Effects of Sodium Silicate Alkali Sludge on the Rheological and Mechanical Properties of an Alkali-Activated Slag System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Specimen Preparation and Test Method
3. Results and Discussion
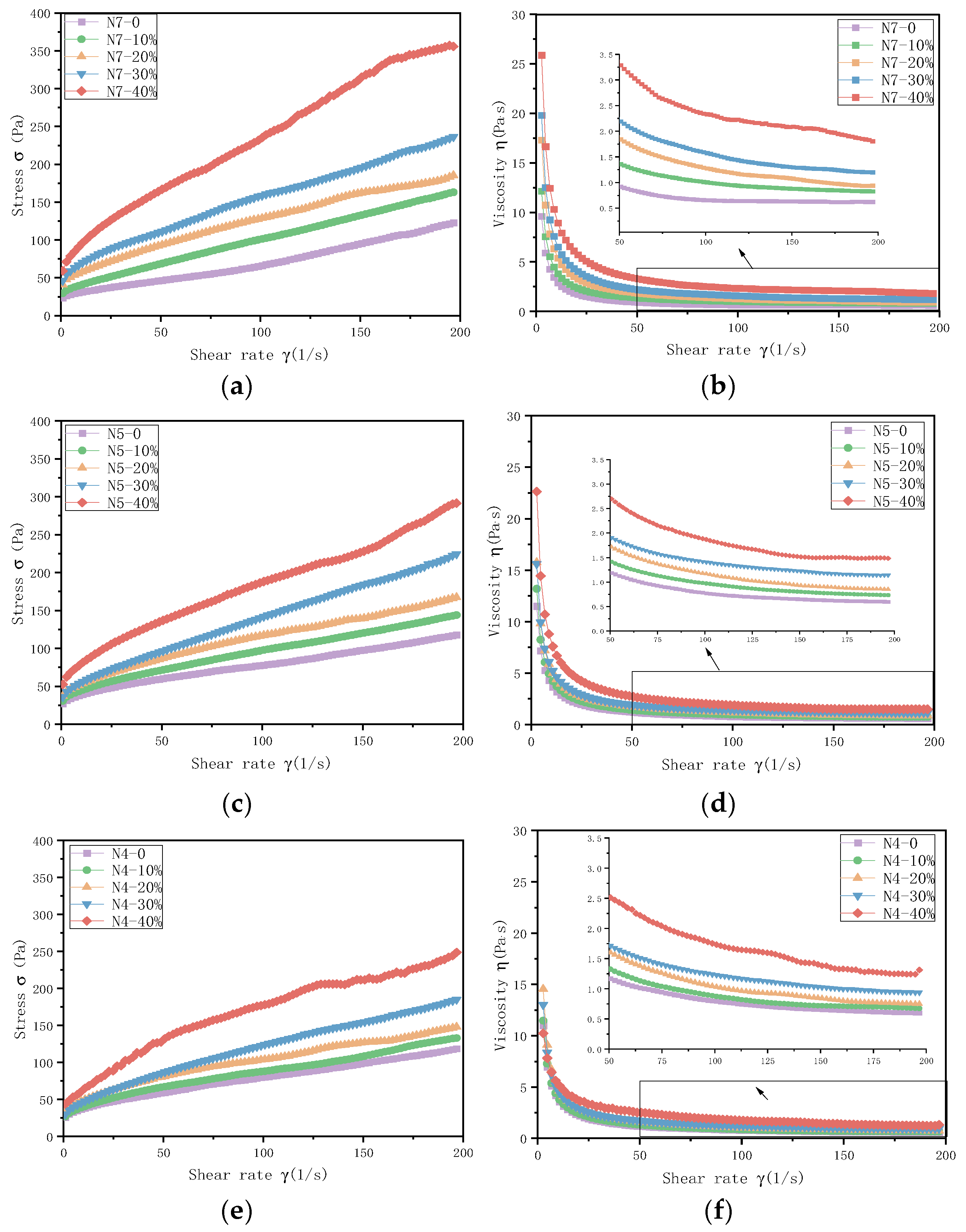
3.1. Fluidity
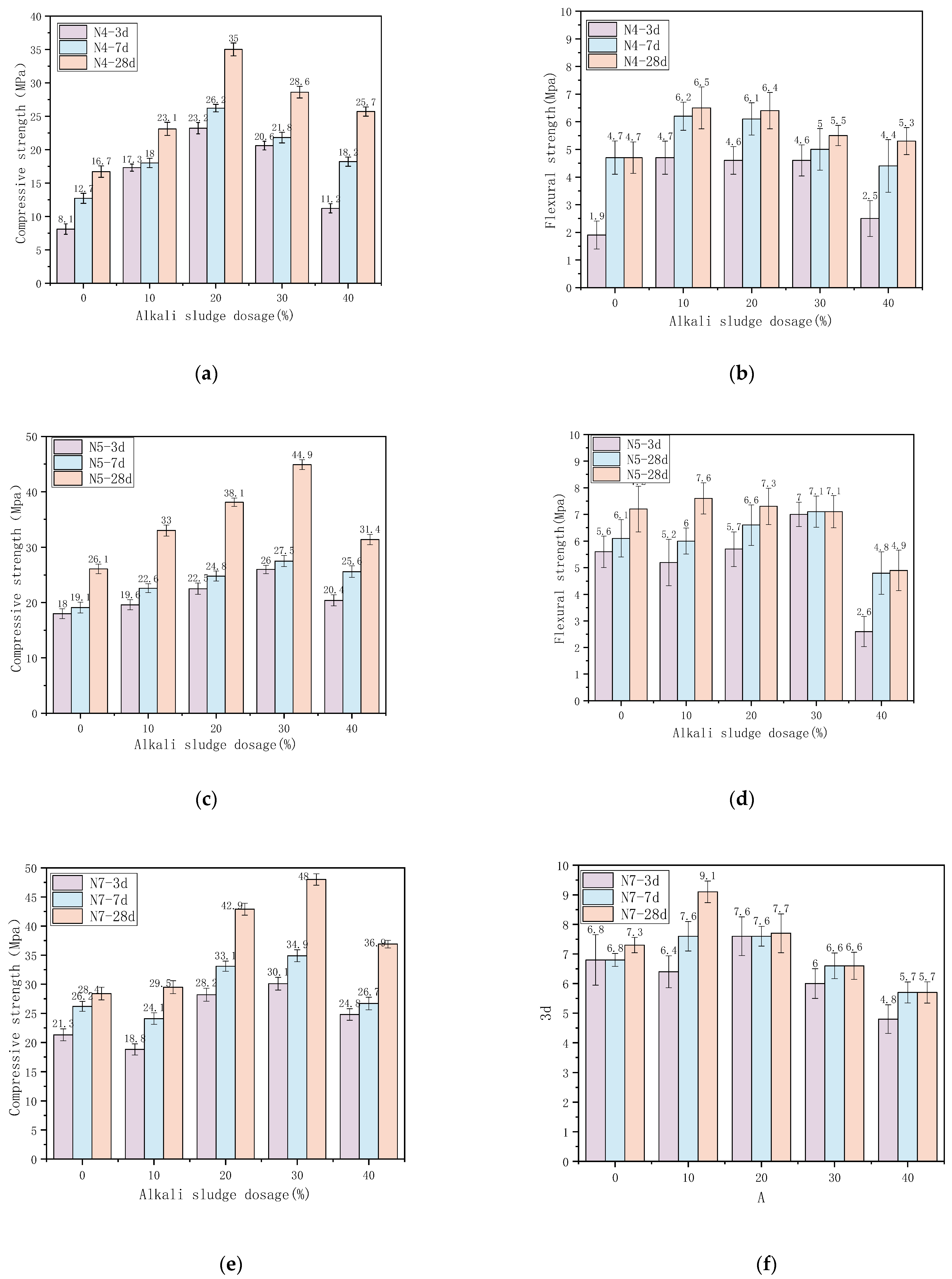
3.2. Mechanical Properties
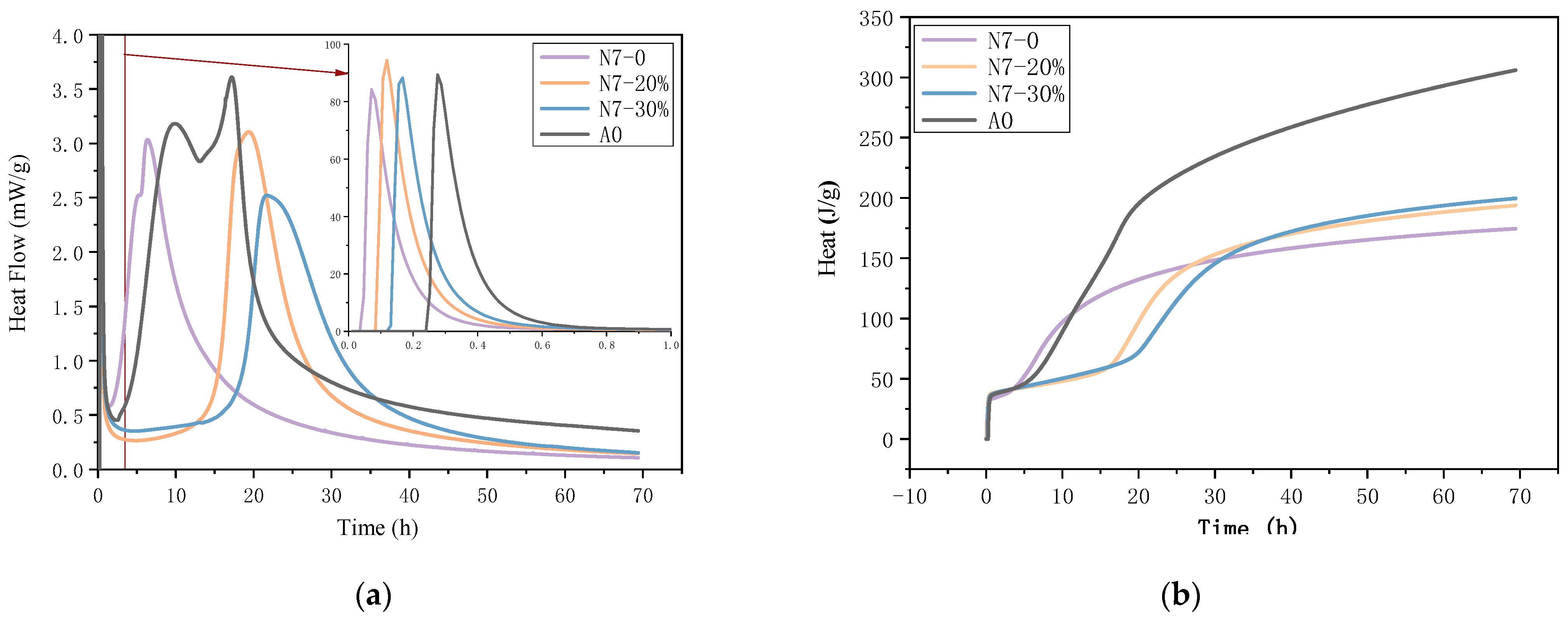
3.3. Heat of Hydration
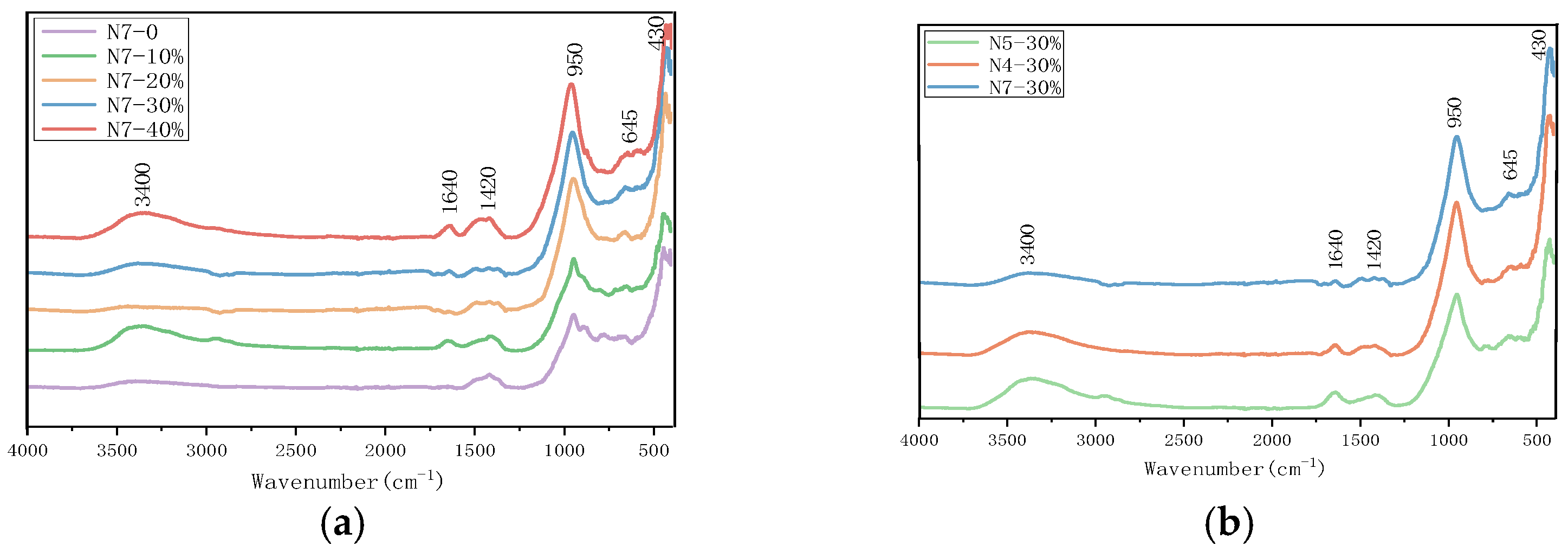
3.4. FTIR Analysis
3.5. SEM Micromorphology
3.6. Sustainable Development and Circular Economy Potentials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, R.; Li, Y.; Meng, X.; Fan, L.; Wu, G.; Li, X.; Jiang, X.; Yu, S.; Hu, Y. Research on the slurrying performance of coal and alkali-modified sludge. Fuel 2021, 294, 120548. [Google Scholar] [CrossRef]
- Lang, L.; Chen, B.; Chen, B. Strength evolutions of varying water content-dredged sludge stabilized with alkali-activated ground granulated blast-furnace slag. Constr. Build. Mater. 2021, 275, 122111. [Google Scholar] [CrossRef]
- Sun, K.; Ali, H.A.; Xuan, D.; Ban, J.; Poon, C.S. Utilization of APC residues from sewage sludge incineration process as activator of alkali-activated slag/glass powder material. Cem. Concr. Compos. 2022, 133, 104680. [Google Scholar] [CrossRef]
- Bayat, A.; Hassani, A.; Yousefi, A. Effects of red mud on the properties of fresh and hardened alkali-activated slag paste and mortar. Constr. Build. Mater. 2018, 167, 775–790. [Google Scholar] [CrossRef]
- Sreelekshmi, S.; Chandran, R.S.; Varghese, S.R.; Naeem; Raju, T.; Ramaswamy, K.P. Rheological and strength behaviour of red mud—Slag geopolymer composites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 989, 012005. [Google Scholar] [CrossRef]
- Li, B.; Li, G. Study on Mechanical Properties of Soda Residue/Fly Ash Composite Cementitious Material. Adv. Mater. Res. 2011, 194–196, 1026–1029. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Wang, L.; Zuo, L.; Zhu, Q.; Ma, W. Physical and mechanical properties and micro characteristics of fly ash-based geopolymers incorporating soda residue. Cem. Concr. Compos. 2019, 98, 125–136. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Q.; Zhang, J.; An, S. Microstructure and composition of hardened paste of soda residue-slag complex binding materials. J. Build. Mater. 2019, 22, 872–877. (In Chinese) [Google Scholar]
- Lin, Y.; Xu, D.; Zhao, X. Experimental research on mechanical property and microstructure of blast furnace slag cementitious materials activated by soda residue. Bull. Chin. Ceram. Soc. 2019, 38, 2876–2881, 2889. (In Chinese) [Google Scholar]
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. Standardization Administration of China: Beijing, China, 2005.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- Li, L.; Lu, J.-X.; Zhang, B.; Poon, C.-S. Rheology behavior of one-part alkali activated slag/glass powder (AASG) pastes. Constr. Build. Mater. 2020, 258, 120381. [Google Scholar] [CrossRef]
- Palacios, M.; Banfill, P. Puertas, Rheology and Setting of Alkali-Activated Slag Pastes and Mortars: Effect of Organic Admixture. ACI Mater. J. 2008, 105, 140–148. [Google Scholar]
- Su, T.; Zhou, Y.; Wang, Q. Recent advances in chemical admixtures for improving the workability of alkali-activated slag-based material systems. Constr. Build. Mater. 2021, 272, 121647. [Google Scholar]
- Xu, W.; Guo, F.; Tian, Q. Rheological properties of cement paste with limestone powder at different dosages of superplasticizers. J. Build. Mater. 2014, 17, 274–279. (In Chinese) [Google Scholar]
- Zhang, S.; He, Y.; Zhang, H.; Chen, J.; Liu, L. Effect of fine sand powder on the rheological properties of one-part alkali-activated slag semi-flexible pavement grouting materials. Constr. Build. Mater. 2022, 333, 127328. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Hu, J.; Yu, Q.; Shi, C. Effects of anionic species of activators on the rheological properties and early gel characteristics of alkali-activated slag paste. Cem. Concr. Res. 2022, 162, 106968. [Google Scholar] [CrossRef]
- Romagnoli, M.; Leonelli, C.; Kamse, E.; Lassinantti Gualtieri, M. Rheology of geopolymer by DOE approach. Constr. Build. Mater. 2012, 36, 251–258. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Wang, T. Influencing factors analysis and optimized prediction model for rheology and flowability of nano-SiO2 and PVA fiber reinforced alkali-activated composites. J. Clean. Prod. 2022, 366, 132988. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; He, X.; Su, Y.; Miao, W.; Strnadel, B.; Huang, X. Hydration and rheology of activated ultra-fine ground granulated blast furnace slag with carbide slag and anhydrous phosphogypsum. Cem. Concr. Compos. 2022, 133, 104727. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; de Schutter, G. Influence of water to binder ratio on the rheology and structural Build-up of Alkali-Activated Slag/Fly ash mixtures. Constr. Build. Mater. 2020, 264, 120253. [Google Scholar] [CrossRef]
- Puertas, F.; Varga, C.; Alonso, M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Al-kroom, H.; Arif, M.A.; Elkhoresy, A.H.; Abd El-Aleem, S.; Mohammed, A.H.; Abd Elrahman, M.; Abdel-Gawwad, H.A. Synergistic positive effects of nano barium silicate on the hydration rate and phase composition of alkali-activated slag. J. Build. Eng. 2022, 59, 105109. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, L.; Cui, X.; He, Y.; Zheng, G.; Shi, C. Effect of limestone on rheological, shrinkage and mechanical properties of alkali—Activated slag/fly ash grouting materials. Constr. Build. Mater. 2018, 191, 1285–1292. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Wang, D.-M.; Liu, Z.; Xie, F.-Z. Rheology, agglomerate structure, and particle shape of fresh geopolymer pastes with different NaOH activators content. Constr. Build. Mater. 2018, 187, 674–680. [Google Scholar] [CrossRef]
- Vance, K.; Dakhane, A.; Sant, G.; Neithalath, N. Observations on the rheological response of alkali activated fly ash suspensions: The role of activator type and concentration. Rheol. Acta 2014, 53, 843–855. [Google Scholar] [CrossRef]
- Fang, S.; Lam, E.S.S.; Li, B.; Wu, B. Effect of alkali contents, moduli and curing time on engineering properties of alkali activated slag. Constr. Build. Mater. 2020, 249, 118799. [Google Scholar] [CrossRef]
- Liew, Y.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.; Heah, C. Optimization of solids-to-liquid and alkali activator ratios of calcined kaolin geopolymeric powder. Constr. Build. Mater. 2012, 37, 440–451. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Lothenbach, B.; Neubauer, J. The early hydration of Ordinary Portland Cement (OPC): An approach comparing measured heat flow with calculated heat flow from QXRD. Cem. Concr. Res. 2012, 42, 134–138. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, Z.; Yang, X.; Li, X.; Li, S.; Zhang, Z. Heat release characteristics of lime and time-dependent rheological behaviors of lime-activated fly ash pastes. Case Stud. Constr. Mater. 2022, 16, e01043. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Adediran, A.; Yliniemi, J.; Luukkonen, T.; Illikainen, M. Effect of organic resin in glass wool waste and curing temperature on the synthesis and properties of alkali-activated pastes. Mater. Des. 2021, 212, 110287. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Chen, L.; Huang, J.; Ma, T. The influence of two types of alkali activators on the microstructure and performance of supersulfated cement concrete: Mitigating the strength and carbonation resistance. Cem. Concr. Compos. 2021, 118, 103947. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, R.; Zhang, J.; Shui, Z. A low-carbon alkali activated slag based ultra-high performance concrete (UHPC): Reaction kinetics and microstructure development. J. Clean. Prod. 2022, 363, 132416. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, Y.; Li, X.; Yang, X.; Rao, F.; Zhong, L. Durability of alkali-activated materials with different C–S–H and N-A-S-H gels in acid and alkaline environment. J. Mater. Res. Technol. 2021, 16, 619–630. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R. 29Si and 17O NMR investigation of the structure of some crystalline calcium silicate hydrates. Adv. Cem. Based Mater. 1996, 3, 133–143. [Google Scholar] [CrossRef]
- Tayeh, B.A.; Zeyad, A.M.; Agwa, I.S.; Amin, M. Effect of elevated temperatures on mechanical properties of lightweight geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00673. [Google Scholar] [CrossRef]
- Shehata, N.; Mohamed, O.; Sayed, E.T.; Abdelkareem, M.A.; Olabi, A. Geopolymer concrete as green building materials: Recent applications, sustainable development and circular economy potentials. Sci. Total Environ. 2022, 836, 155577. [Google Scholar] [CrossRef]
- Carreño-Gallardo, C.; Tejeda-Ochoa, A.; Perez-Ordonez, O.; Ledezma-Sillas, J.; Lardizabal-Gutierrez, D.; Prieto-Gomez, C.; Valenzuela-Grado, J.; Hernandez, F.R.; Herrera-Ramirez, J. In the CO2 emission remediation by means of alternative geopolymers as substitutes for cements. J. Environ. Chem. Eng. 2018, 6, 4878–4884. [Google Scholar] [CrossRef]






| CaO | SiO2 | Al2O3 | MgO | SO3 | Fe2O3 | K2O | Na2O | TiO2 | MnO | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 57.12 | 22.53 | 10.99 | 3.37 | 1.78 | 0.92 | 0.36 | 0.21 | 1.87 | 0.47 | 0.38 |
| Alkali sludge | 3.80 | 74.56 | 5.62 | 0.736 | 0.151 | 0.487 | 1.54 | 12.74 | 0.096 | 0.032 | 0.238 |
| Alkali Equivalent Content | Slag, kg/m3 | Alkali Sludge, kg/m3 | NaOH, kg/m3 | Sand, kg/m3 | |
|---|---|---|---|---|---|
| N4–0 | 4% | 350 | 0 | 171.5 | 744 |
| N4–10% | 315 | 35 | 171.5 | 744 | |
| N4–20% | 280 | 70 | 171.5 | 744 | |
| N4–30% | 245 | 105 | 171.5 | 744 | |
| N4–40% | 210 | 140 | 171.5 | 744 | |
| N5–0 | 5% | 350 | 0 | 171.5 | 744 |
| N5–10% | 315 | 35 | 171.5 | 744 | |
| N5–20% | 280 | 70 | 171.5 | 744 | |
| N5–30% | 245 | 105 | 171.5 | 744 | |
| N5–40% | 210 | 140 | 171.5 | 744 | |
| N7–0 | 7% | 350 | 0 | 171.5 | 744 |
| N7–10% | 315 | 35 | 171.5 | 744 | |
| N7–20% | 280 | 70 | 171.5 | 744 | |
| N7–30% | 245 | 105 | 171.5 | 744 | |
| N7–40% | 210 | 140 | 171.5 | 744 |
| Heat at 72 h | 1st Peak | 2nd Peak | 3rd Peak | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Integral (J/g) | Time | Peak (mW/g) | Integral (J/g) | Time | Peak (mW/g) | Integral (J/g) | Time | Peak (mW/g) | Integral (J/g) | |
| A0 | 305.98 | 17 min | 89.39 | 5.57 | 9 h 52 min | 3.18 | 87.49 | 17 h 11 min | 1031 | 168.47 |
| N7–0 | 174.57 | 4 min | 84.16 | 5.19 | 5 h 19 min | 2.52 | 54.94 | — | — | — |
| N7–20% | 193.82 | 7 min | 94.41 | 7.62 | 19 h 23 min | 3.11 | 90.57 | — | — | — |
| N7–30% | 199.68 | 10 min | 88.31 | 7.88 | 121 h 43 min | 2.52 | 86.30 | — | — | — |
| Element | N7–0 | N7–10% | N7–20% | N7–30% | N7–40% | N4–30% | N5–30% |
|---|---|---|---|---|---|---|---|
| O | 43.83 | 44.73 | 28.17 | 44.29 | 41.85 | 47.41 | 39.54 |
| Na | 6.08 | 6.64 | 8.42 | 10.48 | 12.08 | 6.97 | 10.59 |
| Mg | 3.78 | 4.06 | 0.18 | 0.38 | 3.92 | 0.43 | 5.56 |
| Al | 7.19 | 7.3 | 1.82 | 3.79 | 5.07 | 2.55 | 6.58 |
| Si | 15.52 | 17.95 | 39.44 | 24.84 | 25.27 | 20.76 | 19.71 |
| Ca | 23.61 | 19.08 | 20.81 | 11.86 | 11.15 | 13.87 | 11.81 |
| Ca/Si | 1.06 | 0.74 | 0.53 | 0.33 | 0. | 0.47 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Ren, L.; Wan, X.; Jin, Z.; Wang, H. Effects of Sodium Silicate Alkali Sludge on the Rheological and Mechanical Properties of an Alkali-Activated Slag System. Sustainability 2024, 16, 90. https://doi.org/10.3390/su16010090
Gao L, Ren L, Wan X, Jin Z, Wang H. Effects of Sodium Silicate Alkali Sludge on the Rheological and Mechanical Properties of an Alkali-Activated Slag System. Sustainability. 2024; 16(1):90. https://doi.org/10.3390/su16010090
Chicago/Turabian StyleGao, Liyan, Lijie Ren, Xiaomei Wan, Zuquan Jin, and Hong Wang. 2024. "Effects of Sodium Silicate Alkali Sludge on the Rheological and Mechanical Properties of an Alkali-Activated Slag System" Sustainability 16, no. 1: 90. https://doi.org/10.3390/su16010090






