Short-Term Effects of Tunnel Construction on Soil Organic Carbon and Enzyme Activity in Shrublands in Eastern Tibet Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Soil Sampling
2.3. Laboratory Analyses
2.4. Calculation of Soil Carbon Pool Management Index
2.5. Statistical Analysis
3. Results
3.1. Effects of Tunnel Construction on SOC and Its Fractions
3.2. Effects of Tunnel Construction on Carbon Pool Management Index
3.3. Effect of Tunnel Construction on Soil Enzyme Activity
3.4. Correlations between SOC Fraction and Soil Enzyme Activity
4. Discussion
4.1. SOC Changes
4.2. Change in SOC Fractions Content
4.3. Changes in Soil Enzyme Activities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; Wu, C.; Liu, J.; Jiang, Y. Increasing leaf δ13C values of woody plants in response to water stress induced by tunnel excavation in a karst trough valley: Implication for improving water-use efficiency. J. Hydrol. 2020, 586, 124895. [Google Scholar] [CrossRef]
- Ye, F.; He, C.; Wang, S.-m.; Zhang, J.-l. Landscape design of mountain highway tunnel portals in China. Tunn. Undergr. Space Technol. 2012, 29, 52–68. [Google Scholar] [CrossRef]
- Liu, J.C.; Shen, L.C.; Wang, Z.X.; Duan, S.H.; Wu, W.; Peng, X.Y.; Wu, C.; Jiang, Y.J. Response of plants water uptake patterns to tunnels excavation based on stable isotopes in a karst trough valley. J. Hydrol. 2019, 571, 485–493. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Mu, S.; Ma, Z.; Li, Q.; Li, L. Vertical distributions of soil carbon and nitrogen fractions as affected by land-uses in the Ili River Valley. Chem. Ecol. 2016, 33, 143–155. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Chen, Y.; Liu, G.; Xue, S. Responses of soil enzyme activity and soil organic carbon stability over time after cropland abandonment in different vegetation zones of the Loess Plateau of China. Catena 2021, 196, 104812. [Google Scholar] [CrossRef]
- Pandey, C.B.; Chaudhari, S.K.; Dagar, J.C.; Singh, G.B.; Singh, R.K. Soil N mineralization and microbial biomass carbon affected by different tillage levels in a hot humid tropic. Soil Tillage Res. 2010, 110, 33–41. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Li, L.; Cheng, K.; Zheng, J.; Zhang, X.; Zheng, J.; Joseph, S.; Pan, G. Long-term rice cultivation stabilizes soil organic carbon and promotes soil microbial activity in a salt marsh derived soil chronosequence. Sci. Rep. 2015, 5, 15704. [Google Scholar] [CrossRef] [Green Version]
- Lalnunzira, C.; Tripathi, S.K. Dynamics of Litter Carbon and Nitrogen in Forest Fallows following Shifting Cultivation in Mizoram, Northeast India. Int. J. Plant Environ. 2018, 4, 55–63. [Google Scholar] [CrossRef]
- Hauchhum, R.; Tripathi, S.K. Carbon and nitrogen differences in rhizosphere soil of annual plants in abandoned lands following shifting agriculture in northeast India. Nutr. Cycl. Agroecosyst. 2019, 113, 157–166. [Google Scholar] [CrossRef]
- Manpoong, C.; De Mandal, S.; Bangaruswamy, D.K.; Perumal, R.C.; Benny, J.; Beena, P.S.; Ghosh, A.; Kumar, N.S.; Tripathi, S.K. Linking rhizosphere soil biochemical and microbial community characteristics across different land use systems in mountainous region in Northeast India. Meta Gene 2020, 23, 100625. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-water extractable carbon in soils: A sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 0065–2113. [Google Scholar] [CrossRef]
- Wen, L.; Li, D.; Chen, H.; Wang, K. Dynamics of soil organic carbon in density fractions during post-agricultural succession over two lithology types, southwest China. J. Environ. Manag. 2017, 201, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.; Sun, Q.; Cao, D.; Sun, Y.; Chen, W. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soils Sediments 2017, 18, 1569–1578. [Google Scholar] [CrossRef]
- Hauchhum, R.; Tripathi, S.K. Impact of Rhizosphere Microbes of Three Early Colonizing Annual Plants on Improving Soil Fertility During Vegetation Establishment Under Different Fallow Periods Following Shifting Cultivation. Agric. Res. 2019, 9, 213–221. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of Biochar Application on Soil Organic Carbon Composition and Enzyme Activity in Paddy Soil under Water-Saving Irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Liu, S.; Han, K.; Guan, S.; Zhou, D. Conversion of cropland to forage land and grassland increases soil labile carbon and enzyme activities in northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Barbeta, A.; Peñuelas, J. Sequence of plant responses to droughts of different timescales: Lessons from holm oak (Quercus ilex) forests. Plant Ecol. Divers. 2016, 9, 321–338. [Google Scholar] [CrossRef] [Green Version]
- Hasselquist, N.J.; Allen, M.F.; Santiago, L.S. Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 2010, 164, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Olano, J.M.; Linares, J.C.; Garcia-Cervigon, A.I.; Arzac, A.; Delgado, A.; Rozas, V. Drought-induced increase in water-use efficiency reduces secondary tree growth and tracheid wall thickness in a Mediterranean conifer. Oecologia 2014, 176, 273–283. [Google Scholar] [CrossRef]
- Lalnunzira, C.; Tripathi, S.K. Leaf and root production, decomposition and carbon and nitrogen fluxes during stand development in tropical moist forests, north-east India. Soil Res. 2018, 56, 306–317. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, Y.; Hu, W.; Cao, M.; Mao, Y. A review of the effects of tunnel excavation on the hydrology, ecology, and environment in karst areas: Current status, challenges, and perspectives. J. Hydrol. 2020, 586, 124891. [Google Scholar] [CrossRef]
- Nosrati, K.; Jalali, S. The Effect of Forest Road Construction on Soil Organic Carbon Stock in Mountainous Catchment in Northern Iran. Int. J. Agric. Manag. Dev. 2016, 6, 211–215. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, X.; Tang, Y.; Liu, H.; Wang, M.; Zhang, L. Use of tree rings as indicator for groundwater level drawdown caused by tunnel excavation in Zhongliang Mountains, Chongqing, Southwest China. Environ. Earth Sci. 2017, 76, 1–14. [Google Scholar] [CrossRef]
- Deng, Z.; Chong, Y.; Zhang, D.; Ren, C.; Zhao, F.; Zhang, X.; Han, X.; Yan, G. Temporal Variations in Soil Enzyme Activities and Responses to Land-Use Change in the Loess Plateau, China. Appl. Sci. 2019, 9, 3129. [Google Scholar] [CrossRef] [Green Version]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.-h.; Min, H.; Lü, Z.-h.; Yuan, H.-p. Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2006, 42, 120–126. [Google Scholar] [CrossRef]
- Dilly, O.; Nannipieri, P. Response of ATP content, respiration rate and enzyme activities in an arable and a forest soil to nutrient additions. Biol. Fertil. Soils 2001, 34, 64–72. [Google Scholar] [CrossRef]
- Adamczyk, B.; Kilpeläinen, P.; Kitunen, V.; Smolander, A. Potential activities of enzymes involved in N, C, P and S cycling in boreal forest soil under different tree species. Pedobiologia 2014, 57, 97–102. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2013, 117, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Dell, E.; Shi, W. Chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Raiesi, F. Soil properties and C dynamics in abandoned and cultivated farmlands in a semi-arid ecosystem. Plant Soil 2012, 351, 161–175. [Google Scholar] [CrossRef]
- Shang, Z.-H.; Cao, J.-J.; Guo, R.-Y.; Long, R.-J.; Deng, B. The response of soil organic carbon and nitrogen 10 years after returning cultivated alpine steppe to grassland by abandonment or reseeding. Catena 2014, 119, 28–35. [Google Scholar] [CrossRef]
- Wang, J.N.; Jiang, Y.J.; He, Q.F.; Fan, J.X.; He, R.L.; Wu, C. Response of soil microbial community in grassland to tunnel construction in the karst trough valley, Zhongliang Mountain, Chongqing. Acta Ecol. Sin. 2019, 39, 6136–6145. [Google Scholar] [CrossRef]
- Ding, J.; Chen, L.; Ji, C.; Hugelius, G.; Li, Y.; Liu, L.; Qin, S.; Zhang, B.; Yang, G.; Li, F.; et al. Decadal soil carbon accumulation across Tibetan permafrost regions. Nat. Geosci. 2017, 10, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Fang, J.; Tang, Y.; Ji, C.; Zheng, C.; He, J.; Zhu, B. Storage, patterns and controls of soil organic carbon in the Tibetan grasslands. Glob. Chang. Biol. 2008, 14, 1592–1599. [Google Scholar] [CrossRef]
- Kato, T.; Hirota, M.; Tang, Y.; Wada, E. Spatial variability of CH4 and N2O fluxes in alpine ecosystems on the Qinghai–Tibetan Plateau. Atmos. Environ. 2011, 45, 5632–5639. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Ma, W.; Li, G.; Jiang, Z.; Wu, J.; Chen, G. Response of soil organic carbon to vegetation degradation along a moisture gradient in a wet meadow on the Qinghai-Tibet Plateau. Ecol. Evol. 2018, 8, 11999–12010. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; p. 22162. [Google Scholar]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.B.; Lisle, L. Soil Carbon Fractions Based on their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Guan, S.Y.; Zhang, D.S.; Zhang, Z.M. Soil Enzymes and Research Methods; China Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Gao, Y.Z.; Giese, M.; Lin, S.; Sattelmacher, B.; Zhao, Y.; Brueck, H. Belowground net primary productivity and biomass allocation of a grassland in Inner Mongolia is affected by grazing intensity. Plant Soil 2008, 307, 41–50. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, J.; Li, Y.; Liu, S.; Dong, Q.; Zhou, H.; Yeomans, J.; Li, Y.; Li, S.; Gao, X. Effect of grassland degradation on aggregate-associated soil organic carbon of alpine grassland ecosystems in Qinghai-Tibetan Plateau. Eur. J. Soil Sci. 2019, 71, 69–79. [Google Scholar] [CrossRef]
- Lalnunzira, C.; Brearley, F.Q.; Tripathi, S.K. Root growth dynamics during recovery of tropical mountain forest in North-east India. J. Mt. Sci. 2019, 16, 2335–2347. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.; Zhao, Y.; Chen, H.; Chen, J.; Dyck, M.; Wang, X.; Zhang, J.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. Int. 2017, 24, 10108–10120. [Google Scholar] [CrossRef]
- Sade, N.; Gebremedhin, A.; Moshelion, M. Risk-taking plants: Anisohydric behavior as a stress-resistance trait. Plant. Signal. Behav. 2012, 7, 767–770. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Liu, G.; Li, P.; Xue, S. Effects of short-term elevated CO2 concentration and drought stress on the rhizosphere effects of soil carbon, nitrogen and microbes of Bothriochloa ischaemum. Chin. J. Appl. Ecol. 2017, 28, 3251–3259. [Google Scholar] [CrossRef]
- Emmerling, C.; Schloter, M.; Hartmann, A.; Kandeler, E. Functional diversity of soil organisms-a review of recent research activities in Germany. Soil Sci. 2002, 165, 408–420. [Google Scholar] [CrossRef]
- Ma, W.; Li, G.; Wu, J.; Xu, G.; Wu, J. Response of soil labile organic carbon fractions and carbon-cycle enzyme activities to vegetation degradation in a wet meadow on the Qinghai–Tibet Plateau. Geoderma 2020, 377, 114565. [Google Scholar] [CrossRef]
- Jiang, J.P.; Xiong, Y.C.; Jia, Y.; Li, F.M.; Xu, J.Z.; Jiang, H.M. Soil Quality Dynamics Under Successional Alfalfa Field in the Semi-arid Loess Plateau of Northwestern China. Arid. Land Res. Manag. 2007, 21, 287–303. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Zhao, L.; Ma, Y.; Tong, X.; Deng, J.; Ren, C.; Han, X.; Yang, G. Dynamics of storage and relative availability of soil inorganic nitrogen along revegetation chronosequence in the loess hilly region of China. Soil Tillage Res. 2019, 187, 11–20. [Google Scholar] [CrossRef]
- Patra, A.K.; Abbadie, L.; Clays-Josserand, A.; Degrange, V.; Grayston, S.J.; Guillaumaud, N.; Loiseau, P.; Louault, F.; Mahmood, S.; Nazaret, S.; et al. Effects of management regime and plant species on the enzyme activity and genetic structure of N-fixing, denitrifying and nitrifying bacterial communities in grassland soils. Environ. Microbiol. 2006, 8, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pang, X.; Li, N.; Qi, K.; Huang, J.; Yin, C. Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Collins, S.L.; Sinsabaugh, R.L.; Crenshaw, C.; Green, L.; Porras-Alfaro, A.; Stursova, M.; Zeglin, L.H. Pulse dynamics and microbial processes in aridland ecosystems. J. Ecol. 2008, 96, 413–420. [Google Scholar] [CrossRef]
- An, S.-s.; Huang, Y.-m.; Zheng, F.-l. Evaluation of soil microbial indices along a revegetation chronosequence in grassland soils on the Loess Plateau, Northwest China. Appl. Soil Ecol. 2009, 41, 286–292. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Goncalves, A.P.; Baiao, N.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Assessment of chemical, biochemical and ecotoxicological aspects in a mine soil amended with sludge of either urban or industrial origin. Chemosphere 2008, 72, 1774–1781. [Google Scholar] [CrossRef] [Green Version]
- Dodor, D.E.; Tabatabai, M.A. Effect of cropping systems on phosphatases in soils. J. Plant Nutr. Soil Sci. 2003, 166, 7–13. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Wang, H. The effect of land management on plant community composition, species diversity, and productivity of alpine Kobersia steppe meadow. Ecol. Res. 2006, 21, 181–187. [Google Scholar] [CrossRef]
- Fungo, B.; Lehmann, J.; Kalbitz, K.; Tenywa, M.; Thiongo, M.; Neufeldt, H. Emissions intensity and carbon stocks of a tropical Ultisol after amendment with Tithonia green manure, urea and biochar. Field Crops Res. 2017, 209, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils-A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, W.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci. Total Environ. 2018, 610, 750–758. [Google Scholar] [CrossRef] [PubMed]

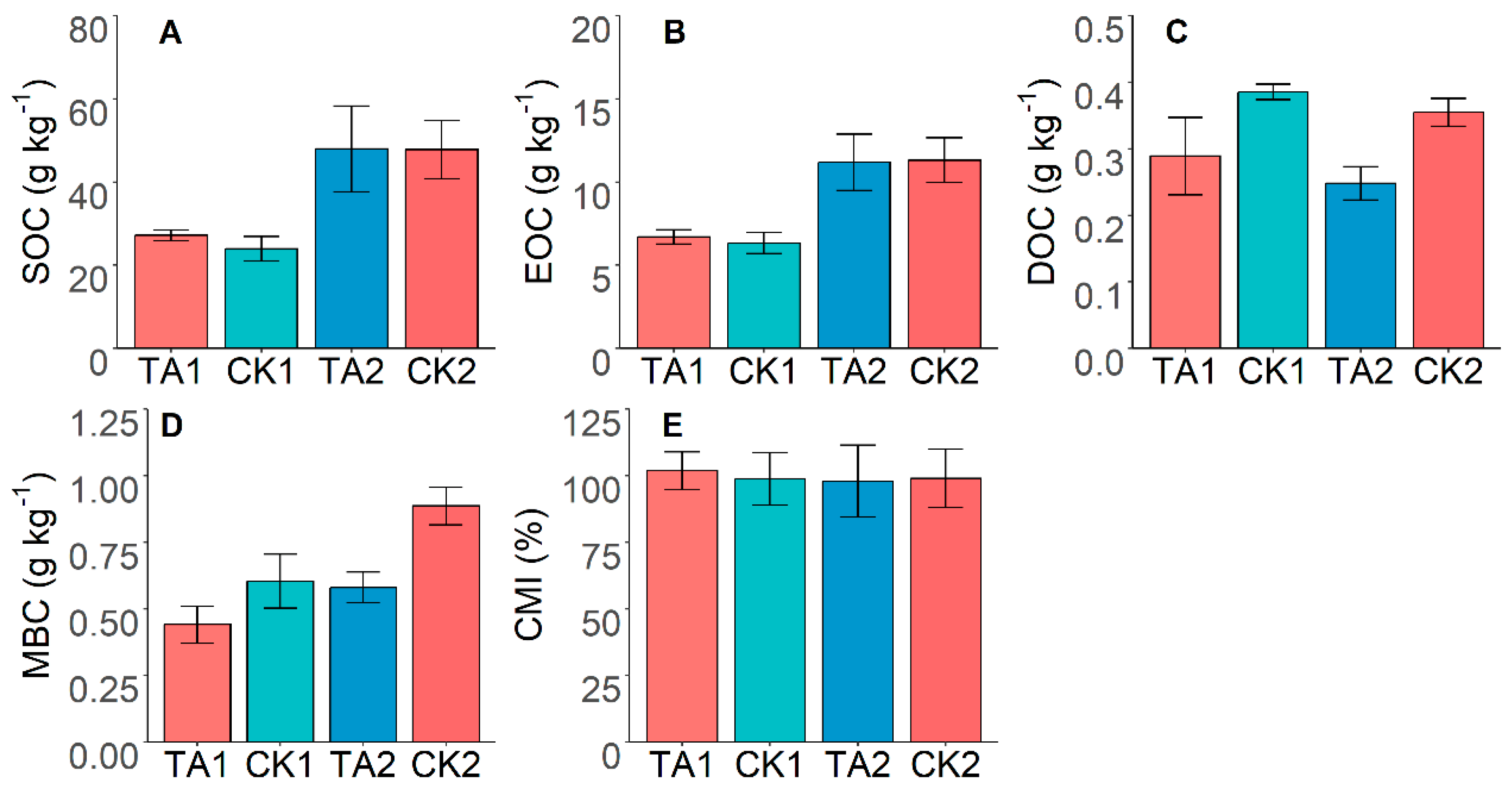
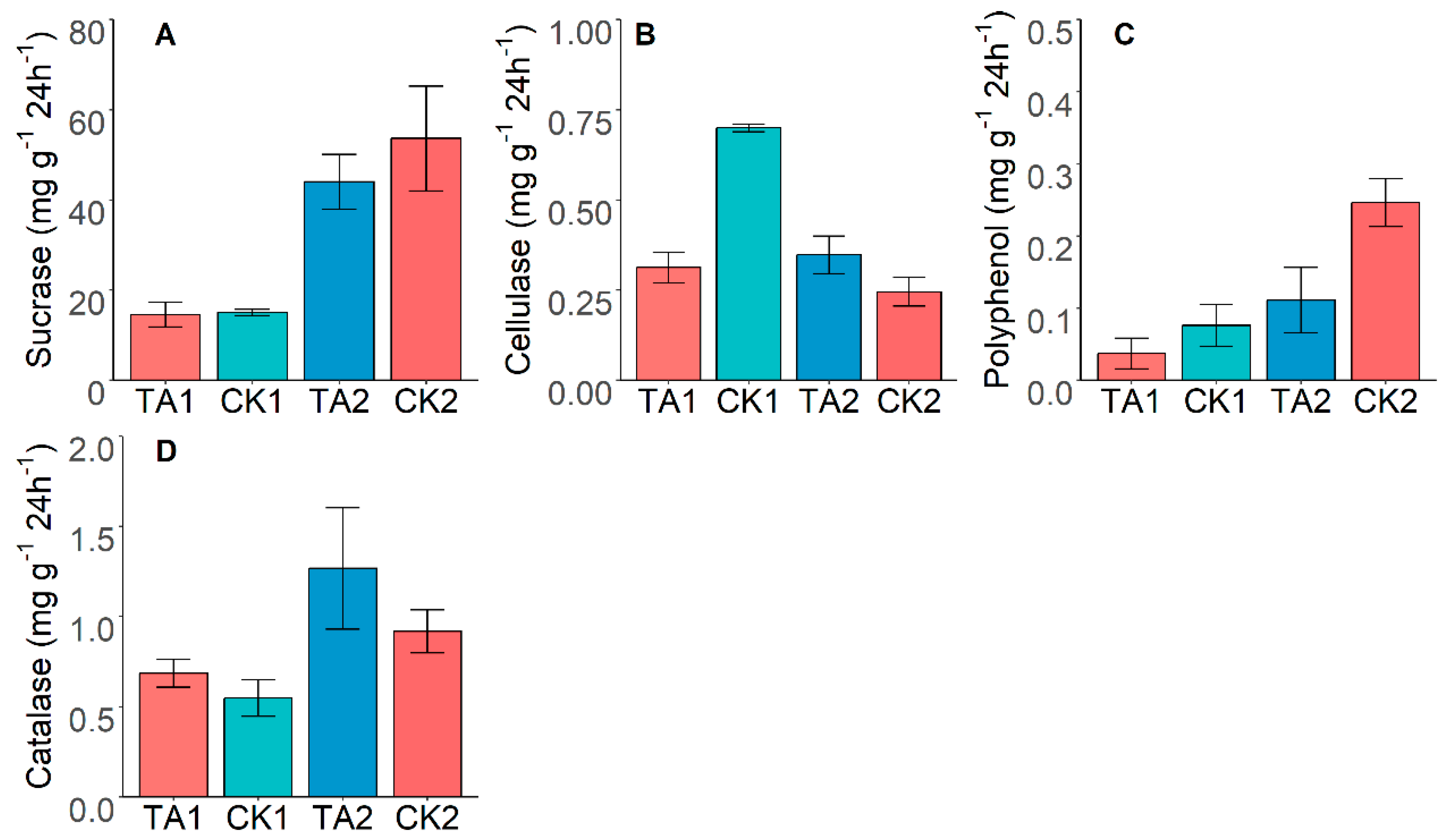
| Variables | pH | SWC (%) | TN (g kg−1) | AGB (kg m−2) | SBD (g cm−3) |
|---|---|---|---|---|---|
| TA1 | 4.95 ± 0.07 a | 0.28 ± 0.01 a | 1.14 ± 0.02 a | 1.24 ± 0.06 a | 0.83 ± 0.04 a |
| CK1 | 4.68 ± 0.07 a | 0.23 ± 0.03 a | 1.13 ± 0.29 a | 1.07 ± 0.14 a | 0.91 ± 0.05 a |
| TA2 | 4.55 ± 0.10 a | 0.31 ± 0.02 a | 2.02 ± 0.08 a | 0.57 ± 0.04 a | 0.72 ± 0.04 a |
| CK2 | 4.33 ± 0.06 a | 0.33 ± 0.02 a | 2.22 ± 0.25 a | 0.49 ± 0.05 a | 0.67 ± 0.05 a |
| Variables | SOC | EOC | DOC | MBC | CMI | SC | CL | PPO | CAT |
|---|---|---|---|---|---|---|---|---|---|
| TC | 0.796 | 0.883 | 0.059 | 0.016 | 0.889 | 0.471 | 0.007 | 0.079 | 0.236 |
| VT | 0.009 | 0.004 | 0.151 | 0.025 | 0.912 | <0.001 | <0.001 | 0.006 | 0.037 |
| TC × VT | 0.820 | 0.865 | 0.660 | 0.380 | 0.890 | 0.514 | <0.001 | 0.184 | 0.594 |
| Variables | SOC | EOC | DOC | MBC | CMI | SC | CL | PPO | CAT | SWC |
|---|---|---|---|---|---|---|---|---|---|---|
| EOC | 0.53 | |||||||||
| DOC | 0.54 | 0.98 ** | ||||||||
| MBC | −0.48 | −0.53 | −0.55 | |||||||
| CMI | −0.10 | 0.40 | 0.42 | 0.01 | ||||||
| SC | −0.12 | −0.08 | −0.02 | −0.39 | −0.24 | |||||
| CL | 0.49 | 0.70 * | 0.72 ** | −0.37 | 0.69 * | −0.15 | ||||
| PPO | −0.13 | −0.42 | −0.42 | 0.53 | −0.23 | 0.01 | −0.47 | |||
| CAT | 0.10 | 0.75 ** | 0.72 ** | −0.07 | 0.77 ** | −0.17 | 0.77 ** | −0.27 | ||
| SWC | 0.71 ** | 0.78 ** | 0.71 ** | −0.48 | 0.19 | −0.20 | 0.59 * | −0.40 | 0.39 | |
| AGB | 0.47 | 0.85 ** | 0.80 ** | −0.54 | 0.33 | −0.18 | 0.65 * | −0.53 | 0.66 * | 0.67 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xiao, Y.; Luo, X.; Ye, C.; Chen, Y.; Xiang, J.; Lei, N.; Song, C.; Pei, X.; Tang, X. Short-Term Effects of Tunnel Construction on Soil Organic Carbon and Enzyme Activity in Shrublands in Eastern Tibet Plateau. Sustainability 2023, 15, 5107. https://doi.org/10.3390/su15065107
Wang X, Xiao Y, Luo X, Ye C, Chen Y, Xiang J, Lei N, Song C, Pei X, Tang X. Short-Term Effects of Tunnel Construction on Soil Organic Carbon and Enzyme Activity in Shrublands in Eastern Tibet Plateau. Sustainability. 2023; 15(6):5107. https://doi.org/10.3390/su15065107
Chicago/Turabian StyleWang, Xiaodong, Yang Xiao, Xinrui Luo, Chenyu Ye, Yuzhuo Chen, Jincheng Xiang, Ningfei Lei, Ci Song, Xiangjun Pei, and Xiaolu Tang. 2023. "Short-Term Effects of Tunnel Construction on Soil Organic Carbon and Enzyme Activity in Shrublands in Eastern Tibet Plateau" Sustainability 15, no. 6: 5107. https://doi.org/10.3390/su15065107





