Utilization of Sludge from African Catfish (Clarias gariepinus) Recirculating Aquaculture Systems for Vermifiltration
Abstract
1. Introduction
2. Materials and Methods
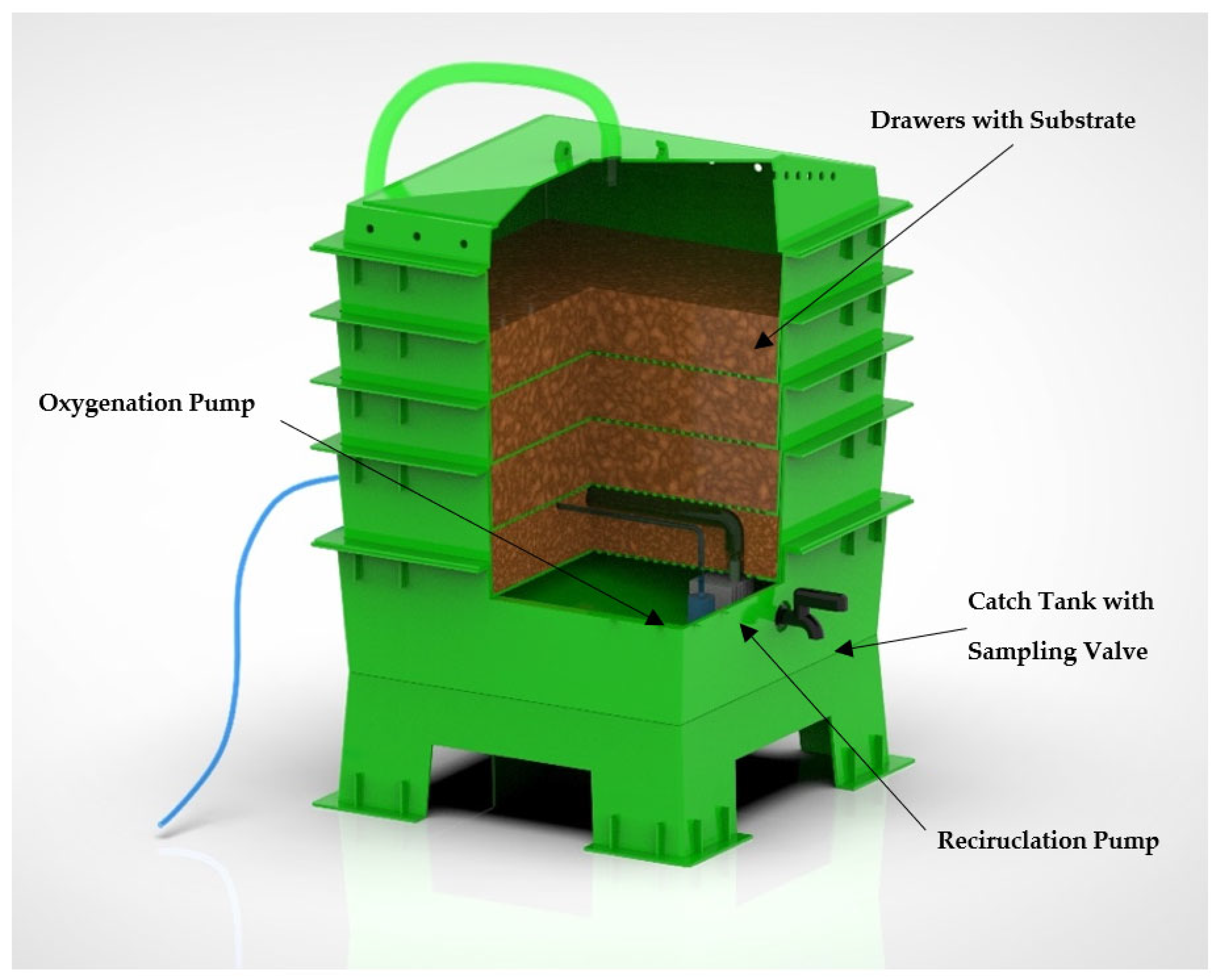
2.1. System Design
2.2. Feed Ration Calculation
2.3. Initial Worm Stocking Weigh-In
2.4. Weigh-Out
2.5. Analysis and Sampling
2.6. Statistical Analysis
3. Results
3.1. Total Worm Biomass and Growth Rate
3.2. Physicochemical Parameters (Influent and Effluent)
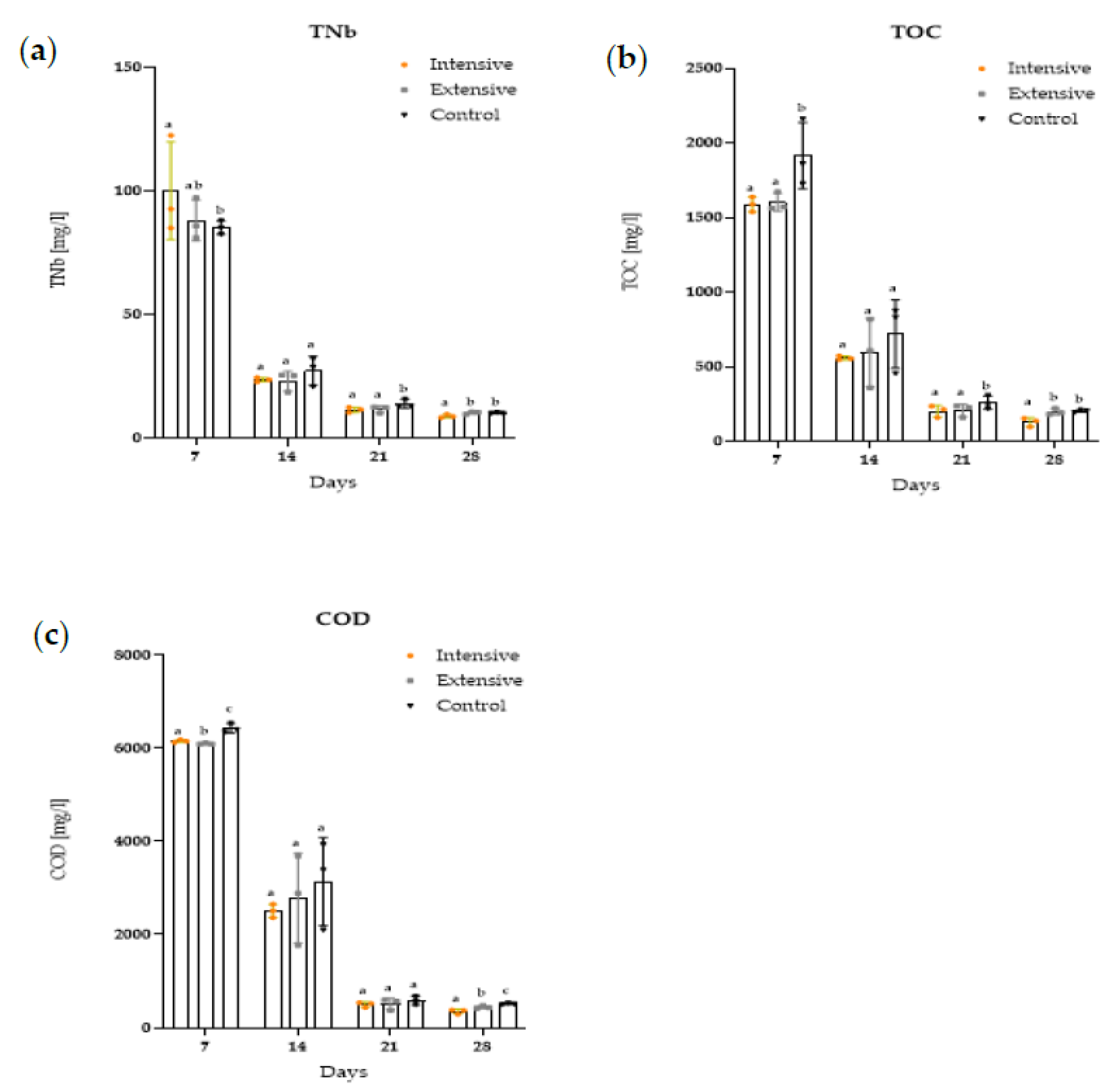
3.3. Organochemical Parameters (TNb, TOC, and COD)
4. Discussion
4.1. Growth Rate
4.2. Physicochemical Parameters: Temperature and pH
4.3. Organochemical Parameters (TNb, TOC, and COD)
4.4. Compost Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture (SOFIA) 2022: Sustainability in Action; FAO: Rome, Italy, 2022; ISBN 9789251326923. [Google Scholar]
- Alltech 2020 Global Feed Survey. 2020, pp. 1–8. Available online: https://www.alltech.com/2020-feed-survey (accessed on 10 July 2021).
- Chen, S.; Malone, R.F. Suspended Solids Control in Recirculating Aquacultural Systems. Aquaculture 1993, 112, 143–155. [Google Scholar] [CrossRef]
- Meriac, A. Smolt production and the potential for solid waste collection in Norway 2019. In Rapport 25-2019; Nofima AS: Tromsø, Norway, 2019. [Google Scholar]
- Britz, P.J.; Hecht, T. Effects of salinity on growth and survival of African sharptooth catfish (clarias gariepinus) larvae. J. Appl. Ichthyol. 1989, 5, 194–202. [Google Scholar] [CrossRef]
- Roques, J.A.C.; Schram, E.; Spanings, T.; van Schaik, T.; Abbink, W.; Boerrigter, J.; de Vries, P.; van de Vis, H.; Flik, G. The impact of elevated water nitrite concentration on physiology, growth and feed intake of African catfish Clarias gariepinus (Burchell 1822). Aquac. Res. 2015, 46, 1384–1395. [Google Scholar] [CrossRef]
- Fauji, H.; Budiardi, T.; Ekasari, J. Growth performance and robustness of African Catfish Clarias gariepinus (Burchell) in biofloc-based nursery production with different stocking densities. Aquac. Res. 2018, 49, 1339–1346. [Google Scholar] [CrossRef]
- Lefevre, S.; Wang, T.; Jensen, A.; Cong, N.V.; Huong, D.T.T.; Phuong, N.T.; Bayley, M. Air-breathing fishes in aquaculture. What can we learn from physiology? J. Fish Biol. 2014, 84, 705–731. [Google Scholar] [CrossRef]
- Fleuren; Noojien, B.V. About Us. Available online: https://www.aquacultureid.com/about-us/ (accessed on 7 May 2022).
- Richards, J.K.G. Carbon and nitrogen dynamics and greenhouse gases emissions in constructed wetlands: A review. Hydrol. Earth Syst. Sci. Discuss. 2014, 11, 7615–7657. [Google Scholar] [CrossRef]
- Turcios, A.; Papenbrock, J.; Turcios, A.E.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents—What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Strauch, S.M.; Wenzel, L.C.; Bischoff, A.; Dellwig, O.; Klein, J.; Schüch, A.; Wasenitz, B.; Palm, H.W. Commercial African Catfish (Clarias gariepinus) recirculating aquaculture systems: Assessment of element and en Aquaponics food production systems ergy pathways with special focus on the phosphorus cycle. Sustainability 2018, 10, 1805. [Google Scholar] [CrossRef]
- Goddek, S.; Appelbaum, S.; Kotzen, B.; Knaus, U.; Palm, H.W.; Haїssam Jijakli, M.; Vermeulen, T.; Strauch, S.M. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Palm, H.W.; Nievel, M.; Knaus, U. Significant factors affecting the economic sustainability of closed aquaponic 678 systems. part III: Plant units. AACL Bioflux 2014, 8, 89–106. [Google Scholar]
- Lobanov, V.P.; Combot, D.; Pelissier, P.; Labbé, L.; Joyce, A.; Martino, C.D.; Del Buono, D. Improving Plant Health through Nutrient Remineralization in Aquaponic Systems. Front. Plant Sci. 2021, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bhunia, P.; Dash, R.R. COD removal index—A mechanistic tool for predicting organics removal performance of vermifilters. Sci. Total Environ. 2018, 643, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Dash, R.R.; Bhunia, P. Effect of hydraulic loading rate and pollutants degradation kinetics in two stage hybrid macrophyte assisted vermifiltration system. Biochem. Eng. J. 2018, 132, 47–59. [Google Scholar] [CrossRef]
- Bajsa, O.; Nair, J.; Mathew, K.; Ho, G.E. Vermiculture as a tool for domestic wastewater management. Water Sci. Technol. 2004, 48, 125–132. [Google Scholar] [CrossRef]
- Sinha, R.K.; Bharambe, G.; Chaudhari, U. Sewage treatment by vermifiltration with synchronous treatment of sludge by earthworms: A low-cost sustainable technology over conventional systems with potential for decentralization. Environmentalist 2008, 28, 409–420. [Google Scholar] [CrossRef]
- Adhami, E.; Ronaghi, A.; Karimian, N.; Molavi, R. Transformation of phosphorus in highly calcareous soils under field capacity and waterlogged conditions. Soil Res. 2012, 50, 249–255. [Google Scholar] [CrossRef]
- Li, Y.S.; Robin, P.; Cluzeau, D.; Bouché, M.; Qiu, J.P.; Laplanche, A.; Hassouna, M.; Morand, P.; Dappelo, C.; Callarec, J. Vermifiltration as a stage in reuse of swine wastewater: Monitoring methodology on an experimental farm. Ecol. Eng. 2008, 32, 301–309. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Thompson, S.A. Effects of C-to-N ratio on vermicomposting of biosolids. Bioresour. Technol. 2000, 75, 7–12. [Google Scholar] [CrossRef]
- Kehres, D.B.; Thelen-Jüngling, M. Methodenbuch zur Analyse Organischer Düngemittel, Bodenverbesserungsmittel und Substrate, 5th ed.; Bundesgütegemeinschaft Kompost e.V.: Cologne, Germany, 2009; ISBN 3-939790-00-1. [Google Scholar]
- SophiéA, A.V.; Reinecke, A.J.; Hartman, L. Life-cycle of the European compost worm Dendrobaena veneta (Oligochaeta). S. Afr. J. Zool. 1991, 26, 43–48. [Google Scholar] [CrossRef]
- Reinecke, S.A.; Reinecke, A.J. The influence of lead and manganese on spermatozoa of Eisenia fetida (Oligochaeta). Soil Biol. Biochem. 1997, 29, 737–742. [Google Scholar] [CrossRef]
- Edwards, C.A.; Bater, J.E. The use of earthworms in environmental management. Soil Biol. Biochem. 1992, 24, 1683–1689. [Google Scholar] [CrossRef]
- Loehr, R.C.; Neuhauser, E.F.; Malecki, M.R. Factors affecting the vermistabilization process. Temperature, moisture content and polyculture. Water Res. 1985, 19, 1311–1317. [Google Scholar] [CrossRef]
- Musyoka, S.N.; Liti, D.M.; Ogello, E.; Waidbacher, H. Utilization of the earthworm, Eisenia fetida (Savigny, 1826) as an alternative protein source in fish feeds processing: A review. Aquac. Res. 2019, 50, 2301–2315. [Google Scholar] [CrossRef]
- Sinha, R.K.; Herat, S.; Valani, D.; Chauhan, K. Earthworms—The environmental engineers: Review of vermiculture technologies for environmental management and resource development. Int. J. Glob. Environ. Issues 2010, 10, 265. [Google Scholar] [CrossRef]
- Dhadse, S.; Satyanarayan, S.; Chaudhari, P.R.; Wate, S.R. Vermifilters: A tool for aerobic biological treatment of herbal pharmaceutical wastewater. Water Sci. Technol. 2010, 61, 2375–2380. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Li, X.; Zheng, W.; Yang, Q.; Ding, Y. A full-scale treatment of freeway toll-gate domestic sewage using ecology filter integrated constructed rapid infiltration. Ecol. Eng. 2010, 36, 827–831. [Google Scholar] [CrossRef]
- Fayolle, L.; Michaud, H.; Cluzeau, D.; Stawiecki, J. Influence of temperature and food source on the life cycle of the earthworm Dendrobaena veneta (Oligochaeta). Soil Biol. Biochem. 1997, 29, 747–750. [Google Scholar] [CrossRef]
- Rorat, A.; Kacprzak, M.; Vandenbulcke, F.; Plytycz, B. Soil amendment with municipal sewage sludge affects the immune system of earthworms Dendrobaena veneta. Appl. Soil Ecol. 2013, 64, 237–244. [Google Scholar] [CrossRef]
- Mallappa Munnoli, P.; Teixeira da Silva, J.A.; Bhosle, S. Dynamic Soil, Dynamic Plant Dynamics of the Soil-Earthworm-Plant Relationship: A Review. Dyn. Soil Dyn. Plant 2015, 4, 1–21. [Google Scholar]
- Greiner, H.G.; Stonehouse, A.M.T.; Tiegs, S.D. Cold tolerance among composting earthworm species to evaluate invasion potential. Am. Midl. Nat. 2011, 166, 349–357. [Google Scholar] [CrossRef]
- Tripathi, G.; Bhardwaj, P. Comparative studies on biomass production, life cycles and composting efficiency of Eisenia fetida (Savigny) and Lampito mauritii (Kinberg). Bioresour. Technol. 2004, 92, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.J.; Nair, J.; Mathew, K.; Ho, G. Toxicity of domestic wastewater pH to key species within an innovative decentralised vermifiltration system. Water Sci. Technol. 2007, 55, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jicong, H.; Yanyun, Q.; Guangqing, L.; Dong, R. The Influence of Temperature, pH and C/N Ratio on the Growth and Survival of Earthworms in Municipal Solid Waste. Int. Comm. Agric. Eng. 2005, 7, 1–6. [Google Scholar]
- Kaplan, D.L.; Hartenstein, R.; Neuhauser, E.F.; Malecki, M.R. Physicochemical requirements in the environment of the earthworm Eisenia foetida. Soil Biol. Biochem. 1980, 12, 347–352. [Google Scholar] [CrossRef]
- Manyuchi, M.M.; Kadzungura, L.; Boka, S. Pilot Scale Studies for Vermifiltration of 1000 m3/day of Sewage Wastewater Treatment. Asian J. Eng. Technol. 2017, 1, 13–19. [Google Scholar]
- Rico, A.L.J. Automated pH Monitoring and Controlling System for Hydroponics under Greenhouse Condition. ARPN J. Eng. Appl. Sci. 2020, 15, 523–528. [Google Scholar] [CrossRef]
- Lennard, W.; Goddek, S. Recirculating Aquaculture Technologies. In Aquaponics Food Production Systems; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Mustapha, A.; Scientific, A. Importance of pH Control in Aquaculture. 2020. Available online: https://www.researchgate.net/publication/340870601 (accessed on 11 July 2022).
- Jiang, L.; Liu, Y.; Hu, X.; Zeng, G.; Wang, H.; Zhou, L.; Tan, X.; Huang, B.; Liu, S.; Liu, S. The use of microbial-earthworm ecofilters for wastewater treatment with special attention to influencing factors in performance: A review. Bioresour. Technol. 2016, 200, 999–1007. [Google Scholar] [CrossRef]
- Singh, R.; D’Alessio, M.; Jahangeer; Meneses, Y.; Bartelt-Hunt, S.; Ray, C. Nitrogen removal in vermifiltration: Mechanisms, influencing factors, and future research needs. J. Environ. Manag. 2021, 281, 111868. [Google Scholar] [CrossRef]
- Cho, S.; Kambey, C.; Nguyen, V.K. Performance of anammox processes for wastewater treatment: A critical review on effects of operational conditions and environmental stresses. Water 2020, 12, 20. [Google Scholar] [CrossRef]
- Arora, S.; Rajpal, A.; Kumar, T.; Bhargava, R.; Kazmi, A.A. Pathogen removal during wastewater treatment by vermifiltration. Environ. Technol. 2014, 35, 2493–2499. [Google Scholar] [CrossRef]
- Lu, X.; Yin, Z.; Sobotka, D.; Wisniewski, K.; Czerwionka, K.; Xie, L.; Zhou, Q.; Makinia, J. Modeling the pH effects on nitrogen removal in the anammox-enriched granular sludge. Water Sci. Technol. 2017, 75, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Egli, K.; Fanger, U.; Alvarez, P.J.J.; Siegrist, H.; Van der Meer, J.R.; Zehnder, A.J.B. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 2001, 175, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Albina, P.; Bertron, A.; Albrecht, A.; Robinet, J.; Erable, B. Nitrate and nitrite bacterial reduction at alkaline pH and high nitrate concentrations, comparison of acetate versus dihydrogen as electron donors. J. Environ. Manag. 2021, 280, 111859. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Sinha, R.K.; Bharambe, G.; Bapat, P. Removal of high BOD and COD loadings of primary liquid waste products from dairy industry by vermi-filtration technology using earthworms. Indian J. Environ. Prot. 2007, 27, 486–501. [Google Scholar]
- Gupta, R.; Garg, V.K. Stabilization of primary sewage sludge during vermicomposting. J. Hazard. Mater. 2008, 153, 1023–1030. [Google Scholar] [CrossRef]
- Kumar, T.; Rajpal, A.; Arora, S.; Bhargava, R.; Hari Prasad, K.S.; Kazmi, A.A. A comparative study on vermifiltration using epigeic earthworm Eisenia fetida and Eudrilus eugeniae. Desalin. Water Treat. 2016, 57, 6347–6354. [Google Scholar] [CrossRef]
- Kumar, T.; Rajpal, A.; Bhargava, R.; Prasad, K.S.H. Performance evaluation of vermifilter at different hydraulic loading rate using river bed material. Ecol. Eng. 2014, 62, 77–82. [Google Scholar] [CrossRef]
- Lourenço, N.; Nunes, L.M. Is filter packing important in a small-scale vermifiltration process of urban wastewater? Int. J. Environ. Sci. Technol. 2017, 14, 2411–2422. [Google Scholar] [CrossRef]
- Lourenço, N.; Nunes, L.M. Optimization of a vermifiltration process for treating urban wastewater. Ecol. Eng. 2017, 100, 138–146. [Google Scholar] [CrossRef]
- Taylor, M.; Clarke, W.P.; Greenfield, P.F. The treatment of domestic wastewater using small-scale vermicompost filter beds. Ecol. Eng. 2003, 21, 197–203. [Google Scholar] [CrossRef]
- Kumar, T.; Bhargava, R.; Prasad, K.S.H.; Pruthi, V. Evaluation of vermifiltration process using natural ingredients for effective wastewater treatment. Ecol. Eng. 2015, 75, 370–377. [Google Scholar] [CrossRef]
- Medina-Sauza, R.M.; Álvarez-Jiménez, M.; Delhal, A.; Reverchon, F.; Blouin, M.; Guerrero-Analco, J.A.; Cerdán, C.R.; Guevara, R.; Villain, L.; Barois, I. Earthworms building up soil microbiota, a review. Front. Environ. Sci. 2019, 7, 81. [Google Scholar] [CrossRef]
- Council of the EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31991L0271 (accessed on 8 May 2022).
- Hillel, D.; Roszenweig, C.; Powlson, D.; Scow, K.; Singer, M.; Sparks, D. Encyclopedia of Soils in the Enviorment; Academic Press: Cambridge, MA, USA, 2004; Volume 1, ISBN 9780123485304. [Google Scholar]
- Brinton, W.F. Compost quality standards & guidelines: An International View. Woods End Res. Lab. Inc. 2000, 10, 1–44. [Google Scholar]
- Tiquia, S.M. Evaluation of organic matter and nutrient composition of partially decomposed and composted spent pig litter. Environ. Technol. 2003, 24, 97–107. [Google Scholar] [CrossRef] [PubMed]


| rmANOVA, p-Value | Week 1 | Week 2 | Week 3 | Week 4 | ||
|---|---|---|---|---|---|---|
| pH | intensive | 0.028 | 7.90 a ± 0.248 | 7.14 b ± 0.038 | 7.09 b ± 0.200 | 7.10 b ± 0.208 |
| extensive | 0.005 | 8.01 a ± 0.114 | 7.34 b ± 0.031 | 7.33 b ± 0.031 | 7.26 c ± 0.458 | |
| control | 0.024 | 7.99 a ± 0.093 | 7.47 b ± 0.057 | 7.47 b ± 0.032 | 7.51 b ± 0.067 | |
| Temp °C | intensive | 0 | 19.83 a ± 0.058 | 21.37 b ± 0.058 | 21.03 b ± 0.058 | 21.17 b ± 0.058 |
| extensive | 0.005 | 19.80 a ± 0.100 | 21.10 b ± 0.100 | 20.97 b ± 0.058 | 21.13 b ± 0.058 | |
| control | 0.001 | 19.63 a ± 0.058 | 20.93 b ± 0.058 | 20.83 b ± 0.058 | 20.90 b ± 0.100 | |
| TnB mg/L | intensive | <0.01 | 100.07 a ± 17.156 | 23.66 b ± 0.795 | 11.47 c ± 0.927 | 8.91 d ± 0.632 |
| extensive | <0.01 | 88.11 a ± 7.215 | 23.14 b ± 3.454 | 11.67 c ± 1.194 | 10.21 c ± 0.436 | |
| control | <0.01 | 85.15 a ± 2.562 | 27.25 b ± 5.065 | 13.84 c ±1.531 | 10.14 d ± 0.160 | |
| TOC mg/L | intensive | <0.01 | 1586.42 a ± 45.302 | 558.53 b ± 12.453 | 202.72 c ± 34.149 | 129.18 a ± 25.881 |
| extensive | <0.01 | 1601.15 a ± 54.970 | 594.95 b ± 199.529 | 207.22 c ± 36.221 | 198.47 c ± 18.349 | |
| control | <0.01 | 1915.61 a ± 191.985 | 719.96 b ± 199.142 | 259.28 c ± 38.414 | 202.39 d ± 7.497 | |
| COD mg/L | intensive | <0.01 | 6157.0 a ± 28.627 | 2510.33 b ± 127.125 | 499.67 c ± 50.712 | 349.67 d ± 46.444 |
| extensive | <0.01 | 6100.33 a ± 27.382 | 2785.33 b ± 835.812 | 514.0 c ± 105.860 | 444.33 c ± 27.249 | |
| control | <0.01 | 6430.67 a ± 88.026 | 3141.67 b ± 815.943 | 585.0 c ± 82.321 | 513.67 c ± 18.974 | |
| Final Total Biomass | Total Weight Gain | Final Weight/Worm | DGR | Mortality | |
|---|---|---|---|---|---|
| g | g | g | mg | % | |
| Int. Group (I) | 609 ± 11.52 | 56–84 | (1.36 ± 0.044)–(1.41 ± 0.025) | 6.79–8.57 | 3.46–5.84 |
| Ext. Group (E) | 432 ± 13.72 | 54–87 | (1.42 ± 0.015)–(−1.52 ± 0.036) | 8.93–(−12.50) | 3.57–5.19 |
| Value in | Value in | ||||
|---|---|---|---|---|---|
| Chemical Parameters | Unit | OS | DM | Quality Requirements BGK | Method |
| Salinity | gKCI/L | 0.73 | EN 13038 /DIN EN 13038:2012-01 | ||
| pH-value | 7.3 | DIN EN 13037: 2012-01 | |||
| Physical Parameters | |||||
| Bulk density | g/L | 1030 | Methods of BGK:2006-09 | ||
| Water content | % | 83.5 | DIN EN 13040: 2008-01 | ||
| Soil improvement | |||||
| C/N-Ratio at 450 °C | 20.7 | >30 | Calculation from single parameters | ||
| Organic matter | % | 11 | 66.4 | DIN EN 13039:2000-02 | |
| Alkaline constituents CaO | % | 0.92 | 5.55 | Methods of BGK: 2006-09 | |
| Plant nutrients | |||||
| Nitrogen total (N) | % | 0.31 | 1.86 | Methods of BGK: 2013-05 | |
| Phosphorus total (P2O5) | % | 0.431 | 2.61 | DIN EN ISO 11885: 2009-09 | |
| Potassium total (K2O) | % | 0.0429 | 0.26 | ||
| Magnesium total (MgO) | % | 0.08 | 0.51 | DIN EN ISO 11885: 2009-09 | |
| Nitrogen CaCl2-soluable | mg/L | 24.5 | Methods of BGK: 2006-09 | ||
| Ammonium (NH4-N) | mg/L | 0.722 | Methods of BGK: 2006-09 | ||
| Nitrate (NO3-N) | mg/L | 23.8 | Methods of BGK: 2006-09 | ||
| Biological Parameters | |||||
| Rotting degree | V | II | Methods of BGK: 2006-09(PT) | ||
| Hygiene | |||||
| Salmonella | in 50g | not found | n.f | Methods of BGK, chapter IV C: 2006-09 | |
| Potential pollutants | Critical limits (mg/kg DM) | ||||
| Lead (Pb) | mg/kg | 0.66 | 4 | 150 | DIN EN ISO 17294-2: 2005-02 |
| Cadmium (Cd) | mg/kg | 0.076 | 0.461 | 1.5 | DIN EN ISO 17294-2: 2017-01 |
| Chromium (Cr) | mg/kg | 0.797 | 4.83 | 100 | DIN EN ISO 17294-2: 2017-01 |
| Copper (Cu) | mg/kg | 8.4 | 51.4 | 100 | DIN EN ISO 17294-2: 2017-01 |
| Nickel (Ni) | mg/kg | 0.67 | 4.06 | 50 | DIN EN ISO 17294-2: 2017-01 |
| Mercury (Hg) | mg/kg | 0.021 | 0.13 | 1 | DIN EN 1483: 2007-07 |
| Zinc (Zn) | mg/kg | 55.9 | 339 | 400 | DIN EN ISO 17294-2: 2017-01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, J.; Schüch, A.; Sandmann, P.; Nelles, M.; Palm, H.W.; Bischoff, A. Utilization of Sludge from African Catfish (Clarias gariepinus) Recirculating Aquaculture Systems for Vermifiltration. Sustainability 2023, 15, 7429. https://doi.org/10.3390/su15097429
Klein J, Schüch A, Sandmann P, Nelles M, Palm HW, Bischoff A. Utilization of Sludge from African Catfish (Clarias gariepinus) Recirculating Aquaculture Systems for Vermifiltration. Sustainability. 2023; 15(9):7429. https://doi.org/10.3390/su15097429
Chicago/Turabian StyleKlein, Jan, Andrea Schüch, Phillip Sandmann, Michael Nelles, Harry Wilhelm Palm, and Adrian Bischoff. 2023. "Utilization of Sludge from African Catfish (Clarias gariepinus) Recirculating Aquaculture Systems for Vermifiltration" Sustainability 15, no. 9: 7429. https://doi.org/10.3390/su15097429
APA StyleKlein, J., Schüch, A., Sandmann, P., Nelles, M., Palm, H. W., & Bischoff, A. (2023). Utilization of Sludge from African Catfish (Clarias gariepinus) Recirculating Aquaculture Systems for Vermifiltration. Sustainability, 15(9), 7429. https://doi.org/10.3390/su15097429








