A Review on Pharmaceuticals and Personal Care Products Residues in the Aquatic Environment and Possibilities for Their Remediation
Abstract
:1. Introduction
2. Materials and Methods
3. Pharmaceutical Residues in Soil, Water and Wastewater
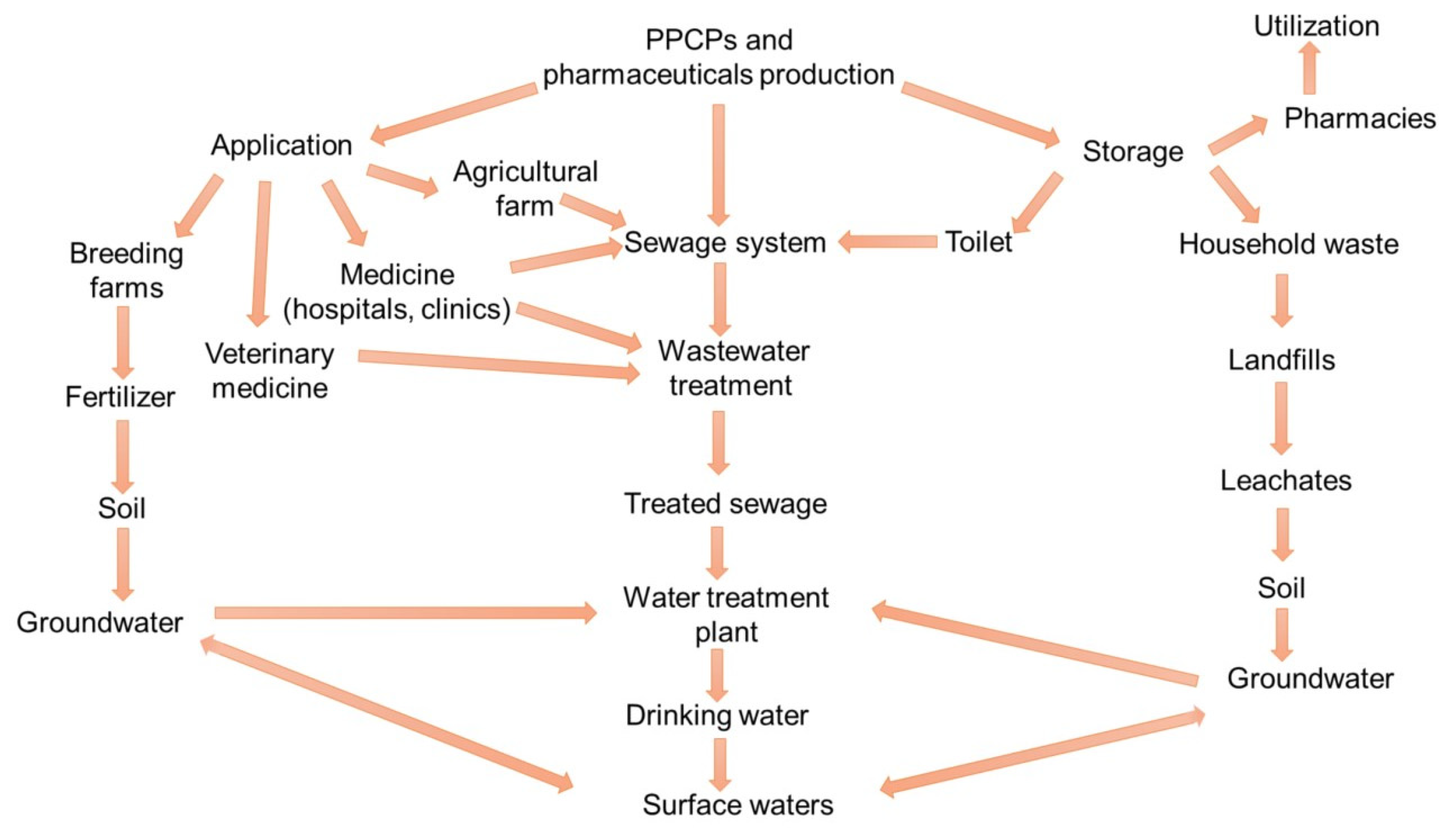
3.1. Sources of PPCPs
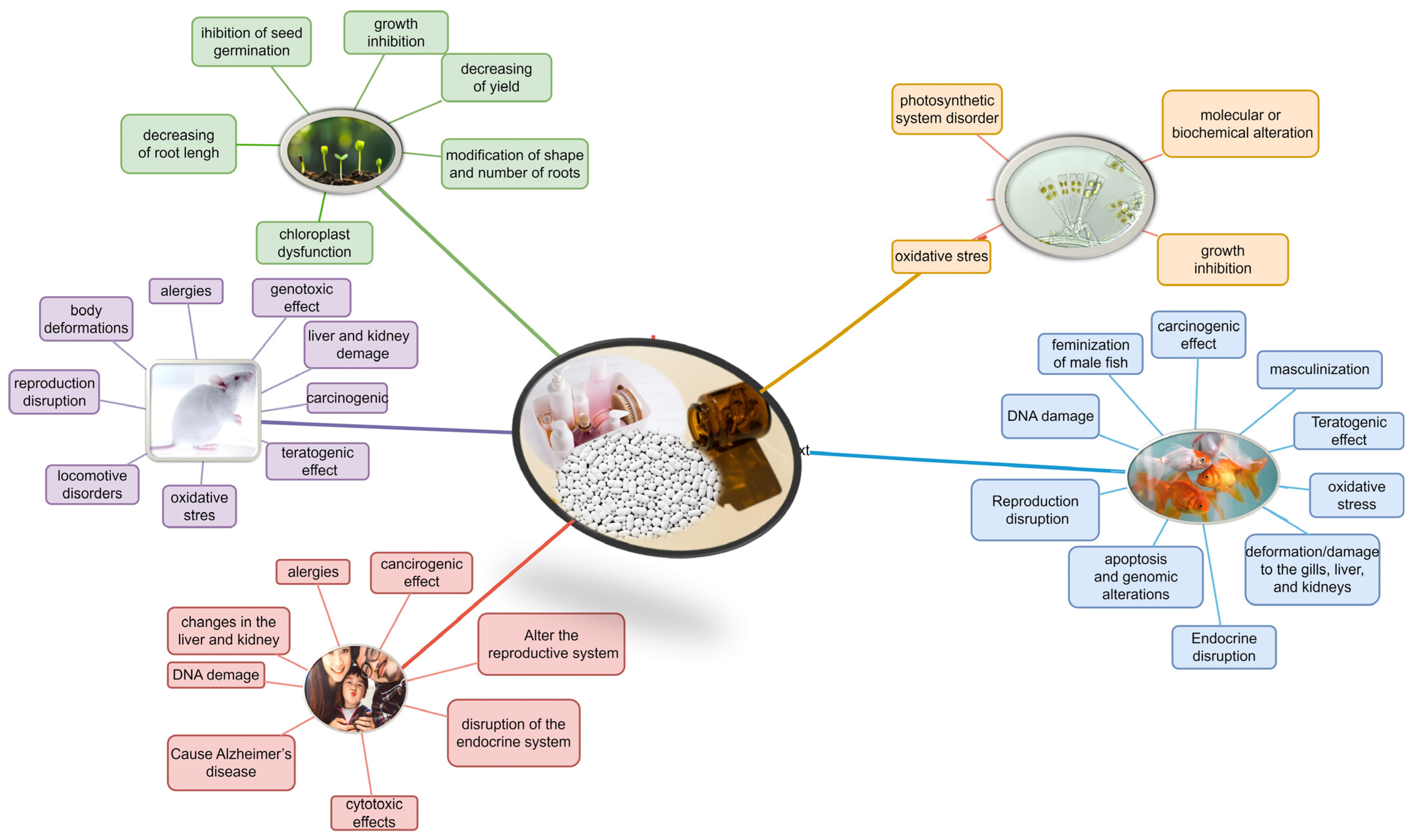
3.2. Types of PPCPs, the Risks They Pose in the Environment and Main Regulations
3.2.1. Non-Steroidal Anti-Inflammatory Drugs and Analgesic Drugs
3.2.2. Drugs Acting on Pathogenic Microorganisms
3.2.3. Hormonal Agents
3.2.4. β-Blockers
3.2.5. Hypolipidemic Drugs (Fat Regulators)
3.2.6. Psychotropic and Antiepileptic Drugs
3.2.7. Others Pharmaceuticals
3.2.8. Personal Care Products
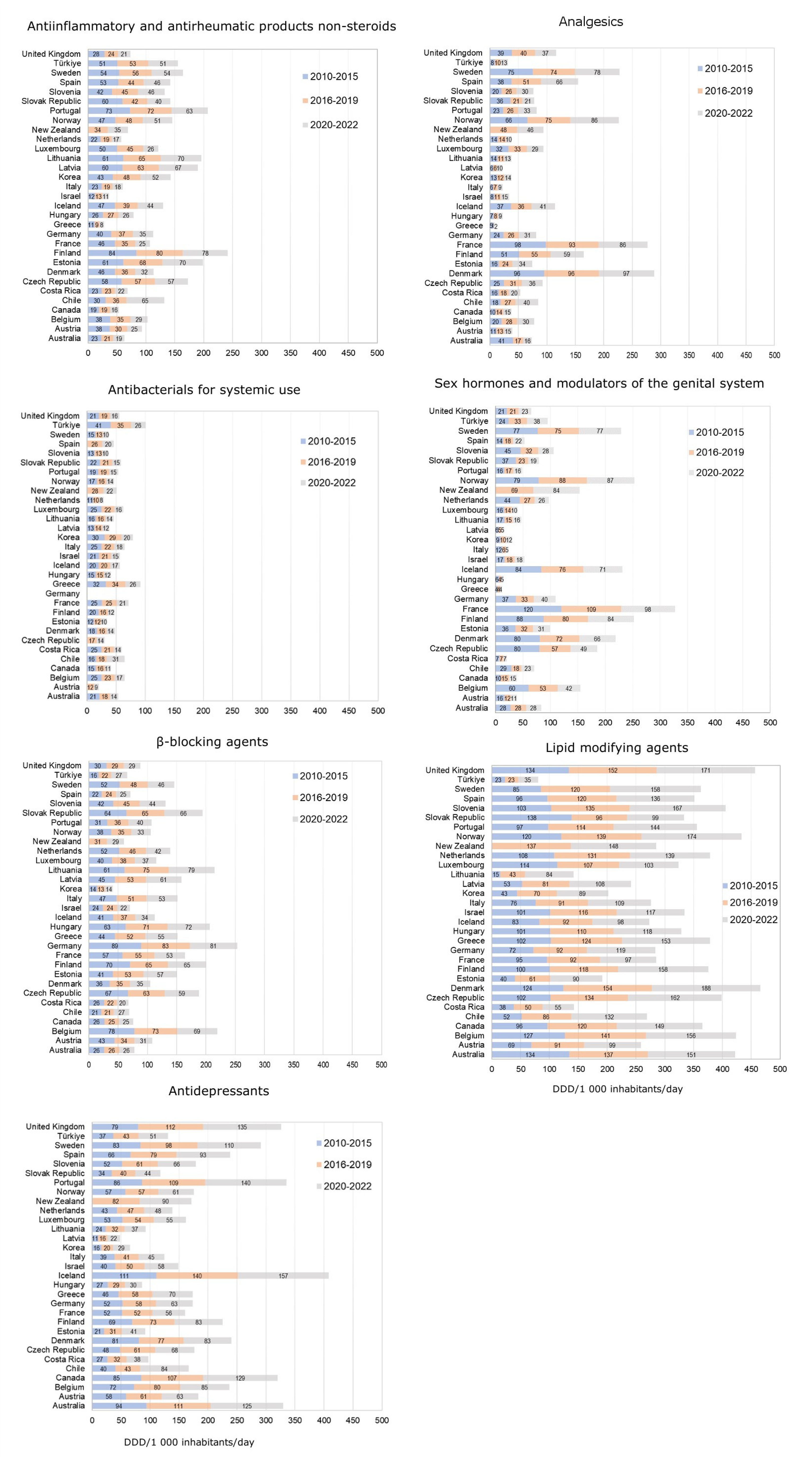
3.2.9. The EU and International Organizations Regulations Regarding the Occurrence of PPCPs in Water and Wastewater
3.3. PPCPs in Water and Wastewater
3.3.1. Concentration of Selected PPCPs in Natural Waters
3.3.2. PPCPs in Wastewater
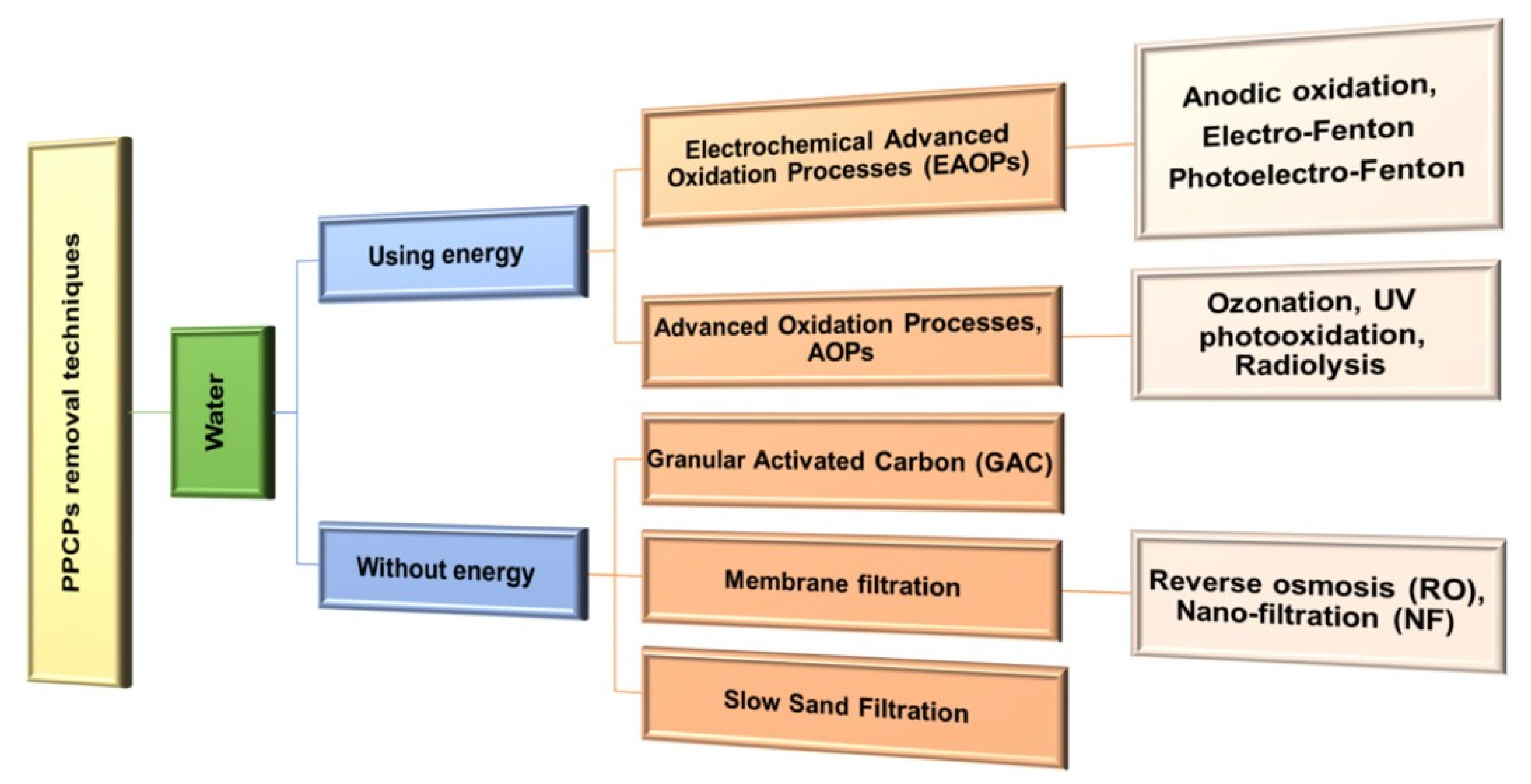
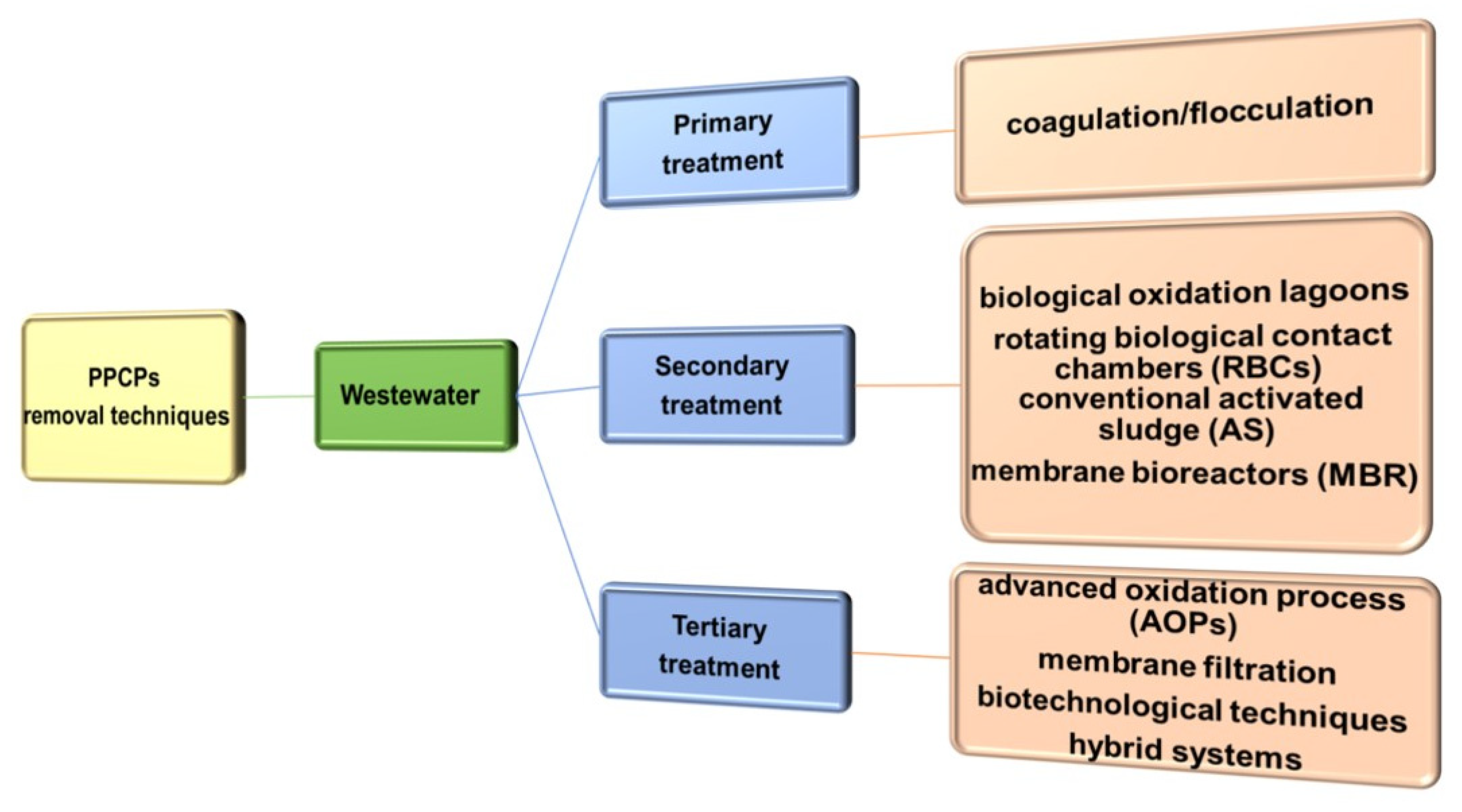
4. An Overview of Methods for Removal of Selected PPCPs Residues from Water and Wastewater
4.1. Techniques Used in Water Treatment
4.1.1. Electrocoagulation
4.1.2. Advanced Oxidation Processes in Water Treatment
4.1.3. Advanced Electrochemical Oxidation Processes in Water Treatment
4.1.4. Adsorption in Water Treatment
4.1.5. Membrane Filtration in Water Treatment
4.1.6. Microalgae and Phytoremediation Technology in Water Treatment
| Methods | Examples of PPCPs | Removal (%) | Energy Consumption (kWh/m3) | References |
|---|---|---|---|---|
| Electrocoagulation with iron anode | Oxytetracycline hydrochloride | 93.2 | 1.4 | [114] |
| Electrocoagulation with aluminum anode | Oxytetracycline hydrochloride | 87.75 | 190 | |
| O3 | Atrazine | 5.1–22.7 | 0.73 | [116] |
| Alachlor | >90 | 0.055 | ||
| Bisphenol A | >90 | 0.041 | ||
| 1,7-α-ethinylestradiol | >90 | 0.084 | ||
| O3/H2O2 | Atrazine | 6.96–92.3 | 5.96 | |
| Alachlor | - | 0.86 | ||
| Bisphenol A | - | 1.29 | ||
| 1,7-α-ethinylestradiol | - | 0.89 | ||
| UV | Atrazine | 5.1 | 499 | |
| Alachlor | 32.5 | 73.1 | ||
| Bisphenol A | 18.1 | 139 | ||
| 1,7-α-ethinylestradiol | 35.6 | 65.5 | ||
| Acyclovir | 32.22 | - | [120] | |
| UV/H2O2 | Atrazine | 7.6–9.1 | 322 | [116] |
| Alachlor | - | 48.2 | ||
| Bisphenol A | - | 102 | ||
| 1,7-α-ethinylestradiol | - | 49.5 | ||
| UV/O3 | Atrazine | 8.61–36.0 | 65.3 | |
| Alachlor | >80 | 4.75 | ||
| Bisphenol A | >80 | 5.27 | ||
| 1,7-α-ethinylestradiol | >80 | 10.6 | ||
| UV/O3/H2O2 | Atrazine | 16–51.1 | 44.1 | |
| Alachlor | >80 | 5.28 | ||
| Bisphenol A | >80 | 13.2 | ||
| 1,7-α-ethinylestradiol | >80 | 8.8 | ||
| Plasma-ozonation | Atrazine | 95.5–99.2 | 19.8 | |
| Alachlor | >80 | 15.7 | ||
| Bisphenol A | 100 | 5.65 | ||
| 1,7-α-ethinylestradiol | 100 | 4.48 | ||
| Membrane capacitive deionization (MCDI) | Acetaminophen Atenolol | 68.1 97.7 | 0.27 (control)—0.03 | [124] |
| Sulfamethoxazole | 93.2 | |||
| Electro-oxidation | 17α-ethinylestradiol (EE2) | 99.9 | - | [122] |
| Cyclodextrin polymers (PolyCyC®)-based adsorption | Progresterone | 92% (for 500 mg adsorbent amount) | - | [127] |
| Magnetic chitosan-based adsorption | Diclofenac and | Capacity: 57.5 mg/g | - | [128] |
| Clofibric acid | Capacity: 191.2 mg/g | - | ||
| Spent coffee-based adsorbents | Sulfamethoxazole | Adsorption capacity: 256 mg/g | [129] | |
| Bisfenol A | Adsorption capacity: 271 mg/g | |||
| Biochar | Diclofenac | Maximum adsorption capacity: 92.7 mg/g | - | [131] |
| Naproxen | 113 mg/g | |||
| Triclosan | 127 mg/g | |||
| Biochar from biowaste coffee grounds | Salicylic acid | 41 mg/g | - | [132] |
| Diclofenac | 39 mg/g | - | ||
| Microalgae | Trimethoprim | 0–10% | - | [137] |
| Sulfamethoxazole | 40% | - | ||
| Carbamazepine | 0–10% | - | ||
| Ciprofloxacin | ~100% | - | ||
| Triclosan | ~100% | - | ||
| Phytoremediation | Acetaminophen | ~51–99% (Phragmites australis) | - | [138] |
| 46.7–99.9% (Typha latifolia) | - |
4.2. Techniques Used in Wastewater Treatment
4.2.1. Primary, Secondary and Tertiary Treatment of Wastewater
4.2.2. Conventional Wastewater Treatment Using Activated Sludge
4.2.3. Adsorption Methods in Wastewater Treatment
4.2.4. AOP Technologies in Wastewater Treatment
| Methods | Examples of PPCPs | Groups | Concentration in Wastewater [ng/L] | Removal Rate [%] | Energy Consumption (kWh/m3) | Comments | References | |
|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | |||||||
| Activated sludge | Naproxen | Analgesics | 0.25 mg/L | - | >87% | - | small-scale pilot wastewater treatment plant | [142] |
| Ketoprofen | - | >87% | - | |||||
| Diclofenac | Anti-inflammatory | - | 49–59% | - | ||||
| Ibuprofen | Non-steroidal | - | >87% | - | ||||
| Clofibric Acid | Lipid regulators | - | 30% | - | ||||
| Musk Ketone | Fragrances | 74.5–161.3 | 6.7–18.5 | <60% | - | - | [153] | |
| Galaxolide | - | 2310–3490 | - | - | - | |||
| Tonalide | - | nd—360 | - | - | ||||
| N,N-diethyl-3-methylbenzamide | Mosquito and insect repellants | 1220–2520 | 574–886 | 40% | - | - | [154] | |
| Flocculation and sedimentation | Erythromycin | Antibiotics | 251–515 | 239–432 | 4.7–16 | - | - | [146] |
| Sulfamethazine | 2.4–3.2 | 0.9–1.4 | 50–72 | - | - | |||
| Roxithromycin | 0.59–1.31 | 0.62–1.36 | −1.3 | - | - | |||
| Carbamazepine | Anticonvulsant | 3.1 | 2.98 | 3.6 | - | - | ||
| Diclofenac | Anti-inflammatory | - | - | 10–38 | - | - | [143] | |
| Ibuprofen | Non-steroidal | - | - | 9–27 | - | - | ||
| Estrone | Hormones | 13–315 | 3–83 | 43–82 | - | - | [149] | |
| 17-β-Estradiol | 20–199 | 4–107 | >52 | - | - | |||
| Progesterone | 163–904 | 0.2–25 | 89–98 | - | - | |||
| Oseltamivir | Antiviral | >42.7 | >17.3 | >42 | - | - | [155] | |
| Methylphenidate | Central nervous system | 50–270 | >50–170 | >81 | - | - | ||
| Paraben | Preservatives | 12–61 | 6.9 | - | - | - | ||
| Triclosan | Soaps and shampoos | 2–98 | >22 | - | - | - | ||
| Synthetic musks | Fragrances | - | - | 61–97 | - | - | [153] | |
| Polycyclic musks | Fragrances | - | - | 46–63% | - | - | ||
| Ozonation | Erythromycin | Antibiotics | - | - | <20 | - | Ozone dosages of 2.5 and 7 g/m3 | [156] |
| Trimethoprim | - | - | >80 | |||||
| Amoxycylin | - | - | - | - | highest removal efficiency at high pH | [152] | ||
| Ketoprofen | Analgesics | 70–220 | 0–80 | 0.73–0.084 | [145] | |||
| Sertraline | Antidepressants | - | - | 20–80 | Ozone dosages of 2.5 and 7 g/m3 | [156] | ||
| Ibuprofen | Non-steroidal | - | - | 20–80 | ||||
| 17-β-Estradiol | Hormones | - | - | >80 | ||||
| Ozonation and granular activated carbons | Erythromycin | Antibiotics | 251–515 | 23–42 | 90–92 | 30% more expensive than ozonation | - | [146] |
| Sulfamethazine | 2.4–3.2 | 0.28–0.33 | 86–91 | - | ||||
| Trimethoprim | - | - | >80 | - | [156] | |||
| Roxithromycin | 0.59–1.31 | 0.5–0.9 | 18–30 | - | [146] | |||
| Carbamazepine | Anticonvulsant | 3.1 | ND | - | - | |||
| Sertraline | Antidepressants | - | - | >80 | - | [156] | ||
| Diclofenac | Anti-inflammatory | 96–98 | [143] | |||||
| Ibuprofen | Non-steroidal | - | - | >80 | - | [156] | ||
| 17-β-Estradiol | Hormones | - | - | >80 | - | |||
| Secondary effluent–ozonation–sand filtration | Atenolol | β-blockers | - | - | 100 | 0.035 | - | [157] |
| Bisphenol A | Sunscreen agents | 834 | 338 | >95 | 0.117 | - | [158] | |
| N,N-diethyl-3-methylbenzamide | Mosquito and insect repellants | 805 | 290 | 48 | - | |||
| Biofiltration–ozonation–soil aquifer treatment | Primidone | Anticonvulsant | - | - | 65 | Ozone dose 10 mg O3·/L | [155] | |
| Iopromide | Agent for intravascular | - | - | 52 | 0.12 | |||
| Activated carbon ultrafiltration (PCA-UF) | Sulfamethoxazole | Antibiotic | - | - | <80% | - | at <5000 BV * | [145] |
| Ciprofloxacin | 2291 | 779 | 63 | - | [158] | |||
| Carbamazepine | Anticonvulsant | - | - | >80% | - | carbon usage of approx. 25–35 g/m3 | [145] | |
| Primidone | - | - | <80% | - | [145] | |||
| Atenolol | β-blockers | 1274 | 682 | 88 | - | - | [158] | |
| 17β-Estradiol | Hormones | 14 | 1.3 | >61 | - | - | ||
| Nanofiltration | Naproxen | Analgesics | - | - | 86.9% | - | Green-synthesized copper nanoparticles (Cu NPs) | [150] |
| Ibuprofen | Non-steroidal | - | - | 74.4% | - | |||
| Ibuprofen | - | - | >90 | - | - | [159] | ||
| Ultrafiltration membrane bioreactor | Metformin | Antihyperglycemic | - | - | 95 | Ultrafiltration required higher costs compared to sand filtration | - | [160,161] |
| Hydroxybupropion | Antidepressant | - | - | 82 | - | |||
| Electrochemical advanced oxidation processes (EAOPs) | Venlafaxine | Antidepressant | 40–2987 | 60–2563 | >68 | [162] | ||
| Metoprolol | β-blockers | 4–810 | 3–435 | >52 | ||||
| Anerobic digestion with algal bioreactor | Diclofenac | Anti-inflammatory | - | - | 50 | - | C. sorokiniana | [133] |
| Ibuprofen | Non-steroidal | - | - | 100 | ||||
| Carbamazepine | Anticonvulsant | 30 | ||||||
4.2.5. Membrane Technology in Wastewater Treatment
4.2.6. Biotechnological Methods in Wastewater Treatment
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, U.; Adelodun, B.; Cabreros, C.; Kumar, P.; Suresh, S.; Dey, A.; Ballesteros, F.; Bontempi, E. Occurrence, Transformation, Bioaccumulation, Risk and Analysis of Pharmaceutical and Personal Care Products from Wastewater: A Review. Environ. Chem. Lett. 2022, 20, 3883–3904. [Google Scholar] [CrossRef] [PubMed]
- Phonsiri, V.; Choi, S.; Nguyen, C.; Tsai, Y.-L.; Coss, R.; Kurwadkar, S. Monitoring Occurrence and Removal of Selected Pharmaceuticals in Two Different Wastewater Treatment Plants. SN Appl. Sci. 2019, 1, 798. [Google Scholar] [CrossRef]
- Adhikari, S.; Kumar, R.; Driver, E.M.; Perleberg, T.D.; Yanez, A.; Johnston, B.; Halden, R.U. Mass Trends of Parabens, Triclocarban and Triclosan in Arizona Wastewater Collected after the 2017 FDA Ban on Antimicrobials and during the COVID-19 Pandemic. Water Res. 2022, 222, 118894. [Google Scholar] [CrossRef] [PubMed]
- Sangion, A.; Gramatica, P. Ecotoxicity Interspecies QAAR Models from Daphnia Toxicity of Pharmaceuticals and Personal Care Products. SAR QSAR Environ. Res. 2016, 27, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Juárez, M.; Linares-Hernández, I.; Martínez-Miranda, V.; Teutli-Sequeira, E.A.; Castillo-Suárez, L.A.; Sierra-Sánchez, A.G. SARS-CoV-2 Pharmaceutical Drugs: A Critical Review on the Environmental Impacts, Chemical Characteristics, and Behavior of Advanced Oxidation Processes in Water. Environ. Sci. Pollut. Res. 2022, 29, 67604–67640. [Google Scholar] [CrossRef] [PubMed]
- González Peña, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public. Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets Ltd. Pharmaceutical Drugs Global Market Report 2023—Research and Markets. Available online: https://www.researchandmarkets.com/reports/5781212/pharmaceutical-drugs-global-market-report (accessed on 6 November 2023).
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and Chemical Analysis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environmental Systems: A Review. Int. J. Environ. Res. Public. Health 2019, 16, 3026. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Ahmed, S.A.; El-Boghdady, N.A.; Metwally, N.S.; Nasr, N.N. Protective Effects of Betanin against Paracetamol and Diclofenac Induced Neurotoxicity and Endocrine Disruption in Rats. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2019, 24, 645–651. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and Removal of Pharmaceuticals in Wastewater Treatment Plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, Fate, and Effects of Pharmaceuticals and Personal Care Products in Aquatic Environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Hu, L.-X.; Zhao, J.-H.; Han, Y.; Liu, Y.-S.; Zhao, J.-L.; Yang, B.; Ying, G.-G. Suspect, Non-Target and Target Screening of Pharmaceuticals and Personal Care Products (PPCPs) in a Drinking Water System. Sci. Total Environ. 2022, 808, 151866. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and Personal Care Products (PPCPs) in the Freshwater Aquatic Environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Encarnação, T.; Palito, C.; Pais, A.A.C.C.; Valente, A.J.M.; Burrows, H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules 2020, 25, 3639. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of Ozonation for Pharmaceuticals and Personal Care Products Removal from Water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Strategic Approach to Pharmaceuticals in the Environment—Thursday, 17 September 2020. Available online: https://www.europarl.europa.eu/doceo/document/TA-9-2020-0226_EN.html (accessed on 6 November 2023).
- Organisation for Economic Co-Operation and Development. OECD Management of Pharmaceutical Household Waste: Limiting Environmental Impacts of Unused or Expired Medicine; Organisation for Economic Co-Operation and Development: Paris, France, 2022.
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Holmström, K.; Gräslund, S.; Wahlström, A.; Poungshompoo, S.; Bengtsson, B.-E.; Kautsky, N. Antibiotic Use in Shrimp Farming and Implications for Environmental Impacts and Human Health. Int. J. Food Sci. Technol. 2003, 38, 255–266. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of Plant–Pharmaceutical Interactions: From Uptake and Effects in Crop Plants to Phytoremediation in Constructed Wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and Removal of Pharmaceuticals in a Municipal Sewage Treatment System in the South of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Fogg, L.A.; Blackwell, P.A.; Kay, P.; Pemberton, E.J.; Croxford, A. Veterinary Medicines in the Environment. Rev. Environ. Contam. Toxicol. 2004, 180, 1–91. [Google Scholar] [CrossRef]
- Ferrey, M.L.; Coreen Hamilton, M.; Backe, W.J.; Anderson, K.E. Pharmaceuticals and Other Anthropogenic Chemicals in Atmospheric Particulates and Precipitation. Sci. Total Environ. 2018, 612, 1488–1497. [Google Scholar] [CrossRef]
- Paíga, P.; Delerue-Matos, C. Determination of Pharmaceuticals in Groundwater Collected in Five Cemeteries’ Areas (Portugal). Sci. Total Environ. 2016, 569–570, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in Drinking Water and Water/Sewage Treatment Plants: A Review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment—Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, V.; Murugan, S.; Kumaresan, R.; Narayanan, M.; Sillanpää, M.; Viet N Vo, D.; Kushwaha, O.S.; Jenis, P.; Potdar, P.; Gadiya, S. Sustainable Adsorbents for the Removal of Pharmaceuticals from Wastewater: A Review. Chemosphere 2022, 300, 134597. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Occurrence, Fate, and Removal of Pharmaceutical Residues in the Aquatic Environment: A Review of Recent Research Data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of Emerging Pollutants in Urban Wastewater and Their Removal through Biological Treatment Followed by Ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Environmental Working Group. Study: Almost Half of All ‘Natural’ Personal Care Products Contain Known Carcinogen. Available online: https://www.ewg.org/news-insights/statement/study-almost-half-all-natural-personal-care-products-contain-known (accessed on 6 November 2023).
- Robinson, P.F.; Liu, Q.-T.; Riddle, A.M.; Murray-Smith, R. Modeling the Impact of Direct Phototransformation on Predicted Environmental Concentrations (PECs) of Propranolol Hydrochloride in UK and US Rivers. Chemosphere 2007, 66, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.C.; Stefan, M.I.; Parnis, J.M.; Metcalfe, C.D. Direct UV Photolysis of Selected Pharmaceuticals, Personal Care Products and Endocrine Disruptors in Aqueous Solution. Water Res. 2015, 84, 350–361. [Google Scholar] [CrossRef]
- O’Flynn, D.; Lawler, J.; Yusuf, A.; Parle-McDermott, A.; Harold, D.; Cloughlin, T.M.; Holland, L.; Regan, F.; White, B. A Review of Pharmaceutical Occurrence and Pathways in the Aquatic Environment in the Context of a Changing Climate and the COVID-19 Pandemic. Anal. Methods 2021, 13, 575–594. [Google Scholar] [CrossRef]
- Lacey, C.; Basha, S.; Morrissey, A.; Tobin, J.M. Occurrence of Pharmaceutical Compounds in Wastewater Process Streams in Dublin, Ireland. Environ. Monit. Assess. 2012, 184, 1049–1062. [Google Scholar] [CrossRef]
- Canosa, P.; Morales, S.; Rodríguez, I.; Rubí, E.; Cela, R.; Gómez, M. Aquatic Degradation of Triclosan and Formation of Toxic Chlorophenols in Presence of Low Concentrations of Free Chlorine. Anal. Bioanal. Chem. 2005, 383, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public. Health 2022, 19, 7717. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Gandhi, K.; Suresh Kumar, M. A Global Overview of Endocrine Disrupting Chemicals in the Environment: Occurrence, Effects, and Treatment Methods. Int. J. Environ. Sci. Technol. 2023, 20, 12875–12902. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Scheil, V.; Schwaiger, J. Ultrastructural Effects of Pharmaceuticals (Carbamazepine, Clofibric Acid, Metoprolol, Diclofenac) in Rainbow Trout (Oncorhynchus Mykiss) and Common Carp (Cyprinus Carpio). Anal. Bioanal. Chem. 2007, 387, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Fort, D.J.; Mathis, M.B.; Hanson, W.; Fort, C.E.; Navarro, L.T.; Peter, R.; Büche, C.; Unger, S.; Pawlowski, S.; Plautz, J.R. Triclosan and Thyroid-Mediated Metamorphosis in Anurans: Differentiating Growth Effects from Thyroid-Driven Metamorphosis in Xenopus laevis. Toxicol. Sci. 2011, 121, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Market: Pharmaceutical Consumption. Available online: https://stats.oecd.org/Index.aspx?QueryId=30135 (accessed on 16 November 2023).
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Multi-Residue Method for the Analysis of Pharmaceutical Compounds in Sewage Sludge, Compost and Sediments by Sonication-Assisted Extraction and LC Determination. J. Sep. Sci. 2010, 33, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Pharmaceutical Residues in Water and Sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere 2015, 134, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Barodia, S.K.; Sengupta, S.; Basu, J.K. Aqueous Degradation Kinetics of Pharmaceutical Drug Diclofenac by Photocatalysis Using Nanostructured Titania–Zirconia Composite Catalyst. Int. J. Environ. Sci. Technol. 2015, 12, 317–326. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Sheikh Abdullah, S.R.; Al-Attabi, A.W.N.; Nash, D.A.H.; Anuar, N.; Rahman, N.A.; Sulistiyaning Titah, H. Removal of Ibuprofen, Ketoprofen, COD and Nitrogen Compounds from Pharmaceutical Wastewater Using Aerobic Suspension-Sequencing Batch Reactor (ASSBR). Sep. Purif. Technol. 2016, 157, 215–221. [Google Scholar] [CrossRef]
- Branco, G.S.; Moreira, R.G.; Borella, M.I.; de Paiva Camargo, M.; Muñoz-Peñuela, M.; Gomes, A.D.; Tolussi, C.E. Nonsteroidal Anti-Inflammatory Drugs Act as Endocrine Disruptors in Astyanax lacustris (Teleostei: Characidae) Reproduction: An Ex Vivo Approach. Aquat. Toxicol. 2021, 232, 105767. [Google Scholar] [CrossRef]
- Lind, D.V.; Main, K.M.; Kyhl, H.B.; Kristensen, D.M.; Toppari, J.; Andersen, H.R.; Andersen, M.S.; Skakkebæk, N.E.; Jensen, T.K. Maternal Use of Mild Analgesics during Pregnancy Associated with Reduced Anogenital Distance in Sons: A Cohort Study of 1027 Mother–Child Pairs. Hum. Reprod. 2017, 32, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Braund, R.; Warren, D.; Peake, B. TiO2-Assisted Photodegradation of Pharmaceuticals—A Review. Open Chem. 2012, 10, 989–1027. [Google Scholar] [CrossRef]
- Watanabe, N.; Bergamaschi, B.A.; Loftin, K.A.; Meyer, M.T.; Harter, T. Use and Environmental Occurrence of Antibiotics in Freestall Dairy Farms with Manured Forage Fields. Environ. Sci. Technol. 2010, 44, 6591–6600. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An Overview on the Environmental Occurrence, Toxicity, Degradation, and Removal Methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence and Risk Assessment of Azole Antifungal Drugs in Water and Wastewater. Ecotoxicol. Environ. Saf. 2020, 187, 109868. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G. Removal of a Synthetic Broad-Spectrum Antimicrobial Agent, Triclosan, in Wastewater Treatment Systems: A Short Review. Environ. Eng. Res. 2015, 20, 111–120. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Galani, A.; Alygizakis, N.; Aalizadeh, R.; Kastritis, E.; Dimopoulos, M.-A.; Thomaidis, N.S. Patterns of Pharmaceuticals Use during the First Wave of COVID-19 Pandemic in Athens, Greece as Revealed by Wastewater-Based Epidemiology. Sci. Total Environ. 2021, 798, 149014. [Google Scholar] [CrossRef]
- Kumari, M.; Kumar, A. Environmental and Human Health Risk Assessment of Mixture of COVID-19 Treating Pharmaceutical Drugs in Environmental Waters. Sci. Total Environ. 2022, 812, 152485. [Google Scholar] [CrossRef]
- Morales-Paredes, C.A.; Rodríguez-Díaz, J.M.; Boluda-Botella, N. Pharmaceutical Compounds Used in the COVID-19 Pandemic: A Review of Their Presence in Water and Treatment Techniques for Their Elimination. Sci. Total Environ. 2022, 814, 152691. [Google Scholar] [CrossRef]
- Teymoorian, T.; Teymourian, T.; Kowsari, E.; Ramakrishna, S. Direct and Indirect Effects of SARS-CoV-2 on Wastewater Treatment. J. Water Process Eng. 2021, 42, 102193. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the Art of Tertiary Treatment Technologies for Controlling Antibiotic Resistance in Wastewater Treatment Plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef] [PubMed]
- Abelenda-Alonso, G.; Padullés, A.; Rombauts, A.; Gudiol, C.; Pujol, M.; Alvarez-Pouso, C.; Jodar, R.; Carratalà, J. Antibiotic Prescription during the COVID-19 Pandemic: A Biphasic Pattern. Infect. Control Hosp. Epidemiol. 2020, 41, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Dou, W.-Y.; Ma, Y.-F.; Liu, Y.-S. Development and Validation of Sensitive Methods for Simultaneous Determination of 9 Antiviral Drugs in Different Various Environmental Matrices by UPLC-MS/MS. Chemosphere 2021, 282, 131047. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Li, C.; Dhangar, K.; Kumar, M. Predicted Occurrence, Ecotoxicological Risk and Environmentally Acquired Resistance of Antiviral Drugs Associated with COVID-19 in Environmental Waters. Sci. Total Environ. 2021, 776, 145740. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Manzoor, M.; Gul, I.; Zafar, R.; Jamil, H.I.; Niazi, A.K.; Ali, M.A.; Park, T.J.; Arshad, M. Phytotoxicity of Different Antibiotics to Rice and Stress Alleviation upon Application of Organic Amendments. Chemosphere 2020, 258, 127353. [Google Scholar] [CrossRef] [PubMed]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Analytical Strategies for the Determination of Antiviral Drugs in the Aquatic Environment. Trends Environ. Anal. Chem. 2019, 24, e00071. [Google Scholar] [CrossRef]
- Schaffner, R.M.D.W. A Meta-Analysis of the Published Literature on the Effectiveness of Antimicrobial Soaps. J. Food Prot. 2011, 74, 1875–1882. [Google Scholar] [CrossRef]
- Alaton, I.A.; Dogruel, S.; Baykal, E.; Gerone, G. Combined Chemical and Biological Oxidation of Penicillin Formulation Effluent. J. Environ. Manag. 2004, 73, 155–163. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Natarajan, L.; Seenivasan, R.; Chandrasekaran, N.; Mukherjee, A. Tetracycline Affects the Toxicity of P25 N-TiO2 towards Marine Microalgae Chlorella sp. Environ. Res. 2019, 179, 108808. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, Y.; Zhang, Y.; Li, Z.; Yu, Y.; Feng, L.; Zhang, S.; Xu, L. Distribution and Human Health Risk Assessment of Antibiotic Residues in Large-Scale Drinking Water Sources in Chongqing Area of the Yangtze River. Environ. Res. 2020, 185, 109386. [Google Scholar] [CrossRef] [PubMed]
- Gee, R.H.; Charles, A.; Taylor, N.; Darbre, P.D. Oestrogenic and Androgenic Activity of Triclosan in Breast Cancer Cells. J. Appl. Toxicol. 2008, 28, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Bina, B.; Ebrahimi, A.; Yavari, Z.; Mohammadi, F.; Rahimi, S. The Occurrence, Fate, and Distribution of Natural and Synthetic Hormones in Different Types of Wastewater Treatment Plants in Iran. Chin. J. Chem. Eng. 2018, 26, 1132–1139. [Google Scholar] [CrossRef]
- Lei, K.; Lin, C.-Y.; Zhu, Y.; Chen, W.; Pan, H.-Y.; Sun, Z.; Sweetman, A.; Zhang, Q.; He, M.-C. Estrogens in Municipal Wastewater and Receiving Waters in the Beijing-Tianjin-Hebei Region, China: Occurrence and Risk Assessment of Mixtures. J. Hazard. Mater. 2020, 389, 121891. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Zheng, X.; Wang, X.; Huang, X. Fate and Removal of Typical Pharmaceuticals and Personal Care Products by Three Different Treatment Processes. Sci. Total Environ. 2013, 447, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; Darisaw, S.; Ehie, O.; Wang, G. Simultaneous Quantification of Polycyclic Aromatic Hydrocarbons (PAHs), Polychlorinated Biphenyls (PCBs), and Pharmaceuticals and Personal Care Products (PPCPs) in Mississippi River Water, in New Orleans, Louisiana, USA. Chemosphere 2007, 66, 1057–1069. [Google Scholar] [CrossRef]
- Flammarion, P.; Brion, F.; Babut, M.; Garric, J.; Migeon, B.; Noury, P.; Thybaud, E.; Palazzi, X.; Tyler, C.R. Induction of Fish Vitellogenin and Alterations in Testicular Structure: Preliminary Results of Estrogenic Effects in Chub (Leuciscus cephalus). Ecotoxicology 2000, 9, 127–135. [Google Scholar] [CrossRef]
- Mina, O.; Gall, H.E.; Elliott, H.A.; Watson, J.E.; Mashtare, M.L.; Langkilde, T.; Harper, J.P.; Boyer, E.W. Estrogen Occurrence and Persistence in Vernal Pools Impacted by Wastewater Irrigation Practices. Agric. Ecosyst. Environ. 2018, 257, 103–112. [Google Scholar] [CrossRef]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP Effluents and Their Solar Photodegradation in Aquatic Environment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef]
- Leyva, E.; Moctezuma, E.; López, M.; Baines, K.M.; Zermeño, B. Photocatalytic Degradation of β-Blockers in TiO2 with Metoprolol as Model Compound. Intermediates and Total Reaction Mechanism. Catal. Today 2019, 323, 14–25. [Google Scholar] [CrossRef]
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and Enantiomer Profiles of β-Blockers in Wastewater and a Receiving Water Body and Adjacent Soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, C.; Woodhouse, A.J.; Miao, X.-S.; Metcalfe, C.D.; Moon, T.W.; Trudeau, V.L. The Human Lipid Regulator, Gemfibrozil Bioconcentrates and Reduces Testosterone in the Goldfish, Carassius Auratus. Aquat. Toxicol. 2005, 73, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shi, X.; Liu, Y.; Feng, L.; Zhang, L. Degradation of Clofibric Acid in UV/Chlorine Disinfection Process: Kinetics, Reactive Species Contribution and Pathways. R. Soc. Open Sci. 2018, 5, 171372. [Google Scholar] [CrossRef] [PubMed]
- Nkoom, M.; Lu, G.; Liu, J.; Yang, H.; Dong, H. Bioconcentration of the Antiepileptic Drug Carbamazepine and Its Physiological and Biochemical Effects on Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 172, 11–18. [Google Scholar] [CrossRef]
- Munari, M.; Marin, M.G.; Matozzo, V. Effects of the Antidepressant Fluoxetine on the Immune Parameters and Acetylcholinesterase Activity of the Clam Venerupis philippinarum. Mar. Environ. Res. 2014, 94, 32–37. [Google Scholar] [CrossRef]
- Malmborg, J.; Magnér, J. Pharmaceutical Residues in Sewage Sludge: Effect of Sanitization and Anaerobic Digestion. J. Environ. Manag. 2015, 153, 1–10. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, K.-H.; Lee, M.; Lee, B.-D. Detection Status and Removal Characteristics of Pharmaceuticals in Wastewater Treatment Effluent. J. Water Process Eng. 2019, 31, 100828. [Google Scholar] [CrossRef]
- Subedi, B.; Kannan, K. Occurrence and Fate of Select Psychoactive Pharmaceuticals and Antihypertensives in Two Wastewater Treatment Plants in New York State, USA. Sci. Total Environ. 2015, 514, 273–280. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative Techniques for Caffeine Removal from Wastewater: An Overview of Opportunities and Challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef]
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and Personal Care Products as Emerging Contaminants: Need for Combined Treatment Strategy. J. Hazard. Mater. Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Alvim, C.B.; Fernández-Navarro, J.; Mompó-Curell, R.; Mendoza-Roca, J.A.; Bes-Piá, A.; Alonso-Molina, J.L.; Amorós-Muñoz, I. Influence of Bisphenol A Occurrence in Wastewaters on Biomass Characteristics and Activated Sludge Process Performance. Sci. Total Environ. 2021, 778, 146355. [Google Scholar] [CrossRef] [PubMed]
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. 2000, Volume 327. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02000L0060-20140101 (accessed on 6 November 2023).
- Parliament, E.U. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 6 November 2023).
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water policy (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:en:PDF (accessed on 6 November 2023).
- Commission Implementing Decision (EU) 2018/ 840—Of 5 June 2018—Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/ 105/ EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495—(Notified under Document C(2018) 3362). Available online: https://eur-lex.europa.eu/eli/dec_impl/2018/840/oj (accessed on 20 December 2023).
- Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document Number C(2020) 5205) (Text with EEA Relevance). 2020, Volume 257. Available online: http://data.europa.eu/eli/dec_impl/2020/1161/oj/eng (accessed on 20 December 2023).
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2022) 5098) (Text with EEA Relevance); 2022; Volume 197. Available online: http://data.europa.eu/eli/dec_impl/2022/1307/oj/eng (accessed on 20 December 2023).
- European Commission. Proposal for a Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (Recast). 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52017PC0753 (accessed on 20 December 2023).
- Ślósarczyk, K.; Jakóbczyk-Karpierz, S.; Różkowski, J.; Witkowski, A.J. Occurrence of Pharmaceuticals and Personal Care Products in the Water Environment of Poland: A Review. Water 2021, 13, 2283. [Google Scholar] [CrossRef]
- Pharmaceuticals in Drinking-Water. Available online: https://www.who.int/publications-detail-redirect/9789241502085 (accessed on 6 November 2023).
- World Health Organization. Pharmaceuticals in Drinking-Water; World Health Organization: Cham, Switzerland, 2012; ISBN 979-11-5621-734-3.
- EFSA. Science, Safe Food, Sustainability. Available online: https://www.efsa.europa.eu/en (accessed on 6 November 2023).
- Ferreiro, C.; Villota, N.; de Luis, A.; Lombraña, J.I.; Etxebarria, N.; Lomas, J.M. Water Reuse Study from Urban WWTPs via C-Ultrafiltration and Ozonation Technologies: Basis for Resilient Cities and Agriculture. Agronomy 2021, 11, 322. [Google Scholar] [CrossRef]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A Review on Pharmaceuticals Removal from Waters by Single and Combined Biological, Membrane Filtration and Ultrasound Systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef] [PubMed]
- Wast Water Treatment Pains. Available online: https://vattenbokhandeln.svensktvatten.se/wp-content/uploads/2021/03/Wast-water-treatment-pains_SvensktVatten_M150.pdf (accessed on 20 December 2023).
- Madikizela, L.M.; Ncube, S. Occurrence and Ecotoxicological Risk Assessment of Non-Steroidal Anti-Inflammatory Drugs in South African Aquatic Environment: What Is Known and the Missing Information? Chemosphere 2021, 280, 130688. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, Interactive Effects and Ecological Risk of Diclofenac in Environmental Compartments and Biota—A Review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef]
- Hernández-Tenorio, R.; González-Juárez, E.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Hernández-Ramírez, A. Review of Occurrence of Pharmaceuticals Worldwide for Estimating Concentration Ranges in Aquatic Environments at the End of the Last Decade. J. Hazard. Mater. Adv. 2022, 8, 100172. [Google Scholar] [CrossRef]
- Mussa, Z.H.; Al-Qaim, F.F.; Jawad, A.H.; Scholz, M.; Yaseen, Z.M. A Comprehensive Review for Removal of Non-Steroidal Anti-Inflammatory Drugs Attained from Wastewater Observations Using Carbon-Based Anodic Oxidation Process. Toxics 2022, 10, 598. [Google Scholar] [CrossRef]
- Kot-Wasik, A.; Jakimska, A.; Śliwka-Kaszyńska, M. Occurrence and Seasonal Variations of 25 Pharmaceutical Residues in Wastewater and Drinking Water Treatment Plants. Environ. Monit. Assess. 2016, 188, 661. [Google Scholar] [CrossRef] [PubMed]
- Ulvi, A.; Aydın, S.; Aydın, M.E. Fate of Selected Pharmaceuticals in Hospital and Municipal Wastewater Effluent: Occurrence, Removal, and Environmental Risk Assessment. Environ. Sci. Pollut. Res. 2022, 29, 75609–75625. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Data on Occurrence and Fate of Emerging Contaminants in a Urbanised Area. Data Brief 2018, 17, 533–543. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Benner, J.; Helbling, D.E.; Kohler, H.-P.E.; Wittebol, J.; Kaiser, E.; Prasse, C.; Ternes, T.A.; Albers, C.N.; Aamand, J.; Horemans, B.; et al. Is Biological Treatment a Viable Alternative for Micropollutant Removal in Drinking Water Treatment Processes? Water Res. 2013, 47, 5955–5976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Q.; Shi, Y.-L.; Chen, Z.; Lu, Y.; Yang, H.-W.; Xie, Y.F.; Hou, L. Study on the Influence of Operational and Management Processes of a Water Reclamation Plant since COVID-19 Situation. Environ. Pollut. 2021, 285, 117257. [Google Scholar] [CrossRef]
- Nariyan, E.; Aghababaei, A.; Sillanpää, M. Removal of Pharmaceutical from Water with an Electrocoagulation Process; Effect of Various Parameters and Studies of Isotherm and Kinetic. Sep. Purif. Technol. 2017, 188, 266–281. [Google Scholar] [CrossRef]
- Pauporté, T.; Rathouský, J. Electrodeposited Mesoporous ZnO Thin Films as Efficient Photocatalysts for the Degradation of Dye Pollutants. J. Phys. Chem. C 2007, 111, 7639–7644. [Google Scholar] [CrossRef]
- Wardenier, N.; Liu, Z.; Nikiforov, A.; Van Hulle, S.W.H.; Leys, C. Micropollutant Elimination by O3, UV and Plasma-Based AOPs: An Evaluation of Treatment and Energy Costs. Chemosphere 2019, 234, 715–724. [Google Scholar] [CrossRef]
- Guo, J.; Dai, Y.; Chen, X.; Zhou, L.; Liu, T. Synthesis and Characterization of Ag3PO4/LaCoO3 Nanocomposite with Superior Mineralization Potential for Bisphenol A Degradation under Visible Light. J. Alloys Compd. 2017, 696, 226–233. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO–Carbon Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Eryildiz, B.; Yavuzturk Gul, B.; Koyuncu, I. A Sustainable Approach for the Removal Methods and Analytical Determination Methods of Antiviral Drugs from Water/Wastewater: A Review. J. Water Process Eng. 2022, 49, 103036. [Google Scholar] [CrossRef]
- Jia, T.-C.; Guo, J.-T.; Wang, Z.; Zhu, X.-S.; Zhang, Q.-X.; Chen, P.; Yao, K.; Lv, W.-Y.; Liu, G.-G. Photodegradation Mechanisms of Acyclovir in Water and the Toxicity of Photoproducts. J. Radioanal. Nucl. Chem. 2019, 320, 823–830. [Google Scholar] [CrossRef]
- Yang, Y.; Hoffmann, M.R. Synthesis and Stabilization of Blue-Black TiO2 Nanotube Arrays for Electrochemical Oxidant Generation and Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11888–11894. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.; Dhawle, R.; Du Pasquier, D.; Tindall, A.J.; Frontistis, Z.; Mantzavinos, D.; de Witte, P.; Cabooter, D. Electrochemical Degradation of 17α-Ethinylestradiol: Transformation Products, Degradation Pathways and In Vivo Assessment of Estrogenic Activity. Environ. Int. 2023, 176, 107992. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E. Remediation of Water Pollution Caused by Pharmaceutical Residues Based on Electrochemical Separation and Degradation Technologies: A Review. Environ. Int. 2012, 40, 212–229. [Google Scholar] [CrossRef]
- Son, M.; Jeong, K.; Yoon, N.; Shim, J.; Park, S.; Park, J.; Cho, K.H. Pharmaceutical Removal at Low Energy Consumption Using Membrane Capacitive Deionization. Chemosphere 2021, 276, 130133. [Google Scholar] [CrossRef]
- Osman, A.I.; Ayati, A.; Farghali, M.; Krivoshapkin, P.; Tanhaei, B.; Karimi-Maleh, H.; Krivoshapkina, E.; Taheri, P.; Tracey, C.; Al-Fatesh, A.; et al. Advanced Adsorbents for Ibuprofen Removal from Aquatic Environments: A Review. Environ. Chem. Lett. 2023. [Google Scholar] [CrossRef]
- Önal, Y.; Akmil-Başar, C.; Sarıcı-Özdemir, Ç. Elucidation of the Naproxen Sodium Adsorption onto Activated Carbon Prepared from Waste Apricot: Kinetic, Equilibrium and Thermodynamic Characterization. J. Hazard. Mater. 2007, 148, 727–734. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Senhadji, O.; Milon, N.; Benamor, M.; Lahiani-Skiba, M. Inclusion and Removal of Pharmaceutical Residues from Aqueous Solution Using Water-Insoluble Cyclodextrin Polymers. Chem. Eng. Res. Des. 2015, 97, 145–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Dai, C.; Zhou, X. Removal of Selected Pharmaceuticals from Aqueous Solution Using Magnetic Chitosan: Sorption Behavior and Mechanism. Environ. Sci. Pollut. Res. 2014, 21, 12780–12789. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.A.; Islam, M.T.; Imam, M.A.; Hyder, A.H.M.G.; Jabbari, V.; Dominguez, N.; Noveron, J.C. Biosorption of Bisphenol A and Sulfamethoxazole from Water Using Sulfonated Coffee Waste: Isotherm, Kinetic and Thermodynamic Studies. J. Environ. Chem. Eng. 2018, 6, 6602–6611. [Google Scholar] [CrossRef]
- Bielská, L.; Škulcová, L.; Neuwirthová, N.; Cornelissen, G.; Hale, S.E. Sorption, Bioavailability and Ecotoxic Effects of Hydrophobic Organic Compounds in Biochar Amended Soils. Sci. Total Environ. 2018, 624, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Czech, B.; Kończak, M.; Rakowska, M.; Oleszczuk, P. Engineered Biochars from Organic Wastes for the Adsorption of Diclofenac, Naproxen and Triclosan from Water Systems. J. Clean. Prod. 2021, 288, 125686. [Google Scholar] [CrossRef]
- Zungu, V.; Hadebe, L.; Mpungose, P.; Hamza, I.; Amaku, J.; Gumbi, B. Fabrication of Biochar Materials from Biowaste Coffee Grounds and Assessment of Its Adsorbent Efficiency for Remediation of Water-Soluble Pharmaceuticals. Sustainability 2022, 14, 2931. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and Pesticides in Reclaimed Water: Efficiency Assessment of a Microfiltration–Reverse Osmosis (MF–RO) Pilot Plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Kuttiani Ali, J.; Abi Jaoude, M.; Alhseinat, E. Polyimide Ultrafiltration Membrane Embedded with Reline-Functionalized Nanosilica for the Remediation of Pharmaceuticals in Water. Sep. Purif. Technol. 2021, 266, 118585. [Google Scholar] [CrossRef]
- Zhou, A.; Jia, R.; Wang, Y.; Sun, S.; Xin, X.; Wang, M.; Zhao, Q.; Zhu, H. Abatement of Sulfadiazine in Water under a Modified Ultrafiltration Membrane (PVDF-PVP-TiO2-Dopamine) Filtration-Photocatalysis System. Sep. Purif. Technol. 2020, 234, 116099. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Algae-Mediated Removal of Selected Pharmaceutical and Personal Care Products (PPCPs) from Lake Mead Water. Sci. Total Environ. 2017, 581–582, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Verlicchi, P.; Young, T.M. Paracetamol Removal in Subsurface Flow Constructed Wetlands. J. Hydrol. 2011, 404, 130–135. [Google Scholar] [CrossRef]
- Jesus, F.; Domingues, E.; Bernardo, C.; Pereira, J.L.; Martins, R.C.; Gomes, J. Ozonation of Selected Pharmaceutical and Personal Care Products in Secondary Effluent—Degradation Kinetics and Environmental Assessment. Toxics 2022, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Lema, J.M. Removal of Cosmetic Ingredients and Pharmaceuticals in Sewage Primary Treatment. Water Res. 2005, 39, 4790–4796. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of Pharmaceutical Residues in a Pilot Wastewater Treatment Plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Wontorska, K. The problematic aspects of removal pharmaceuticals in wastewater treatment processes. GAZ WODA Tech. Sanit. 2018, 1, 32–38. [Google Scholar] [CrossRef]
- Huang, S.; Yu, J.; Li, C.; Zhu, Q.; Zhang, Y.; Lichtfouse, E.; Marmier, N. The Effect Review of Various Biological, Physical and Chemical Methods on the Removal of Antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Altmann, J.; Rehfeld, D.; Träder, K.; Sperlich, A.; Jekel, M. Combination of Granular Activated Carbon Adsorption and Deep-Bed Filtration as a Single Advanced Wastewater Treatment Step for Organic Micropollutant and Phosphorus Removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Fu, W.; Fu, J.; Li, X.; Li, B.; Wang, X. Occurrence and Fate of PPCPs in Typical Drinking Water Treatment Plants in China. Environ. Geochem. Health 2019, 41, 5–15. [Google Scholar] [CrossRef]
- Yang, X.; Flowers, R.C.; Weinberg, H.S.; Singer, P.C. Occurrence and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in an Advanced Wastewater Reclamation Plant. Water Res. 2011, 45, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved Removal of Estrogenic and Pharmaceutical Compounds in Sewage Effluent by Full Scale Granular Activated Carbon: Impact on Receiving River Water. J. Hazard. Mater. 2011, 185, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Omotola, E.O.; Olatunji, O.S. Pharmaceuticals and Personal Care Products in Water and Wastewater: A Review of Treatment Processes and Use of Photocatalyst Immobilized on Functionalized Carbon in AOP Degradation. BMC Chem. 2020, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Puga, A.; Meijide, J.; Pazos, M.; Rosales, E.; Sanromán, M.A. Electric Field as a Useful Tool to Improve the Poor Adsorption Affinity of Pollutants on Carbonaceous Aerogel Pellets. J. Mol. Liq. 2022, 366, 120269. [Google Scholar] [CrossRef]
- Silva, B.S.; Ribeiro, M.C.B.; Ramos, B.; de Castro Peixoto, A.L. Removal of Amoxicillin from Processing Wastewater by Ozonation and UV-Aided Ozonation: Kinetic and Economic Comparative Study. Water 2022, 14, 3198. [Google Scholar] [CrossRef]
- Homem, V.; Silva, J.A.; Ratola, N.; Santos, L.; Alves, A. Long Lasting Perfume—A Review of Synthetic Musks in WWTPs. J. Environ. Manag. 2015, 149, 168–192. [Google Scholar] [CrossRef]
- Mukarunyana, B.; Boman, C.; Kabera, T.; Lindgren, R.; Fick, J. The Ability of Biochars from Cookstoves to Remove Pharmaceuticals and Personal Care Products from Hospital Wastewater. Environ. Technol. Innov. 2023, 32, 103391. [Google Scholar] [CrossRef]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and Removal of Transformation Products of PPCPs and Illicit Drugs in Wastewaters: A Review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review. Membranes 2023, 13, 158. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration. Available online: https://pubs.acs.org/doi/epdf/10.1021/es9014629 (accessed on 29 September 2023).
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of Micropollutants in Municipal Wastewater: Ozone or Powdered Activated Carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Khan, S.J.; McDonald, J.A.; Kandasamy, J.; Vigneswaran, S. Enhanced Nanofiltration Rejection of Inorganic and Organic Compounds from a Wastewater-Reclamation Plant’s Micro-Filtered Water Using Adsorption Pre-Treatment. Sep. Purif. Technol. 2021, 260, 118207. [Google Scholar] [CrossRef]
- Gerrity, D.; Gamage, S.; Holady, J.C.; Mawhinney, D.B.; Quiñones, O.; Trenholm, R.A.; Snyder, S.A. Pilot-Scale Evaluation of Ozone and Biological Activated Carbon for Trace Organic Contaminant Mitigation and Disinfection. Water Res. 2011, 45, 2155–2165. [Google Scholar] [CrossRef]
- Mousel, D.; Bastian, D.; Firk, J.; Palmowski, L.; Pinnekamp, J. Removal of Pharmaceuticals from Wastewater of Health Care Facilities. Sci. Total Environ. 2021, 751, 141310. [Google Scholar] [CrossRef]
- Roberts, J.; Kumar, A.; Du, J.; Hepplewhite, C.; Ellis, D.J.; Christy, A.G.; Beavis, S.G. Pharmaceuticals and Personal Care Products (PPCPs) in Australia’s Largest Inland Sewage Treatment Plant, and Its Contribution to a Major Australian River during High and Low Flow. Sci. Total Environ. 2016, 541, 1625–1637. [Google Scholar] [CrossRef]
- Yuan, X.; Lacorte, S.; Cristale, J.; Dantas, R.F.; Sans, C.; Esplugas, S.; Qiang, Z. Removal of Organophosphate Esters from Municipal Secondary Effluent by Ozone and UV/H2O2 Treatments. Sep. Purif. Technol. 2015, 156, 1028–1034. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, M.; Hu, A.; Yang, X.; Yu, C.-P. Seasonal Variation in the Occurrence and Removal of Pharmaceuticals and Personal Care Products in a Wastewater Treatment Plant in Xiamen, China. J. Hazard. Mater. 2014, 277, 69–75. [Google Scholar] [CrossRef]
- Zucker, I.; Mamane, H.; Cikurel, H.; Jekel, M.; Hübner, U.; Avisar, D. A Hybrid Process of Biofiltration of Secondary Effluent Followed by Ozonation and Short Soil Aquifer Treatment for Water Reuse. Water Res. 2015, 84, 315–322. [Google Scholar] [CrossRef]
- Svebrant, S.; Spörndly, R.; Lindberg, R.H.; Olsen Sköldstam, T.; Larsson, J.; Öhagen, P.; Söderström Lindström, H.; Järhult, J.D. On-Site Pilot Testing of Hospital Wastewater Ozonation to Reduce Pharmaceutical Residues and Antibiotic-Resistant Bacteria. Antibiotics 2021, 10, 684. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(Electro)Catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Ji, R.; Chen, J.; Liu, T.; Zhou, X.; Zhang, Y. Critical Review of Perovskites-Based Advanced Oxidation Processes for Wastewater Treatment: Operational Parameters, Reaction Mechanisms, and Prospects. Chin. Chem. Lett. 2022, 33, 643–652. [Google Scholar] [CrossRef]
- Aguilar-Pérez, K.M.; Avilés-Castrillo, J.I.; Ruiz-Pulido, G. Nano-Sorbent Materials for Pharmaceutical-Based Wastewater Effluents—An Overview. Case Stud. Chem. Environ. Eng. 2020, 2, 100028. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of Micropollutants during Post-Treatment of Hospital Wastewater with Powdered Activated Carbon, Ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.-Q.; Ying, G.-G.; Yang, B.; Liu, S.; Lai, H.-J.; Liu, Y.-S.; Chen, Z.-F.; Zhou, G.-J. Biotransformation of Progesterone and Norgestrel by Two Freshwater Microalgae (Scenedesmus obliquus and Chlorella pyrenoidosa): Transformation Kinetics and Products Identification. Chemosphere 2014, 95, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Hom-Diaz, A.; Llorca, M.; Rodríguez-Mozaz, S.; Vicent, T.; Barceló, D.; Blánquez, P. Microalgae Cultivation on Wastewater Digestate: β-Estradiol and 17α-Ethynylestradiol Degradation and Transformation Products Identification. J. Environ. Manag. 2015, 155, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of Selected Pharmaceutical and Personal Care Products (PPCPs) by White-Rot Fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Kurade, M.B.; Ha, Y.-H.; Xiong, J.-Q.; Govindwar, S.P.; Jang, M.; Jeon, B.-H. Phytoremediation as a Green Biotechnology Tool for Emerging Environmental Pollution: A Step Forward towards Sustainable Rehabilitation of the Environment. Chem. Eng. J. 2021, 415, 129040. [Google Scholar] [CrossRef]
- Kurade, M.B.; Xiong, J.-Q.; Govindwar, S.P.; Roh, H.-S.; Saratale, G.D.; Jeon, B.-H.; Lim, H. Uptake and Biodegradation of Emerging Contaminant Sulfamethoxazole from Aqueous Phase Using Ipomoea Aquatica. Chemosphere 2019, 225, 696–704. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Hua, T.; Gersberg, R.M.; Zhu, J.; Ng, W.J.; Tan, S.K. Carbamazepine and Naproxen: Fate in Wetland Mesocosms Planted with Scirpus validus. Chemosphere 2013, 91, 14–21. [Google Scholar] [CrossRef]



| Group | Classification | Examples of Compound | Country /Region | Natural Water | Wastewater (ng/L) | References | ||
|---|---|---|---|---|---|---|---|---|
| Type | Concentration (ng/L) | Influent/Raw Wastewater | Effluent | |||||
| Pharmaceuticals | NSAID and analgesic drugs | Naproxen | South Africa | Surface water | 23,000 | 159,000 | 91,100 | [106] |
| South Korea | Surface water | 230 | [105] | |||||
| USA | river | 0–135.2 | [71] | |||||
| Ibuprofen | Global | Surface water | 445–689 | 1060 | 1380 | [103] | ||
| USA | river | 0–34.0 | [71] | |||||
| China | Surface water | 10−180 | [105] | |||||
| Spain | Surface water | 2.5–650 | [105] | |||||
| Diclofenac | Poland | Groundwater | 114 | 556–4001 | 743–5402 | [107] | ||
| - | Drinking water | 1–10 (max. 56) | [104] | |||||
| Surface water | 0.2–193,000 | [105] | ||||||
| 836,000 | [104] | |||||||
| Antimicrobial agents | Erythromycin | Groundwater | 12.3–443 | [11] | ||||
| Freshwater | 0.02–362.49 | [11] | ||||||
| Tetracyclines | Freshwater | 5–712.40 | [11] | |||||
| Fluconazole | South Africa | 9959 | [50] | |||||
| Favipiravir | Japan | Surface water | 40–60 | [55] | ||||
| Hormones | 17β-estradiol (E2) | USA | River | 0–4.5 | [71] | |||
| EE2 | 2193 | 549 | [70] | |||||
| β-blockers | Atenolol | Freshwater | 0.25–1237 | [11] | ||||
| Groundwater | 0.8–106 | [11] | ||||||
| Propranolol | 8–76 | [13] | ||||||
| Hypolipidemic drugs (fat regulators) | Gemfibrozil | Global | Groundwater | 1.2–1950 | [11] | |||
| <76,000 | [10] | |||||||
| Clofibric acid | Freshwater | 0.01–450 | <2593 | [11] | ||||
| USA | River | 3.2–26.7 | [71] | |||||
| Bezafibrate | 3445 | [10] | ||||||
| Psychotropic drugs | Carbamazepine | Groundwater | 2–900 | [11] | ||||
| Seawater | 0.02–310 | [11] | ||||||
| Turkey | 136 | 101 | [108] | |||||
| Others | Caffeine | England | Freshwater | 11–44,179 | 150,413 | [11] | ||
| Groundwater | 56.8–16,249 | [11] | ||||||
| Personal care products | Plasticizer | Bisphenol A | USA | River | 147 | [71] | ||
| Italy | 326.9–1317 | <70 | [109] | |||||
| Insect repellent | DEET | River | 22–94 | [8] | ||||
| 600–1200 | 40–624 | [25] | ||||||
| Disinfectant | Triclosan | USA | River | 8.8–26.3 | [71] | |||
| Italy | 505–2210 | <390 | [109] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wydro, U.; Wołejko, E.; Luarasi, L.; Puto, K.; Tarasevičienė, Ž.; Jabłońska-Trypuć, A. A Review on Pharmaceuticals and Personal Care Products Residues in the Aquatic Environment and Possibilities for Their Remediation. Sustainability 2024, 16, 169. https://doi.org/10.3390/su16010169
Wydro U, Wołejko E, Luarasi L, Puto K, Tarasevičienė Ž, Jabłońska-Trypuć A. A Review on Pharmaceuticals and Personal Care Products Residues in the Aquatic Environment and Possibilities for Their Remediation. Sustainability. 2024; 16(1):169. https://doi.org/10.3390/su16010169
Chicago/Turabian StyleWydro, Urszula, Elżbieta Wołejko, Linda Luarasi, Klementina Puto, Živilė Tarasevičienė, and Agata Jabłońska-Trypuć. 2024. "A Review on Pharmaceuticals and Personal Care Products Residues in the Aquatic Environment and Possibilities for Their Remediation" Sustainability 16, no. 1: 169. https://doi.org/10.3390/su16010169
APA StyleWydro, U., Wołejko, E., Luarasi, L., Puto, K., Tarasevičienė, Ž., & Jabłońska-Trypuć, A. (2024). A Review on Pharmaceuticals and Personal Care Products Residues in the Aquatic Environment and Possibilities for Their Remediation. Sustainability, 16(1), 169. https://doi.org/10.3390/su16010169









