Beneficial Microorganisms in the Anaerobic Digestion of Cattle and Swine Excreta
Abstract
:1. Introduction
2. Materials and Methods
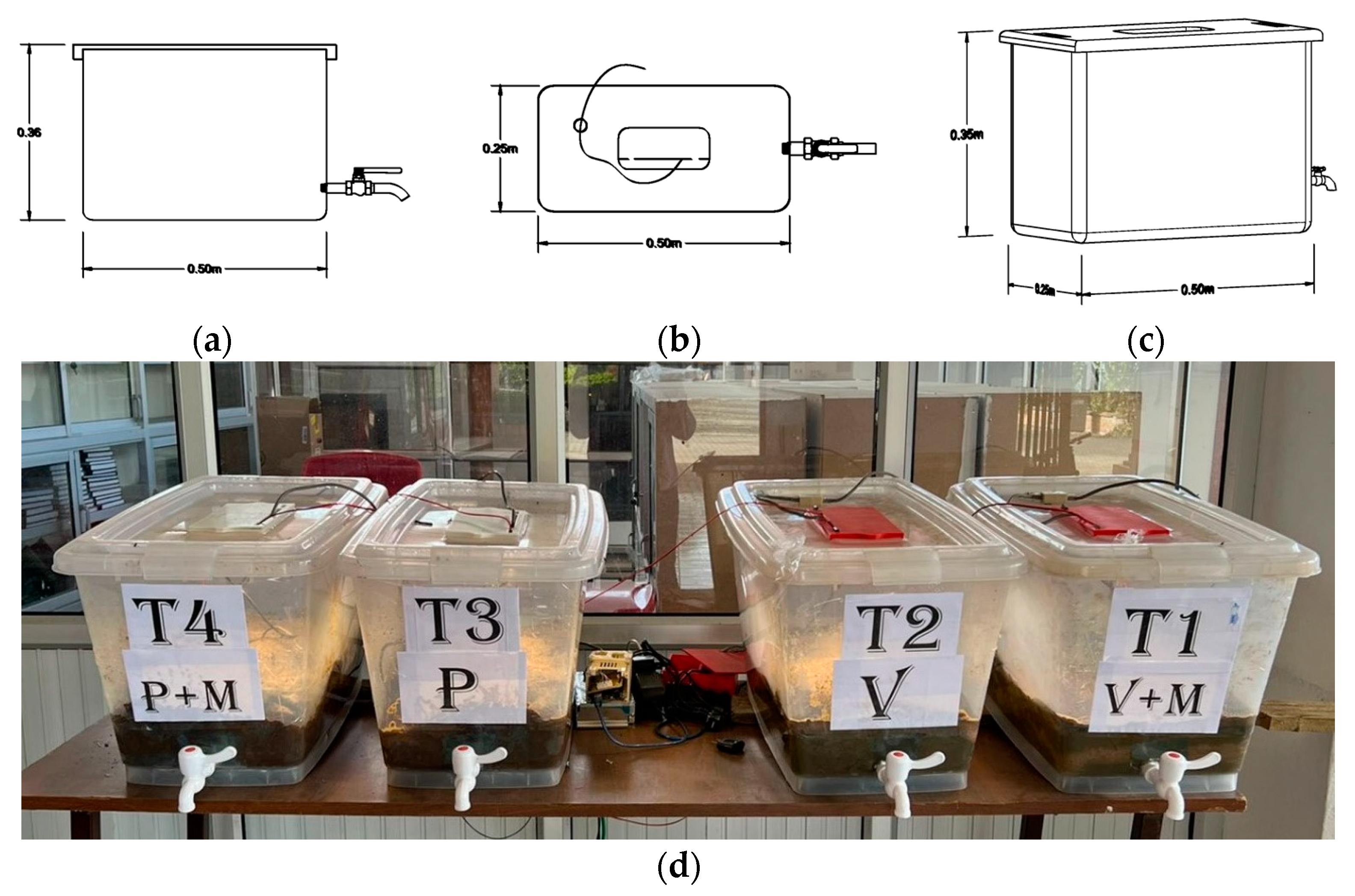
2.1. Bioreactors for the Biological Treatment of Manure
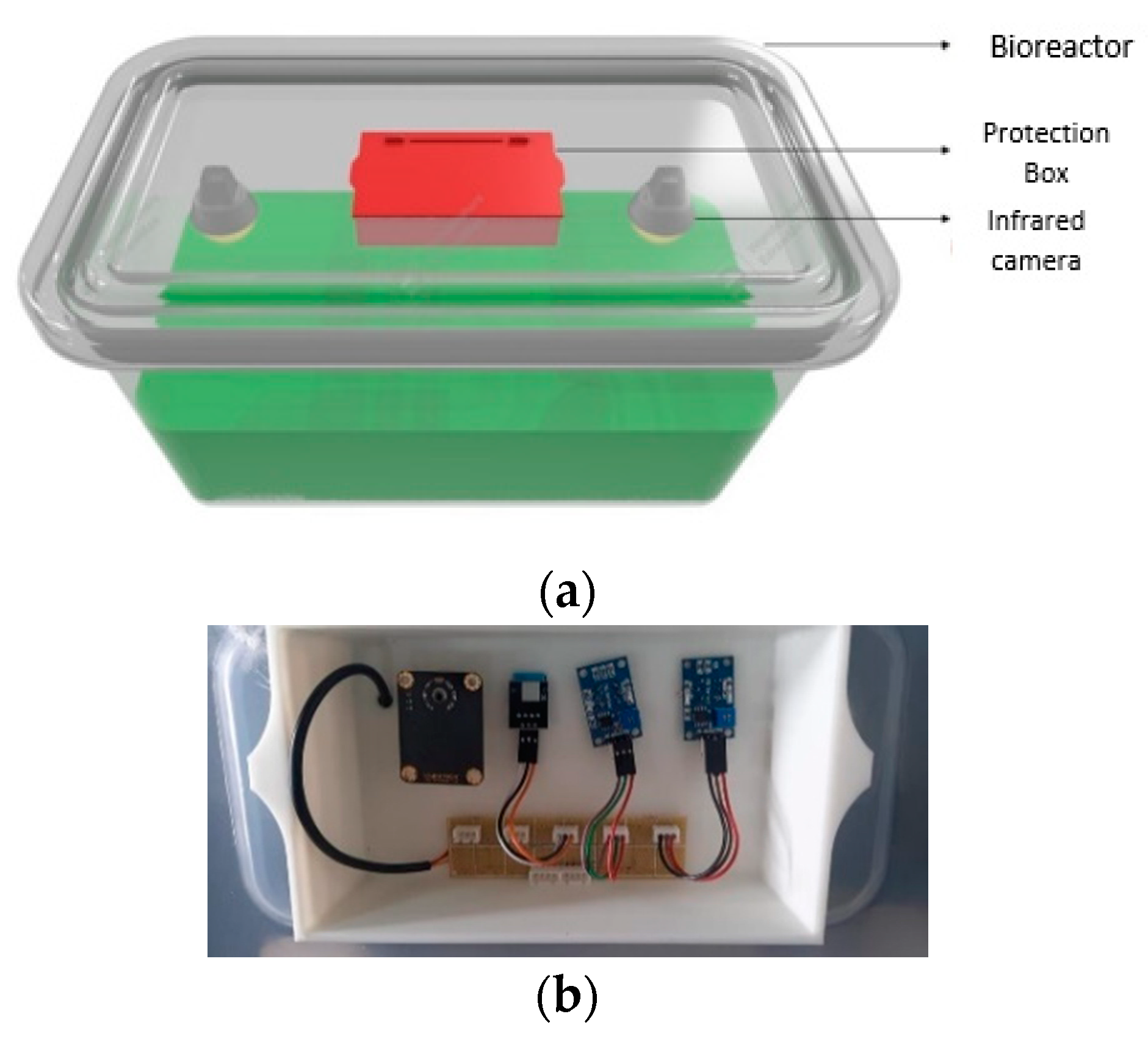
2.2. Electronic Data Processing and Monitoring System
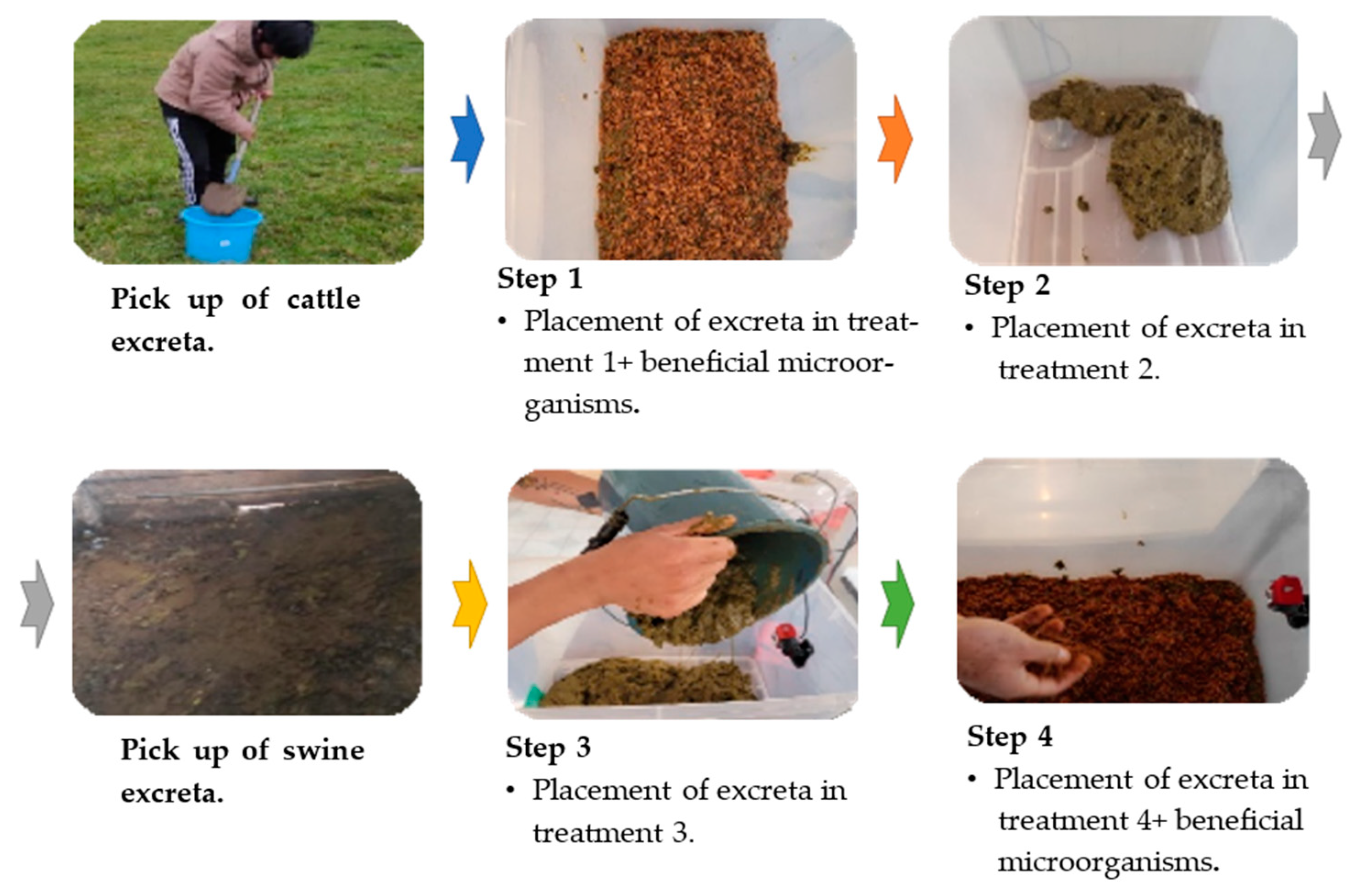
2.3. Biomass Collection
2.4. Immobilization of Beneficial Microorganisms in Inert Material
2.5. Adequacy of the Biomass in the Containers and the Application of Beneficial Microorganisms
2.6. Data Collection, Analysis, and Interpretation
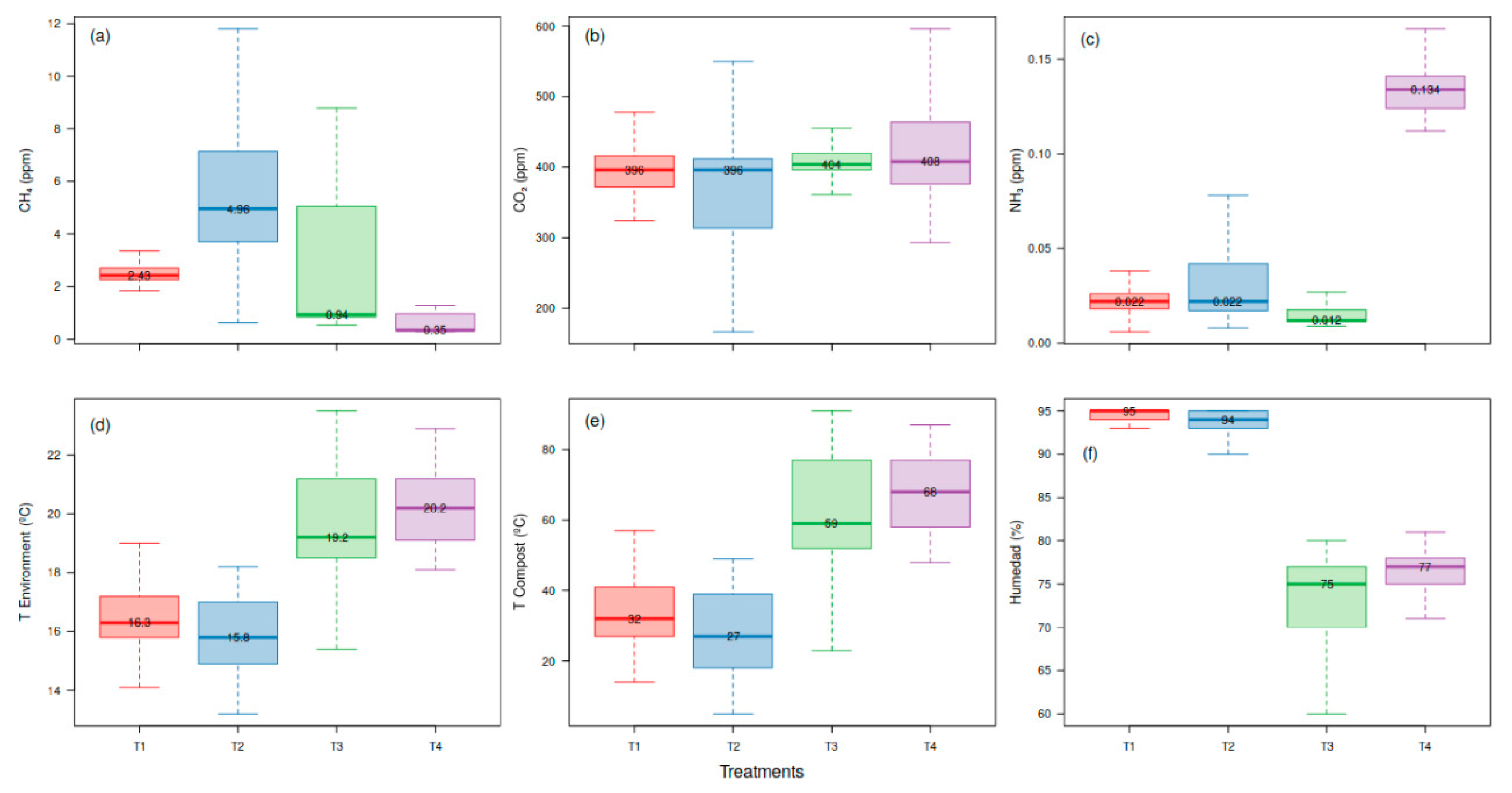
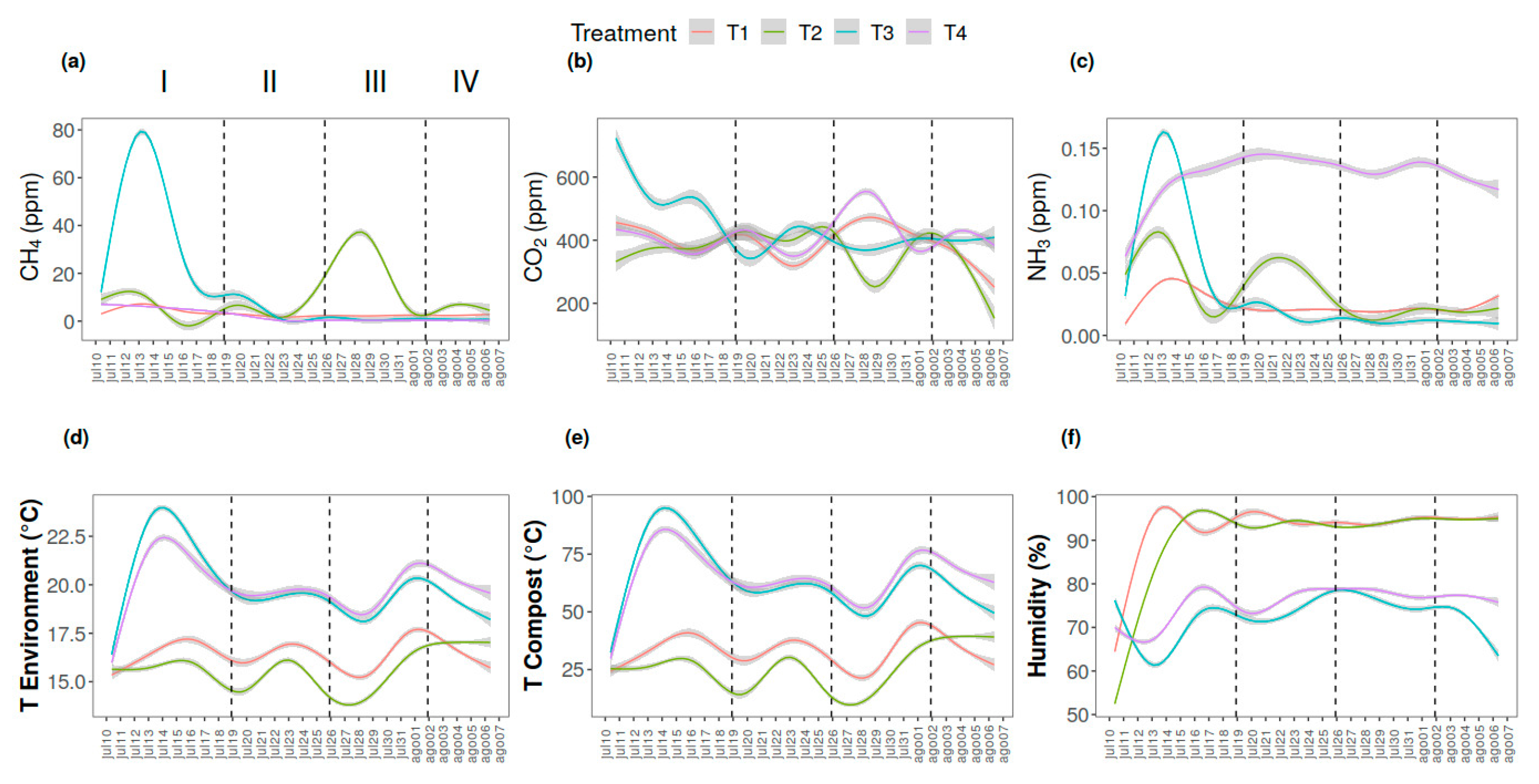
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ropero-Portillo, S. ¿Las Vacas Contaminan? Available online: https://www.ecologiaverde.com/las-vacas-contaminan-3233.html (accessed on 6 April 2023).
- Herrero, M.A.; Gil, S.B. Consideraciones Ambientales de La Intensificación En Producción Animal. Ecol. Austral 2008, 18, 273–289. [Google Scholar]
- Burkholder, J.A.; Libra, B.; Weyer, P.; Heathcote, S.; Kolpin, D.; Thorne, P.S.; Wichman, M. Impacts of Waste from Concentrated Animal Feeding Operations on Water Quality. Environ. Health Perspect. 2007, 115, 308–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Blas, C.; García-Rebollar, P.; Cambra-López, M.; Torres, A.G. Contribución de Los Rumiantes a Las Emisiones de Gases Con Efecto Invernadero. In XXIV Curso de Especialización FEDNA; Editorial FEDNA: Madrid, Spain, 2008; pp. 121–150. [Google Scholar]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 9251055718. [Google Scholar]
- Sutaryo, S.; Ward, A.J.; Møller, H.B. Thermophilic Anaerobic Co-Digestion of Separated Solids from Acidified Dairy Cow Manure. Bioresour. Technol. 2012, 114, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, W.B. Biodiversidad y Cambio Climático. Apunt. Cienc. Soc. 2015, 5, 5–10. [Google Scholar]
- Directorate-General JRC. Integrated Pollution Prevention and Control (IPPC) Reference Document on Best Available Techniques for Intensive Rearing of Poultry and Pigs Draft Dated July 2001; Citeseer: University Park, PA, USA, 2001. [Google Scholar]
- FAO. El Papel de La FAO En La Producción Animal. Available online: https://www.fao.org/animal-production/es#:~:text=La FAO trabaja para mejorar,vista económico%2C social y ambiental (accessed on 6 April 2023).
- Safieddin Ardebili, S.M. Green Electricity Generation Potential from Biogas Produced by Anaerobic Digestion of Farm Animal Waste and Agriculture Residues in Iran. Renew Energy 2020, 154, 29–37. [Google Scholar] [CrossRef]
- Khalil, M.; Berawi, M.A.; Heryanto, R.; Rizalie, A. Waste to Energy Technology: The Potential of Sustainable Biogas Production from Animal Waste in Indonesia. Renew. Sustain. Energy Rev. 2019, 105, 323–331. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Sielski, J.; Grzesik, M.; Romanowska-Duda, Z.; Piotrowski, K.; Lewandowska, W. Acquisition of Torrefied Biomass from Jerusalem Artichoke Grown in a Closed Circular System Using Biogas Plant Waste. Molecules 2020, 25, 3862. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Teoh, Y.H.; Ud Din, Z.; Sabah, N.U.; Jamil, M.A.; Mujtaba, M.A.; Abid, A. The Potential of Sustainable Biogas Production from Biomass Waste for Power Generation in Pakistan. J. Clean. Prod. 2021, 307, 127250. [Google Scholar] [CrossRef]
- Berra, G. La “Vaca Mochilera”, Un Invento Argentino Contra El Cambio Climático. Available online: https://www.agenciacyta.org.ar/2010/01/la-“vaca-mochilera”-un-invento-argentino-contra-el-cambio-climatico/ (accessed on 6 April 2023).
- Lan, W.; Yang, C. Ruminal Methane Production: Associated Microorganisms and the Potential of Applying Hydrogen-Utilizing Bacteria for Mitigation. Sci. Total Environ. 2019, 654, 1270–1283. [Google Scholar] [CrossRef]
- Cai, C.; Leu, A.O.; Xie, G.J.; Guo, J.; Feng, Y.; Zhao, J.X.; Tyson, G.W.; Yuan, Z.; Hu, S. A Methanotrophic Archaeon Couples Anaerobic Oxidation of Methane to Fe(III) Reduction. ISME J. 2018, 12, 1929–1939. [Google Scholar] [CrossRef]
- Sandoval Pelcastre, A.A.; Ramirez Mella, M.; Rodríguez Ávila, N.L.; Candelaria Martinez, B. Tropical Trees and Shrubs with Potential to Reduce the Production of Methane in Ruminants. Trop. Subtrop. Agroecosyst. 2020, 23, 33. [Google Scholar]
- Eugène, M.; Klumpp, K.; Sauvant, D. Methane Mitigating Options with Forages Fed to Ruminants. Grass Forage Sci. 2021, 76, 196–204. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Ramírez, J.M. Incluencia de Saccharomyces Cerevisiae En La Eficiencia Ambiental de La Producción de Metano En Ganado Lehero y Ganado de Engorda: Meta-Análisis; Universidad Autónoma del Estado de México: Toluca, Mexico, 2018. [Google Scholar]
- Castillo-Lopez, E.; Clark, K.; Dominguez-Ordonez, M.; Kononoff, P. Salud Y Enfermedades Metabólicas Relacionados Con Factores Nutricionales En Vacas Lecheras (G1743S). Available online: https://extensionpubs.unl.edu/publication/9000018609715/salud-y-enfermedades-metabolicas-relacionados-con-factores-nutricionales-en-vacas-lecheras-g1743s/ (accessed on 1 April 2023).
- Chinwendu, S.; Chibueze, U.; Tochukwu, E. Anaerobic Digester Considerations of Animal Waste. Am. J. Biochem. 2013, 2013, 93–96. [Google Scholar] [CrossRef]
- Reyes-Ordoñez, H.R.; Ortiz-Torres, J.I.; Álvarez-Vera, M.S.; Cobos-Torres, J.C. Evaluación de La Degradación de Materia Orgánica Mediante Técnicas de Visión Artificial y Sensores. Rev. Arbitr. Interdiscip. Koinonía 2020, 5, 421–436. [Google Scholar] [CrossRef]
- Tortosa, G.; Alburquerque, J.A.; Ait-Baddi, G.; Cegarra, J. The Production of Commercial Organic Amendments and Fertilisers by Composting of Two-Phase Olive Mill Waste (“alperujo”). J. Clean. Prod. 2012, 26, 48–55. [Google Scholar] [CrossRef]
- Moreno Ayala, L.; Cadillo Castro, J. Uso Del Estiércol Porcino Sólido Como Abono Orgánico En El Cultivo Del Maíz Chala. An. Cient. 2018, 79, 415. [Google Scholar] [CrossRef] [Green Version]
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. Available online: https://fishr-core-team.github.io/FSA/ (accessed on 1 April 2023).
- Im, S.; Petersen, S.O.; Lee, D.; Kim, D.H. Effects of Storage Temperature on CH4 Emissions from Cattle Manure and Subsequent Biogas Production Potential. Waste Manag. 2020, 101, 35–43. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.; Zhao, D.; Fu, K. Enhancing the CH4 Yield of Anaerobic Digestion via Endogenous CO2 Fixation by Exogenous H2. Chemosphere 2015, 140, 34–39. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The Anaerobic Digestion of Solid Organic Waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Rodhe, L.K.K.; Ascue, J.; Willén, A.; Persson, B.V.; Nordberg, Å. Greenhouse Gas Emissions from Storage and Field Application of Anaerobically Digested and Non-Digested Cattle Slurry. Agric. Ecosyst. Env. 2015, 199, 358–368. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Asad, F.; Huang, D. Influence of Bio-Fertilizer Containing Beneficial Fungi and Rhizospheric Bacteria on Health Promoting Compounds and Antioxidant Activity of Spinacia oleracea L. Bot. Stud. 2017, 58, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessuto, J.; Scopel, B.S.; Perondi, D.; Godinho, M.; Dettmer, A. Enhancement of Biogas and Methane Production by Anaerobic Digestion of Swine Manure with Addition of Microorganisms Isolated from Sewage Sludge. Process Saf. Environ. Prot. 2016, 104, 233–239. [Google Scholar] [CrossRef]
- Salomoni, C.; Caputo, A.; Bonoli, M.; Francioso, O.; Rodriguez-Estrada, M.T.; Palenzona, D. Enhanced Methane Production in a Two-Phase Anaerobic Digestion Plant, after CO2 Capture and Addition to Organic Wastes. Bioresour. Technol. 2011, 102, 6443–6448. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, S.; Qv, M.; Dai, D.; Liu, D.; Wang, W.; Tang, C.; Zhu, L. Microalgal Cultivation for the Upgraded Biogas by Removing CO2, Coupled with the Treatment of Slurry from Anaerobic Digestion: A Review. Bioresour. Technol. 2022, 364, 128118. [Google Scholar] [CrossRef]
- Xu, S.; Qiao, Z.; Luo, L.; Sun, Y.; Wong, J.W.C.; Geng, X.; Ni, J. On-Site CO2 Bio-Sequestration in Anaerobic Digestion: Current Status and Prospects. Bioresour. Technol. 2021, 332, 125037. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the Methane Fermentation Process with Particular Emphasis on the Microbiological Aspect: A Review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Neibling, H. Anaerobic Digestion Basics; University of Idaho Extension: Moscow, ID, USA, 2014; Volume 6, pp. 1–6. [Google Scholar]
- Mlinar, S.; Weig, A.R.; Freitag, R. Influence of NH3 and NH4+ on Anaerobic Digestion and Microbial Population Structure at Increasing Total Ammonia Nitrogen Concentrations. Bioresour. Technol. 2022, 361, 127638. [Google Scholar] [CrossRef]
- Shehu, M.S.; Abdul Manan, Z.; Wan Alwi, S.R. Optimization of Thermo-Alkaline Disintegration of Sewage Sludge for Enhanced Biogas Yield. Bioresour. Technol. 2012, 114, 69–74. [Google Scholar] [CrossRef]
- Lee, J.J.; Choi, C.U.; Lee, M.J.; Chung, I.H.; Kim, D.S. A Study of NH3-N and P Refixation by Struvite Formation in Hybrid Anaerobic Reactor. Water Sci. Technol. 2004, 49, 207–214. [Google Scholar] [CrossRef]
- De La Rubia, M.A.; Perez, M.; Romero, L.I.; Sales, D. Anaerobic Mesophilic and Thermophilic Municipal Sludge Digestion. Chem. Biochem. Eng. Q. 2002, 16, 119–124. [Google Scholar]
- Bouallagui, H.; Rachdi, B.; Gannoun, H.; Hamdi, M. Mesophilic and Thermophilic Anaerobic Co-Digestion of Abattoir Wastewater and Fruit and Vegetable Waste in Anaerobic Sequencing Batch Reactors. Biodegradation 2009, 20, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Riau, V.; De la Rubia, M.Á.; Pérez, M. Temperature-Phased Anaerobic Digestion (TPAD) to Obtain Class A Biosolids: A Semi-Continuous Study. Bioresour. Technol. 2010, 101, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A. Biogas Production: Microbiology and Technology. In Anaerobes in Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 195–234. ISBN 978-3-319-45651-5. [Google Scholar]
- Babaee, A.; Shayegan, J.; Roshani, A. Anaerobic Slurry Co-Digestion of Poultry Manure and Straw: Effect of Organic Loading and Temperature. J. Environ. Health Sci. Eng. 2013, 11, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzcinski, A.P.; Stuckey, D.C. Treatment of Municipal Solid Waste Leachate Using a Submerged Anaerobic Membrane Bioreactor at Mesophilic and Psychrophilic Temperatures: Analysis of Recalcitrants in the Permeate Using GC-MS. Water Res. 2010, 44, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of Temperature and Hydraulic Retention Time on Anaerobic Digestion of Food Waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, G.; An, C.; Chen, X.; Zhang, P.; Xin, X.; Shen, J.; Agnew, J. Anaerobic Digestion of Livestock Manure in Cold Regions: Technological Advancements and Global Impacts. Renew. Sustain. Energy Rev. 2020, 119, 109494. [Google Scholar] [CrossRef]
- Kashyap, D.R.; Dadhich, K.S.; Sharma, S.K. Biomethanation under Psychrophilic Conditions: A Review. Bioresour. Technol. 2003, 87, 147–153. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water 2019, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Castillo M., E.F.; Cristancho, D.E.; Victor Arellano, A. Study of the Operational Conditions for Anaerobic Digestion of Urban Solid Wastes. Waste Manag. 2006, 26, 546–556. [Google Scholar] [CrossRef]
- Fezzani, B.; Cheikh, R. Ben Two-Phase Anaerobic Co-Digestion of Olive Mill Wastes in Semi-Continuous Digesters at Mesophilic Temperature. Bioresour. Technol. 2010, 101, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castillo, R.; Llabrés-Luengo, P.; Mata-Alvarez, J. Temperature Effect on Anaerobic Digestion of Bedding Straw in a One Phase System at Different Inoculum Concentration. Agric. Ecosyst. Env. 1995, 54, 55–66. [Google Scholar] [CrossRef]
- Briski, E.; VanStappen, G.; Bossier, P.; Sorgeloos, P. Laboratory Production of Early Hatching Cysts of Artemia Sp. by Selection. In Departmental Seminar; Great Lakes Institute for Environmental Research, University of Windsor: Windsor, ON, Canada, 2007. [Google Scholar]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and Biogas Production Potential of Municipal Solid Wastes Pretreated with a Rotary Drum Reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Berriel, M.C.; Márquez-Benavides, L.; González-Pérez, D.J.; Buenrostro-Delgado, O. The Effect of Moisture Regimes on the Anaerobic Degradation of Municipal Solid Waste from Metepec (México). Waste Manag. 2008, 28, 14–20. [Google Scholar] [CrossRef]
- Bouallagui, H.; Ben Cheikh, R.; Marouani, L.; Hamdi, M. Mesophilic Biogas Production from Fruit and Vegetable Waste in a Tubular Digester. Bioresour. Technol. 2003, 86, 85–89. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, S.; Li, F.; Wu, B.; Yang, X.; Li, X. Coupling Electrokinetics with Microbial Biodegradation Enhances the Removal of Cycloparaffinic Hydrocarbons in Soils. J. Hazard. Mater. 2016, 320, 591–601. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 06, 205–212. [Google Scholar] [CrossRef] [Green Version]


| Sensor | Gases | Unit | Range | Principle | Voltage | Output |
|---|---|---|---|---|---|---|
| MQ4 | Methane | ppm | 200–10,000 | Resistive | 5 V | Analog |
| MQ135 | Ammonia | ppm | 100–300 | Resistive | 5 V | Analog and Digital |
| MG811 | Carbon dioxide | ppm | 0–10,000 | Resistive | 5 V | Analog and Digital |
| DHT11 | Temperature and humidity | °C and % | 0–50 °C | NTC and resistive | 5 V | Analog and Digital |
| DS18B20 | Relative temperature | °C | −55–125 °C | NTC and resistive | 5 V | Analog |
| MO % | Humidity % | pH | EC dS/m | N % | P2O5 % | K2O % | CaO% | MgO % | Na % | Fe ppm | Cu ppm | Zn ppm | Mn ppm | B ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 80.86 | 26.23 | 6.36 | 6.27 | 2.04 | 6.1 | 1.63 | 3.98 | 2.0 | 0.23 | 29 | 385 | 1580 | 488 | 1417 |
| N° | Microorganism | CFU/mL |
|---|---|---|
| 1 | Acinetobacter sp. | 0.34298 |
| 2 | Aeromonas sp. | 129.475 |
| 3 | Alcaligenes sp. | 28.472 |
| 4 | Bacillus cereus | 202.941 |
| 5 | Bacillus subtilis | 121.231 |
| 6 | Candida sp. | 0.98246 |
| 7 | Clostridium sp. | 190.928 |
| 8 | Lactobacillus sp. | 0.39875 |
| 9 | Listeria monocytogenes | 0.45922 |
| 10 | Micrococcus sp. | 213.476 |
| 11 | Pseudomonas aeruginosa | 0.6439 |
| 12 | Pseudomonas fluorescens | 0.27424 |
| 13 | Pseudomonas putida | 162.572 |
| 14 | Saccharomyces cerevisiae | 274.925 |
| 15 | Salmonella sp. | 0.29289 |
| 16 | Yarrowia lipolytica | 123.827 |
| Post Hoc (Dunn’s Test) | ||||||
|---|---|---|---|---|---|---|
| Kruskal-Wallis Model | T1–T2 | T1–T3 | T2–T3 | T1–T4 | T2–T4 | T3–T4 |
| Temperature ~ Treatment | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.061 |
| Humidity ~ Treatment | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.014 |
| CO2 ~ Treatment | 0.024 | <0.001 | <0.001 | 0.3 | 0.174 | <0.001 |
| CH4 ~ Treatment | 0.002 | <0.001 | <0.001 | <0.001 | 0.036 | <0.001 |
| NH3 ~ Treatment | <0.001 | 0.016 | <0.001 | <0.001 | <0.001 | <0.001 |
| Symbology | Phase | Time (Days) |
|---|---|---|
| PI | Hydrolysis | 9 |
| PII | Acidogenesis | 7 |
| PIII | Acetogenesis | 7 |
| PIV | Methanogenesis | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Espinosa, P.-S.; Alvarez-Vera, M.; Cárdenas, A.; Cobos-Torres, J.-C. Beneficial Microorganisms in the Anaerobic Digestion of Cattle and Swine Excreta. Sustainability 2023, 15, 6482. https://doi.org/10.3390/su15086482
Vidal-Espinosa P-S, Alvarez-Vera M, Cárdenas A, Cobos-Torres J-C. Beneficial Microorganisms in the Anaerobic Digestion of Cattle and Swine Excreta. Sustainability. 2023; 15(8):6482. https://doi.org/10.3390/su15086482
Chicago/Turabian StyleVidal-Espinosa, Paulina-Soledad, Manuel Alvarez-Vera, Andrés Cárdenas, and Juan-Carlos Cobos-Torres. 2023. "Beneficial Microorganisms in the Anaerobic Digestion of Cattle and Swine Excreta" Sustainability 15, no. 8: 6482. https://doi.org/10.3390/su15086482





