Abstract
Environmental pollution caused by increasing levels of heavy metals (HM) is a pressing problem throughout the world. Phytoremediation is considered a prospective remediation approach for HM-contaminated soil, but more research is required to enhance remediation efficiency. Biochar is a promising bio-residue material that can be used for the sustainable remediation of heavy metal-contaminated soil. In this study, a pot experiment was conducted to investigate the effects of biochar from different bio-substrates (digestate, waste of biodiesel production from rapeseed, corn stalk) on HM (Cr, Cd, Cu, Ni, Pb, and Zn) accumulation in buckwheat and white mustard cultivated in sewage sludge-contaminated soil. The total amount of HM in soil, plant above- and below-ground biomass, leachate, and bioconcentration and translocation factors were studied to explore the mechanism of how the different bio-substrates’ biochar affects HM accumulation in selected plants. It was observed that rapeseed biochar showed the greatest significant effect in reducing the HM content in soil, plant biomass, and lysimetric water. Meanwhile, the incorporation of digestate biochar significantly increased the HM content in all the soil-plant systems and affected the HM leaching from the soil. The concentration of HM in the leachate decreased from 2.5 to 10 times. It was determined that phytostabilization is the core process of HM accumulation in buckwheat, in contrast to mustard, where the mechanism is phytoextraction. This study confirmed that biochar addition enhances the phytoremediation efficiency in soil, which can potentially improve the development of ecofriendly in-situ bioremediation technology for HM-contaminated sites.
1. Introduction
Soil is essential for the terrestrial ecosystem’s maintenance and preservation of ecological stability and agricultural productivity []. Due to the world’s rapid expansion of industrial activity, inaccurate use of synthetic pesticides and fertilizers, and waste management, soil contamination with heavy metals (HMs) has become a serious environmental concern [,]. The environmental pollution caused by growing levels of HM is becoming increasingly serious worldwide and has thus become a hot spot in current research [,]. As HM could be easily uptaken by plants, they enter the food chain and could be accumulated in live organisms, where they can be potentially dangerous [,]. Plant uptake of HM is influenced by a variety of factors, making the remediation of heavy metal-polluted soils a challenging task. Furthermore, the HM contamination in soil is persistent []. Some research has gone into creating efficient remediation methods, such as thermal treatment, soil washing, excavation of contaminated soils, and phytoremediation, to reduce the threat of heavy metals in contaminated soil [].
The use of biochar, a solid carbonaceous substance, is a cost-efficient and sustainable remediation technique. Biochar could be used as an amendment to stabilize heavy metals in soil and prevent plants from accumulating HM [,,,]. Immobilization of HMs in soil using biochar is described by several mechanisms: (i) electrostatic attraction; (ii) heavy metal electron transfer with calcium, magnesium, and other cations linked to biochar; this is explained by the process of leaching, which also forms inner complex molecules with humic substances and mineral oxides in biochar (iii) the relationship between HMs and numerous functional groups, inner-sphere complexes, and surface complex substances associated with the mineral oxides; (iv) the formation of precipitates; and (v) the inferred relationships between biochar and HMs []. Furthermore, the sorption mechanisms of biochar in contaminated soils may differ depending on the type of soil and the cations found in both the soil and the biochar. This could have different effects on metal remediation in contaminated soils [,]. The immobilization of HM in soil treated with biochar can be impacted by changes in soil parameters, particularly an increase in pH, which lowers the metals’ availability to plants [,]. However, many variables, including the type of metal, pyrolysis options, biochar feedstock, and quantity of biochar applied to the soil, affect how readily available heavy metals are to plants after being added to the soil. For instance, corn-straw-derived biochar showed higher efficiency in reducing the availability of HM to plants than wood-derived biochar because it was characterized by having more oxygen-containing groups on its surface. In terms of the pyrolysis temperature, biochar produced at high temperatures (700 °C) was found to significantly reduce HM bioavailability and leachability compared to biochar produced at low temperatures (300 °C) []. Consequently, there is considerable variation regarding the effect of biochar on crop productivity, heavy metal immobilization, and soil characteristics [,]. Thus, before applying biochar on a large scale, it is essential to thoroughly assess each unique site’s biochar’s effectiveness in immobilizing heavy metals in contaminated soil and lowering their phytoavailability. This will help to confirm that using biochar in the remediation of contaminated sites is feasible. Research gaps still exist in the development of practical methods for preparing and applying different biochar for site-specific conditions. As the remediation effect depends on the characteristics of biochar, soil, and plants and their interactions, the effects of biochar on the soil ecosystem and effective methods to relieve the negative impacts are far from clear. These gaps may impede the understanding of the risks of metals in biochar-amended soils and the application of biochar for remediation purposes.
This study has selected three kinds of biochar produced from different bio-substrates and applied to soil contaminated by sewage sludge. This study aimed to investigate the effects of different types of biochar on plants’ growth (common buckwheat and white mustard), the accumulation of heavy metals in soil and plants’ parts, as well as the leachability of HM to the groundwater. As common buckwheat and white mustard are recognized as hyperaccumulators, studies concerning the tolerance of these plants to soil HM in polluted areas are essential, both in phytoremediation practices and in crop production planning and management. Understanding the capability of these plants to absorb heavy metals from contaminated soils is also important for ecology, public health, and safety. This research provides background data and recommendations for field experiments in the future regarding reducing the toxicity of heavy metals in polluted soils using the phytoremediation approach.
2. Materials and Methods
2.1. Biochar Production and Its Properties Evaluation
The production of biochar was implemented at the laboratories of the Marine Technology and Natural Sciences Faculty of Klaipeda University, Lithuania. Three bio-substrates were used as biomass raw materials: corn stalks, waste from biodiesel production from rapeseed (rapeseed biochar), and digestate from biogas production from sewage sludge (digestate biochar) (Figure 1). Corn stalks were chopped with a chopper “Bosh 2500” to <3 cm particles. All substrates were dried to a constant weight before the pyrolysis process. Later, the biomass was slowly pyrolyzed in a batch reactor.

Figure 1.
The bio-substrates and biochars are: (a) digestate; (b) waste of biodiesel production from rapeseed; and (c) corn stalks, where on the left side of pictures—bio—substrate and on the right side—prepared biochar.
Pyrolysis of corn stalks. In order to obtain the required amount of biochar, three pyrolysis steps were performed. For one pyrolysis process, 400 g of corn stalks (in particles less than 3 cm long) were used. The average temperature rise rate was 1.588 °C per minute. The duration of the process was 4 h and 20 min. The final pyrolysis temperature achieved was 450 °C.
Pyrolysis of rapeseed. To extract the required amount of rapeseed biochar, 3 kg of rapeseed pellets were used, and pyrolysis was performed twice. The average temperature rise rate was 1.804 °C per minute. The duration of the process was 4 h. Pyrolysis was completed when the reactor reached a temperature of 450 °C.
Pyrolysis of dried sewage sludge. For the dried digestate pyrolysis, 2 kg of digestate was used, and pyrolysis was performed twice. The average temperature rise rate was 1.792 °C per minute. The duration of the process was 4 h. Pyrolysis was completed when the reactor reached a temperature of 450 °C.
Half of each substrate’s extracted biochar was heated in a heating furnace at a temperature of 700 °C for one hour in a tightly closed metal container.
Biochar characterization. For determination of the CHN amount in biochar, an elemental analyzer CHNS-O was employed, applying “Duma’s method,” which implies the total and simultaneous oxidation of the sample by “flash combustion,” to calculate the percentages of carbon, hydrogen, and nitrogen. The sample (~10 mg) was burned by helium (He) gas combustion. Bulk density was obtained as an unsaturated substance measurement of volume (cm3) and mass (g). A pH meter was used to measure the soil acidity in the biochar and water solution. The phosphorous content was determined using an ICP mass spectrometer in the range of 500–2000 µg/L. The amount of organic carbon in biochar was estimated using ISO 10694:1995.
The characterization of the biochar used in the experiment is presented in Table 1.

Table 1.
Elemental composition of biochar from different bio-substrates at different temperatures.
The initial amounts of heavy metals in biochar are presented in Table 2.

Table 2.
Heavy metal distribution in biochar.
2.2. Pot Experiment Instalation
Seeking to evaluate heavy metal accumulation by plants and how biochar could enhance the potential of phytoremediation, the pots’ experiment under local climatic conditions was established at the Vėžaičiai Branch of the LAMMC. The experiment was performed using forty-two plastic vegetative containers with an overall capacity of 6.12 L and an outer diameter of 19.50 cm. The pots’ top and bottom widths were equally in the range of 20.0 cm. The pots’ experiment was prepared as follows (Figure 2): soil (6882.4 g) was combined with 117.6 g of wastewater sludge and differently produced biochar (210 g, 3%), except for the control treatment, where 7000 g of natural acid soil without any amendments was added. The soil used for the pots’ experiment was naturally acid moraine loam, Bathygleyic Dystric Glossic Retisol (WRB, 2014), with a clay content (<0.002 mm) of 15.0%, sand (0.05–2.0 mm)—51.1%, and silt (0.05–0.002 mm)—33.6%.

Figure 2.
Preparation of the pots’ experiment: preparation of substrate, soil compaction, pots preparation for planting, sowing (from left to right).
The following soil parameters were determined before the installation of the pots’ experiment and are given in Table 3.

Table 3.
Experimental soil parameters.
After the soil was mixed, the substrates were well compacted to achieve a density of 1.35 g/cm3—optimal for plant growth due to moisture and aeration. With a special tool, 50 holes were made for the seeds so that the plants could grow at the optimal distance. Buckwheat and mustard seeds were put into the substrate. Then, 100 g of quartz sand was poured in, which helped to retain moisture.
The detailed experiment scheme is presented in Figure 3. A total of fourteen treatments were selected for this experiment that affected differently fertilized soil: untreated soil, soil treated with sewage sludge, and soil treated with sludge and biochar. Two plants were cultivated in the experiment with these substrates for growth: buckwheat (Fagopyrum esculentum) and white mustard (Sinapis alba). Sewage sludge for this experiment originated from JSC “Klaipeda’s water.” Every container was seeded with experimental plants (white mustard and buckwheat). Seeds were evenly spaced at a depth of 1 cm in a growth substrate. The pots’ experiment was conducted in the local climate with no ability to regulate moisture, sunlight, or warmth. The climate is moderately warm and humid. The mean annual precipitation rate is~1200 mm, and the average air temperature is 7.2 °C. The experiment was conducted during the summer period (July–August), when the average air temperature was 19.8 °C (2.6° higher than the standard climate rate). In addition, experimental plants were manually watered in accordance with the weather at the time. Since it was a hot summer, it was necessary to water the plants with 0.5–1 L of water every 2–3 days. For every treatment, three repetitions were carried out.
Figure 3.
Scheme of the pots’ experiment. Note: Control—soil without any amendments; SS—sewage sludge; RB450—rapeseed biochar at 450 °C; RB700—rapeseed biochar at 700 °C; DB450—digestate biochar at 450 °C; DB700—digestate biochar at 700 °C; CB450 and 700—corn stalk biochar at 450 °C; and 700 °C.
All planted vegetative pots were covered. After two days, when seeds germinated, pots were uncovered and watered with 0.5 L of water. Buckwheat and white mustard grew for exactly two months. As it was a hot summer period, it was necessary to water plants with 0.5–1 L of water every 2–3 days. Weeds were removed every certain period of time, and the plants were thinned. Every week, a certain number of plants (growing too close to each other) were uprooted, until eventually there were 12 plants in one pot.
Soil, plant, and leachate samples were taken after the plants’ vegetation. The soil samples were about 10 g, collected with a soil drill, air-dried, and passed through 2.0 mm sieves for the determination of heavy metals. For plant analysis, the dry roots and aerial parts of each pot were taken separately after drying, chopped, and ground with a coffee grinder into flour-like particles. The water that was leached from the soil was collected from the bottom plates of the vegetation pots.
2.3. Determination of Total Amount of Heavy Metals
An automated CEM MARS 6® (Matthews, NC, USA) digestion system was used to perform microwave-assisted extraction r to ascertain the total concentration of heavy metals (Cd, Cr, Cu, Ni, Pb, and Zn) in soil, biochar, and lysimeter water samples []. Approximately 0.3 g of the homogenized and dried sample was accurately weighed into a Teflon vessel and digested using a nitric (HNO3) and hydrochloric (HCl) acid mixture (5:1). Digestion was performed under the following conditions: temperature—180 °C; pressure—800 psi; ramp time—20 min; hold time—20 min; microwave power—800 W. Then, the digested sample was cooled down, thoroughly transferred into a 100 mL volumetric flask and diluted using bidistilled water.
To determine the total amount of heavy metals (Cd, Cr, Cu, Ni, Pb, Zn), the digested samples were analyzed using inductively coupled plasma mass spectrometry (ICP-MS) analysis. With the use of an external multi-element calibration curve, the amounts of the examined heavy metals (Cd, Cr, Cu, Ni, Pb, and Zn) were determined within a range of 20–1000 µg/L. The accuracy and precision of the method were assessed by recovery experiments using SQC001-certified reference material. The obtained recovery for individual heavy metals was Cd 89.1 ± 0.10%, Cu 90.5 ± 0.12%, Ni 91.3 ± 0.19%, Pb 91.0 ± 0.03%, Zn 96.7 ± 0.09%, and Cr 104.2 ± 0.02%.
2.4. Calculation of Translocation and Bioconcentration Factors
The translocation factor (TF) is a significant factor in calculating the capacity of any plant species to relocate heavy metals from the roots to the aerial part of the plant []:
The capacity of buckwheat and white mustard to absorb heavy metals from soil was estimated by calculating the bioconcentration factor (BCF) [].
2.5. Statistical Analysis
The results of this study were displayed as the three repetitions’ arithmetic average ± standard deviation, which was computed using Microsoft Excel 2018.
3. Results and Discussion
3.1. Effect of Biochar Addition on Heavy Metal Concentration in Soil, Following the Growth of Buckwheat and White Mustard
Due to its special porous structure and abundance of oxygen-containing functional groups on its surface, biochar can immobilize contaminants in soil and act as a soil conditioner, providing nutrients that promote plant development. Additionally, biochar can retain nutrients in such a way that it improves the physical and biological characteristics of soil []. The control soil’s heavy metal content was categorized in a subsequent order: Zn > Cr > Cu > Pb > Ni > Cd (Figure 4). The amount of Cd was found below the limit of quantitation in all studied soil variants after harvesting white mustard and buckwheat. When comparing the heavy metal sequence under other examined treatments to the sequence perceived in control, it was observed that Zn ranked higher due to its higher concentration.
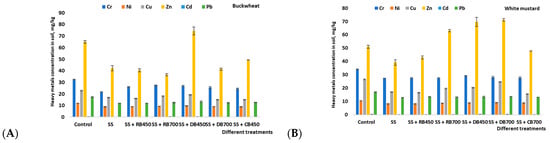
Figure 4.
The concentration of heavy metals (mean ± standard deviation) in the soil after growing buckwheat (A) and white mustard (B). Note: SS—sewage sludge; SS + RB450—sewage sludge + rapeseed biochar at 450 °C; SS + RB700—sewage sludge + rapeseed biochar at 700 °C; SS + DB450—sewage sludge + digestate biochar at 450 °C; SS + DB700—sewage sludge + digestate biochar at 700 °C; SS + CB450 and 700—sewage sludge + corn stalk biochar at 450 °C; and 700 °C.
Heavy metal concentrations tended to increase in this study. Studies conducted on the same type of soil showed that this could be related to the pH of the analyzed soil, which slightly decreased during this study period. In relation to those heavy metals that are more accessible at a lower pH, they may be excessively absorbed by plants. This increase in soil acidity may explain the increased heavy metal accumulation in non-fertilized soil. Furthermore, with increasing soil acidity, the mobility of Cr and Pb also increases, so migration into underlying soil layers is possible. Studies by other scientists reported similar findings. Metals, as natural components of the soil mineral fraction, are important components of clay and minerals. One important aspect is that the soil for this study was taken from a crop rotation field where fertilization, in particular with phosphorus fertilizers and pesticides, was used for more than 50 years. Therefore, heavy metals could be naturally found in the soil. Heavy metals are also present in the solid part of the soil, and under favorable conditions, in this case, with an increase in soil acidity, heavy metals could be transferred to the soil solution.
It was determined that incorporation of biochar had a notable effect on reducing heavy metal content in soil after buckwheat vegetation compared to the control. In addition, differences in the assessment of heavy metal accumulation were observed between biochar types. The maximum effect was found after the insertion of rapeseed and cornstalk biochar produced at 450 °C. The rapeseed biochar (450 °C) showed the greatest significant decrease over the control, which was 19.3%, 24.8%, 29.5%, 37.8%, and 30.6% for Cr, Ni, Cu, Zn, and Pb, respectively (Figure 4). This could be related to the better immobilization of available heavy metals in soil. The results are based on the data provided by Xu et al. [], Soudek et al. [] and Erdem []. The functional groups found on the surface of biochar, such as carboxyl, hydroxyl, phenol, alcohol, carbonyl, or enol, might possess the ability to chelate metals and serve as crucial for the complexation of heavy metals onto the surface and interior pores of biochar [,]. The opposite effect on the accumulation of Zn in soil was observed after the addition of digestate’s biochar produced at 450 °C. The Zn amount in the soil was increased by 14.5%. This could be related to the fact that Zn is a frequently detected metal with a large amount in sewage sludge, so the long-term application of digestate biochar produced from sewage sludge could cause additional Zn accumulation in soil [].
Similar tendencies were found in white mustard vegetation. The amount of Cr, Ni, and Pb decreased in all study treatments. This decrease could be explained by biochar’s high surface area, complexation, surface precipitation, electrostatic interaction, ion-metal exchange, different functional groups, physical sorption processes, and large pore volume [,]. Also, after the cultivation of buckwheat, the rapeseed biochar (450 °C) showed the biggest effect on reducing the amount of heavy metals in the soil where the white mustard was grown. Incorporation of rapeseed biochar (450 °C) decreased the amount of Cr, Ni, Cu, Zn, and Pb by 19.3%, 21.8%, 37.7%, 15.7%, and 20.6%, respectively. These data are confirmed by other researchers who found that the incorporation of biochar induced the reduction of these HMs in soil: Ni, Cu, and Pb. The explanation for this could be related to the biochar sorption properties when HMs are strongly sorbed by the biochar’s surface to the complex of organic carbon contained in this amendment [,]. Other applied treatments were less effective in decreasing heavy metals’ content in soil. It was found that the incorporation of digestate biochar produced at both temperatures (450 °C and 700 °C) contributed to the higher Zn accumulation than the incorporation of biochar from other bio-substrates. Therefore, the addition of Zn through digestate biochar emerges as a significant factor contributing to the elevated Zn concentrations observed in all the soil-plant systems in this study. Generally, optimizing the dose of applied digestate’s biochar could be a good strategy for treating zinc surplus or shortage in agricultural soils. It was found that the sewage sludge from which digestate’s biochar was produced in this study contained a lot of Zn, Cu, and Ni and was not very suitable for soil amendment and remediation.
3.2. Accumulation of Heavy Metals in Plants Belowground Biomass after Biochar Incorporation
Heavy metal accumulation in plants is a significant environmental issue, especially when biowaste-based fertilizers are employed. The heavy metals’ content in buckwheat and white mustard roots after biochar addition is presented in Figure 5. Application of different types of biochar showed significant changes in heavy metal accumulation in both plants’ (buckwheat and white mustard) belowground biomass. The results of this study showed that the addition of all types of biochar exerted an accumulation of all investigated HMs (Cr, Ni, Cu, Zn, and Pb) in plant roots, except for Cd. Heavy metals in buckwheat roots were distributed as follows: Zn > Cr > Ni > Cu > Pb, expect the treatments where digestate’s biochar was produced at 700 °C and corn stalk’s biochar was added. In these treatments, buckwheat roots mainly accumulated Cr and Ni. The amount of Cd in all investigated treatments was found to be below the quantification limit (<0.007 mg/kg) because of the low amount found in the control soil as well as in biochar and sewage sludge. Application of different bio-substrates, such as biochar, to the soil particularly promoted the accumulation of Cr and Ni in buckwheat roots compared to the control. The highest concentrations of the HM were determined after sewage sludge + digestate’s biochar addition was produced at 700 °C. The content of Cr, Ni, and Cu in buckwheat roots increased 10 and 12 times, respectively, which resulted from the raw material from which they were produced. As sewage sludge provides between 60 and 70 percent of the Cr and 70 to 80 percent of the Ni in the fertilizers that were evaluated, it could explain the high doses of these HMs in buckwheat roots. Several researchers have observed that sewage sludge has a high potential bioavailability of these metals based on pots’ experiment results [,].
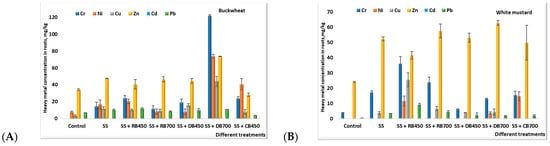
Figure 5.
The heavy metal concentration (mean ± standard deviation) in buckwheat (A) and white mustard (B) roots after biochar incorporation. Note: SS—sewage sludge; SS + RB450—sewage sludge + rapeseed biochar at 450 °C; SS + RB700—sewage sludge + rapeseed biochar at 700 °C; SS + DB450—sewage sludge + digestate biochar at 450 °C; SS + DB700—sewage sludge + digestate biochar at 700 °C; SS + CB450 and 700—sewage sludge + corn stalk biochar at 450 °C; and 700 °C.
Our study showed that the origin of biochar influenced the accumulation of heavy metals in buckwheat roots. The highest HMs accumulation potential was observed after the incorporation of digestate biochar, followed by biochar produced from rapeseed waste and biochar from corn stalks’ residue. It should be highlighted that the amount of heavy metals in the buckwheat roots decreased after the incorporation of rapeseed biochar produced at a higher temperature (700 °C). Buckwheat roots showed a higher affinity for Ni after corn stalk biochar addition when compared with results obtained for the control treatment.
Incorporation of different-origin biochar showed an unequal effect on the content of heavy metals in white mustard belowground biomass compared to buckwheat (Figure 5). The obtained results confirm other researchers’ statements about the notable variations in the amount of HMs that plants were able to absorb after adding biochar, depending on the type of plant. Different plants’ physiology and soil characteristics may be attributed to the diverse impacts of biochar on plant HM absorption [,]. Differently from buckwheat growing conditions, the amounts of Ni, Cu, and Cd were found to be below the quantification limit in the control treatment. The addition of different-origin biochar significantly increased the amount of Zn and Cr in white mustard roots. The higher amount of Cr could be explained by Cr accumulation in the vacuoles of the root cells, which may be a natural toxicity response of the plants []. The amount of Zn in white mustard roots increased twice in all treatments where biochar was added. White mustard roots accumulate a significantly higher amount of Zn in their root system compared to the aboveground biomass, especially if they are grown in soils that are enriched with Zn. This may be attributed to the slow translocation of Zn from roots to the aerial parts []. Our study showed that rapeseed biochar produced at 450 °C and incorporated into the soil promoted a higher ability to accumulate HMs in white mustard roots compared to the other types of biochar. In this treatment, the highest concentrations of Cr, Ni, Cu, and Pb were determined. Higher concentrations of these heavy metals in roots than in aboveground plant biomass may be attributed to the fact that roots are the first target organ to come in contact with metals; therefore, higher accumulation has occurred in the root tissues [,].
3.3. Accumulation of Heavy Metals in Plants Aboveground Biomass after Biochar Incorporation
According to the literature, both buckwheat and white mustard have been observed to serve as efficient phytoremediators for heavy metal extraction from soil []. Results from our study showed that plants’ roots act as a buffer and hold back contamination from the aerial parts. In both plants, only two heavy metals—Cu and Zn—were detected in the aboveground biomass; as opposed to the data observed in these plants’ roots (Figure 6). Being exposed to trace elements, Cu and Zn happen to be more extensively absorbed from the substrate by crops than the remaining elements. Studies have identified that heavy metals are less efficiently transferred into plants because they are more tightly bound by the soil absorption complex. The obtained findings are in line with the research carried out by Patra et al. [], which states that Zn and Cu constitute components of a series of high and moderate accumulations.
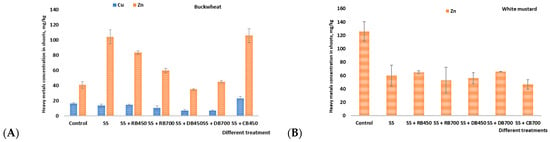
Figure 6.
The heavy metals concentration (mean ± standard deviation) in buckwheat (A) and white mustard (B) shoots after biochar incorporation. Note: SS—sewage sludge; SS + RB450—sewage sludge + rapeseed biochar at 450 °C; SS + RB700—sewage sludge + rapeseed biochar at 700 °C; SS + DB450—sewage sludge + digestate biochar at 450 °C; SS + DB700—sewage sludge + digestate biochar at 700 °C; SS + CB450 and 700—sewage sludge + corn stalk biochar at 450 °C; and 700 °C.
These statements are also consistent with the results of our study.
Cu was found only in buckwheat shoots, and incorporation of all types of biochar showed a tendency to decrease the amount of Cu in buckwheat shoots, except for the treatment where corn stalk’s biochar was added. The lower concentration of Cu in shoots might be due to the low concentration of bioavailable Cu in the soil []. Among all types of biochar, the digestate’s biochar produced at both temperatures (450 °C and 700 °C) has reduced the Cu uptake by 57.6% and 55.8%, respectively. Karami et al. [] also reported that biochar addition has reduced the uptake of Cu in plant shoots. The incorporation of different-origin biochar significantly increased the amount of Zn, especially in these treatments where rapeseed and corn stalk biochar were added. When Zn concentration varies from 200 to 300 mg/kg, this amount of Zn is not harmful to plants and does not interfere with basic physiological processes. It should be emphasized that the concentration of Zn in buckwheat could increase after fertilization with different types of fertilizers [].
In contrast to buckwheat, only Zn was determined in white mustard shoots, and the incorporation of all types of biochar caused a significant reduction of this metal. Uptake of Zn decreased from 47.8% after the addition of rapeseed biochar to 62.8% after the incorporation of corn stalk’s biochar. Our findings correspond with the findings reported by Ahmad et al. [] and Park et al. [], who observed that applying biochar significantly increased the immobilization of heavy metals in soil, resulting in a decrease in the absorption of HMs by plants. Several investigations demonstrate a connection between increases in soil pH, soil organic carbon, and decreased heavy metal uptake by plants treated with biochar. According to Mohamed et al. [], there could be three possible reasons for this decrease in HM uptake by plants: increased dry biomass production, plant selectivity, and metal immobilization in the soil. Furthermore, these metals may form less-soluble complexes [] or stable metal-organic complexes that immobilize metals in soil [,].
3.4. Effect of Biochar on Heavy Metals Concentration in Lysimetric Water
Heavy metals from contaminated soils can leach into groundwater and threaten the natural environment, particularly in acidic conditions. One potential solution for cleaning up polluted soils is biochar. Our study showed that only two of the six investigated heavy metals—Cu and Zn—were found in the lysimetric water samples (Figure 7). Other investigated heavy metals (Cr, Ni, Cd, and Pb) were found to be below the quantification limit (Cr < 0.06; Ni and Pb < 0.01; Cd < 0.007). The addition of biochar to the soil reduced the amount of Cu and Zn in the leachate, except for the treatments where digestate biochar was added. The addition of digestate biochar increased Zn mobility, and the amount of Zn in the leachate was 43.2% higher compared to the control. The main reason for this increase is that digestate’s biochar contains a large amount of Zn [,,]. Second, approximately 50% of the Zn in digestate biochar exists in relatively more active forms, such as the carbonate-bound state and the Fe-Mn oxide-bound state [,].
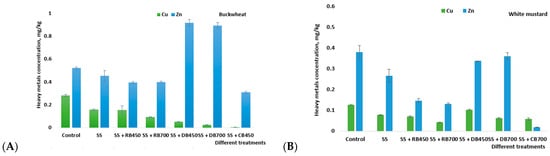
Figure 7.
The heavy metals concentration (mean ± standard deviation) in lysimetric water, growing buckwheat (A) and white mustard (B). Note: SS—sewage sludge; SS + RB450—sewage sludge + rapeseed biochar at 450 °C; SS + RB700—sewage sludge + rapeseed biochar at 700 °C; SS + DB450—sewage sludge + digestate biochar at 450 °C; SS + DB700—sewage sludge + digestate biochar at 700 °C; SS + CB450 and 700—sewage sludge + corn stalk biochar at 450 °C; and 700 °C.
In assessing the Cu amount in leachate, lower levels of heavy metals were detected in each leachate sample after biochar was applied to the soil, suggesting that the remedy for Cu using biochar is promising. The use of biochar significantly decreased the amount of Cu leaching. Following the addition of biochar, the reduction of Cu in the leachate ranged from 66.9 to 91.4 percent. Bessley and Marmiroli [], who observed reductions in Cu and Zn leached from contaminated soil treated with biochar, found similar results. The decrease of these metals in the leachate, compared to the control treatment, suggests that the increased sorption of Cu, Zn, and Pb on the surface of biochar may have also contributed to these metals’ reductions. The biochar pH also played a very important role in the leaching behavior of Cu and Zn, possibly combined with the dilution effect due to mixing with soils containing much lower levels of the metals. Zinc is a rather dynamic element as compared to other HMs, making it considerably easier to alter the level of absorption. Earlier studies conducted in this type of soil revealed that the incorporation of biochar increased Zn bioavailability due to the increased nutrient amount. The authors also note that increased soil pH (pH > 6) could also increase Zn bioavailability in plants [].
3.5. Effects of Biochar Bioconcentration and Translocation Factors
Excessive accumulation of heavy metals in soils can potentially put human health at risk by entering the food chain. Therefore, it is particularly important to choose the right measures and plants suitable for soil remediation. This is also very important when deciding on the correct use/utilization of plants contaminated with heavy metals [].
Some plant species are better suited to phytoremediation than others are. Two coefficients are important to discuss in assessing plant phytoremediation potential: the translocation factor (TF) and the bioconcentration factor (BCF). The bioconcentration factor shows the ability of plants to extract HM compounds from the soil. The BCF evaluates the plant’s HM content in relation to the soil’s HM content. A BCF of less than 1.0 means that a plant is able to absorb HM but cannot accumulate and store it. A value higher than one BCF indicates that a plant is a hyperaccumulator. Meanwhile, the translocation factor represents the heavy metal’s capacity to move from plant roots to other organs. TF values can be utilized for determining the effectiveness of plants in HM transfer from the root system to the above-ground plant biomass. A TF greater than 1.0 shows that the plant successfully transferred the HM from the root to the shoot. On the other hand, TF < 1 denotes poor metal transmission, suggesting that the roots of these plants contain a higher amount of metal than the shoots or/and leaves. So the plant could be classified as a hyperaccumulator; both ratios are required to be greater than one [,,]. Plants are suitable for phytostabilization when they have BCF > 1 and TF < 1, while plants with BCF < 1 and TF > 1 are suitable for phytoextraction [].
The results of our study demonstrated that plant species have a significant impact on the efficiency of heavy metal uptake and phytoremediation capacity. Two selected indicators (BCF and TF) represent the capacity of buckwheat and white mustard to absorb heavy metals (Table 4). Based on the obtained results, buckwheat showed a higher ability to accumulate heavy metals in its roots compared to white mustard. Buckwheat is unique in that its root system, although less developed than other agricultural plants, has a much stronger sorption capacity. Buckwheat roots, exuding organic acids (formic, vinegar, and limonene), are able to absorb various elements from hard-to-dissolve compounds in the soil. Thanks to this property, buckwheat, in addition to the main nutrients (nitrogen, phosphorus, and potassium), is able to absorb heavy metals naturally present in the soil to a greater extent than other agricultural plants.

Table 4.
Effect of biochar on bioconcentration (BCF) and translocation factor (TF).
The data presented in Table 4 demonstrates that Ni and Zn are mainly accumulated in buckwheat roots, while Cu is more accumulated in shoots. The incorporation of biochar significantly promoted the accumulation of all examined heavy metals in buckwheat roots. In these treatments, BCF values were greater than 1, but TF values were quite low, demonstrating that the addition of biochar promotes HM accumulation in plant roots. This finding reveals that phytostabilization is the main method through which HMs are accumulated in buckwheat. Plants can precipitate HMs, decrease their valence in the rhizosphere, sequestrate them within the root tissue, or adsorb them onto the root cell wall. This simultaneously reduces the plant’s uptake [,]. The presence of heavy metals in soil might hinder the plant root apex, hence decreasing its potential to transmit heavy metals from roots to the areal components []. Furthermore, apo-plastic barriers can appear close to the apex of the root, lowering the quantity of HM that migrates from roots to shoots [].The greatest translocation factors (TF > 1) were observed for Cu and Zn for root-shoot translocation in these treatments.
Growing white mustard showed the opposite result for heavy metal accumulation. The data showed that the main mechanism for the accumulation of HMs in white mustard are phytoextraction. The BCF and TF factors indicate that white mustard is capable of absorbing but not accumulating HMs in its roots. In all studied treatments, high root—shoot translocation was observed. The addition of rapeseed biochar produced at 450 °C showed the greatest effect in accumulating Cr, Ni, and Cu in white mustard roots and greater root—shoot translocation for Zn. Pb translocation was low in all study treatments, as the mechanism of Pb adsorption by biochar is fundamentally related to the soil pH. In addition, it has also been suggested that Pb could bind to the functional groups (such as carboxyl and phenolic functional groups) on the surface of the biochar and reduce the mobility of Pb in soil and plants [].
4. Conclusions
The effects of biochar derived from various bio-substrates on the accumulation of heavy metals in soil and buckwheat and white mustard biomass were studied during a pots’ experiment. The incorporation of biochar significantly reduces the heavy metal content of the soil. It was found that zinc had the most mobility among the metals, whereas nickel and lead had the lowest. The rapeseed’s biochar produced at 450 °C showed the greatest significant effect in decreasing the heavy metal content in soil, buckwheat biomass, and lysimetric water. The opposite effect was determined for the use of digestate’s biochar. The incorporation of this type of biochar significantly increased the HMs content in all the soil-plant systems, as well as promoted the HMs leaching from the soil. Based on the obtained data, it can be stated that sewage sludge with high Cu/Zn concentrations are not suitable for soil amendment and remediation. Our study showed that plant roots act as a buffer and hold back contamination from the aerial parts. Only two heavy metals—Cu and Zn—were detected in the buckwheat and white mustard aboveground biomass. The application of biochar helps to immobilize heavy metals in the soil, thereby reducing the HMs leaching from the soil. The use of biochar significantly decreased the amount of Zn and Cu leaching, and the reduction varied from 25 to 91% depending on the origin of biochar. Our study revealed that phytostabilization is the main method through which HMs are accumulated in buckwheat, unlike in mustard cultivation, where the main mechanism of accumulation of HMs is phytoextraction. The BCF and TF factors indicate that white mustard is capable of absorbing but not accumulating HMs in its roots.
According to our research, biochar holds great potential for reducing the mobility of heavy metals in soil, especially in regions where acidifying soil conditions are common. However, it is crucial to explore the long-term effects of biochar on metal immobilization in addition to investigating key mechanisms such as pH, functional groups, and biotic and abiotic surface oxidation. More research is needed to ensure that the mechanisms and processes underlying the interaction between heavy metals and biochar from biosubstrates can be safely used for a circular economy.
Author Contributions
Conceptualization, O.A. and D.K.; methodology, O.A.; software, I.M.; validation, O.A. and D.K.; formal analysis, I.M.; investigation, O.A., G.Ž. and K.B.; resources, O.A.; data curation, D.K., R.R. and K.B.; writing—original draft preparation, I.M.; writing—review and editing, O.A., D.K., R.R., G.Š., K.B. and G.Ž.; visualization, I.M.; supervision, O.A.; project administration, O.A. and D.K.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Regional Development Fund under the Interreg V-A South Baltic Cross-Border Co-operation Program 2014–2020 NR. STHB. 02.02.00–SE–0155/18 Baltic Phytoremediation (BARP) project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lin, X.; Zhou, F.; Qin, J.; Li, H. Biochar and crushed straw additions affect cadmium absorption in cassava-peanut intercropping system. Ecotoxicol. Environ. Saf. 2019, 167, 520–530. [Google Scholar] [CrossRef]
- Oladoye, P.O. Natural, low-cost adsorbents for toxic Pb(II) ion sequestration from (waste)water: A state-of-the-art review. Chemosphere 2022, 287, 132130. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.X.; Al-Abed, S.R.; Reisman, D.J. Biosorption of heavy metals from mining influenced water onto chitin products. Chem. Eng. J. 2011, 166, 1002–1009. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Han, Z.; Xie, B.; Wu, S. The effects of leaching methods on the combustion characteristics of rice straw. Biomass Bioenergy 2013, 49, 22–27. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef]
- Perez-Esteban, J.; Escolastico, C.; Moliner, A.; Masaguer, A.; Ruiz-Fernandez, J. Phytostabilization of metals in mine soils using Brassica juncea in combination with organic amendments. Plant Soil. 2014, 377, 97–109. [Google Scholar] [CrossRef]
- Chou, J.; Wey, M.; Chang, S.H. Study on Pb and PAHs emission levels of heavy metals- and PAHs-contaminated soil during thermal treatment process. J. Environ. Eng. 2010, 136, 112–118. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Lomaglio, T.; Hattab-Hambli, N.; Miard, F.; Lebrun, M.; Nandillon, R.; Trupiano, D.; Scippa, G.S.; Gauthier, A.; Motelica-Heino, M.; Bourgerie, S.; et al. Cd, Pb, and Zn mobility and (bio)availability in contaminated soils from a former smelting site amended with biochar. Environ. Sci. Pollut. Res. 2017, 25, 25744–25756. [Google Scholar] [CrossRef] [PubMed]
- Poucke, R.V.; Ainsworth, J.; Maeseele, M.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Chemical stabilization of Cd-contaminated soil using biochar. Appl. Geochem. 2018, 88, 122–130. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Strezov, V.; Kan, T.; Weldekidan, H.; Asumadu-Sarkodie, S.; Kumar, R. Effect of temperature on heavy metal(loid) deportment during pyrolysis of Avicennia marina biomass obtained from phytoremediation. Bioresour. Technol. 2019, 278, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, Y.Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Wat Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Vaněk, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jimenez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochar’s potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Shen, X.; Huang, D.-Y.; Ren, X.-F.; Zhu, H.-H.; Wang, S.; Xu, C.; He, Y.-B.; Luo, Z.-C.; Zhu, Q.-H. Phytoavailability of Cd and Pb in crop straw biochar-amended soil is related to the heavy metal content of both biochar and soil. J. Environ. Manag. 2016, 168, 245–251. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Zhang, M.; Moon, D.H.; Alwabel, M.I.; Lee, S.S. Impact of soybean stover- and pine needle-derived biochar’s on Pb and As mobility, microbial community, and carbon stability in a contaminated agricultural soil. J. Environ. Manag. 2016, 166, 131–139. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Qasim, B.; Razzak, A.A.; Rasheed, R.T. Effect of biochar amendment on mobility and plant uptake of Zn, Pb and Cd in contaminated soil. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012082. [Google Scholar] [CrossRef]
- Barcauskaite, K.; Mažeika, R. Chemical composition and risk assessment of spring barley grown in artificially contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 21684–21695. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007. [Google Scholar]
- Kalavrouziotis, I.K.; Kostakioti, E.; Koukoulakis, P.H.; Papadopoulos, A.H.; Leotsinidis, M.; Sakazli, E. The impact of Cl-Cd interrelationship on planning wastewater reuse on cabbage. Water Air Soil. Pollut. 2011, 214, 565–573. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, Y.F.; Chang, S.X.; Zhang, J.J.; Jiang, P.K.; Zhou, G.M.; Shen, Z.M. Contrasting effects of bamboo leaf and its biochar on soil CO2 efflux and labile organic carbon in an intensively managed Chinese chestnut plantation. Biol. Fertil. Soils 2014, 50, 1109–1119. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotox. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Soudek, P.; Rodriguez, I.M.; Petrova, S.; Song, J.; Vanek, T. Characteristics of different types of biochar and effects on the toxicity of heavy metals to germinating sorghum seeds. J. Geochem. Explor. 2017, 182, 157–165. [Google Scholar] [CrossRef]
- Erdem, H. The effects of biochar’s produced in different pyrolysis temperatures from agricultural wastes on cadmium uptake of tobacco plant. Saudi J. Biol. Sci. 2021, 28, 3965–3971. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Jung, M.C. The effects of biochar and inorganic amendments on soil remediation in the presence of hyperaccumulator plant. Int. J. Energy Environ. Eng. 2017, 8, 317–329. [Google Scholar] [CrossRef]
- Yang, X.; Igalavithana, A.D.; Oh, S.E.; Nam, H.; Zhang, M.; Wang, C.H.; Kwon, E.E.; Tsang, D.C.W.; Ok, Y.S. Characterization of bioenergy biochar and its utilization for metal/metalloid immobilization in contaminated soil. Sci. Total Environ. 2018, 640–641, 704–713. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gong, Y.; Liu, Q.; Yang, S.; Ma, J.; Zhao, L.; Hou, H. Status of copper accumulation in agricultural soils across China (1985–2016). Chemosphere 2020, 244, 125516. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Hussain, Q.; Ullah, H.; Al-Wabel, M.; Ahmad, M.; Kong, J. High efficiency remediation of cadmium (Cd2+) from aqueous solution using poultry manure- and farmyard manure-derived biochar’s. Separ. Sci. Technol. 2016, 51, 2307–2317. [Google Scholar] [CrossRef]
- Kelly, C.N.; Peltz, C.D.; Stanton, M.; Rutherford, D.W.; Rostad, C.E. Biochar application to hardrock mine tailings: Soil quality, microbial activity, and toxic element sorption. Appl. Geochem. 2014, 43, 35–48. [Google Scholar] [CrossRef]
- Nguyen, T.X.T.; Amyot, M.; Labrecque, M. Differential effects of plant root systems on nickel, copper and silver bioavailability in contaminated soil. Chemosphere 2017, 168, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Vavva, C.; Voutsas, E.; Magoulas, K. Process development for chemical stabilization of fly ash from municipal solid waste incineration. Chem. Eng. Res. Des. 2017, 125, 57–71. [Google Scholar] [CrossRef]
- Tytła, M. Identification of the chemical forms of heavy metals in municipal sewage sludge as a critical element of ecological risk assessment in terms of its agricultural or natural use. J. Environ. Res. Publ. Health 2020, 17, 4640. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, S.; Fu, T.; Li, J.; Zhou, X.; Xue, Y.; Hou, H. Effects of electromagnetic induction on migration and speciation of heavy metals in drying sewage sludge: Mechanistic insights. Waste Manag. 2020, 109, 192–201. [Google Scholar] [CrossRef]
- Albert, H.A.; Li, X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J.; et al. Influence of biochar and soil properties on soil and plant tissue concentrations of Cd and Pb: A meta-analysis. Sci. Total Environ. 2021, 755, 142582. [Google Scholar] [CrossRef]
- Nigam, N.; Khare, P.; Ahsan, M.; Yadav, V.; Shanker, K.; Singh, R.P.; Pandey, V.; Das, P.; Anupama Yadav, R.; Tripathi, P.; et al. Biochar amendment reduced the risk associated with metal uptake and improved metabolite content in medicinal herbs. Physiol. Plantarum 2021, 173, 321–339. [Google Scholar] [CrossRef]
- Nayek, S.; Gupta, S.; Saha, R.N. Metal accumulation and its effects in relation to biochemical response of vegetables irrigated with metal contaminated water and wastewater. J. Hazard. Mater. 2010, 178, 588–595. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Jabeen, R.; Ahmad, A.; Iqbal, M. Phytoremediation of Heavy Metals: Physiological and Molecular Mechanisms. Bot. Rev. 2009, 75, 339–364. [Google Scholar] [CrossRef]
- Patra, D.K.; Pradhan, C.; Patra, H.K. Toxic metal decontamination by phytoremediation approach: Concept, challenges, opportunities and future perspectives. Environ. Technol. Innova. 2020, 18, 100672. [Google Scholar] [CrossRef]
- Karak, T.; Bhattacharyya, P.; Das, T.; Paul, R.K.; Bezbaruah, R. Non-segregated municipal solid waste in an open dumping ground: A potential contaminant in relation to environmental health. Int. J. Environ. Sci. Technol. 2013, 10, 503–518. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass (Lolium perenne). J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Mocek, A.; Magdziak, Z.; Gasecka, M.; Mocek-Płóciniak, A. Impact of Metal/Metalloid-Contaminated Areas on Plant Growth. In Plant-Based Remediation Processes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 79–100. [Google Scholar]
- Ahmad, M.; Usman, A.R.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil. 2011, 348, 439. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.; Shi Li Guo, Z.; Liu, Y.; Chen, F.; Dai, K. Ecological restoration of an acidic Cd contaminated soil using bamboo biochar application. Ecol. Eng. 2015, 84, 67–76. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Arowolo, T.A.; Bamgbose, O. Cadmium, copper, and nickel levels in vegetables from industrial and residential areas of Lagos City, Nigeria. Food Chem. Toxicol. 2003, 41, 375–378. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.-P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, L.; Liu, X.; Wu, J.; Brookes, P.C.; Xu, J. Physicochemical properties of biochar produced from aerobically composted swine manure and its potential use as an environmental amendment. Bioresour. Technol. 2013, 142, 641–646. [Google Scholar] [CrossRef]
- Wang, K.; Peng, N.; Lu, G.; Dang, Z. Effects of pyrolysis temperature and holding time on physicochemical properties of swine-manure-derived biochar. Waste Biomass Valori 2020, 11, 613–624. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, X.; Wang, L.; Chen, Q.; Fang, J.; Shen, X.; Lou, L.; Tian, G. The speciation, leachability and bioaccessibility of Cu and Zn in animal manure derived biochar: Effect of feedstock and pyrolysis temperature. Front. Environ. Sci. Eng. 2017, 11, 5. [Google Scholar] [CrossRef]
- Tian, R.; Li, C.; Xie, S.; You, F.; Cao, Z.; Xu, Z.; Yu, G.; Wang, Y. Preparation of biochar via pyrolysis at laboratory and pilot scales to remove antibiotics and immobilize heavy metals in livestock feces. J. Soils Sediments 2019, 19, 2891–2902. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium, and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef]
- Barcauskaite, K.; Anne, O.; Mockeviciene, I.; Repšiene, R.; Šiaudinis, G.; Karcauskiene, D. Determination of Heavy Metals Immobilization by Chemical Fractions in Contaminated Soil Amended with Biochar. Sustainability 2023, 15, 8677. [Google Scholar] [CrossRef]
- Szarek-Łukaszewska, G. Plants That Hyperaccumulate Metals. Kosmos 2014, 63, 443–453. [Google Scholar]
- Dahlawi, S.; Sadiq, M.; Sabir, M.; Farooqi, Z.U.R.; Saifullah; Qadir, A.A.; Faraj, T.K. Differential Response of Brassica Cultivars to Potentially Toxic Elements and Their Distribution in Different Plant Parts Irrigated with Metal-Contaminated Water. Sustainability 2023, 15, 1966. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Murtaza, G.; Bibi, S.; Sabir, M.; Owens, G.; Saifullah; Ahmad, I.; Zeeshan, N. Immobilization of Cadmium in Soil-Plant System through Soil and Foliar Applied Silicon. Int. J. Phytoremed. 2022, 24, 1193–1204. [Google Scholar] [CrossRef]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of Metal and Metalloid Trace Elements: Facts and Fiction. Plant Soil. 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Cheraghi, M.; Lorestani, B.; Khorasani, N.; Yousefi, N.; Karami, M. Findings on the Phytoextraction and Phytostabilization of Soils Contaminated with Heavy Metals. Biol. Trace Elem. Res. 2011, 144, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Oladoye, P.O.; Olowe, O.M.; Asemoloye, M.D. Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literature. Chemosphere 2022, 288, 132555. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; An, J.; Yin, Y.; Gao, C.; Wang, B.; Wei, S. Heavy metals uptake and translocation of typical wetland plants and their ecological effects on the coastal soil of a contaminated bay in Northeast China. Sci. Total Environ. 2022, 803, 149871. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Sun, H.; Chen, Y.; Pan, H.; Yang, X.; Rafiq, T. Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS ONE 2014, 9, e108568. [Google Scholar] [CrossRef]
- Pajevič, S.; Borišev, M.; Nikolič, N.; Arsenov, D.D.; Orlovič, S.; Župunski, M. Phytoextraction of heavy metals by fast-growing trees: A review. In Phytoremediation; Springer: New York, NY, USA, 2016; pp. 29–64. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).