Processing and Food Applications. Carbohydr. Polym. 2023, 299, 120142. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Q.; Lin, Z.; Liu, S.; Su, Q.; Pan, Y. Therapeutic effects of chitin from Pleurotus eryngii on high-fat diet induced obesity in rats. Acta Sci. Pol. Technol. Aliment. 2020, 19, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Konti, A.; Mamma, D.; Scarlat, N.; Damigos, D. The Determinants of the Growth of the European Bioplastics Sector—A Fuzzy Cognitive Maps Approach. Sustainability 2022, 14, 6035. [Google Scholar] [CrossRef]
- Nachod, B.; Keller, E.; Hassanein, A.; Lansing, S. Assessment of Petroleum-Based Plastic and Bioplastics Degradation Using Anaerobic Digestion. Sustainability 2021, 13, 13295. [Google Scholar] [CrossRef]
- Negi, H.; Verma, P.; Singh, R.K. A Comprehensive Review on the Applications of Functionalized Chitosan in Petroleum Industry. Carbohydr. Polym. 2021, 266, 118125. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M. Oil Structuring Using Polysaccharides. Curr. Opin. Food Sci. 2019, 27, 29–35. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and Chitosan: Prospective Biomedical Applications in Drug Delivery, Cancer Treatment, and Wound Healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2010, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- el Hadrami, A.; Adam, L.R.; el Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Hadwiger, L.A. Multiple Effects of Chitosan on Plant Systems: Solid Science or Hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a Promising Natural Compound to Enhance Potential Physiological Responses in Plant: A Review. Ind. J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Veliz, E.A.; Martínez-Hidalgo, P.; Hirsch, A.M. Chitinase-Producing Bacteria and Their Role in Biocontrol. AIMS Microbiol. 2017, 3, 689. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B.; Nishant Bhanu, A. A Future Perspective in Crop Protection: Chitosan and Its Oligosaccharides Pigeonpea View Project Scaling City Institutions for India (SCI-FI): Sanitation, Centre for Policy Research, New Delhi View Project; MedCrave: Edmond, OK, USA, 2014. [Google Scholar] [CrossRef]
- Hasan, S.; Boddu, V.M.; Viswanath, D.S.; Ghosh, T.K. Chitosan Uses in Cosmetics; Springer: Cham, Switzerland, 2022; pp. 377–404. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; de los Gandía, M.L.; Caballero, A.H. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Caner, C. The Effect of Edible Eggshell Coatings on Egg Quality and Consumer Perception. J. Sci. Food Agric. 2005, 85, 1897–1902. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of Chitin- and Chitosan-Derivatives for the Detoxification of Water and Wastewater—A Short Review. Adv. Colloid Int. Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano)Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kishor, R. Chitin and Chitosan: Origin, Properties, and Applications. In Handbook of Chitin and Chitosan: Volume 1: Preparation and Properties; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 9780128179703. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and Properties of Chitosan. Appl. Chitin Chitosan 2020, 7, 3–29. [Google Scholar] [CrossRef]
- Amar Cheba, B.; Khan, F.I.; Ali, S. Chitin and Chitosan: Marine Biopolymers with Unique Properties and Versatile Applications. Glob. J. Biotechnol. Biochem. 2011, 6, 149–153. [Google Scholar]
- Rajiv Gandhi, M.; Kousalya, G.N.; Meenakshi, S. Removal of Copper(II) Using Chitin/Chitosan Nano-Hydroxyapatite Composite. Int. J. Biol. Macromol. 2011, 48, 119–124. [Google Scholar] [CrossRef]
- Silvianti, F.; Siswanta, D.; Aprilita, N.H.; Kiswandono, A.A. Adsorption Characteristic of Iron onto Poly[Eugenol-Co-(Divinyl benzene)] from Aqueous Solution. J. Nat. 2017, 17, 108. [Google Scholar] [CrossRef]
- Pinto, P.X.; Al-Abed, S.R.; Reisman, D.J. Biosorption of Heavy Metals from Mining Influenced Water onto Chitin Products. Chem. Eng. J. 2011, 166, 1002–1009. [Google Scholar] [CrossRef]
- Tang, H.; Chang, C.; Zhang, L. Efficient Adsorption of Hg2+ Ions on Chitin/Cellulose Composite Membranes Prepared via Environmentally Friendly Pathway. Chem. Eng. J. 2011, 173, 689–697. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, W.; Zhang, L. Adsorption Isotherms and Kinetics Studies of Malachite Green on Chitin Hydrogels. J. Hazard. Mater. 2012, 209–210, 218–225. [Google Scholar] [CrossRef]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a Circular Economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Lawal, U.; Valapa, R.B. Bioplastics: An Introduction to the Role of Eco-Friendly Alternative Plastics in Sustainable Packaging. In Bio-Based Packaging; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Jankowska, E.; Gorman, M.R.; Frischmann, C.J. Transforming the Plastic Production System Presents Opportunities to Tackle the Climate Crisis. Sustainability 2022, 14, 6539. [Google Scholar] [CrossRef]
- The New Industrial Strategy for Europe. Intereconomics 2021, 56, 132. [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Ezgi, B.A.; Havva, D.O. A Review: Investigation of Bioplastics. J. Civ. Eng. Archit. 2015, 9, 188–192. [Google Scholar] [CrossRef]
- Kumar, S.; Thakur, K. Bioplastics—Classification, Production and Their Potential Food Applications. J. Hill Agric. 2017, 8, 118–129. [Google Scholar] [CrossRef]
- Faizan, M.; Nadeem, H.; Arif, A.; Zaheer, W. Bioplastics from Biopolymers: An Eco-Friendly and Sustainable Solution of Plastic Pollution. Polym. Sci.–Ser. C 2021, 63, 47–63. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical Properties of the Edible Films from the Blends of High Methoxyl Apple Pectin and Chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- Fernandez-Saiz, P.; Lagarón, J.M.; Ocio, M.J. Optimization of the Film-Forming and Storage Conditions of Chitosan as an Antimicrobial Agent. J. Agric. Food Chem. 2009, 57, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; Sobral, P.J.d.A. Production and Characterization of Films Based on Blends of Chitosan from Blue Crab (Callinectes sapidus) Waste and Pectin from Orange (Citrus sinensis Osbeck) Peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Martins da Costa, J.C.; Lima Miki, K.S.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, e03718. [Google Scholar] [CrossRef] [PubMed]
- Samsalee, N.; Sothornvit, R. Development and Characterization of Porcine Plasma Protein-Chitosan Blended Films. Food Packag. Shelf Life 2019, 22, 100406. [Google Scholar] [CrossRef]
- Wu, Y.; Ying, Y.; Liu, Y.; Zhang, H.; Huang, J. Preparation of Chitosan/Poly Vinyl Alcohol Films and Their Inhibition of Biofilm Formation against Pseudomonas Aeruginosa PAO1. Int. J. Biol. Macromol. 2018, 118, 2131–2137. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Y.; Yang, J.; Kennedy, J.F.; Wang, X.; Wang, L. Synthesis, Characterization and Antibacterial Activity of Guanidinylated Chitosan. Carbohydr. Polym. 2007, 67, 66–72. [Google Scholar] [CrossRef]
- Hamdi, M.; Hammami, A.; Hajji, S.; Jridi, M.; Nasri, M.; Nasri, R. Chitin Extraction from Blue Crab (Portunus segnis) and Shrimp (Penaeus kerathurus) Shells Using Digestive Alkaline Proteases from P. Segnis Viscera. Int. J. Biol. Macromol. 2017, 101, 455–463. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Y.; Lin, X.; Yue, F.; Liu, M. Sustainable, High-Performance, and Biodegradable Plastic Made from Chitin. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Jarolimkova, V. Preparation and Characterization of Antimicrobial Packaging Films from Cricket Chitosan Enriched with Schisandra Chinensis Extract; Lunds University: Lund, Sweden, 2015. [Google Scholar]
- Liceaga, A.A.; San Martin, F.; Jones, O.; Garcia Bravo, J.M.; Kaplan, I.; Bhunia, A. Purification and Characterization of Acheta Domesticus and Gryllodes Sigillatus Cricket Chitin and Chitosan for Bioactive and Biodegradable Food Packaging Applications. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2021. [Google Scholar] [CrossRef]
- Malm, M.; Liceaga, A.M. Physicochemical Properties of Chitosan from Two Commonly Reared Edible Cricket Species, and Its Application as a Hypolipidemic and Antimicrobial Agent. Polysaccharides 2021, 2, 339–353. [Google Scholar] [CrossRef]
- Wei, X.Y.; Xia, W.; Zhou, T. Antibacterial Activity and Action Mechanism of a Novel Chitosan Oligosaccharide Derivative against Dominant Spoilage Bacteria Isolated from Shrimp Penaeus Vannamei. Lett. Appl. Microbiol. 2022, 74, 268–276. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chen, C.Y. Antibacterial Characteristics and Activity of Acid-Soluble Chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Yeh, J.Y.; Tsai, C.F. Antibacterial Characteristics and Activity of Water-Soluble Chitosan Derivatives Prepared by the Maillard Reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia illucens. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Zhang, H.; Wang, J.; Fu, Q.; Qiao, H.; Wang, Q. Insight into Antibacterial Mechanism of Polysaccharides: A Review. LWT 2021, 150, 111929. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Eltaweil, A.S.; Elshishini, H.M.; Hosny, M.; Abou Alsoaud, M.M.; Attia, N.F.; El-Subruiti, G.M.; Omer, A.M. Sustainable Adsorptive Removal of Antibiotic Residues by Chitosan Composites: An Insight into Current Developments and Future Recommendations. Arab. J. Chem. 2022, 15, 103743. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Rezazadeh, N.; Kianvash, A. Preparation, Characterization, and Antibacterial Activity of Chitosan/Silicone Rubber Filled Zeolite, Silver, and Copper Nanocomposites against Pseudomonas aeruginosa and Methicillin-Resistant Staphylococcus aureus. J. Appl. Polym. Sci. 2021, 138, 50552. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; De Yao, K. Antibacterial Action of Chitosan and Carboxymethylated Chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan: Drug Delivery and Biomedical Applications; Springer: Singapore, 2020; pp. 457–489. ISBN 9789811502637. [Google Scholar]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Chandrika, K.S.V.P.; Prasad, R.D.; Godbole, V. Development of Chitosan-PEG Blended Films Using Trichoderma: Enhancement of Antimicrobial Activity and Seed Quality. Int. J. Biol. Macromol. 2019, 126, 282–290. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Feng, X.-Q.; Yang, S.; Fu, G.-Q.; Wang, T.-P.; Su, Z.-X. Chitosan Kills Escherichia coli through Damage to Be of Cell Membrane Mechanism. Carbohydr. Polym. 2010, 79, 493–499. [Google Scholar] [CrossRef]
- Lou, M.M.; Zhu, B.; Muhammad, I.; Li, B.; Xie, G.L.; Wang, Y.L.; Li, H.Y.; Sun, G.C. Antibacterial Activity and Mechanism of Action of Chitosan Solutions against Apricot Fruit Rot Pathogen Burkholderia seminalis. Carbohydr. Res. 2011, 346, 1294–1301. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef]
- Jing, Y.J.; Hao, Y.J.; Qu, H.; Shan, Y.; Li, D.S.; Du, R.Q. Studies on the Antibacterial Activities and Mechanisms of Chitosan Obtained from Cuticles of Housefly Larvae. Acta Biol. Hung. 2007, 58, 75–86. [Google Scholar] [CrossRef]
- Ali, U.; Baffa, M.U.; Shamsuddeen, Y. Physicochemical and Functional Characterization of Chitosan Prepared from Schistocerca gregaria (Desert Grasshopper) and the Investigation of Its Antimicrobial Activity. Bayero J. Pure Appl. Sci. 2021, 12, 104–110. [Google Scholar] [CrossRef]



| # | Method | Methods of Treatment | Advantages and Disadvantages |
|---|---|---|---|
| 1 | Chemical extraction | Demineralization: by acidic treatment using HCl, HNO3, H2SO4.Deproteinization: by alkaline treatment using NaOH or KOH.Decoloration: acetone or organic solvent.Deacetylation: by alkaline treatment using a strong NaOH or KOH |
|
| 2 | Biological extraction | Demineralization: Bacteria-produced lactic acid is used in demineralization.Deproteinization: Fermentation media proteases are released into the medium to deproteinize the cultureDecoloration: Acetone or organic solvents are effective decolorizers.Deacetylation: Bacteria produce chitin deacetylase, which deacetifies chitin. |
|
| # | Source | Stage | Steps Involved in Extraction | Chitin Yield | References |
|---|---|---|---|---|---|
| 1 | Hermetia illucens | Larvae | Demineralization (1:10, m/v with HCl 1 M at room temperature for 1 h), deproteinization (1 M NaOH treatment 1:25, m/v, 1 h at 80 °C), filtration (pore size 25 μm, 49 PA, 25/14, Solana), and washing with demineralized water until neutral pH and dried at 105 °C for 48 h | 9.5% | [106] |
| Prepupae | 9.1% | ||||
| Pupae | 10.3% | ||||
| Shedding | 31.1% | ||||
| Cocoon | 23.8% | ||||
| Flies | 5.6% | ||||
| Prepupae | Defatting (with petroleum ether), deproteinization (1 M NaOH) | 11.7–14.6% | [107] | ||
| Larvae | Acidic hydrolysis (1 M NaOH), deproteinization (1 M NaOH) | 8.50% | [108] | ||
| Demineralization (HCOOH), deproteinization (1.9–2 M NaOH) | 8.3–8.7% | [104] | |||
| Larvae | Depigmentation (3.6% HCl, NaClO), demineralization (2 M HCl), deproteinization (2 M NaOH) | 3.6% | [100] | ||
| Prepupae | 3.1% | ||||
| Pupae | 14.1% | ||||
| Adult | 2.9% | ||||
| Larvae | Defatting (C6H14), demineralization (1 M HCl), deproteinization (1 M NaOH) | 5.4% | [109] | ||
| Defatting (CHCl3, CH3OH), demineralization (2% HCl), deproteinization (5% NaOH) | 4.6% | [110] | |||
| Defatting (CHCl3:CH3OH, 7:3), demineralization (2% HCl), deproteinization (5%, w/w NaOH) | 7% | [111] | |||
| Pupae exuviae | Deproteinization (1 M NaOH), demineralization (1 M HCl solution) | 9% | [112] | ||
| Imago | 23% | ||||
| Dead flies | Demineralisation (HCl at 2 h), deproteinization (NaOH at 90 °C for 3 h) | 21.3% | [113] | ||
| 2 | Musca domestica | Pupae | Demineralisation (3 h in 500 mL of 2 N HCl solution at room), deproteinization (500 mL of 1.25 N NaOH at 95 °C for 3 h) | 8.02% | [114] |
| Larvae | Deproteinization (1 mol/l NaOH solution at 100 °C for 3 h) | 9.1% | [115] | ||
| Deproteinization (100 mL of 1 mol/L NaOH at 95 °C for 6 h), decoloration (10 mg/mL KMnO4 for 4 h) | Not determined (ND) | [116] | |||
| 3 | Tenebrio molitor | Larvae exuviae | Decalcified (2 N HCl at 20 °C, the exuviae were decalcified for 3 h), deproteinization (500 mL of 5% NaOH at 95 °C) | 18.01% | [117] |
| Whole body | Decalcification (3 h in 500 mL of 2 N HCl at 20 °C), deproteinization (3 h at 95 °C in 500 mL of 1.25 N NaOH) | 4.92% | |||
| Larvae | Deproteinization (400 mL of 1.25 M NaOH solution at 80 °C for 24 h), demineralization (1.5 M HCl solution, 1:10 w/v in a shaker 120 rpm, 6 h, at 20 °C) | 4.72% | [118] | ||
| Demineralization (2 N HCl, room temp., 3 h), deproteinization (5% NaOH, w/w for 3.5 h at 70 °C), decolorization (3% H2O2 for 1.5 h at 80 °C) | 6.82% | [119] | |||
| 4 | Zophobas morio | Larvae | Deproteinization (10%, w/v NaOH at80 °C for 24 h), demineralization (7%, v/v HCl at 25 °C for 24 h) | 4.60% | [120] |
| Adult | 8.40% | ||||
| Larvae | Demineralization (1.0 M of HCl in 35 °C), deproteinization (0.5 M, 1.0 M and 2.0 M NaOH in 80 °C for 20 h), decoloration (glacial acetone for 30 min) | 5.43% | [121] | ||
| 5 | Acheta domesticus | Adult | Deproteinization (1 M NaOH at 95 °C for 6 h), demineralization (Oxalic acid for 3 h at room temperature), decoloration (1% sodium hypochlorite for 3 h) | 4.3–7.1% | [122] |
| Deproteinization (NaOH, 1 M, s/l ratio = 1:50), demineralization (HCl, 1 M, s/l ratio 1:30) | 7.34 | [123] | |||
| 6 | Gryllus bimaculatus | Adult | Deproteinization (1.25 M NaOH), demineralization (2 N HCl). | 20.9–23.3% | [105] |
| 7 | Schistocerca gregaria | Adult | Deproteinization (1 M NaOH),demineralization (1 N HCl) | 22.5% | [124] |
| Deproteinization (1.0 M NaOH at 100 °C for 8 h), demineralization (1 M HCl) | 12.2% | [125] | |||
| 8 | Bombyx mori | Pupae exuviae | Defatting (acetone / alcohol), deproteinization (5–7% NaOH), demineralization (2% HCl), depigmentation (H2O2) | 3.6% | [60,126] |
| Pupae | Demineralization (1 N HCl), deproteinization (1 N NaOH) | 15–20% | [127] | ||
| Chrysalides | Demineralization (1 M HCl), deproteinization (1 M NaOH) | 2.6–4.2% | [128] | ||
| Egg Shell | Demineralization (7%, v/v HCl), deproteinization (10%, w/w NaOH) | 6% | [129] | ||
| Pupae | 18% |
| # | Source | Steps Involved in Extraction | Chitin to Chitosan Yield | References |
|---|---|---|---|---|
| 1 | Hermetia illucens | Demineralization (1:10, m/v with HCl 1 M at room temperature for 1 h), deproteinization (1 M NaOH treatment 1:25, m/v, 1 h at 80 °C), filtration (pore size 25 μm, 49 PA, 25/14, Solana), and washing with demineralized water (until neutral pH and dried at 105 °C for 48 h), deacetylation(1:30 m:v sample in 50 m% NaOH, 90 °C, 1 or 3 h) | ND, whereas the degree of deacetylation of chitin was determined (89%) | [106] |
| Demineralization (HCOOH), deproteinisation (1.9–2 M NaOH), deacetylation (10–12 M NaOH) | 13–43% | [104] | ||
| Defatting (C6H14), demineralisation (1 M HCl), deproteinisation (1 M NaOH), deacetylation (NaOH, NaBH4) | ND, whereas the degree of deacetylation of chitin was determined (66.11%) | [109] | ||
| Deacetylation (30% NaOH), chitin precipitation (85% H3PO4) | 32% | [111] | ||
| Demineralization (2% HCl), deproteinisation (5% NaOH), deacetylation (50% NaOH), defatting (CHCl3, CH3OH) | 53% | [110] | ||
| Deproteinisation (30% NaOH), defatting ((C2H5)2O), demineralisation (1% HCl), deacetylation (50% NaOH) | 18–29% | [60] | ||
| 2 | Musca domestica | Deproteinization (1 mol/L NaOH solution at 100 °C for 3 h), deacetylation (NaOH, 50% w/v at 125 °C for 4 h) | 60–70% | [115] |
| Decalcified (3 h in 500 mL of 2 N HCl solution at room temperature), deproteinization (500 mL of 1.25 N NaOH at 95 °C for 3 h), deacetylation (50% NaOH at 105 °C for 3 h) | 5.9% | [114] | ||
| Deproteinization (100 mL of 1 mol/L NaOH at 95 °C for 6 h), decoloration (10 mg/mL KMnO4 for 4 h), deacetylation (400 mg/mL NaOH at 70 °C for 8 h) | ND, whereas the degree of deacetylation of chitin was determined (90.3%) | [116] | ||
| 3 | Tenebrio molitor | Deproteinization (500 mL 5% NaOH at 95 °C for 3) demineralization (3 h in 1500 mL 2 N HCl at 20 C), deacetylation (500 mL of NaOH at 95 or 105 °C for 3 h or 5 h) | 13.07–14.48% | [117] |
| Demineralization (2 N HCl, room temp., 3 h), deproteinization (5% NaOH, w/w for 3.5 h at 70 °C), decolorization (3% H2O2 for 1.5 h at 80 °C), deacetylation (50% NaOH, w/w for 5 h at 105 °C) | 50% | [119] | ||
| Deproteinization (enzymatic hydrolysis started by adding the alcalase enzyme in a proportion of 2%, w/w; enzyme/substrate), deacetylation (NaOH 40% w/v solution at 90 °C under 500 rpm, 8 h), demineralization (suspension was neutralized to pH 7.0 with HCl (1 M) and filtered again to separate the supernatant) | 31.9% | [14] | ||
| Deproteinization (400 mL of 1.25 M NaOH solution and maintained at 80 °C for 24 h), demineralization (71.5 M HCl solution, 1:10, w/v, and shaken at 20 °C in a shaker, 120 rpm, 6 h)), deacetylation (50% NaOH at 80 °C for 4 h) | ND, whereas the degree of deacetylation of chitin was determined (95.02%) | [118] | ||
| 4 | Zophobas morio | Deproteinization (0.5 M, 1.0 M and 2.0 M NaOH in °C for 20 h), demineralization (1.0 M of HCl in 35 °C), decoloration (glacial acetone for 30 min), deacetylation (50 wt % NaOH in 90 °C for 30 h) | 65.84% | [121] |
| Demineralization (1 M HCl); deproteinization (0.5–2 M NaOH); depigmentation ((CH3)2CO), deacetylation (50% NaOH) | 78–83% | [120] | ||
| 5 | Acheta domesticus | Deproteinization (1 M NaOH at 95 °C for 6 h), demineralization (Oxalic acid for 3 h at room temperature), decoloration (1% sodium hypochlorite for 3 h), deacetylation (50% (w/v) NaOH in 121 °C for 5 h) | 2.4–5.8% | [122] |
| Deproteinization (1.0 M NaOH at 100 °C for 8 h), demineralization (1 M HC), deacetylation (50% NaOH (15 mL/g) at 100 °C for 8 h) | ND, whereas the degree of deacetylation of chitin was determined (98%) | [124] | ||
| 6 | Gryllus bimaculatus | Deproteinization (1.25 M NaOH), demineralization(2 N HCl), deacetylation (50% NaOH (w/v)) | 79.1–94.2% | [105] |
| 7 | Schistocerca gregaria | Deproteinization (1.0 M NaOH at 100 °C for 8 h), demineralization (1 M HC), deacetylation (50% NaOH (15 mL/g) at 100 °C for 8 h) | 55% | [125] |
| 8 | Bombyx mori | Demineralization (1 M HCl), deproteinization (1 M NaOH), deacetylation (NaOH, NaBH4) | 73–97% | [128] |
| Demineralization (7% (v/v) HCl), deproteinization (10% (w/w) NaOH), deacetylation (55% (w/v) NaOH) | 4.4–16% | [129] | ||
| Demineralization (1 N HCl), deproteinization (1 N NaOH), deacetylation (40% NaOH, NaBH4) | 70–80% | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, K.u.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.u.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability 2023, 15, 4864. https://doi.org/10.3390/su15064864
Rehman Ku, Hollah C, Wiesotzki K, Heinz V, Aganovic K, Rehman Ru, Petrusan J-I, Zheng L, Zhang J, Sohail S, et al. Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability. 2023; 15(6):4864. https://doi.org/10.3390/su15064864
Chicago/Turabian StyleRehman, Kashif ur, Clemens Hollah, Karin Wiesotzki, Volker Heinz, Kemal Aganovic, Rashid ur Rehman, Janos-Istvan Petrusan, Longyu Zheng, Jibin Zhang, Summar Sohail, and et al. 2023. "Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector" Sustainability 15, no. 6: 4864. https://doi.org/10.3390/su15064864
APA StyleRehman, K. u., Hollah, C., Wiesotzki, K., Heinz, V., Aganovic, K., Rehman, R. u., Petrusan, J.-I., Zheng, L., Zhang, J., Sohail, S., Mansoor, M. K., Rumbos, C. I., Athanassiou, C., & Cai, M. (2023). Insect-Derived Chitin and Chitosan: A Still Unexploited Resource for the Edible Insect Sector. Sustainability, 15(6), 4864. https://doi.org/10.3390/su15064864














