Effect of Modified Illite on Cd Immobilization and Fertility Enhancement of Acidic Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Reagents and Soil Samples
2.2. Preparation of Amendment
2.3. Adsorption Experiments
2.4. Characterization
2.5. Soil Remediation Experiments
2.6. Pot Experiment
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Illite before and after Modification
3.2. Mechanism of Cadmium Stabilization
3.2.1. Adsorption Isotherms of Amended Soil
3.2.2. Characterization of MIT after Adsorption
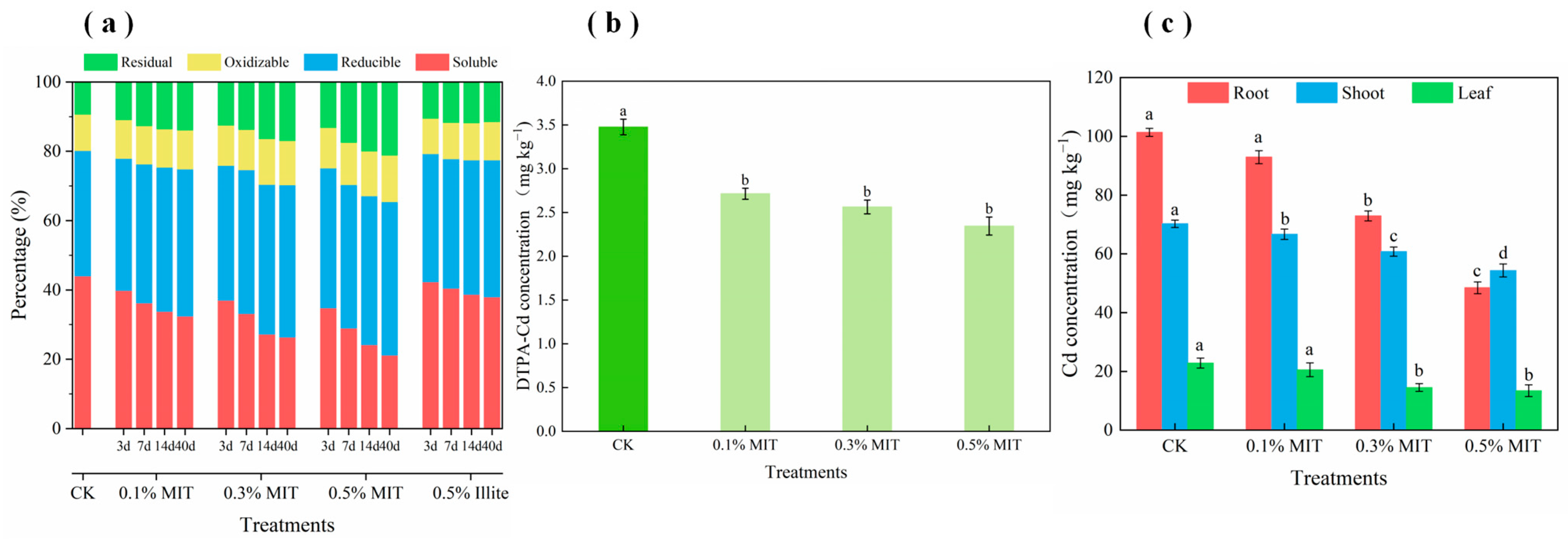
3.2.3. Analysis of Soil Remediation
3.2.4. Stabilization Mechanism
3.3. Enhancement of Soil Fertility
3.3.1. Effect on Plant Growth
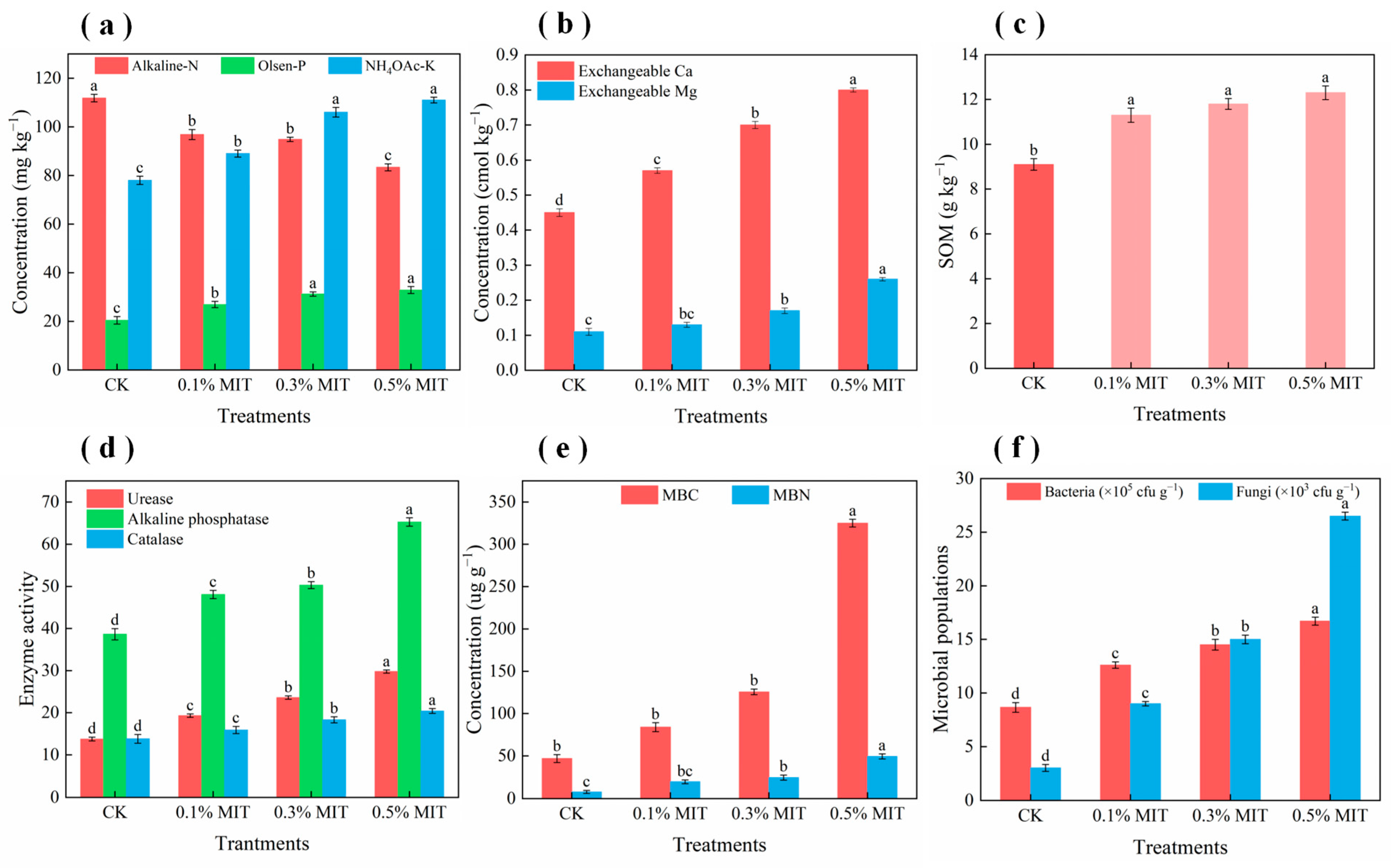
3.3.2. Enrichment of Soil Nutrients
3.3.3. Improvement of Soil Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Hu, L.; Zhou, Y.; Min, X.; She, S.; Chen, S.; Huang, M.; et al. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Yuan, X.H.; Xue, N.D.; Han, Z.G. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.W.; Zia-ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.C.; Cannata, M.G.; Carvalho, R.; Ribeiro Bastos, A.R.; Freitas, M.P.; Augusto, A.d.S. Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: Nutrient contents and translocation. Ecotoxicol. Environ. Saf. 2012, 86, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hediji, H.; Djebali, W.; Belkadhi, A.; Cabasson, C.; Moing, A.; Rolin, D.; Brouquisse, R.; Gallusci, P.; Chaibi, W. Impact of long-term cadmium exposure on mineral content of Solanum lycopersicum plants: Consequences on fruit production. S. Afr. J. Bot. 2015, 97, 176–181. [Google Scholar] [CrossRef]
- Jinadasa, N.; Collins, D.; Holford, P.; Milham, P.J.; Conroy, J.P. Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): Is cadmium tolerance necessarily desirable in food crops? Environ. Sci. Pollut. Res. Int. 2016, 23, 5296–5306. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, Y.; Zeng, G.-M.; Zhang, J.-C.; Chen, Y.-N.; Wang, L.; Zhang, W.-J. Speciation of Cadmium and Changes in Bacterial Communities in Red Soil Following Application of Cadmium-Polluted Compost. Environ. Eng. Sci. 2010, 27, 1019–1026. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Derakhshan Nejad, Z.; Jung, M.C.; Kim, K.H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods—A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef]
- Ahumada, I.; Sepúlveda, K.; Fernández, P.; Ascar, L.; Pedraza, C.; Richter, P.; Brown, S. Effect of biosolid application to Mollisol Chilean soils on the bioavailability of heavy metals (Cu, Cr, Ni, and Zn) as assessed by bioassays with sunflower (Helianthus annuus) and DGT measurements. J. Soils Sediments 2014, 14, 886–896. [Google Scholar] [CrossRef]
- Mohamed, I.; Ahamadou, B.; Li, M.; Gong, C.; Cai, P.; Liang, W.; Huang, Q. Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. J. Soils Sediments 2010, 10, 973–982. [Google Scholar] [CrossRef]
- Nyambaka, H.; Nyaenya, N.; Murungi, J. Use of Low Cost Soil Amendments Reduces Uptake of Cadmium and Lead by Tobacco (Nicotiana tabacum) Grown in Medially Polluted Soils. J. Environ. Hum. 2014, 2014, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Antonious, G.; Dennis, S.O.; Unrine, J.M.; Snyder, J.C. Heavy metals uptake in plant parts of sweet potato grown in soil fertilized with municipal sewage sludge. Int. J. Geol. 2011, 5, 14–20. [Google Scholar]
- Bernal, M.; Clemente, R.; Walker, D.J. The role of organic amendments in the bioremediation of heavy metal-polluted soils. Environ. Res. Lead. Edge 2007, 1–57. [Google Scholar]
- Clemente, R.; Pardo, T.; Madejón, P.; Madejón, E.; Bernal, M.P. Food byproducts as amendments in trace elements contaminated soils. Food Res. Int. 2015, 73, 176–189. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J. Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol. Eng. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Wang, B.; Xie, Z.; Chen, J.; Jiang, J.; Su, Q. Effects of field application of phosphate fertilizers on the availability and uptake of lead, zinc and cadmium by cabbage (Brassica chinensis L.) in a mining tailing contaminated soil. J. Environ. Sci. 2008, 20, 1109–1117. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef]
- Sharma, A.; Nagpal, A.K. Soil amendments: A tool to reduce heavy metal uptake in crops for production of safe food. Rev. Environ. Sci. Bio Technol. 2017, 17, 187–203. [Google Scholar] [CrossRef]
- Yuan, G.D.; Theng, B.K.G.; Churchman, G.J.; Gates, W.P. Chapter 5.1—Clays and Clay Minerals for Pollution Control. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 587–644. [Google Scholar]
- Jiang, J.; Ayaz, T.; Jiang, Z.; Lei, M. Green Remediation of Heavy Metal Polluted Water and Soil Using Clay Minerals: A Review. In Proceedings of the Asia Conference on Geological Research and Environmental Technology (GRET), Kamakura City, Japan, 10–11 October 2020. [Google Scholar]
- Wu, Y.J.; Zhou, H.; Zou, Z.J.; Zhu, W.; Yang, W.T.; Peng, P.Q.; Zeng, M.; Liao, B.H. A three-year in-situ study on the persistence of a combined amendment (limestone+sepiolite) for remedying paddy soil polluted with heavy metals. Ecotoxicol. Environ. Saf. 2016, 130, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Ji, X.; Liu, Y.; Lin, Z.; Lin, Z.; Xiao, S.; Peng, B.; Tan, C.; Zhang, X. Effect of a novel Ca-Si composite mineral on Cd bioavailability, transport and accumulation in paddy soil-rice system. J. Environ. Manag. 2019, 233, 802–811. [Google Scholar] [CrossRef]
- Sedmale, G.; Randers, M.; Rundans, M.; Seglins, V. Application of differently treated illite and illite clay samples for the development of ceramics. Appl. Clay Sci. 2017, 146, 397–403. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Shafi, M.; Sun, Y.; Li, Y.; Chen, Z.; Xiang, Z.; Jin, G.; Zhong, B.; Ye, Z.; et al. Effects of adsorption characteristics of different amendments on heavy metals (Pb, Zn, and Cd). J. Soils Sediments 2020, 20, 2868–2876. [Google Scholar] [CrossRef]
- Ruiz-García, M.; Villalobos, M.; Antelo, J.; Martínez-Villegas, N. Tl(I) adsorption behavior on K-illite and on humic acids. Appl. Geochem. 2022, 138, 105220. [Google Scholar] [CrossRef]
- Siroux, B.; Wissocq, A.; Beaucaire, C.; Latrille, C.; Petcut, C.; Calvaire, J.; Tabarant, M.; Benedetti, M.F.; Reiller, P.E. Adsorption of strontium and caesium onto an Na-illite and Na-illite/Na-smectite mixtures: Implementation and application of a multi-site ion-exchange model. Appl. Geochem. 2018, 99, 65–74. [Google Scholar] [CrossRef]
- Wick, S.; Baeyens, B.; Marques Fernandes, M.; Voegelin, A. Thallium Adsorption onto Illite. Environ. Sci. Technol. 2018, 52, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Silicate minerals—Potential source of potash—A review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Sun, K.; Shi, Y.; Chen, H.; Wang, X.; Li, Z. Extending surfactant-modified 2:1 clay minerals for the uptake and removal of diclofenac from water. J. Hazard. Mater. 2017, 323, 567–574. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, E.; Lee, Y.-S. Direct fluorination as a novel organophilic modification method for the preparation of Illite/polypropylene nanocomposites. J. Mater. Sci. 2011, 47, 1046–1053. [Google Scholar] [CrossRef]
- Mohamed, A.M.G.; Mohamed, M.M.A.; Farrag, A.; Ali, A.R.M. Novel elimination method of iron and manganese ions from drinkable groundwater in Assiut, Egypt, by using sodalite-bearing modified illite. Environ. Sci. Pollut. Res. 2022, 29, 26850–26859. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, Z.; Wang, Y. Mechanochemical preparation of ternary polyethyleneimine modified magnetic illite/smectite nanocomposite for removal of Cr(VI) in aqueous solution. Appl. Clay Sci. 2020, 198, 105832. [Google Scholar] [CrossRef]
- Qu, J.; Dong, M.; Bi, F.; Tao, Y.; Wang, L.; Jiang, Z.; Zhang, G.; Zhang, B.; Zhang, Y. Microwave-assisted one-pot synthesis of β-cyclodextrin modified biochar for stabilization of Cd and Pb in soil. J. Clean. Prod. 2022, 346, 131165. [Google Scholar] [CrossRef]
- Niu, W.; Qiu, X.; Wu, P.; Guan, W.; Zhan, Y.; Jin, L.; Zhu, N. Unrolling the tubes of halloysite to form dickite and its application in heavy metal ions removal. Appl. Clay Sci. 2023, 231, 106748. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Malima, N.M.; Owonubi, S.J.; Lugwisha, E.H.; Mwakaboko, A.S. Thermodynamic, isothermal and kinetic studies of heavy metals adsorption by chemically modified Tanzanian Malangali kaolin clay. Int. J. Environ. Sci. Technol. 2021, 18, 3153–3168. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, D.; Zhang, D.; Yan, C.; Zhang, J. Crystal transformation of calcium silicate minerals synthesized by calcium silicate slag and silica fume with increase of C/S molar ratio. J. Mater. Res. Technol. 2021, 15, 4185–4192. [Google Scholar] [CrossRef]
- Zhen, R.; Chi, Q.; Wang, X.; Yang, K.; Jiang, Y.; Li, F.; Xue, B. Crystallinity, ion conductivity, and thermal and mechanical properties of poly(ethylene oxide)–illite nanocomposites with exfoliated illite as a filler. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jiang, T.; Li, G.; Qiu, G.; Fan, X.; Huang, Z. Thermal activation and alkali dissolution of silicon from illite. Appl. Clay Sci. 2008, 40, 81–89. [Google Scholar] [CrossRef]
- Tavangarian, F.; Zolko, C.A.; Fahami, A.; Forghani, A.; Hayes, D. Facile synthesis and structural insight of nanostructure akermanite powder. Ceram. Int. 2019, 45, 7871–7877. [Google Scholar] [CrossRef]
- Ptáček, P.; Opravil, T.; Šoukal, F.; Havlica, J.; Holešinský, R. Kinetics and mechanism of formation of gehlenite, Al–Si spinel and anorthite from the mixture of kaolinite and calcite. Solid State Sci. 2013, 26, 53–58. [Google Scholar] [CrossRef]
- Han, J.; Xu, Y.; Liang, X.; Xu, Y. Sorption Stability and Mechanism Exploration of Palygorskite as Immobilization Agent for Cd in Polluted Soil. Water Air Soil Pollut. 2014, 225, 2160. [Google Scholar] [CrossRef]
- Escamilla-Roa, E.; Nieto, F.; Sainz-Díaz, C.I. Stability of the Hydronium Cation in the Structure of Illite. Clays Clay Miner. 2016, 64, 413–424. [Google Scholar] [CrossRef]
- Harrison, J.L.; Murray, H.H. Clay Mineral Stability and Formation During Weathering. Clays Clay Miner. 1957, 6, 144–153. [Google Scholar] [CrossRef]
- Zviagina, B.B.; Drits, V.A.; Dorzhieva, O.V. Distinguishing Features and Identification Criteria for K-Dioctahedral 1M Micas (Illite-Aluminoceladonite and Illite-Glauconite-Celadonite Series) from Middle-Infrared Spectroscopy Data. Minerals 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Yuan, X.; Xiong, T.; Tan, Y.Z.; Wang, H. Physicochemical properties, metal availability and bacterial community structure in heavy metal-polluted soil remediated by montmorillonite-based amendments. Chemosphere 2020, 261, 128010. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Dai, C.; Huang, L.; Li, W.; Chen, W. Self-assembly of cadmium metasilicate nanowires as a broadband optical limiter. Opt. Mater. 2016, 54, 50–56. [Google Scholar] [CrossRef]
- Tonk, S.; Aradi, L.E.; Kovács, G.; Turza, A.; Rápó, E. Effectiveness and Characterization of Novel Mineral Clay in Cd2+ Adsorption Process: Linear and Non-Linear Isotherm Regression Analysis. Water 2022, 14, 279. [Google Scholar] [CrossRef]
- Ali, A.M.; Padmanabhan, E.; Mijinyawa, A.; Kwaya, M.Y. Effect of pH on the stability of quartz in a multi-phase system of kaolinite, hydrous Al (hydr)oxide and quartz. SN Appl. Sci. 2019, 1, 388. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.-J.; Chiu, Y.-L.; Chen, H.-L.; Lai, H.-Y. Effects of Sludge and pH Adjustment on Cd Speciation in Soil and Growth and Cd Accumulation in Pak Choi. Soil Sediment Contam. Int. J. 2012, 21, 510–524. [Google Scholar] [CrossRef]
- Lu, H.-L.; Li, K.-W.; Nkoh, J.N.; Shi, Y.-X.-X.; He, X.; Hong, Z.-N.; Xu, R.-K. Effects of the increases in soil pH and pH buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere 2022, 301, 134674. [Google Scholar] [CrossRef]
- Wang, N. Advances in research on repairing heavy metal pollution in soil by clay minerals. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 42017. [Google Scholar]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105, 200–206. [Google Scholar] [CrossRef]
- Shah, K.J.; Pan, S.-Y.; Shukla, A.D.; Shah, D.O.; Chiang, P.-C. Mechanism of organic pollutants sorption from aqueous solution by cationic tunable organoclays. J. Colloid Interface Sci. 2018, 529, 90–99. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Vivanco, J.M.; Manter, D.K. Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl. Soil Ecol. 2016, 107, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Spohn, M. Increasing the organic carbon stocks in mineral soils sequesters large amounts of phosphorus. Glob. Chang. Biol. 2020, 26, 4169–4177. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I.I. Potassium and Potassium-Permeable Channels in Plant Salt Tolerance. In Ion Channels and Plant Stress Responses; Demidchik, V., Maathuis, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 87–110. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Wang, Z.; Ul Hassan, M.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium Fertilization Improves Crop Yield in Most Production Systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.H.; Islam, S.; Mohammad, F. Sulphur as a dynamic mineral element for plants: A review. J. Soil Sci. Plant Nutr. 2022, 22, 2118–2143. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculik, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Kopittke, P.M.; Dalal, R.C.; Hoeschen, C.; Li, C.; Menzies, N.W.; Mueller, C.W. Soil organic matter is stabilized by organo-mineral associations through two key processes: The role of the carbon to nitrogen ratio. Geoderma 2020, 357, 113974. [Google Scholar] [CrossRef]
- Churchman, G.J.; Singh, M.; Schapel, A.; Sarkar, B.; Bolan, N. Clay Minerals as the Key to the Sequestration of Carbon in Soils. Clays Clay Miner. 2020, 68, 135–143. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.Y.; Shao, H.B.; Shao, M.A. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Zhang, S.; Xing, Y.; Wang, R.; Liang, W. Organic amendment effects on aggregate-associated organic C, microbial biomass C and glomalin in agricultural soils. Catena 2014, 123, 188–194. [Google Scholar] [CrossRef]
- Nuzzo, A.; Satpute, A.; Albrecht, U.; Strauss, S.L. Impact of Soil Microbial Amendments on Tomato Rhizosphere Microbiome and Plant Growth in Field Soil. Microb. Ecol. 2020, 80, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Nunan, N.; Hirsch, P.R.; Sun, B.; Zhou, J.; Liang, Y. Theory of microbial coexistence in promoting soil–plant ecosystem health. Biol. Fertil. Soils 2021, 57, 897–911. [Google Scholar] [CrossRef]
- Wan, P.; He, R.; Wang, P.; Cao, A. Implementation of different forest management methods in a natural forest: Changes in soil microbial biomass and enzyme activities. For. Ecol. Manag. 2022, 520, 120409. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H.; Whalen, J.K. Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Agric. Ecosyst. Environ. 2016, 232, 199–207. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Contreras-Cornejo, H.A.; Macias-Rodriguez, L.; Lopez-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Herrera Paredes, S.; Lebeis, S.L.; Bailey, J.K. Giving back to the community: Microbial mechanisms of plant–soil interactions. Funct. Ecol. 2016, 30, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214 Pt 1, 113821. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, T.; Peng, O.; Chen, A.; Tie, B.; Shao, J. Responses of microbial community and soil enzyme to heavy metal passivators in cadmium contaminated paddy soils: An in situ field experiment. Int. Biodeterior. Biodegrad. 2021, 164, 105292. [Google Scholar] [CrossRef]
- Yu, Y.; Li, L.; Yu, L.; Lin, B.; Chen, X.; Li, H.; Han, Q.; Ge, Q.; Li, H. Effect of exposure to decabromodiphenyl ether and tetrabromobisphenol A in combination with lead and cadmium on soil enzyme activity. Int. Biodeterior. Biodegrad. 2017, 117, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Aponte, H.; Medina, J.; Butler, B.; Meier, S.; Cornejo, P.; Kuzyakov, Y. Soil quality indices for metal(loid) contamination: An enzymatic perspective. Land Degrad. Dev. 2020, 31, 2700–2719. [Google Scholar] [CrossRef]

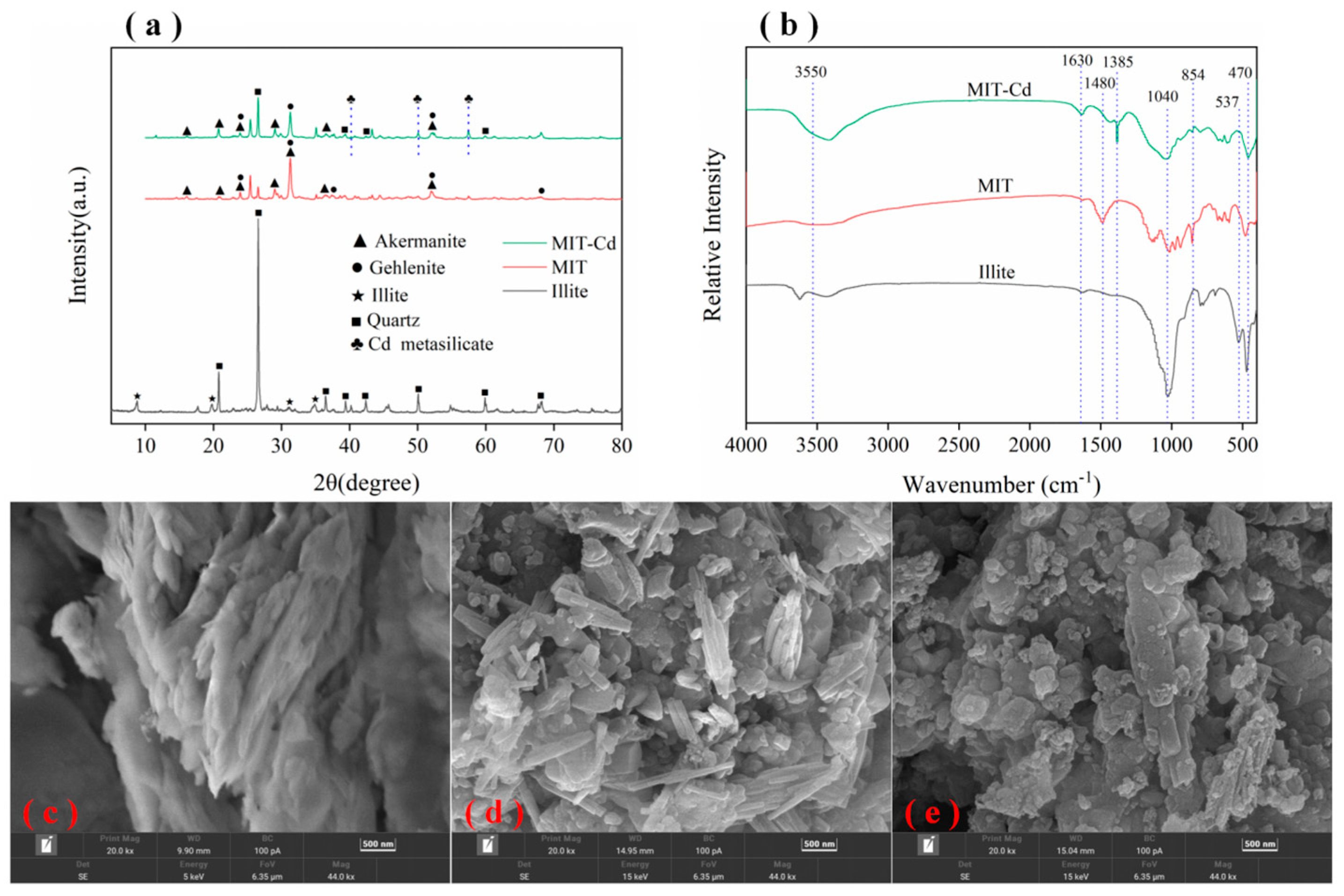



| Parameter | Illite | MIT |
|---|---|---|
| pH | 6.8 | 11.2 |
| CEC | 23.11 | 98.37 |
| BET surface area (m2/g) | 10.32 | 37.49 |
| Adsorption average pore diameter (nm) | 6.51 | 10.81 |
| Single point adsorption total pore volume (cm3/g) | 0.017 | 0.101 |
| Treatments | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qe (mg g−1) | KL (L mg−1) | R2 | 1/n | KF (L mg−1) | R2 | |
| CK | 3.66 | 0.347 | 0.94 | 0.532 | 1.30 | 0.97 |
| 0.1% MIT | 3.87 | 0.283 | 0.93 | 0.588 | 1.27 | 0.98 |
| 0.3% MIT | 4.05 | 0.348 | 0.95 | 0.564 | 1.42 | 0.98 |
| 0.5% MIT | 4.30 | 0.412 | 0.95 | 0.545 | 1.62 | 0.98 |
| 0.5% Illite | 3.84 | 0.252 | 0.94 | 0.603 | 1.21 | 0.96 |
| Dosage | Fresh Biomass (g plant−1) | Size (cm plant−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Leaves | Root | Shoot | Leaves | |||
| Length | Diameter | Length | Width | |||||
| CK | 0.24 c | 3.03 c | 1.29 c | 5.53 d | 59.13 b | 0.36 d | 6.58 d | 1.70 c |
| 0.1% | 0.58 bc | 4.81 b | 2.20 b | 9.45 c | 63.73 b | 0.46 c | 8.16 c | 2.21 b |
| 0.3% | 0.87 b | 6.37 a | 3.30 a | 11.76 b | 74.32 a | 0.51 b | 9.03 b | 2.54 ab |
| 0.5% | 1.91 a | 6.59 a | 3.54 a | 17.59 a | 74.37 a | 0.56 a | 10.15 a | 2.58 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Shi, L.; Chen, R.; Yuan, J. Effect of Modified Illite on Cd Immobilization and Fertility Enhancement of Acidic Soils. Sustainability 2023, 15, 4950. https://doi.org/10.3390/su15064950
Huang H, Shi L, Chen R, Yuan J. Effect of Modified Illite on Cd Immobilization and Fertility Enhancement of Acidic Soils. Sustainability. 2023; 15(6):4950. https://doi.org/10.3390/su15064950
Chicago/Turabian StyleHuang, Haoyong, Lin Shi, Rui Chen, and Jie Yuan. 2023. "Effect of Modified Illite on Cd Immobilization and Fertility Enhancement of Acidic Soils" Sustainability 15, no. 6: 4950. https://doi.org/10.3390/su15064950







