The Combination of Anaerobic Digestion and Electro-Oxidation for Efficient COD Removal in Beverage Wastewater: Investigation of Electrolytic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characteristics of Beverage Wastewater
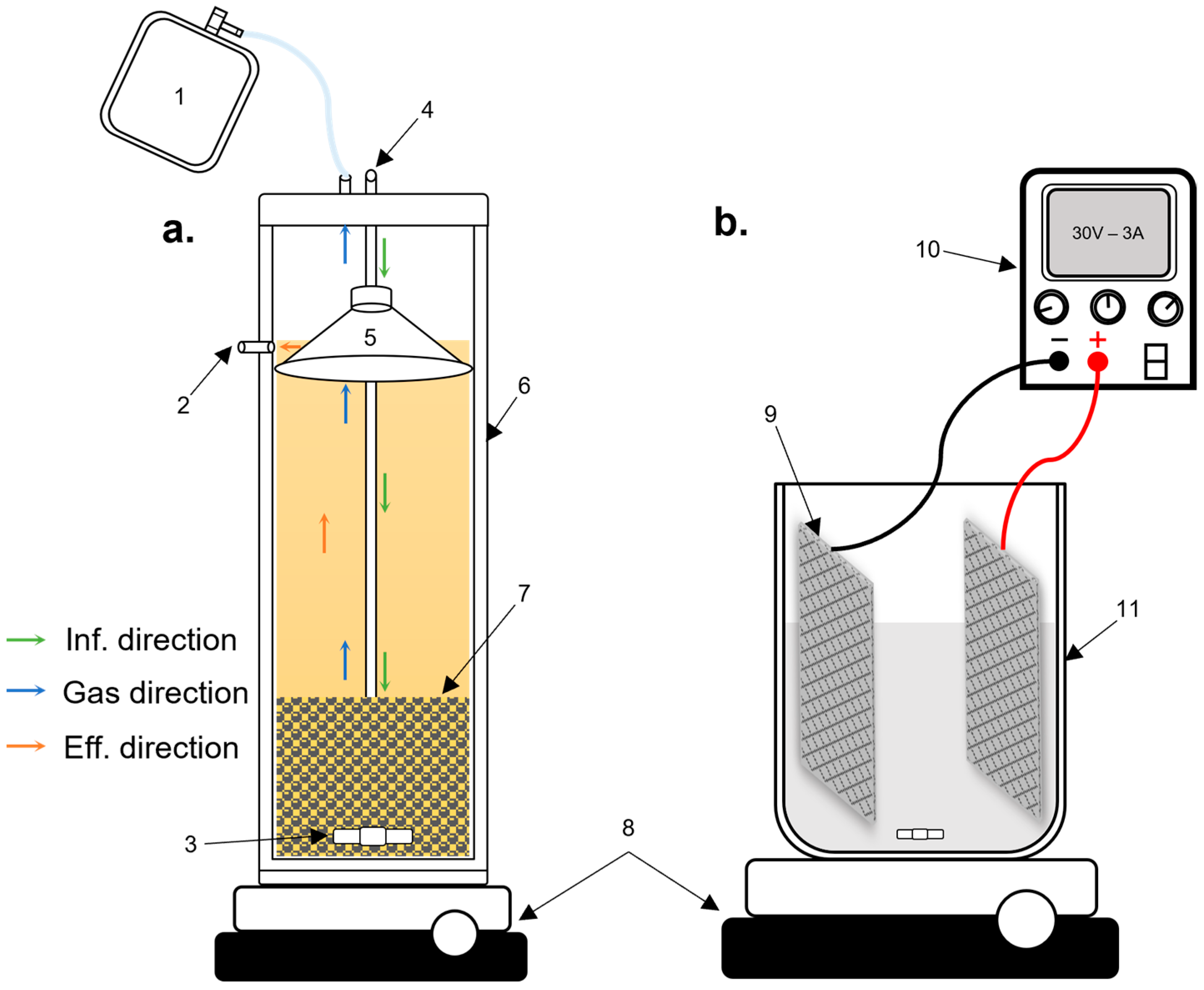
2.3. Experimental Design
2.3.1. Stage 1: USAB Digester
2.3.2. Stage 2: Electrolytic Cell
2.4. Analytical Procedure
3. Results and Discussion
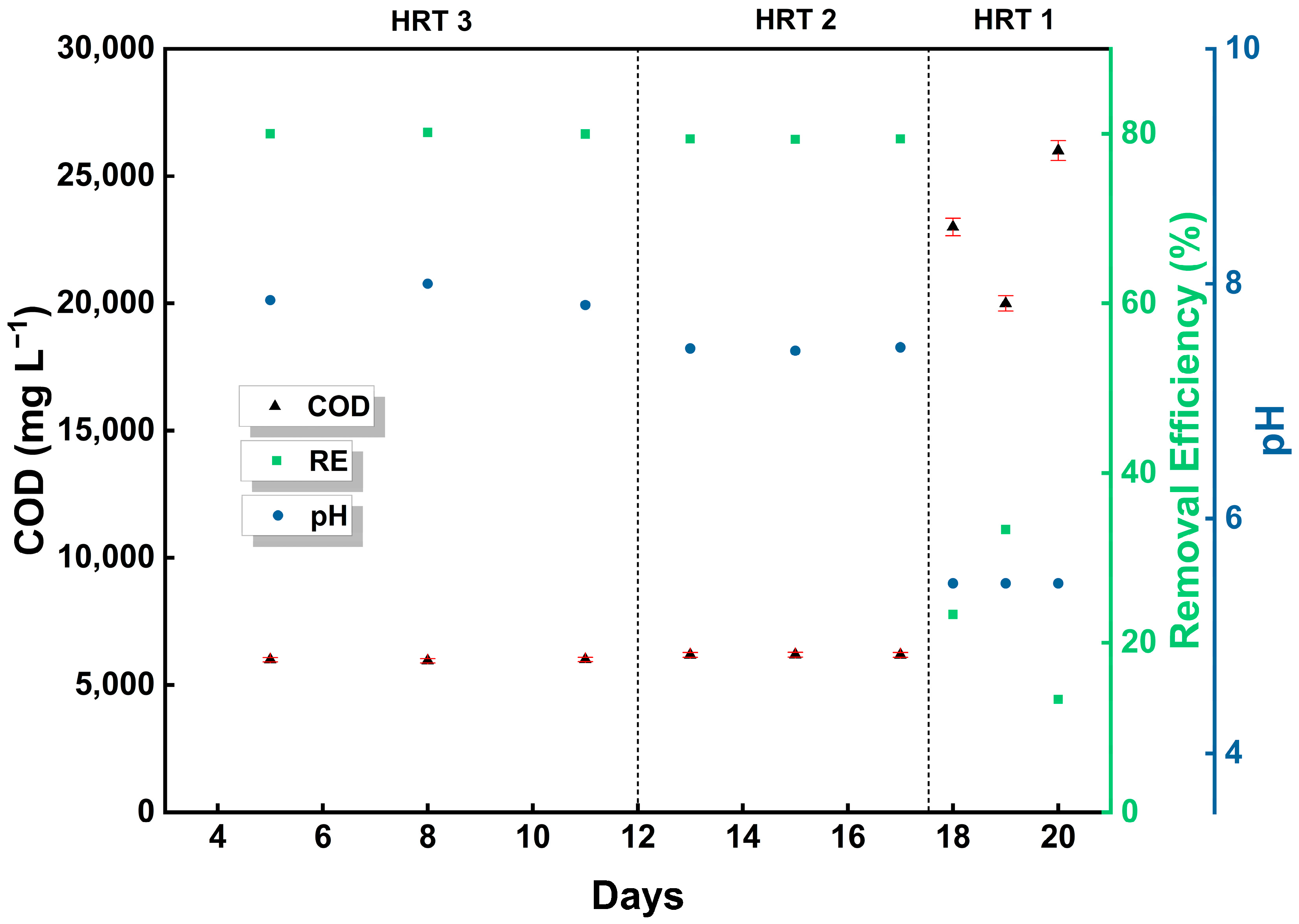
3.1. The effect of HRT on Anaerobic Digestion
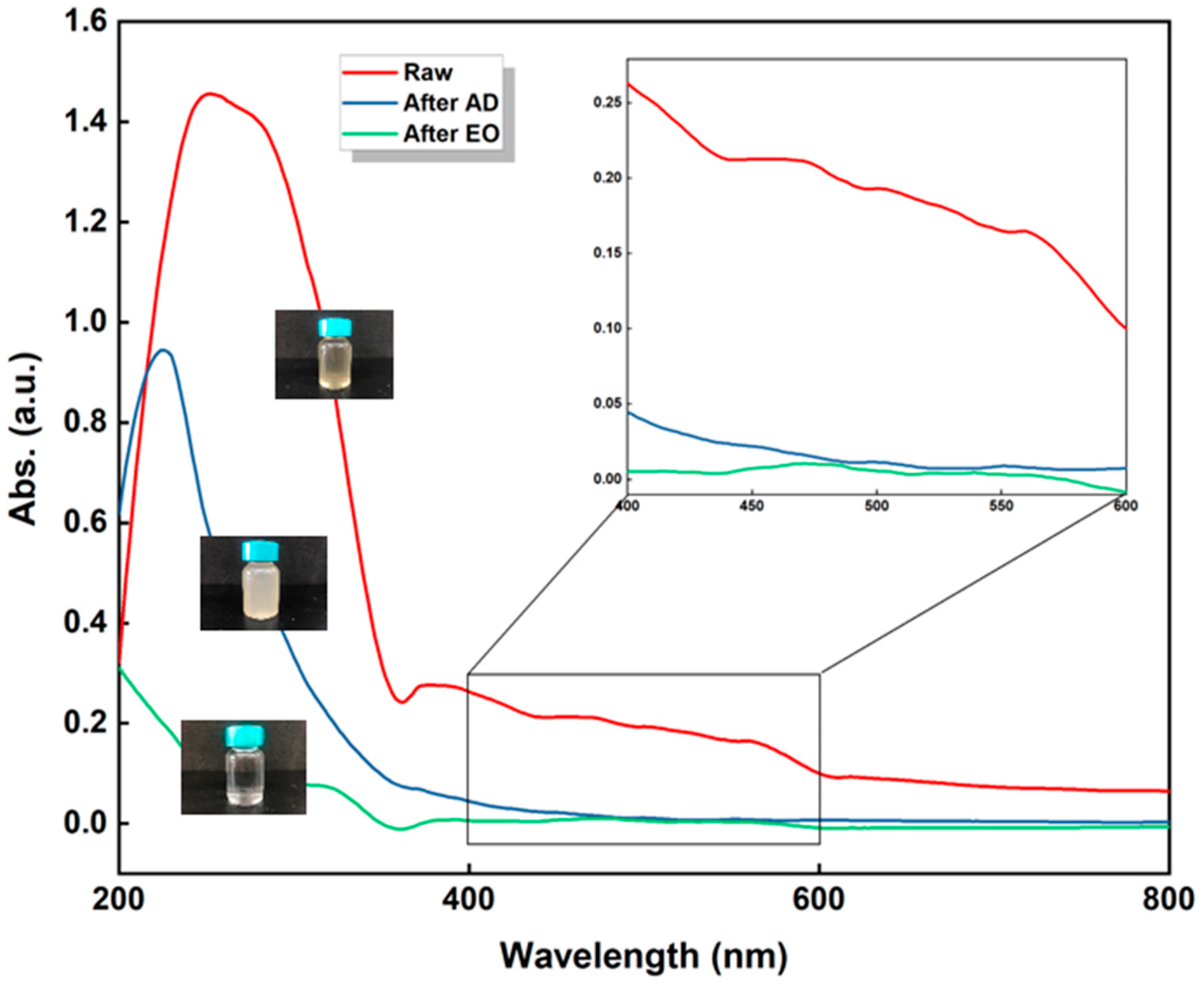
3.2. Electro-Oxidation as a Post-Treatment
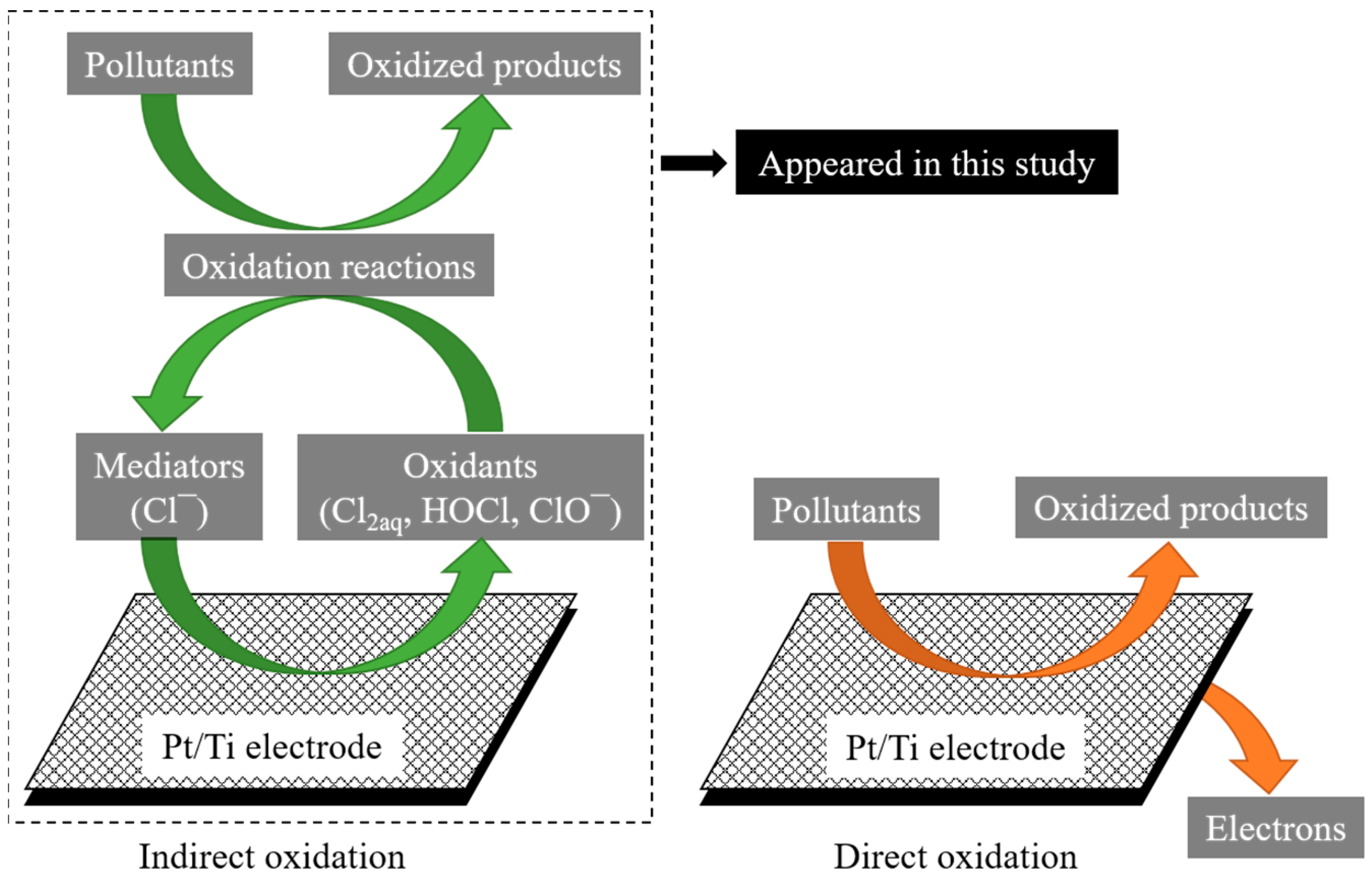
3.2.1. Mechanisms
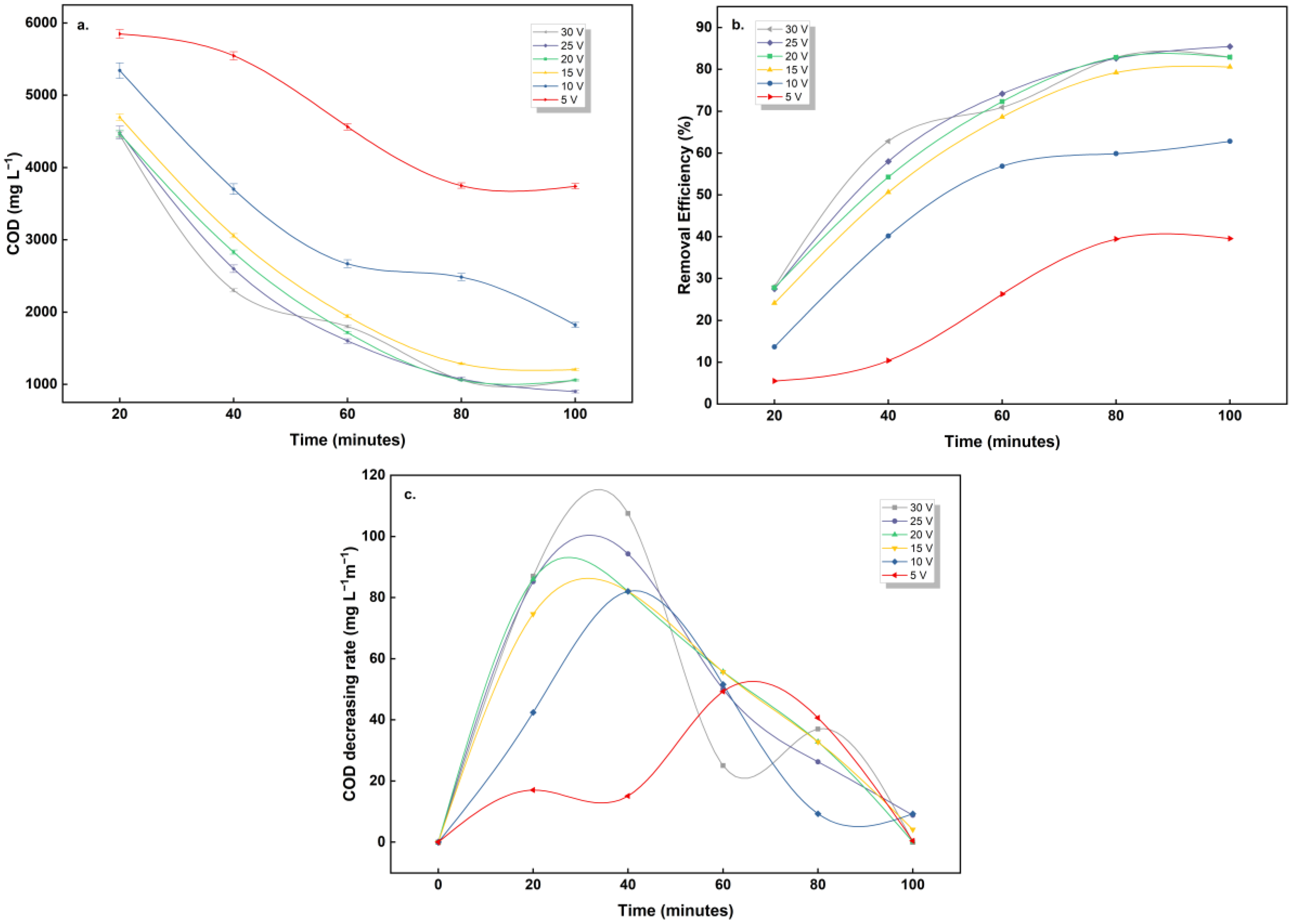
3.2.2. The Effect of Electrolysis Time
3.2.3. The Effect of NaCl Dosage
3.2.4. The Effect of Initial pH
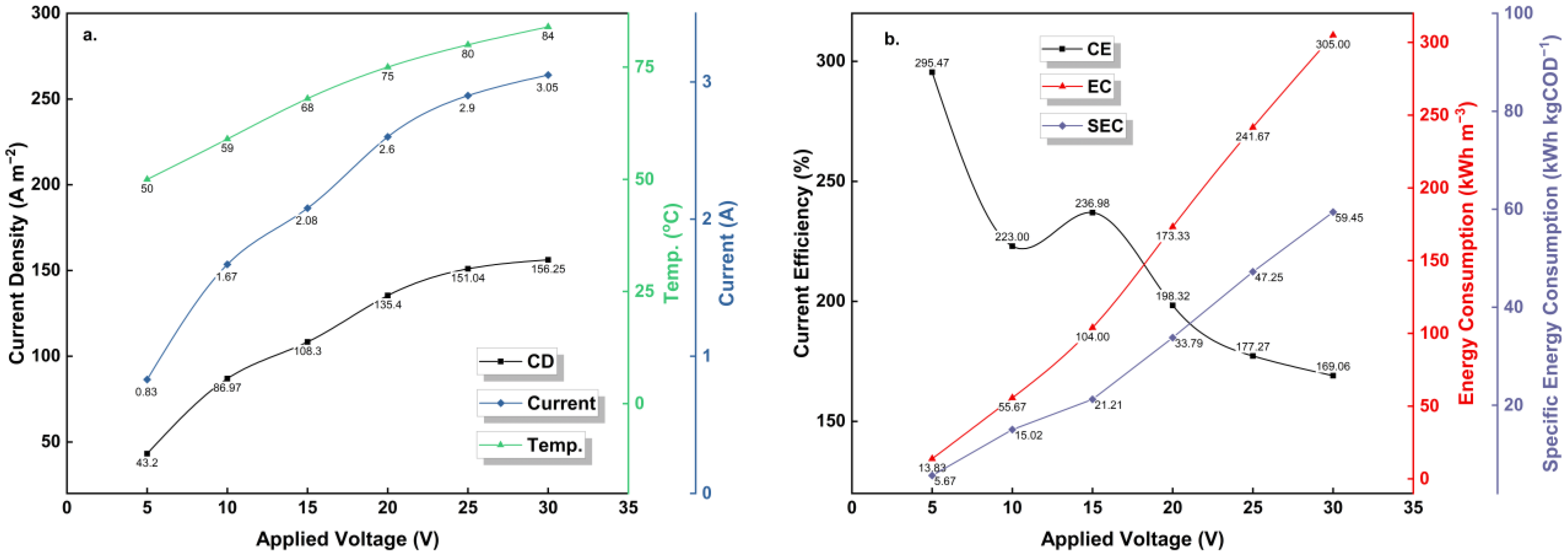
3.2.5. Technical Investigation—Electro-Properties
3.3. Summary of the Combination Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohtashami, R.; Shang, J.Q. Electroflotation for Treatment of Industrial Wastewaters: A Focused Review. Environ. Process. 2019, 6, 325–353. [Google Scholar] [CrossRef]
- Gumbo, B.; Mlilo, S.; Broome, J.; Lumbroso, D. Industrial water demand management and cleaner production potential: A case of three industries in Bulawayo, Zimbabwe. Phys. Chem. Earth Parts A/B/C 2003, 28, 797–804. [Google Scholar] [CrossRef]
- E.C. European Integrated Pollution Prevention and Control Bureau (EIPPCB). Reference Document on Best Available Techniques (BAT) in the Food, Drink and Milk Industries; EIPPCB: Seville, Spain, 2017.
- Li, Y.; Yu, T.; Kang, D.; Shan, X.; Zheng, P.; Hu, Z.; Ding, A.; Wang, R.; Zhang, M. Sources of anammox granular sludge and their sustainability in treating low-strength wastewater. Chemosphere 2019, 226, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Martín-Marroquín, J.M. 1—Adding Sustainability to the Beverage Industry Through Nature-Based Wastewater Treatment. In Processing and Sustainability of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–36. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef]
- Ghosh Ray, S.; Ghangrekar, M.M. Comprehensive review on treatment of high-strength distillery wastewater in advanced physico-chemical and biological degradation pathways. Int. J. Environ. Sci. Technol. 2019, 16, 527–546. [Google Scholar] [CrossRef]
- Li, W.c.; Chen, H.; Jin, Y.; Zhang, H.; Niu, Q.; Qi, W.; Zhang, Y.; Li, Y.-Y.; Gao, Y. Treatment of 3,4,5-trimethoxybenzaldehyde and Di-bromo-aldehyde manufacturing wastewater by the coupled Fenton pretreatment and UASB reactor with emphasis on optimization and chemicals analysis. Sep. Purif. Technol. 2015, 142, 40–47. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Chen, X.; Cai, Y.; Zhang, X.; Qian, M.; Chen, X.; Zhao, H.; Sheng, M.; Cao, G.; et al. Removal of veterinary antibiotics from swine wastewater using anaerobic and aerobic biodegradation. Sci. Total Environ. 2020, 709, 136094. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Ta, D.-T.; Lin, C.-Y.; Chu, C.-Y.; Ta, T.-M.-N. Biohythane production from swine manure and pineapple waste in a single-stage two-chamber digester using gel-entrapped anaerobic microorganisms. Int. J. Hydrogen Energy 2022, 47, 25245–25255. [Google Scholar] [CrossRef]
- Sani, K.; Kongjan, P.; Pakhathirathien, C.; Cheirsilp, B.; O-Thong, S.; Raketh, M.; Kana, R.; Jariyaboon, R. Effectiveness of using two-stage anaerobic digestion to recover bio-energy from high strength palm oil mill effluents with simultaneous treatment. J. Water Process Eng. 2021, 39, 101661. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Electrochemical oxidation for landfill leachate treatment. Waste Manag. 2007, 27, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent developments of electro-oxidation in water treatment—A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Durán, F.E.; de Araújo, D.M.; do Nascimento Brito, C.; Santos, E.V.; Ganiyu, S.O.; Martínez-Huitle, C.A. Electrochemical technology for the treatment of real washing machine effluent at pre-pilot plant scale by using active and non-active anodes. J. Electroanal. Chem. 2018, 818, 216–222. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Klidi, N.; Clematis, D.; Delucchi, M.; Gadri, A.; Ammar, S.; Panizza, M. Applicability of electrochemical methods to paper mill wastewater for reuse. Anodic oxidation with BDD and TiRuSnO2 anodes. J. Electroanal. Chem. 2018, 815, 16–23. [Google Scholar] [CrossRef]
- Cifcioglu-Gozuacik, B.; Ergenekon, S.M.; Ozbey-Unal, B.; Balcik, C.; Karagunduz, A.; Dizge, N.; Keskinler, B. Efficient removal of ammoniacal nitrogen from textile printing wastewater by electro-oxidation considering the effects of NaCl and NaOCl addition. Water Sci. Technol. 2021, 84, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Barrios, J.A.; Cano, A.; Rivera, F.F.; Cisneros, M.E.; Durán, U. Efficiency of integrated electrooxidation and anaerobic digestion of waste activated sludge. Biotechnol. Biofuels 2021, 14, 81. [Google Scholar] [CrossRef]
- Fontmorin, J.-M.; Huguet, S.; Fourcade, F.; Geneste, F.; Floner, D.; Amrane, A. Electrochemical oxidation of 2,4-Dichlorophenoxyacetic acid: Analysis of by-products and improvement of the biodegradability. Chem. Eng. J. 2012, 195–196, 208–217. [Google Scholar] [CrossRef]
- Popat, A.; Nidheesh, P.V.; Anantha Singh, T.S.; Suresh Kumar, M. Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological processes. Chemosphere 2019, 237, 124419. [Google Scholar] [CrossRef]
- Lay, C.-H.; Vo, T.-P.; Lin, P.-Y.; Abdul, P.M.; Liu, C.-M.; Lin, C.-Y. Anaerobic hydrogen and methane production from low-strength beverage wastewater. Int. J. Hydrogen Energy 2019, 44, 14351–14361. [Google Scholar] [CrossRef]
- Ni, J.; Ji, J.; Li, Y.-Y.; Kubota, K. Microbial characteristics in anaerobic membrane bioreactor treating domestic sewage: Effects of HRT and process performance. J. Environ. Sci. 2022, 111, 392–399. [Google Scholar] [CrossRef]
- Charalambous, P.; Shin, J.; Shin, S.G.; Vyrides, I. Anaerobic digestion of industrial dairy wastewater and cheese whey: Performance of internal circulation bioreactor and laboratory batch test at pH 5-6. Renew. Energy 2020, 147, 1–10. [Google Scholar] [CrossRef]
- Comninellis, C. Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim. Acta 1994, 39, 1857–1862. [Google Scholar] [CrossRef]
- Bonfatti, F.; Ferro, S.; Lavezzo, F.; Malacarne, M.; Lodi, G.; De Battisti, A. Electrochemical Incineration of Glucose as a Model Organic Substrate. II. Role of Active Chlorine Mediation. J. Electrochem. Soc. 2000, 147, 592. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.; Ferro, S.; Reyna, S.; Cerro-López, M.; De Battisti, A.; Quiroz, M.A. Electrochemical oxidation of oxalic acid in the presence of halides at boron doped diamond electrode. J. Braz. Chem. Soc. 2008, 19, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, M.A.; Espinoza, L.C.; Coreño, O.; García, V.; Fuentes, R.; Thiam, A.; Salazar, R. A comparative study of anodic oxidation and electrocoagulation for treating cattle slaughterhouse wastewater. J. Environ. Chem. Eng. 2022, 10, 108306. [Google Scholar] [CrossRef]
- Tran, T.-K.; Leu, H.-J.; Chiu, K.-F.; Lin, C.-Y. Electrochemical Treatment of Heavy Metal-containing Wastewater with the Removal of COD and Heavy Metal Ions. J. Chin. Chem. Soc. 2017, 64, 493–502. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Jiang, J.; Xia, G.; Chen, J. Process Optimization of Electrochemical Treatment of COD and Total Nitrogen Containing Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 850. [Google Scholar] [CrossRef]
- Candia-Onfray, C.; Espinoza, N.; Sabino da Silva, E.B.; Toledo-Neira, C.; Espinoza, L.C.; Santander, R.; García, V.; Salazar, R. Treatment of winery wastewater by anodic oxidation using BDD electrode. Chemosphere 2018, 206, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, C.; Chen, S.; Mei, R.; Wang, X.; Cao, J.; Ma, L.; Zhou, B.; Wei, Q.; Ouyang, G.; et al. Ultrasound enhanced electrochemical oxidation of Alizarin Red S on boron doped diamond(BDD) anode: Effect of degradation process parameters. Chemosphere 2018, 209, 685–695. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. An overview on the elimination of organic contaminants from aqueous systems using electrochemical advanced oxidation processes. J. Water Process Eng. 2021, 41, 102040. [Google Scholar] [CrossRef]
- Trasatti, S. Progress in the understanding of the mechanism of chlorine evolution at oxide electrodes. Electrochim. Acta 1987, 32, 369–382. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef]
- Ghimire, U.; Jang, M.; Jung, S.P.; Park, D.; Park, S.J.; Yu, H.; Oh, S.-E. Electrochemical Removal of Ammonium Nitrogen and COD of Domestic Wastewater using Platinum Coated Titanium as an Anode Electrode. Energies 2019, 12, 883. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Lu, J.; Zhuo, Q.; Ren, X.; Qiu, Y.; Li, Y.; Chen, Z.; Huang, K. Treatment of wastewater from adhesive-producing industries by electrocoagulation and electrochemical oxidation. Process Saf. Environ. Prot. 2022, 157, 527–536. [Google Scholar] [CrossRef]
- Fornaciari, J.C.; Weng, L.-C.; Alia, S.M.; Zhan, C.; Pham, T.A.; Bell, A.T.; Ogitsu, T.; Danilovic, N.; Weber, A.Z. Mechanistic understanding of pH effects on the oxygen evolution reaction. Electrochim. Acta 2022, 405, 139810. [Google Scholar] [CrossRef]
- Zou, J.; Peng, X.; Li, M.; Xiong, Y.; Wang, B.; Dong, F.; Wang, B. Electrochemical oxidation of COD from real textile wastewaters: Kinetic study and energy consumption. Chemosphere 2017, 171, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Muthuchamy, M. Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng. 2018, 6, 7358–7367. [Google Scholar] [CrossRef]
- Sun, D.; Hong, X.; Cui, Z.; Du, Y.; Hui, K.S.; Zhu, E.; Wu, K.; Hui, K.N. Treatment of landfill leachate using magnetically attracted zero-valent iron powder electrode in an electric field. J. Hazard. Mater. 2020, 388, 121768. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Anodic oxidation of phenol for waste water treatment. J. Appl. Electrochem. 1991, 21, 703–708. [Google Scholar] [CrossRef]
- Kumar, A.; Nidheesh, P.V.; Suresh Kumar, M. Composite wastewater treatment by aerated electrocoagulation and modified peroxi-coagulation processes. Chemosphere 2018, 205, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Güven, G.; Perendeci, A.; Özdemir, K.; Tanyolaç, A. Specific energy consumption in electrochemical treatment of food industry wastewaters. J. Chem. Technol. Biotechnol. 2012, 87, 513–522. [Google Scholar] [CrossRef]
- Nurhayati, E.; Juang, Y.; Huang, C. The kinetics, current efficiency, and power consumption of electrochemical dye decolorization by BD-NCD film electrode. AIP Conf. Proc. 2017, 1855, 050005. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, P.V.; Kumar, A.; Syam Babu, D.; Scaria, J.; Suresh Kumar, M. Treatment of mixed industrial wastewater by electrocoagulation and indirect electrochemical oxidation. Chemosphere 2020, 251, 126437. [Google Scholar] [CrossRef]
- Chen, Y.-T. The Factors Affecting Electricity Consumption and the Consumption Characteristics in the Residential Sector—A Case Example of Taiwan. Sustainability 2017, 9, 1484. [Google Scholar] [CrossRef] [Green Version]
- Linares Hernández, I.; Barrera Díaz, C.; Valdés Cerecero, M.; Almazán Sánchez, P.T.; Castañeda Juárez, M.; Lugo Lugo, V. Soft drink wastewater treatment by electrocoagulation–electrooxidation processes. Environ. Technol. 2017, 38, 433–442. [Google Scholar] [CrossRef]
- Chakchouk, I.; Elloumi, N.; Belaid, C.; Mseddi, S.; Chaari, l.; Kallel, M. A combined electrocoagulation-electrooxidation treatment for dairy wastewater. Braz. J. Chem. Eng. 2017, 34, 109–117. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kwarciak, A. The application of hybrid system UASB reactor-RO in landfill leachate treatment. Desalination 2008, 222, 128–134. [Google Scholar] [CrossRef]
- Güneş, E.; Gönder, Z.B. Evaluation of the hybrid system combining electrocoagulation, nanofiltration and reverse osmosis for biologically treated textile effluent: Treatment efficiency and membrane fouling. J. Environ. Manag. 2021, 294, 113042. [Google Scholar] [CrossRef]
- Kyriacou, A.; Lasaridi, K.E.; Kotsou, M.; Balis, C.; Pilidis, G. Combined bioremediation and advanced oxidation of green table olive processing wastewater. Process Biochem. 2005, 40, 1401–1408. [Google Scholar] [CrossRef]
- Khoufi, S.; Aloui, F.; Sayadi, S. Pilot scale hybrid process for olive mill wastewater treatment and reuse. Chem. Eng. Process. Process Intensif. 2009, 48, 643–650. [Google Scholar] [CrossRef]
- Carvalho, C.; Fernandes, A.; Lopes, A.; Pinheiro, H.; Gonçalves, I. Electrochemical degradation applied to the metabolites of Acid Orange 7 anaerobic biotreatment. Chemosphere 2007, 67, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, M.; Gutiérrez, M.-C.; López-Grimau, V.; López-Mesas, M.; Crespi, M. Biological Treatment of a Textile Effluent After Electrochemical Oxidation of Reactive Dyes. Water Environ. Res. 2010, 82, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, T.W.; Rogers, T.S.; Stoner, M.H.; Sellgren, K.L.; Lynch, B.J.; Forbis-Stokes, A.A.; Stoner, B.R.; Hawkins, B.T. A granular activated carbon/electrochemical hybrid system for onsite treatment and reuse of blackwater. Water Res. 2018, 144, 553–560. [Google Scholar] [CrossRef]
- Abdur Rawoof, S.A.; Kumar, P.S.; Vo, D.-V.N.; Devaraj, T.; Subramanian, S. Biohythane as a high potential fuel from anaerobic digestion of organic waste: A review. Renew. Sustain. Energy Rev. 2021, 152, 111700. [Google Scholar] [CrossRef]
- Lee, I.-S.; Parameswaran, P.; Rittmann, B.E. Effects of solids retention time on methanogenesis in anaerobic digestion of thickened mixed sludge. Bioresour. Technol. 2011, 102, 10266–10272. [Google Scholar] [CrossRef]
- Ta, D.-T.; Lin, C.-Y.; Ta, T.-M.-N.; Chu, C.-Y. Effect of pH shock on single-stage biohythane production using gel-entrapped anaerobic microorganisms. Int. J. Hydrogen Energy 2022, 47, 3679–3689. [Google Scholar] [CrossRef]
- He, T.; Kar, M.; McDaniel, N.D.; Randolph, B.B. Electrochemical Hydrogen Production. In Springer Handbook of Electrochemical Energy; Breitkopf, C., Swider-Lyons, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 897–940. [Google Scholar] [CrossRef]

| Electrode Specification | |
|---|---|
| Material | Titanium—Grade: GR1 |
| Platinum coating thickness | 200 nm |
| Mesh hole size | 3 mm × 2 mm |
| Mesh thickness | 0.5 mm |
| No. of electrodes | 2 |
| Shape | Rectangular |
| Size | 6 cm × 10 cm |
| Arrangement | Parallel |
| Exposed surface area | 48 cm2 |
| Electrolytic cell | |
| Electrode gap | 4 cm |
| Reactor | Beaker—500 mL |
| Volume | 400 mL |
| Type | Batch |
| Stirring | Magnetic bar—100 rpm |
| DC supply | |
| Voltage | 0–30 V |
| Current | 0–3 A |
| Connector | Electrical wire with alligator clip |
| Parameter | Before Treatment | AD Treatment | EO Treatment |
|---|---|---|---|
| COD (mg L−1) | 30,000 | 6190 | 1059.73 |
| Removal efficiency (%) | - | 79.37 | 96.47 |
| Color | Brown-yellow | Light brown-yellow | Colorless |
| Conductivity (mS cm−1) | 0.0094 | 0.0094 | 10.31 |
| Temperature (°C) | 25 | - | - |
| Treatment Method | Type of Wastewater | Initial COD | COD Removal Efficiency | Reference |
|---|---|---|---|---|
| Pre-treatment: AD Post-treatment: EO | Beverage wastewater | 30,000 mg L−1 | 96.47% | This work |
| Pre-treatment: EC 1 Post-treatment: EO | Soft drink wastewater | 4300 mg L−1 | 85% | [49] |
| Pre-treatment: EC Post-treatment: EO | Dairy wastewater | 4010 mg L−1 | 60% | [50] |
| Pre-treatment: UASB reactor Post-treatment: RO 2 | Landfill leachate | 4200 mg L−1 | 95.4% | [51] |
| Pre-treatment: EC Post-treatment: NF 3, RO | Textile effluent | 370 mg L−1 | 93% | [52] |
| Pre-treatment: Fungal Post-treatment: EO | Green table olive processing wastewater | 11,750 mg L−1 | 96% | [53] |
| Pre-treatment: Electro-Fenton Post-treatment: AF 4, ultrafiltration | Olive mill wastewater | 95,000 mg L−1 | 88.4% | [54] |
| Pre-treatment: EO Post-treatment: UASB | Textile dye | 847.5 mg L−1 | 70% | [55] |
| Pre-treatment: Electrochemical Post-treatment | Textile effluent | 1201 mg L−1 | 72% | [56] |
| Pre-treatment: GAC 5 Post-treatment: Electrochemical cell | Blackwater | 1732 mg L−1 | 69% | [57] |
| Biogas from AD | |
|---|---|
| H2 (%) | 10.8 |
| CH4 (%) | 8.2 |
| CO2 (%) | 1.9 |
| Theoretical Gas from EO | |
| H2 (g) | 0.012826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, H.N.Q.; Leu, J.H.; Nguyen, V.N.D. The Combination of Anaerobic Digestion and Electro-Oxidation for Efficient COD Removal in Beverage Wastewater: Investigation of Electrolytic Cells. Sustainability 2023, 15, 5551. https://doi.org/10.3390/su15065551
Phan HNQ, Leu JH, Nguyen VND. The Combination of Anaerobic Digestion and Electro-Oxidation for Efficient COD Removal in Beverage Wastewater: Investigation of Electrolytic Cells. Sustainability. 2023; 15(6):5551. https://doi.org/10.3390/su15065551
Chicago/Turabian StylePhan, Huy N. Q., Jyh Hoang Leu, and Vi N. D. Nguyen. 2023. "The Combination of Anaerobic Digestion and Electro-Oxidation for Efficient COD Removal in Beverage Wastewater: Investigation of Electrolytic Cells" Sustainability 15, no. 6: 5551. https://doi.org/10.3390/su15065551






