Experimental Investigation on the Effect of Salt Solution on the Soil Freezing Characteristic Curve for Expansive Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
3. Results and Discussion
3.1. The T2 Distribution Curves for Soils at Different Temperatures
3.2. The Influence of Salt Solution Concentration on the Freezing Characteristic Curve
3.3. Correlations among Soil Sample Freezing Point, Concentration of Salt Solution, and Soil Properties
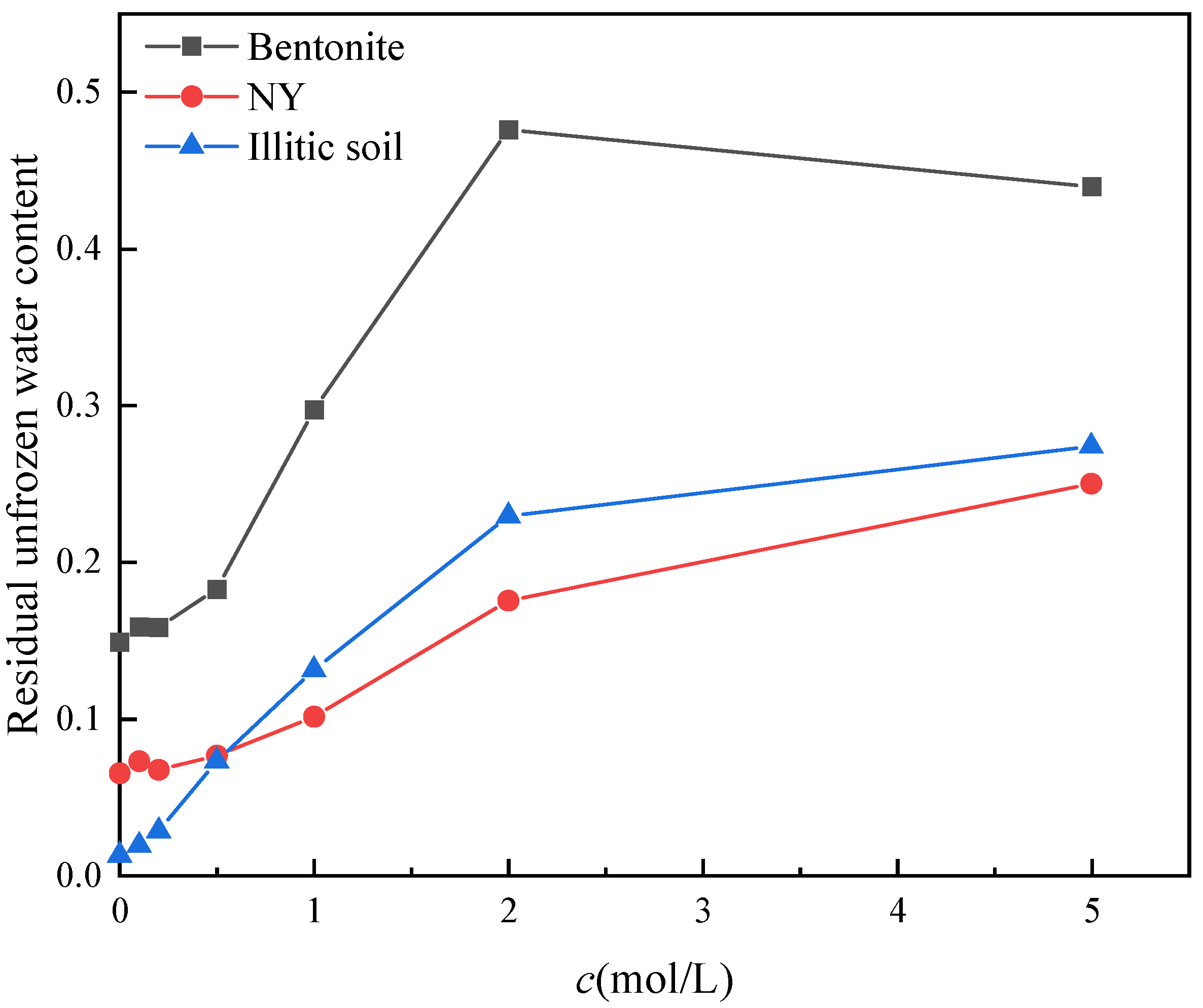
3.4. Influence of Salt Solution Concentration and Soil Properties on the Residual Unfrozen Water Content
4. Simulation of Soil Freezing Characteristic Curve
5. Conclusions
- (1)
- The freezing characteristic curve for expansive soil is very similar to the water retention curve. When the temperature reaches the freezing point, the soil sample begins to freeze; as the temperature further decreases, a large amount of pore water begins to freeze, and the unfrozen water content rapidly decreases; when the temperature further decreases, the speed at which the unfrozen water content decreases slows down.
- (2)
- When soil samples are saturated with salt solutions of different concentrations, the freezing point of the soil decreases with increasing salt concentration. At a given negative temperature, there is more unfrozen pore water in saline soil. Therefore, under the same conditions, the presence of salt promotes thawing in frozen areas. During thawing, saltwater freeze zones provide more liquid water than non-saltwater freeze zones. The higher the montmorillonite content in soil samples, the lower the freezing temperature of the soil samples and the higher the residual unfrozen water content. This conclusion can explain the existence of winter thawing areas in Nanyang.
- (3)
- Based on the similarity between soil freezing characteristic curves and soil–water characteristic curves, a unified model in the literature was used to simulate the experimental results for the three soils under different salt contents. Simulation results show that freezing characteristic curves under different salt contents can be simulated using one set of parameters, indicating that the effects of the salt solution and capillary action are independent. At the same time, when the salt solution concentration is large, it deviates from the ideal solution assumption and requires correction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Wang, J.; Zhang, L. Physics of Frozen Soil; Science Press: Beijing, China, 2001. [Google Scholar]
- Wei, M.; Da-yan, W. Studies on frozen soil mechanics in China in past 50 years and their prospect. Chin. J. Geotech. Eng. 2012, 34, 625–640. [Google Scholar]
- Shi, G.; Zhao, S.; Li, X.; Niu, Y. The frost heaving deformation of high-speed railway subgrades in cold regions: Monitoring and analyzing. J. Glaciol. Geocryol. 2014, 36, 360–368. [Google Scholar]
- Li, X.; Cheng, G.; Jin, H.; Kang, E.; Che, T.; Jin, R.; Wu, L.; Nan, Z.; Wang, J.; Shen, Y. Cryospheric change in China. Glob. Planet Change 2008, 62, 210–218. [Google Scholar] [CrossRef]
- Hua, L.; Fujun, N.; Yonghong, N.; Xifeng, Y. Study on thermal regime of roadbed–culvert transition section along a high speed railway in seasonally frozen regions. Cold Reg. Sci. Technol. 2014, 106, 216–231. [Google Scholar] [CrossRef]
- Tai, B.; Yue, Z.; Qi, S.; Wang, P. Experimental and numerical investigation on thermal-moisture-mechanical behaviors on a new anti-frost cutting bed of high-speed railway in deep seasonally frozen ground regions under extreme climate. Comput. Geotech. 2021, 136, 104251. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, Z.; Sun, D.; Lv, H. Comparative study on thermal properties of undisturbed and compacted lateritic soils subjected to drying and wetting. Eng. Geol. 2020, 277, 105800. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, X.; Zhao, Y.; Lu, H. Performance of a Ground-Source Heat Pump System and the Heat-Moisture Migration of Surrounding Soils in Karst Areas. J. Perform. Constr. Fac. 2017, 31, 04017050. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, Y.; Lu, H.; Wei, C. Experimental performance study of ground-coupled heat pump system for cooling and heating provision in karst region. Energy Build. 2018, 158, 971–986. [Google Scholar] [CrossRef]
- Batbaatar, J.; Gillespie, A.R.; Sletten, R.S.; Mushkin, A.; Amit, R.; Trombotto Liaudat, D.; Liu, L.; Petrie, G. Toward the Detection of Permafrost Using Land-Surface Temperature Mapping. Remote Sens. 2020, 12, 695. [Google Scholar] [CrossRef]
- Spaans, E.J.; Baker, J.M. The soil freezing characteristic: Its measurement and similarity to the soil moisture characteristic. Soil Sci. Soc. Am. J. 1996, 60, 13–19. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, C.; Lai, Y.; Wei, H.; Tian, H. Application of the generalized Clapeyron equation to freezing point depression and unfrozen water content. Water Resour. Res. 2018, 54, 9412–9431. [Google Scholar] [CrossRef]
- Marion, G.M. Freeze-Thaw Processes and Soil Chemistry; Special Report; Cold Regions Research and Engineering Laboratory: Hanover, NH, USA, 1995. [Google Scholar]
- Corte, A.E. Experimental Formation of Sorted Patterns in Gravel Overlying a Melting Ice Surface; U.S. Army Snow, Ice, and Permafrost Research Establishment: Wilmette, IL, USA, 1959.
- Corte, A.E. Vertical migration of particles in front of a moving freezing plane. J. Geophys. Res. 1962, 67, 1085–1090. [Google Scholar] [CrossRef]
- Corte, A.E. Relationship between Four Ground Patterns, Structure of the Active Layer, and Type and Distribution of Ice in the Permafrost; US Army, Corps of Engineers: Hanover, NH, USA, 1962; p. 79.
- Corte, A.E. Particle sorting by repeated freezing and thawing. Biuletyn Peryglacjalny 1966, 15, 174–240. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. Observations of soil freezing and frost heave at Inuvik, Northwest Territories, Canada. Can. J. Earth Sci. 1985, 22, 283–290. [Google Scholar] [CrossRef]
- Williams, P.J.; Smith, M.W. The Frozen Earth: Fundamentals of Geocryology; Cambridge University Press: Cambridge, NY, USA, 1989; p. 306. [Google Scholar]
- Flerchinger, G.; Seyfried, M.; Hardegree, S. Using soil freezing characteristics to model multi-season soil water dynamics. Vadose Zone J. 2006, 5, 1143–1153. [Google Scholar] [CrossRef]
- Yan, R.; Yu, H.; Yang, D.; Tang, H.; Zhang, Q. Shear strength and pore pressure characteristics of methane hydrate-bearing soil under undrained condition. Int. J. Hydrogen Energy 2023, 48, 12240–12256. [Google Scholar] [CrossRef]
- Yang, D.; Yan, R.; Yan, M.; Lu, D.; Wei, C. Geomechanical properties of artificial methane hydrate-bearing fine-grained sediments. Gas Sci. Eng. 2023, 109, 104852. [Google Scholar] [CrossRef]
- Bertouille, H. Effets du Gel sur les Sols Fins; Institut Francais des Sciences et Technologies des Transports, de l’Aménagement et des Réseaux (IFSTTAR): Paris, France, 1972; Volume 21. [Google Scholar]
- Watanabe, K.; Wake, T. Measurement of unfrozen water content and relative permittivity of frozen unsaturated soil using NMR and TDR. Cold Reg. Sci. Technol. 2009, 59, 34–41. [Google Scholar] [CrossRef]
- Koopmans, R.W.R.; Miller, R. Soil freezing and soil water characteristic curves. Soil Sci. Soc. Am. J. 1966, 30, 680–685. [Google Scholar] [CrossRef]
- Tsytovich, N.A. The Mechanics of Frozen Ground; McGraw-Hill: New York, NY, USA, 1975. [Google Scholar]
- Wang, C.; Li, S.; Lai, Y.; Chen, Q.; He, X.; Zhang, H.; Liu, X. Predicting the Soil Freezing Characteristic From the Particle Size Distribution Based on Micro-Pore Space Geometry. Water Resour. Res. 2022, 58, e2021WR030782. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Wang, J.; Zhao, Y.; Dou, Z. Quantification and division of unfrozen water content during the freezing process and the influence of soil properties by low-field nuclear magnetic resonance. J. Hydrol. 2021, 602, 126719. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Wei, C.; Zhang, K.; Shen, Z.; Yang, Z. Effects of salinity on soil freezing temperature and unfrozen water content. Rock Soil Mech. 2020, 41, 952–960. [Google Scholar]
- Xiao, Z.; Zhu, L.; Hou, Z. The change mechanism and a prediction model of unfrozen water content in sodium chloride soil. Geoderma 2022, 419, 115881. [Google Scholar] [CrossRef]
- Anderson, D.M.; Morgenstern, N. Physics, chemistry and mechanics of frozen ground: A review. In Proceedings of the Permafrost, Second International Conference, Yakutsk, Russia, 13–28 July 1973; National Academy of Sciences: Washington, DC, USA, 1973; pp. 257–288. [Google Scholar]
- Lewis, G.N.; Randall, M. Thermodynamics; McGraw-Hill: New York, NY, USA, 1961. [Google Scholar]
- Pusch, R.; Yong, R.N. Microstructure of Smectite Clays and Engineering Performance; CRC Press: London, UK, 2006. [Google Scholar]
- Tan, L.; Wei, C.; Tian, H.; Zhou, J.; Wei, H. Experimental study of unfrozen water content of frozen soils by low-field nuclear magnetic resonance. Rock Soil Mech. 2015, 36, 1566–1572. [Google Scholar]
- Dastidar, R. Nuclear Magnetic Resonance (NMR) Study of Freezing and Thawing of Saturated Porous Media and Application to Shale and Pore Volume Compressibility Estimation. Ph.D. Thesis, The University of Oklahoma, Norman, OK, USA, 2007. [Google Scholar]
- Cowan, B.; Cowan, B. Nuclear Magnetic Resonance and Relaxation; Cambridge University Press: Cambridge, NY, USA, 1997; Volume 427. [Google Scholar]
- Tian, H.; Wei, C.; Lai, Y.; Chen, P. Quantification of water content during freeze–thaw cycles: A nuclear magnetic resonance based method. Vadose Zone J. 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Qi, J.; Ma, W.; Song, C. Influence of freeze–thaw on engineering properties of a silty soil. Cold Reg. Sci. Technol. 2008, 53, 397–404. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, G.; Han, B.; Li, J. Experimental study on pore structure and freezing characteristics of graded soils based on NMR. Chin. J. Geotech. Eng. 2022, 44, 178–182. [Google Scholar]
- Liu, Y.; Deng, H.; Xu, J.; Tian, G.; Deng, J. Association Study on the Pore Structure and Mechanical Characteristics of Coarse-Grained Soil under Freeze–Thaw Cycles. Minerals 2022, 12, 314. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Wu, X. Experimental study on pore fluid characteristics of fractured sandstone based on nuclear magnetic resonance technology. Sci. Eng. Ethics 2022, 214, 110408. [Google Scholar] [CrossRef]
- Wei, C. A theoretical framework for modeling the chemomechanical behavior of unsaturated soils. Vadose Zone J. 2014, 13, 1–21. [Google Scholar] [CrossRef]
- Brooks, R.H. Hydraulic Properties of Porous Media; Colorado State University: Fort Collins, CO, USA, 1965. [Google Scholar]
- Perez, M. Gibbs–Thomson effects in phase transformations. Scr. Mater. 2005, 52, 709–712. [Google Scholar] [CrossRef]
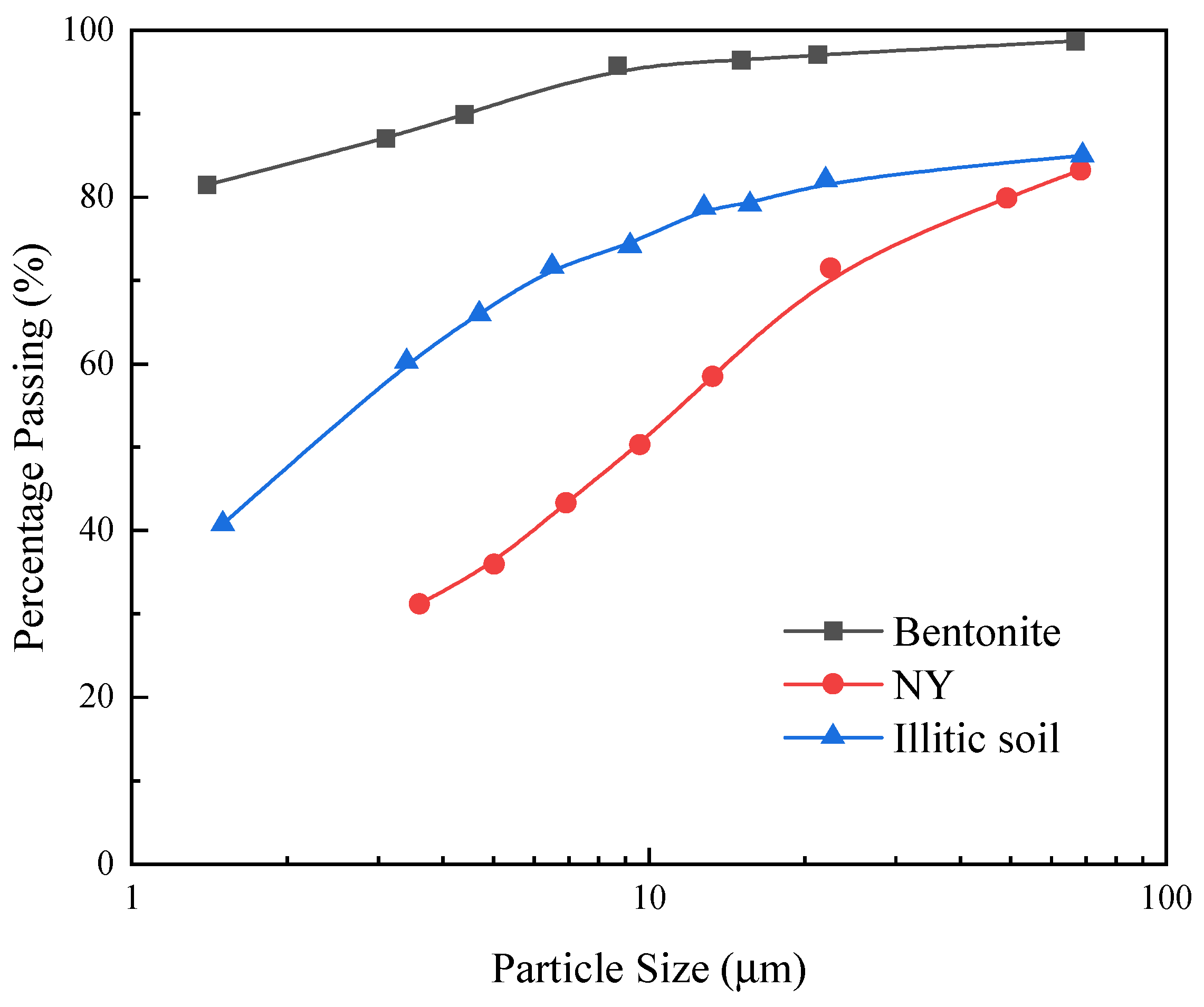








| Soil Type | Liquid Limit/% | Plastic Limit/% | Plasticity Index | Specific Gravity |
|---|---|---|---|---|
| Bentonite | 176.61 | 41.46 | 135.16 | 2.72 |
| NY | 57.4 | 26.5 | 30.9 | 2.73 |
| Illitic soil | 40.62 | 23.61 | 17.01 | 2.72 |
| Soil Type | Clay Mineral Composition/% | |||||
|---|---|---|---|---|---|---|
| Montmorillonite | Quartz | Illite | Albite | Microcline | Anorthite | |
| Bentonite | 68.3 | 0.8 | - | - | - | 30.9 |
| NY | 33.7 | 29.7 | - | 24.9 | 11.7 | - |
| Illitic soil | - | 18.2 | 49.0 | 7.9 | 24.9 | - |
| Soil Type | k | Tf/°C |
|---|---|---|
| Bentonite | 0.7 | −6.5 |
| NY | 0.7 | −2 |
| Illitic soil | 3 | −1.5 |
| Soil Type | A | B | ||
|---|---|---|---|---|
| Bentonite | 6.94 × 1020 | 26.16 | 0.148 | 0.98 |
| NY | 90.36 | 8.19 | 0.077 | 0.96 |
| Illitic soil | 2.55 | 5.23 | 0.02 | 0.98 |
| Solution Concentration /(mol∙L−1) | Coefficient of Determination | ||
|---|---|---|---|
| Bentonite | NY | Illitic Soil | |
| 0 | 0.98 | 0.96 | 0.98 |
| 0.1 | 0.98 | 0.96 | 0.89 |
| 0.2 | 0.94 | 0.91 | 0.89 |
| 0.5 | 0.87 | 0.83 | 0.95 |
| 1 | 0.79 | 0.86 | 0.96 |
| 2 | - | 0.96 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Hao, F.; Yi, P.; Zhang, Q.; Ma, T. Experimental Investigation on the Effect of Salt Solution on the Soil Freezing Characteristic Curve for Expansive Soils. Sustainability 2024, 16, 363. https://doi.org/10.3390/su16010363
Yu H, Hao F, Yi P, Zhang Q, Ma T. Experimental Investigation on the Effect of Salt Solution on the Soil Freezing Characteristic Curve for Expansive Soils. Sustainability. 2024; 16(1):363. https://doi.org/10.3390/su16010363
Chicago/Turabian StyleYu, Haiwen, Fengfu Hao, Panpan Yi, Qin Zhang, and Tiantian Ma. 2024. "Experimental Investigation on the Effect of Salt Solution on the Soil Freezing Characteristic Curve for Expansive Soils" Sustainability 16, no. 1: 363. https://doi.org/10.3390/su16010363






