Characterization of the Tunisian Phosphate Rock from Metlaoui-Gafsa Basin and Bio-Leaching Assays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Characterization of the Phosphate Rock
2.1.1. Sampling
2.1.2. Characterization of Phosphate Rock
2.2. Bio-Treatment of the Phosphate Rock
2.2.1. The Used Bacteria
2.2.2. Bioleaching In Vitro Assay
2.2.3. Cell Viability Essay
2.2.4. Biofilm Formation Assays
Qualitative Biofilm Study
Characterization of Semi-Quantitative Slime-Producing Bacteria
2.2.5. Hydrophobicity Study
2.2.6. Atomic Absorption Spectroscopy
2.3. Statistical Analysis
3. Results
3.1. Characterization of Phosphate Size Fractions
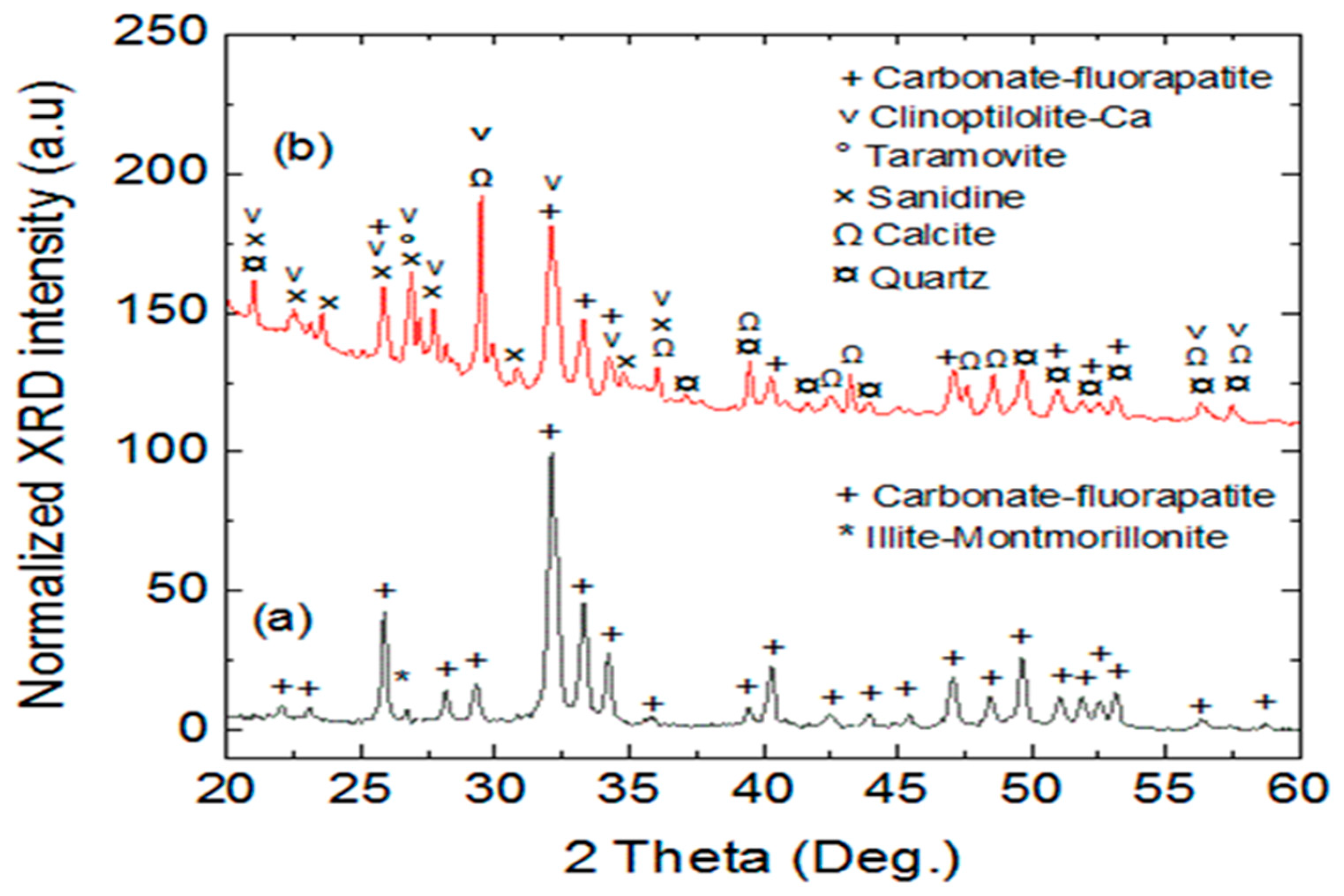
3.1.1. X-ray Powder Diffraction Analysis
3.1.2. Chemical Analysis
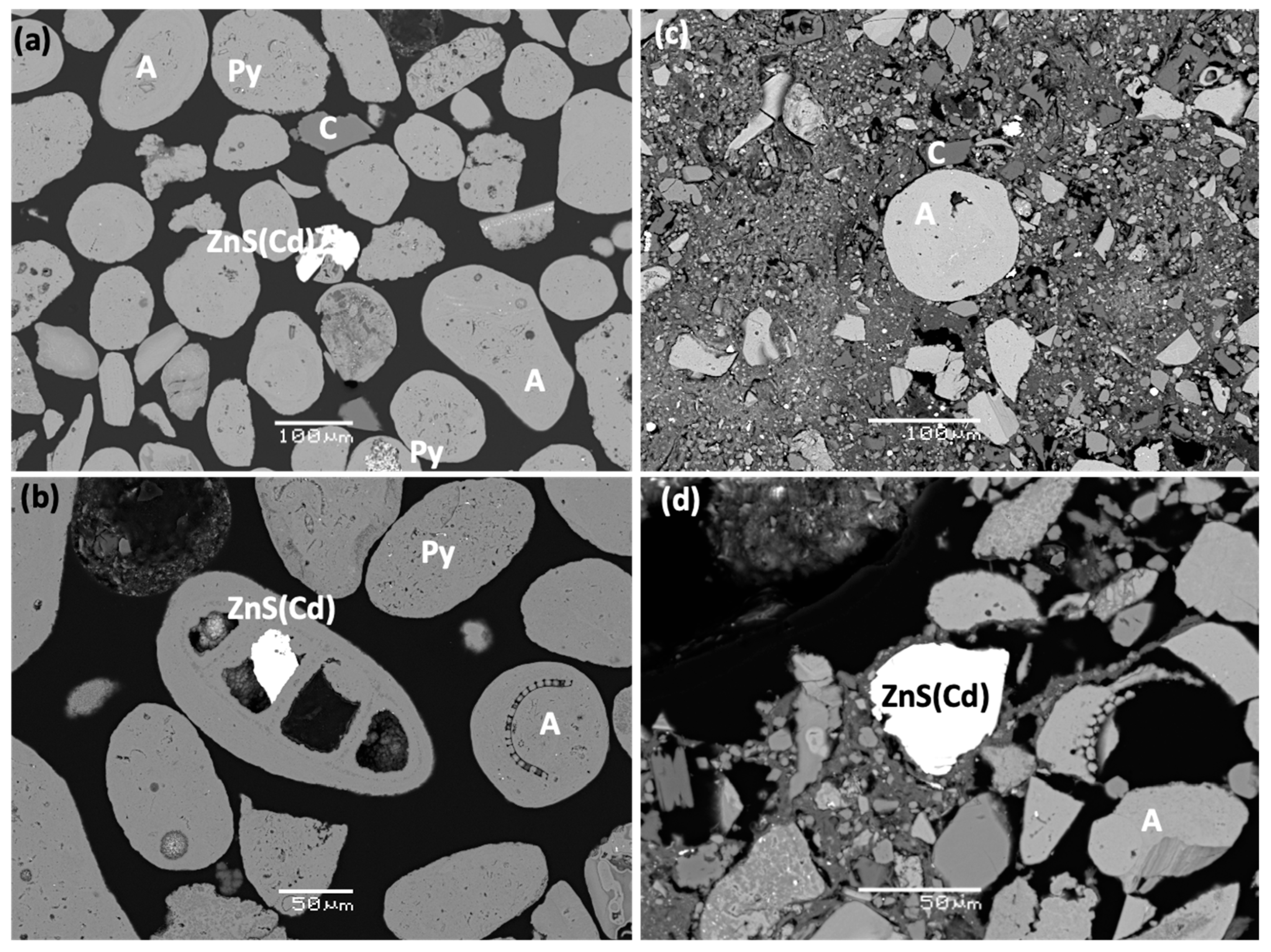
3.1.3. Electron Microscopy
3.2. Bioleaching Procedure
3.2.1. Characterization of the Newly Isolated Bacteria
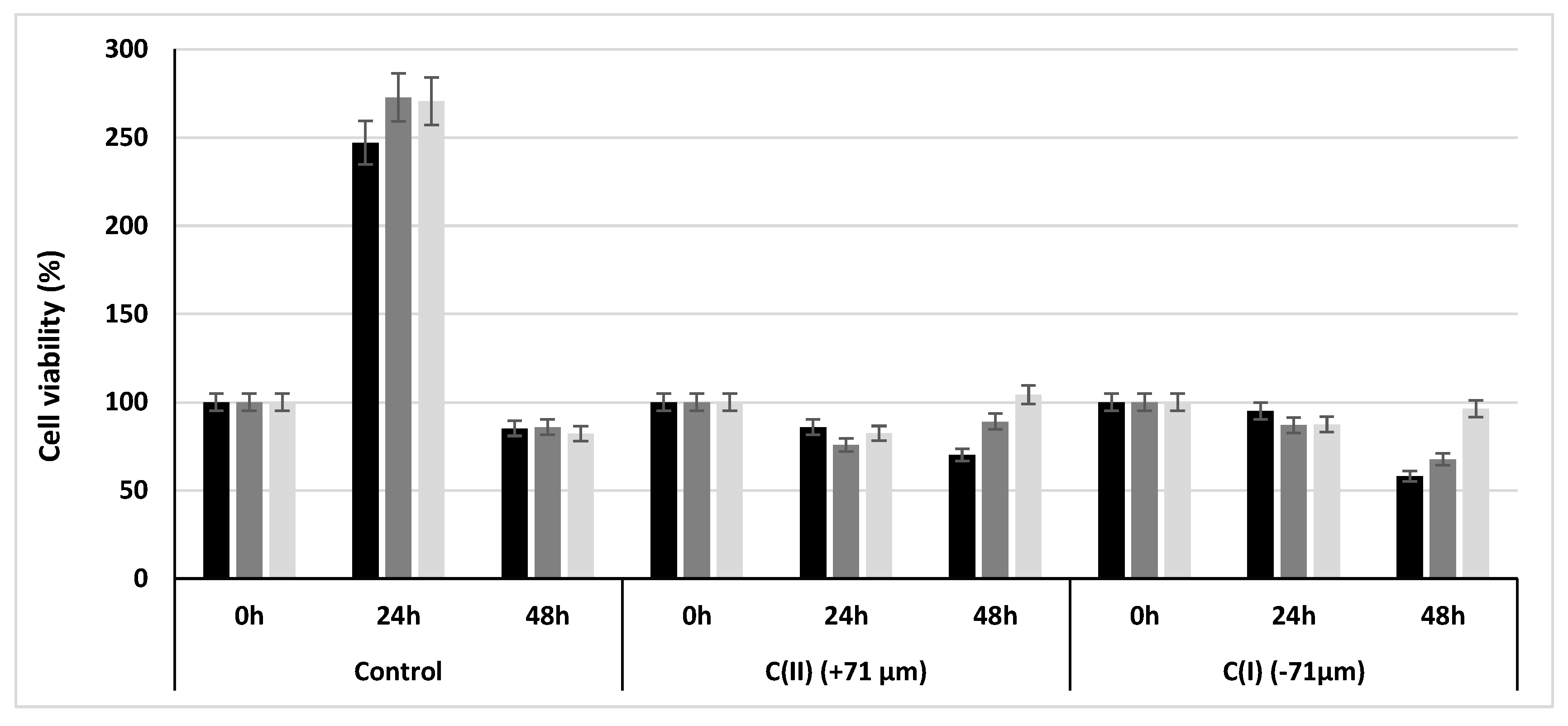
3.2.2. Cell Viability
3.2.3. Biofilm Formation
3.3. Cell Surface Hydrophobicity
3.4. Effect of Bacterial Treatment on Soluble Cd Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brahmi, M.; Ghorbel-Zouari, S. Historical timeline, global positioning and economic performance: The case of a Tunisia public mining firm. Int. Res. J. Geol. Min. 2012, 2, 88–102. [Google Scholar]
- Stemer, E. Les pays du Maghreb producteurs d’engrais phosphatés. Maghreb Machrek 1973, 59, 34–37. [Google Scholar] [CrossRef]
- Potvin, R. Réduction de la Toxicité des Effluents des Mines de Métaux de Base et Précieux à l’Aide de Méthodes de Traitement Biologique; Université du Québec en Abitibi-Témiscamingue: Rouyn-Noranda, QC, USA, 2004; 50p. [Google Scholar]
- Azzi, V.; Kanso, A.; Kaspard, V.; Kobeissi, A.; Lartiges, B.; El Samarani, A. Lactuca sativa growth in compacted and non-compacted semi-arid alkaline soil under phosphate fertilizer treatment and cadmium contamination. Soil Tillage Res. 2017, 165, 1–10. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Xiao, C.Q.; Chi, R.A. Remediation of soil cadmium pollution by biomineralization using microbial-induced precipitation: A review. World J. Microbiol. Biotechnol. 2021, 37, 208–223. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Yu, S.Y.; Li, Y.Z.; Peng, J.; Yu, J.X.; Chi, R.A.; Xiao, C.Q. Efficient bioimmobilization of cadmium contamination in phosphate mining wastelands by the phosphate solubilizing fungus Penicillium oxalicum ZP6. Biochem. Eng. J. 2022, 187, 108667. [Google Scholar] [CrossRef]
- Pérez, A.L.; Anderson, K.A. DGT estimates cadmium accumulation in wheat and potato from phosphate fertilizer applications. Sci. Total Environ. 2009, 407, 5096–5103. [Google Scholar] [CrossRef]
- Saqib, B.; Shahid Rizwan, M.; Abdus, S.; Qingling, F.; Jun, Z.; Shaaban, M.; Hongqing, H. Cadmium Immobilization Potential of Rice Straw-Derived Biochar, Zeolite and Rock Phosphate: Extraction Techniques and Adsorption Mechanism. Bull. Environ. Contam. Toxicol. 2018, 100, 727–732. [Google Scholar]
- Hamzah, A.; Hapsari, R.I.; Wisnubroto, E.I. Phytoremediation of Cadmium-contaminated agricultural land using indigenous plants. Int. J. Environ. Agric. Res. 2016, 2, 8–14. [Google Scholar]
- El Ouardia, E. Étude de la calcination du phosphate clair de youssoufia (Maroc). Afrique Sci. Rev. Int. Sci. Technol. 2010, 4, 199–211. [Google Scholar] [CrossRef]
- Sajjad, W.; Zheng, G.; Din, G.; Ma, X.; Rafiq, M.; Wang, X. Metals Extraction from Sulfide Ores with Microorganisms: The Bioleaching Technology and Recent Developments. Trans. Indian Inst. Met. 2019, 72, 559–579. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial technologies for heavy metal remediation: Effect of process conditions and current practices. Clean Technol. Environ. Policy, 2021; in press. [Google Scholar] [CrossRef]
- Taieb, I.; Ben Younes, S.; Messai, B.; Mnif, S.; Mzoughi, R.; Bakhrouf, A.; Jabeur, C.; Ayala Serrano, J.A.; Ellafi, A. Isolation, characterization and identification of a new lysinibacillus fusiformis strain zc from metlaoui phosphate laundries wastewater: Bio-treatment assays. Sustainability 2021, 13, 10072. [Google Scholar] [CrossRef]
- Boughzala, K.; Fattah, N.; Bouzouita, K.; Ben Hassine, H. Mineralogical and Chemistry study of natural phosphate of Oum El Khecheb (Gafsa, Tunisia). Rev. Sci. Matériaux 2015, 6, 11–29. [Google Scholar]
- Chaieb, K.; Chehab, O.; Zmantar, T.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. In vitro effect of pH and ethanol on biofilm formation by clinical ica-positive Staphylococcus epidermidis strains. Ann. Microbiol. 2007, 57, 431–437. [Google Scholar] [CrossRef]
- Min, S.C.; Schraft, H.; Hansen, L.T.; Mackereth, R. Effects of physicochemical surface characteristics of Listeria monocytogenes strains on attachment to glass. Food Microbiol. 2006, 23, 250–259. [Google Scholar]
- El Haddi, H.; Benbouziane, A.; Mouflih, M.; Jourani, E.; Amaghzaz, M. Siliceous forms of phosphate deposits of Cretaceous age in Oulad Abdoun basin (Central Morocco). Mineralogy, geochemistry and diagenetic phenomena. Procedia Eng. 2014, 83, 60–69. [Google Scholar] [CrossRef]
- Bounemia, L.; Mellah, A. Characterization of crude and calcined phosphates of Kef Essennoun (Djebel Onk, Algeria). J. Therm. Anal. Calorim. 2021, 146, 2049–2057. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Mezghache, H.; Hani, A. Typologie chimique des phosphates du gisement de Djemi-Djema -bassin de Djebel Onk (Algérie orientale). Géologie Méditerranéenne 2000, 27, 95–106. [Google Scholar] [CrossRef]
- Nathan, Y.; Benalioulhaj, N.; Prévôt, L.; Lucas, J. The geochemistry of cadmium in the phosphate-rich and organic-rich sediments of the Oulad-abdoun and Timahdit basins (Morocco). J. African Earth Sci. 1996, 22, 17–27. [Google Scholar] [CrossRef]
- Galai, H.; Sliman, F. Mineral characterization of the Oum El Khacheb phosphorites (Gafsa-Metlaoui basin; S Tunisia). Arab. J. Chem. 2019, 12, 1607–1614. [Google Scholar] [CrossRef]
- Gossiaux, L. Géopolitique du Phosphore au Sein de l’Union Européenne; Louvain School of Management: Ottignies Louvain, Belgium, 2020. [Google Scholar]
- Ben Younes, S.; Bouallagui, Z.; Sayadi, S. Catalytic behaviour and detoxifying ability of an atypical homotrimeric laccase from the thermophilic strain Scytalidium thermophilum on selected Azo and Triarylmethane dyes. J. Mol. Catal. B Enzym. 2012, 79, 41–48. [Google Scholar] [CrossRef]
- Ben Younes, S.; Dallali, C.; Ellafi, A.; Feriani, A.; Bouslama, L.; Sayadi, S. Extracellular Enzymatic Activities of bacterial Strains Isolated from Tunisian biotopes: Decolorization and detoxification of Indigo carmine. Catal. Lett. 2020, 151, 1248–1261. [Google Scholar] [CrossRef]
- Lejri, R.; Ben Younes, S.; Ellafi, A.; Bouallegue, A.; Moussaoui, Y.; Chaieb, M.; Mekki, A. Physico-chemical, microbial and toxicity assessment of industrial effluents from the southern Tunisian tannery. J. Water Process Eng. 2022, 47, 102686–102697. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef]
- Ellafi, A.; Lagha, R.; Ben Abdallah, F.; Bakhrouf, A. Biofilm production, adherence and hydrophobicity of starved Shigella in seawater. Afr. J. Microbiol. Res. 2012, 6, 4355–4359. [Google Scholar] [CrossRef]
- Kouidhi, B.; Zmantar, T.; Mahdouani, K.; Hentati, H.; Bakhrouf, A. Antibiotic resistance and adhesion properties of oral Enterococci associated to dental caries. BMC Microbiol. 2011, 11, 155. [Google Scholar] [CrossRef]
- Baumgarten, T.; Sperling, S.; Seifert, J.; Von Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane vesicle formation as a multiple-stress response mechanism enhances pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef]
- Chaney, R.L. Food safety issues for mineral and organic fertilizers. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2012; Volume 117. [Google Scholar]
- Kouzbour, S.; Gourich, B.; Grosb, F.; Vialb, C.; Allama, F.; Stiribac, Y. Comparative analysis of industrial processes for cadmium removal from phosphoric acid: A review. Hydrometallurgy 2019, 188, 222–247. [Google Scholar] [CrossRef]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Lemire, J.; Mailloux, R.; Auger, C.; Whalen, D.; Appanna, V.D. Pseudomonas fluorescens orchestrates a fine metabolic-balancing act to counter aluminium toxicity. Environ. Microbiol. 2010, 12, 1384–1390. [Google Scholar]

| LOG | Power Ratings | Layers | Descriptions |
|---|---|---|---|
 | 4.56 | C(I) | Pelphospharenite, coprolite, bioclastic, very slightly lithoclastic |
| 0.95 | m 1-2 | Olive-green marl | |
| 2.40 | C(II) | Homogeneous phospharenite, rich in tricalcium phosphate, carbonate | |
| 0.82 | m 2-3 | Greenish marls Pelphospharenite very marly, grey | |
| 0.40 | II | ||
| 0.33 | m 3-4 | Greenish grey marls Coarse, carbonated pelphospharenite | |
| 0.23 | C(IV) a | ||
| 0.37 | m 4a-b | ||
| 0.38 | C(IV) b | Grey pelphospharenite, very hard, very coprolite | |
| 4.13 | m 4-5 | Greenish grey marls, carbonate banks, very hard whitish ones | |
| 1.06 | C(V) | Grey Pelphospharenite | |
| 0.73 | m 5-6 | Greenish, hard marls | |
| 1.27 | C(VI) | Grey Pelphospharenite | |
| 3.60 | m 6-7a | Cherts greyish white | |
| 0.43 | C(VII) a | Carbonate, magnesian | |
| 0.72 | m 7a-b | Greenish-grey marl limestone | |
| 0.76 | C(VII) b | Grey pelphospharenites, not very marly | |
| 0.74 | m 7b-8 | Continuous carbonate benches, very hard blankets | |
| 1.19 | C(VIII) | Soft, bioclastic pelphospharenite | |
| Base shell |
| Layers | Meshes | Weight (g) | Weight Yields% | P2O5 % | CaO % | SiO2 % | MgO % | CO2 % | COrg % | Cd ppm | Zn ppm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C(I) | −71 µm | 281 ± 12 | 13.59 ± 0.023 | 11.30 ± 1.73 | 22.98 ± 1.49 | 24.10 ± 0.98 | 1.69 ± 0.07 | 13.10 ± 1.06 | 3.31 ± 0.57 | 81.38 ± 4.75 | 572 ± 19.33 |
| C(II) | +71 µm | 1670 ± 24.90 | 67.26 ± 4.51 | 29.09 ± 2.13 | 45.83 ± 2.15 | 4.97 ± 0.83 | 0.59 ± 0.08 | 10.08 ± 1.37 | 2.85 ± 0.96 | 67.23 ± 3.72 | 500 ± 24.21 |
| Strains | Isolation Temperature | Shape | Gram Staining | Catalase | Oxidase |
|---|---|---|---|---|---|
| S1 | 30 °C | Bacile | Gram+ | + | − |
| S2 | 30 °C | Bacile | Gram+ | − | − |
| S3 | 30 °C | Bacile | Gram+ | − | − |
| Control | C(II) (+71 µm) | C(I) (−71 µm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Strains | S1 | S2 | S3 | S1 | S2 | S3 | S1 | S2 | S3 |
| OD570 | 0.04 ± 0.003 | 0.04 ± 0.002 | 0.04 ± 0.003 | 0.10± 004 | 0.24 ± 0.009 | 0.30 ± 0.012 | 0.1 ± 0.0017 | 0.21 ± 0.009 | 0.35 ± 0.011 |
 |  |  | |||||||
| Strains | Control | C(II) (+71 µm) | C(I) (−71 µm) |
|---|---|---|---|
| S1 | 28 ± 2.82% | 69 ± 2.49% | 73 ± 2.07% |
| S2 | 39 ± 1.77% | 66 ± 1.95% | 77 ± 1.45% |
| S3 | 42 ± 2.80% | 82 ± 2.43% | 86 ± 2.64% |
| Strains | Cd Content (ppm) | |
|---|---|---|
| C(II) (+71 µm) | C(I) (−71 µm) | |
| Control | 67.23 ± 8.12 | 81.30 ± 9.94 |
| S1 | 59.78 ± 7.75 | 73.21 ± 9.49 |
| S2 | 61.68 ± 6.30 | 72.60 ± 7.72 |
| S3 | 58.28 ± 7.13 | 70.63 ± 8.73 |
| S1 + S2 | 57.54 ± 7 | 69.67 ± 8.57 |
| S1 + S3 | 55.43 ± 7.14 | 67.80 ± 8.74 |
| S2 + S3 | 54.24 ± 7.27 | 66.84 ± 8.90 |
| S1 + S2 + S3 | 47.68 ± 4.57 | 55.60 ± 5.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messai, B.; Taieb, I.; Ben Younes, S.; Lartiges, B.; Ben Salem, E.; Ellafi, A. Characterization of the Tunisian Phosphate Rock from Metlaoui-Gafsa Basin and Bio-Leaching Assays. Sustainability 2023, 15, 7204. https://doi.org/10.3390/su15097204
Messai B, Taieb I, Ben Younes S, Lartiges B, Ben Salem E, Ellafi A. Characterization of the Tunisian Phosphate Rock from Metlaoui-Gafsa Basin and Bio-Leaching Assays. Sustainability. 2023; 15(9):7204. https://doi.org/10.3390/su15097204
Chicago/Turabian StyleMessai, Boutheina, Ines Taieb, Sonia Ben Younes, Bruno Lartiges, Ezzedine Ben Salem, and Ali Ellafi. 2023. "Characterization of the Tunisian Phosphate Rock from Metlaoui-Gafsa Basin and Bio-Leaching Assays" Sustainability 15, no. 9: 7204. https://doi.org/10.3390/su15097204







