Abstract
Plant growth-promoting rhizobacteria (PGPRs) represent a promising strategy for enhancing plant resilience and yields under drought-stress conditions. This study isolated and characterized PGPR from wheat rhizosphere soil in Egypt. Four PGPR strains were evaluated for an array of plant growth-promoting traits, including IAA production, biofilm formation, siderophore production, nitrogen fixation, ACC deaminase activity, phosphate solubilization, and antagonistic potential. Molecular identification via 16S rRNA sequencing classified three isolates (MMH101, MMH102, and MMH103) within the Bacillus genus and one isolate (MMH104) as Myroides sp. Greenhouse experiments examined the effects of PGPR inoculation on the drought-stressed Egyptian wheat cultivar, Gimmeza-9. Wheat plants inoculated with PGPR isolates showed dramatic improvements in growth parameters and stress tolerance indicators compared to non-inoculated controls when subjected to a 10-day drought period, with Bacillus rugosus (MMH101) inoculation resulting in increases of 61.8% in fresh biomass, 77.2% in dry biomass, 108.5% shoot length, and 134.9% root length. PGPR treatments also elevated the chlorophyll and proline content while reducing malondialdehyde levels. The findings demonstrate the effectiveness of PGPR inoculation in enhancing the morphology, physiology, and drought stress resilience of wheat. Isolated PGPR strains hold promise as biofertilizers for improving cereal productivity under water-deficit conditions.
1. Introduction
In dry and semi-dry areas worldwide, drought stress poses a significant agricultural challenge, continuously reducing crop productivity [1,2]. These harsher drought conditions pose a significant threat to food production in affected countries [3]. Water is crucial for various plant functions, such as growth, development, and metabolism. It makes up a significant part, ranging from 80% to 95% of a plant’s fresh biomass [4,5]. Due to water scarcity, many experts argue that drought is the main environmental stressor, particularly in regions prone to limited water resources. Drought has historically led to severe famines [6].
To tackle this important problem, various methods have been developed to enhance a plant’s resilience to drought stress. Water-saving irrigation practices, conventional breeding techniques, and genetic engineering to create drought-tolerant transgenic plants have been explored. Unfortunately, these methods require significant expertise and effort, making their application in practical, real-world situations a significant challenge [7]. Plant growth-promoting rhizobacteria (PGPRs) represent a promising avenue for mitigating drought stress and promoting sustainable agriculture. These bacteria, through various mechanisms, enhance plant growth and shield plants from diseases and abiotic stresses [8]. The assessment of crucial bacterial traits such as biological nitrogen fixation, phosphate solubilization, ACC deaminase activity, and the production of siderophores and phytohormones has highlighted their potential to facilitate plant development across diverse environments [9].
The direct processes through which plant growth-promoting bacteria foster plant growth encompass phytohormone production and regulation, the release of volatile compounds, and improvements in plant nutrient absorption, including nitrogen, phosphorus, and iron [10]. In parallel, indirect mechanisms involve inhibiting pathogen-produced enzymes or toxins, triggering host-induced systemic resistance, and suppressing harmful microorganisms and plant pathogens [11]. This is primarily achieved by PGPRs through the production of antimicrobial metabolites, hydrolytic enzymes, competition for nutrients, and space within the rhizosphere [12]. Gene expression plays a crucial role in the intricate interaction between plants and plant growth-promoting rhizobacteria (PGPR). This interaction, at the molecular level, involves a complex network of genetic responses that governs the plant’s ability to establish beneficial relationships with these rhizospheric bacteria [13,14].
Wheat (Triticum aestivum L.) is one of the world’s staple crops, serving as a primary source of sustenance for millions of people. However, wheat cultivation is often challenged by drought stress [15], particularly in regions characterized by arid and semiarid climates. An increasingly prominent strategy for enhancing wheat drought stress tolerance involves the use of plant growth-promoting rhizobacteria (PGPR). These beneficial soil bacteria play a pivotal role in improving plant growth and stress resilience through diverse mechanisms. Notably, PGPR possesses ACC deaminase activity, as demonstrated [9,16], enabling them to enzymatically reduce elevated ethylene levels induced by drought conditions, thereby mitigating the growth-inhibiting effects of this stress hormone.
For successful PGPR application, especially in soils facing drought challenges, the introduced bacteria must demonstrate resilience to drought stress. Another important feature is their capacity to establish themselves in the rhizosphere and outcompete the native microflora [17]. Consequently, drought-tolerant rhizobacteria hold a competitive advantage, as they demonstrate the capacity to flourish in novel drought-prone environments, ensuring their presence in sufficient numbers to confer tangible benefits to plants [18].
In this context, our investigation aims to evaluate the impact of inoculating native rhizosphere bacteria on the growth and production of Egyptian wheat (Triticum aestivum L.), particularly under conditions of drought stress. Through this comprehensive evaluation, we aim to characterize the diverse plant growth-promoting attributes of our isolated bacterial strains and delineate how they may synergistically boost drought tolerance in wheat. The identification of beneficial PGPR with positive effects on plants holds the potential for successful applications, offering a promising avenue to enhance crop resilience and productivity.
2. Materials and Methods
2.1. Isolation and Screening of PGPR
Rhizosphere soil samples were taken from the “Giza 168” wheat cultivar cultivated in the fields of the Agricultural Genetic Engineering Research Institute, Giza, Egypt (coordinates: 30°01′25″ N, 31°12′11″ E). The soil composition in this region exhibits a clay loam texture, comprised of 35% clay, 37.9% silt, 16.7% fine sand, and 10.1% coarse sand. Using a serial dilution technique, rhizobacteria were isolated from rhizosphere soil taken from the Giza 168 wheat cultivar. Morphologically different bacterial colonies were purified, kept on slants at 4 °C, and stored in 50% glycerol stock at −80 °C for further use. To examine the drought resistance of isolated bacteria, we formulated a distinct nutritional broth (NB) medium containing varying concentrations of polyethylene glycol (PEG 6000), specifically (0%, 10%, 15%, and 20%). A volume of 100 μL from each bacterial isolate, with a concentration of 1 × 107 colony-forming units (CFU) per milliliter, determined via optical density (OD) measurement at 600 nm, was introduced into the test tubes containing 5 mL of NB. The optical density at 600 nm was determined using a spectrophotometer following an overnight incubation in an orbital shaker at 30 °C with a rotation speed of 150 rpm. The impact of different levels of PEG on the growth of bacterial cultures was assessed [19].
2.2. Evaluation of Plant Growth-Promoting (PGP) Characteristics
2.2.1. Production of Indole Acetic Acid (IAA)
To determine the concentration of IAA, 1.5 mL of the extract without bacteria was mixed with 4 mL of Salkowski reagent, which consisted of 12 g of FeCl3 per liter in 7.9 M H2SO4. The mixture was then incubated in the dark for 24 h. The intensity of the pink color that formed was measured at a wavelength of 520 nm using a spectrophotometer. The IAA concentration was determined by comparing the measurement to a standard curve of known IAA concentrations as previously outlined by [20].
2.2.2. Biofilm Production
Bacterial isolates were cultured aerobically overnight in a nutrient broth (NB) medium. Subsequently, these bacterial cultures were diluted with fresh NB medium to achieve an optical density of 0.02 at 600 nm. The cultures were then dispensed into a 96-well polystyrene plate, with each well receiving 160 µL of the bacterial culture. The plates were incubated at 28 °C for 48 h. Following incubation, biofilm development was assessed using crystal violet staining [21]. To measure biofilm formation, the wells of the microtiter plate were emptied, gently washed with distilled water (dd H2O) at least five times to remove weakly attached bacterial cells, and then left at room temperature for 30 min. Each well was stained with 200 µL of 0.1 percent crystal violet and incubated for 20 min at 28 °C. After cleaning the plate’s wells, 70 percent ethanol was added to each dry well to quantify the crystal violet staining. The absorbance at 590 nm was determined using a plate reader after a 20 min incubation of the samples. Five replicates were performed for each sample.
2.2.3. Production of Siderophores
We used a universal chrome azurol S (CAS) and hexadecyltrimethylammonium bromide (HDTMA) agar plate to qualitatively detect siderophore production by the bacterial strains. Produced siderophores were quantified by scoring the size of the halo zones for each of the isolates, as previously outlined by [22].
2.2.4. ACC Deaminase Activity
Bacterial isolates were initially plated on LB agar plates and incubated for 24 h at 28 °C. Subsequently, these isolates were subcultured onto sterile minimum DF (Dworkin and Foster) salt media, with a modification involving the substitution of (NH4)2SO4 with 3 mM ACC (1-aminocyclopropane-1-carboxylate) as the sole nitrogen source, following the methods outlined by [23,24]. Over three days, the inoculated plates were incubated at 28 °C, and daily measurements of growth were recorded. Colonies that exhibited growth on these plates upon subculturing were identified as ACC deaminase producers, as per the criteria established by [25].
2.2.5. Phosphate Solubilization
To assess phosphate-solubilizing abilities, spot inoculations of the bacterial isolates were performed on Pikovskaya’s agar medium, as described by [26]. This medium was modified by the inclusion of 2% (w/v) tricalcium phosphate (TCP). Following inoculation, the plates were cultured at 28 °C for seven days. The appearance of clear zones surrounding the bacterial colonies was indicative of effective phosphate solubilization.
2.2.6. Nitrogen Fixation
To identify bacterial isolates with nitrogen-fixing capabilities, we conducted a qualitative assessment by cultivating these bacteria in a nitrogen-free (NF) medium. This medium consisted of the following components per liter: 20 g mannitol, 0.2 g K2HPO4, 0.2 g NaCl, 0.2 g MgSO4·7H2O, 0.1 g K2SO4, 5 g CaCO3, and 20 g agar, following the method outlined by reference [27]. After 48 h of incubation at 28 °C, we observed and monitored the growth of the isolates to ascertain their nitrogen-fixing activity.
2.2.7. Antagonism Assay
To assess the antagonistic activity of bacterial isolates against Rhizoctonia solani, dual culture tests were carried out following the procedure described by [28]. In these tests, PDA (Potato Dextrose Agar) plates were inoculated by streaking bacterial suspensions parallel to the edge of the plates. Following bacterial inoculation, a mycelial plug of Rhizoctonia solani was placed in the center of the plate. Control plates containing only Rhizoctonia solani or only bacterial isolates were also prepared. Subsequently, all plates were incubated at 25 °C for 7–10 days.
2.3. 16S rDNA Gene Analysis of Bacterial Isolates
Following the manufacturer’s instructions, total genomic DNA was extracted using the G-spin™ Genomic DNA Extraction Kit (iNtRON Biotechnology, Inc., Seongnam, Kyonggi-do, Republic of Korea) and utilized as a DNA template in PCR reactions. To amplify the 16S rDNA region, we employed the 16S rDNA bacterial universal primers, forward (5′-AGAGTTTGATCCTGGCTCAG-3′) and reverse (5′-TACGGYTACCTTGTTACGACTT-3′), as previously described by [29]. The PCR reactions were carried out in a 25 μL reaction mixture, comprising 30 ng of genomic DNA (gDNA) as the template, 5 μL of (5X) PCR buffer, 2.5 μL of a primer mix (10 μM of each primer), 1.5 μL of (25 mM) MgCl2, 0.5 μL of (10 mM) dNTPs, 0.5 μL of (50 units/μL) Taq polymerase, and the final volume was adjusted to 25 μL using nuclease-free water. The PCR amplification was carried out using a thermal cycler (BIO-RAD C1000TM Thermal Cycler, Bio-Rad, CA, USA) with the following conditions: initial DNA denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 57 °C for 1 min, extension at 72 °C for 1.5 min, and a final elongation step at 72 °C for 7 min. Subsequently, the amplified products were purified using the QIAquick PCR Purification Kit, and their analysis was conducted via gel electrophoresis. The purified DNA products were sent to Macrogen Inc. in Seoul, South Korea for DNA sequencing.
The obtained sequences were subsequently subjected to analysis through the BLAST Sequence Similarity Search to identify the most closely related members in the NCBI GenBank DNA database (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 29 August 2023). The 16S rDNA sequences of the PGPR strains have been submitted to the NCBI database, and their corresponding accession numbers have been assigned. Sequence alignment and the analysis of evolutionary relationships with the most closely related bacterial sequences retrieved from the NCBI database were conducted using MEGA X, employing the neighbor-joining approach, as described by [30].
2.4. Pot Experiment and Drought Treatment
Surface-sterilized wheat cultivar Gimmeza-9 seeds were divided into two groups: untreated (control) and PGPR-treated with strains MMH101 to MMH104. These seeds were sown in standard pots, each filled with a mixture of three and a half kilograms of sterile soil, sand, and peat. The experiment encompassed five treatments: T1 (drought-stressed, uninoculated), T2 (drought-stressed, inoculated with MMH101), T3 (drought-stressed, inoculated with MMH102), T4 (drought-stressed, inoculated with MMH103), and T5 (drought-stressed, inoculated with MMH104). Each treatment was replicated three times, with six plants per pot, facilitating the investigation of the influence of drought stress and PGPR treatment on the growth and performance of the wheat cultivar Gimmeza-9. Seedlings that were ten days old and had been treated with PGPR were given a 1% bacterial solution (~106 CFU mL−1). On the other hand, untreated control plants received the same amount of MS media. Both the PGPR-treated and control plants were watered every other day to maintain soil moisture at 60% of field capacity for three weeks. Afterward, all plants were exposed to ten days of drought stress by withholding water. Non-stressed plants were watered as usual. To recover from the drought, plants were rehydrated for three days.
2.5. Evaluating the Impact of PGPR on Wheat Drought Tolerance
Following a period of 10 days of induced drought stress and subsequent 3 days of drought recovery, we assessed the morphological traits, chlorophyll content, proline levels, and malondialdehyde (MDA) content in each plant. This analysis aimed to evaluate the impact of PGPR isolates on the drought tolerance of wheat plants.
2.5.1. Morphological Traits Measurement
After a predetermined period of exposure to drought stress, morphological traits were assessed. Measurements included fresh biomass, determined by weighing the entire seedling immediately after harvesting; dry biomass, obtained by drying the seedlings to a constant weight; shoot length, measured from the base of the stem to the tip of the longest leaf; and root length, measured from the base of the root system to the tip of the longest root.
2.5.2. Chlorophyll Content
For each treatment, a leaf sample weighing 100 mg was collected and homogenized with 80% acetone (v/v) to extract chlorophyll content. The resulting homogenate was then filtered through filter paper. To determine the amounts of chlorophylls a and b, the absorbance of the solution was measured using a spectrophotometer at 663 and 645 nm wavelengths, respectively, following the method of [31]. The total chlorophyll content (Chlorophyll a + b) was calculated using the following formulas [32]:
- Chlorophyll a (mg/g FW) = [{(12.7 × OD663) − (2.69 × OD645)} × V × 1000 × W]
- Chlorophyll b (mg/g FW) = [{(22.9 × OD645) − (4.68 × OD663)} × V × 1000 × W]
where OD663 and OD645 represent the spectrophotometer readings at 663 and 645 nm, respectively, V is the volume of the homogenate, and W is the weight of the leaf sample in grams.
2.5.3. Proline
The proline amino acid content was quantified using a colorimetric assay based on ninhydrin. Plant leaves were first homogenized with 3% sulfosalicylic acid while kept on ice and then centrifuged for 5 min. Next, 100 μL of the resulting supernatant was mixed with an additional 100 μL of 3% sulfosalicylic acid, 200 μL of glacial acetic acid, and 200 μL of acidic ninhydrin. This mixture was incubated for 1 h at 96 °C, after which the reaction was halted by placing the samples on ice. To facilitate phase separation, 1 mL of toluene was added, followed by vortexing for 30 s and allowing the organic and aqueous phases to separate for 5 min. The absorbance of the chromophore-containing toluene layer was then measured at 520 nm, using toluene as a reference, as described by [33].
2.5.4. Malondialdehyde (MDA) Content
To determine the level of lipid peroxidation in wheat shoots, we employed thiobarbituric acid.
(TBA) method, as originally outlined by Heath and Packer, 1968. In brief, 500 mg of wheat shoots were meticulously homogenized using a mortar and pestle in 1 mL of 0.1% trichloroacetic acid (TCA, w/v). Subsequently, the homogenate underwent centrifugation at 10,000× g for 20 min, and 0.5 mL of the resulting supernatant was combined with 1 mL of 0.5% TBA dissolved in 20% TCA. The resulting mixture was then subjected to heat in a boiling water bath for 1 h. To halt the reaction, the tubes were transferred to an ice bath for a 10 min cooling period and, thereafter, centrifuged for an additional 10 min at 10,000× g. The absorbance of the supernatant was recorded at 532 nm and 600 nm, with the non-specific absorption at 600 nm being subtracted. The MDA-TBA complex concentration was determined using the extinction coefficient of 155 mM−1 cm−1 [34].
2.6. Statistical Analysis
The statistical analysis was conducted using the ANOVA function in SPSS software version 19.0. To compare the means, we used Tukey’s multiple range test with a significance level of p ≤ 0.05. The results were reported as mean ± SD. GraphPad Prism 9 was used to calculate the standard error and mean and create graphs.
3. Results
3.1. Isolation and Identification of Bacteria
In this study, four rhizobacteria were obtained from the rhizospheric soil of the Giza 168 wheat cultivar. Each of these isolates exhibited distinct colony shapes. Subsequently, their capacity to promote plant growth (PGP potential) was assessed through functional validation.
3.2. Qualitative Evaluation of Plant Growth-Regulating Traits
The results of various plant growth-promoting traits and antagonistic activities exhibited by four PGPR isolates (MMH 101, MMH102, MMH 103, and MMH 104) are summarized in Table 1. These isolates were evaluated for siderophore production, nitrogen fixation ability, ACC deaminase activity, and antagonism. Among the isolates, MMH 101 and MMH 104 displayed strong siderophore production abilities, with MMH 102 showing slightly lower production and MMH 103 exhibiting a moderate level. Regarding nitrogen fixation, MMH 101 and MMH 103 demonstrated high capabilities, while MMH 102 had a lower capacity, and MMH 104 showed moderate ability. ACC deaminase activity was detected in MMH 101 and MMH 103, with MMH 102 and MMH 104 exhibiting lower levels of this activity. In contrast, none of the microbial isolates displayed phosphate solubilization activity in the assessments conducted. In terms of antagonistic behavior, MMH 101 and MMH 103 inhibited potential plant pathogens, while MMH 102 and MMH 104 did not exhibit such behavior. These findings suggest that MMH 101 and MMH 103 possess a range of beneficial plant growth-promoting traits, including siderophore production, nitrogen fixation, and ACC deaminase activity, along with the ability to inhibit plant pathogens.

Table 1.
Overview of PGPR traits across the evaluated isolates, encompassing characteristics such as siderophore production, nitrogen fixation, ACC deaminase production, phosphate solubilization, and antagonism.
3.3. Quantitative Evaluation of Plant Growth-Regulating Traits
The results of indole-3-acetic acid (IAA) production by the four PGPR isolates are presented in Figure 1. Notably, MMH 101 demonstrated the highest IAA production, registering a concentration of 31.90 μg/mL. In contrast, MMH 102 exhibited a lower IAA production level, measuring 12.12 μg/mL, while MMH 103 and MMH 104 displayed even lower IAA production, with concentrations of 9.11 μg/mL and 6.01 μg/mL, respectively. These findings underscore the considerable variation in IAA production among the isolates, with MMH 101 emerging as the most prolific producer of this crucial plant growth-promoting phytohormone.
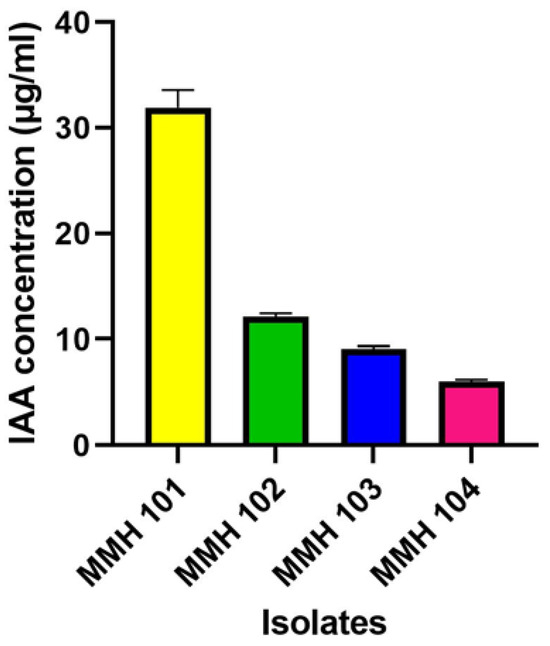
Figure 1.
The assessment of indole acetic acid (IAA) production by 4 PGPR isolates. Each value corresponds to the average ± standard deviation of five replicates.
The results of biofilm production, measured in terms of optical density (OD), are presented for the four PGPR isolates in Figure 2. Among these isolates, MMH 101 exhibited the highest biofilm production with an OD reading of 0.323, indicating a relatively robust biofilm-forming ability. In contrast, MMH 102 displayed a lower level of biofilm production, recording an OD of 0.196, while MMH 103 demonstrated a similar, though slightly higher, biofilm production with an OD of 0.333. Notably, MMH 104 stood out as the most prolific biofilm producer among the isolates, showcasing significantly higher biofilm production with an OD of 0.826.
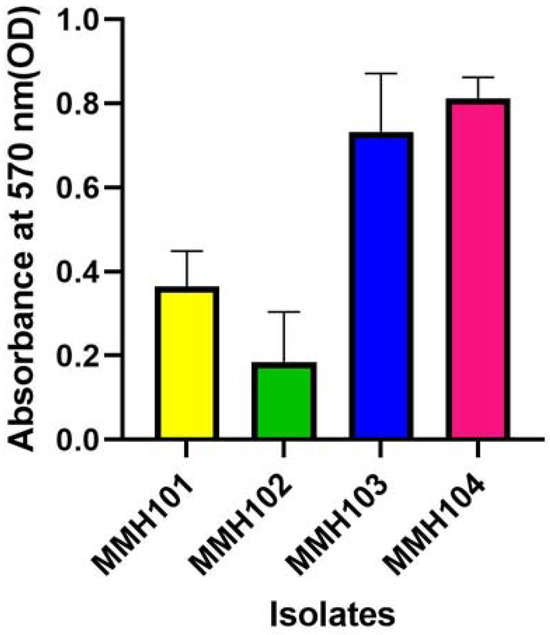
Figure 2.
The assessment of biofilm production by 4 PGPR isolates. Each value corresponds to the average ± standard deviation of five replicates.
3.4. Molecular Identification of PGPR Isolates
A 1500-base-pair segment of the 16S rRNA gene was isolated and sequenced for analysis from four distinct isolates, as shown in Figure 3. The sequencing results revealed that three of these bacterial strains were classified within the phylum Firmicutes, while the fourth belonged to the phylum Bacteroidota. Specifically, three of the microbial isolates were identified as belonging to the genus Bacillus, with the following designations: MMH101 (identified as Bacillus rugosus), MMH102 (identified as Bacillus licheniformis), and MMH103 (identified as Bacillus halotolerans). The fourth isolate, MMH104, was classified as a member of the genus Myroides. To visually represent the evolutionary relationships between these isolates and other related bacteria, a phylogenetic tree was constructed using the 16S rDNA data, as depicted in Figure 4. The 16S rDNA sequences of these plant growth-promoting rhizobacteria (PGPR) strains have been deposited in the NCBI database and have been assigned the following accession numbers for reference: Bacillus rugosus strain MMH101 (accession number: OQ073496), Bacillus licheniformis strain MMH102 (accession number: OQ073497), Bacillus halotolerant strain MMH103 (accession number: OQ073498), and Myroides sp. strain MMH104 (accession number: OQ073499).
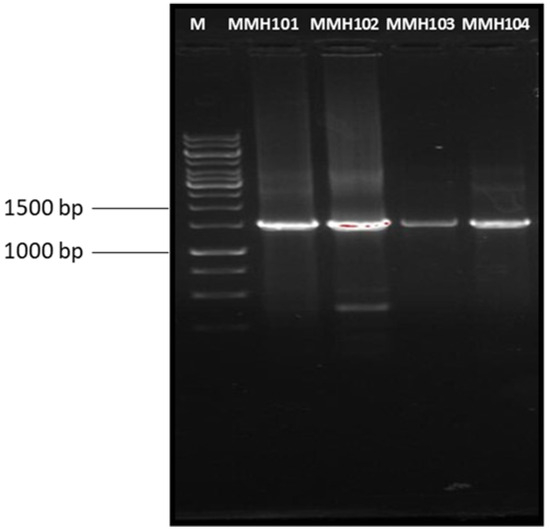
Figure 3.
1% agarose electrophoresis gel showing the PCR product of 16S gene amplification. (M) Thermo Scientific™ GeneRuler 1 kb DNA Ladder (Waltham, MA, USA).
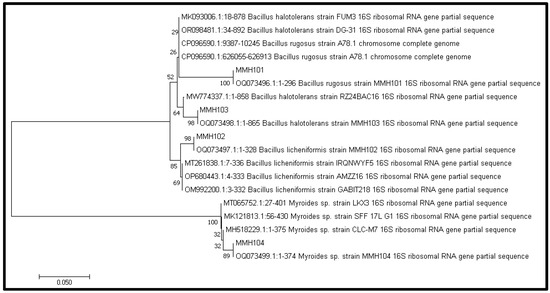
Figure 4.
Construction of a phylogenetic tree utilizing 16S rRNA gene sequences from isolates and their closest relatives.
3.5. Assessment of Inoculated Wheat Morphological Traits under Drought Stress
Leaf rolling and wilting were visually apparent in all drought-treated plants beginning 10 days after stress initiation. By the end of the trial, droughted plants had exhibited complete leaf rolling. In addition to rolling and wilting, leaf chlorosis and senescence were visibly enhanced in plants under drought conditions. Quantifying the timing, extent, and progression of changes in leaf morphology provides insights into drought tolerance mechanisms and the systematic impacts of water deficits on plant health and growth. The assessment of average fresh biomass in wheat plants subjected to drought stress, as depicted in Figure 5A. Treatments T2, T3, T4, and T5, representing wheat plants inoculated with various plant growth-promoting rhizobacteria (PGPR), were compared to the control group (T1) after exposure to drought stress. T2 exhibited the highest average fresh biomass at 251.36 mg, representing a significant increase compared to the control’s average fresh biomass of 155.36 mg. This indicates a substantial improvement of 61.8% in fresh biomass when inoculated with the PGPR. Treatments T3, T4, and T5, while displaying lower average fresh biomass values (215.52 mg, 191.248 mg, and 196.16 mg, respectively), also showed positive percentage changes from the control, with improvements of 38.7%, 23.8%, and 26.2%, respectively. Treatment T2 displayed the highest average dry biomass at 17.1 mg, signifying a considerable augmentation compared to the control’s average dry biomass of 9.664 mg (Figure 5B). This translates to a substantial improvement of 77.2% in dry biomass when subjected to the PGPR treatment. Treatments T3, T4, and T5, while exhibiting lower average dry biomass values (14.8 mg, 12.3 mg, and 12.2 mg, respectively), also demonstrated positive percentage changes from the control, with enhancements of 53.4%, 27.2%, and 26.1%, respectively.
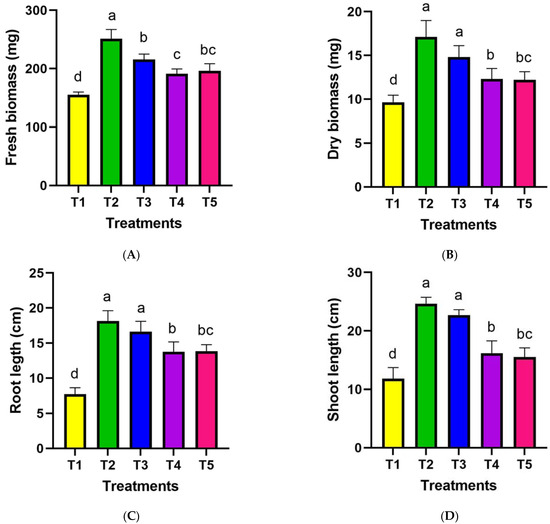
Figure 5.
Impact of drought stress and PGPR application on morphological wheat traits: (A) fresh biomass; (B) dry biomass; (C) shoot length; (D) root length. The data represent the mean values of five replications ± SD. Lowercase letters placed above the bars indicate statistically significant differences between the treatments at a significance level of p < 0.05.
For shoot length (Figure 5C), Treatment T2 exhibited the highest average at 24.6 cm, representing a substantial increase compared to the control’s average shoot length of 11.8 cm. This corresponds to a notable improvement of 108.5% in shoot length due to the application of PGPR. Treatments T3, T4, and T5, while displaying lower average shoot lengths (22.7 cm, 16.2 cm, and 15.5 cm, respectively), also demonstrated positive percentage changes from the control, with enhancements of 92.4%, 37.3%, and 31.4%, respectively. Regarding root length (Figure 5D), Treatment T2 again exhibited the highest average at 18.146 cm, showcasing a substantial increase compared to the control’s average root length of 7.734 cm. This results in a remarkable improvement of 134.9% in root length due to the PGPR treatment. Treatments T3, T4, and T5, with lower average root lengths (16.654 cm, 13.772 cm, and 13.866 cm, respectively), still manifested positive percentage changes from the control, showing enhancements of 114.1%, 77.7%, and 78.5%, respectively.
3.6. Assessment of Inoculated Wheat Physiological Traits under Drought Stress
Data on chlorophyll levels in wheat samples are represented in Figure 6A, with means derived from three replicates. In the control group, chlorophyll levels were recorded at 0.5637 mg/g FW (milligrams per gram of fresh weight). Notably, the wheat samples inoculated with PGPR isolates displayed significant variation in chlorophyll levels. T2 plants exhibited the highest chlorophyll content among the isolates, measuring 0.8557 mg/g FW, signifying a substantial improvement compared to the control group. T3 plants also demonstrated enhanced chlorophyll levels, at 0.7177 mg/g FW. Meanwhile, T4 and T5 plants recorded chlorophyll levels of 0.6800 mg/g FW and 0.6460 mg/g FW, respectively, both showing enhancements over the control group.
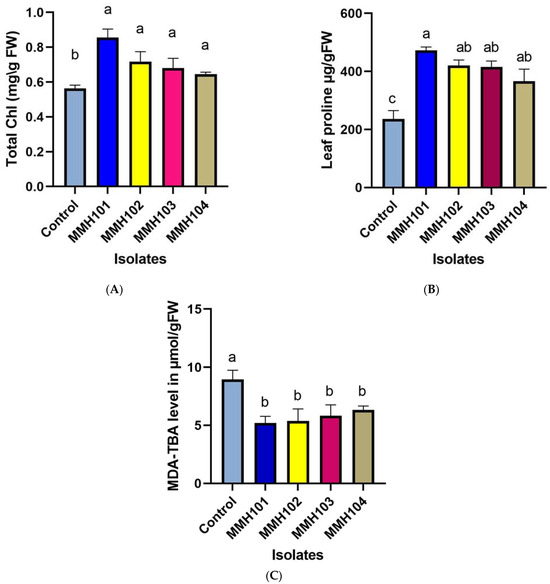
Figure 6.
Impact of drought stress and PGPR application on physiological wheat traits: (A) chlorophyll; (B) proline; (C) MDA. The data represent the mean values of five replications ± SD. Lowercase letters placed above the bars indicate statistically significant differences between the treatments at a significance level of p < 0.05.
The proline levels in wheat plants are represented in Figure 6B. In the control group, the proline level was measured at 236 µg/g FW (micrograms per gram of fresh weight). In contrast, the wheat samples inoculated with PGPR isolates displayed varying proline levels. T2 plants exhibited the highest proline content among the isolates, recording 472 µg/g FW, indicating a significant increase compared to the control group. T3 and T4 plants also displayed elevated proline levels, with values of 420.67 µg/g FW and 415 µg/g FW, respectively. T5 plants showed a proline level of 366 µg/g FW.
MDA levels in wheat following inoculation with PGPR isolates, represented in Figure 6C, were compared to a control group of non-inoculated wheat. In the control group, the MDA level was measured at 8.9567 µmol/g FW. However, the wheat samples inoculated with PGPR isolates displayed varying degrees of reduction in MDA levels. Ranked by their performance in reducing MDA levels, T2 plants exhibited the most substantial reduction with an MDA level of 5.2 µmol/g FW, followed by T3 plants at 5.37 µmol/g FW. T4 and T5 plants also demonstrated reductions, with MDA levels of 5.83 µmol/g FW and 6.3433 µmol/g FW, respectively.
4. Discussion
The results of our study highlight the potential of four rhizobacteria isolates (MMH 101, MMH 102, MMH 103, and MMH 104) as plant growth-promoting rhizobacteria (PGPR). These isolates exhibited distinct functional traits and were subsequently assessed for their effects on wheat plants, particularly under drought stress conditions. The results of this study are in line with previous research on the PGP potential of rhizobacteria. For example, a study by [35] found that the rhizobacterium Azospirillum sp. B515CD-1 was able to promote plant growth and drought tolerance in maize plants. Another study by [36] found that various PGPR strains were able to improve plant growth and drought tolerance in crops grown under drought-stressed conditions. The findings of this study have important implications for sustainable agriculture. The four rhizobacteria isolates identified in this study could be used as biofertilizers to promote plant growth and yield while also reducing the need for chemical fertilizers. This would be beneficial for the environment and for farmers’ bottom lines.
The functional validation of PGPR isolates revealed a range of beneficial traits related to plant growth promotion and antagonistic activity. Siderophore production, an important trait for iron acquisition, was evident in all four isolates. High siderophore production was observed in MMH 101 and MMH 104, while MMH 102 displayed slightly lower production and MMH 103 exhibited a moderate level. This is consistent with the role of siderophores in improving iron availability for plants [37]. Regarding nitrogen fixation, an essential process for providing plants with a crucial nutrient, MMH 101 and MMH 103 exhibited high capabilities. MMH 102 showed a lower capacity, while MMH 104 displayed a moderate ability. Nitrogen-fixing PGPR can contribute to enhanced plant growth and reduce the need for synthetic nitrogen fertilizers [38]. ACC deaminase activity, which reduces plant stress by lowering ethylene levels [39], was observed in MMH 101 and MMH 103, with MMH 102 and MMH 104 displaying lower levels of activity. This activity can play a role in alleviating plant stress and enhancing growth [40]. On the other hand, the absence of phosphate solubilization activity in all microbial isolates is an interesting finding. Phosphate solubilization is a trait often associated with PGPR, and its absence in our isolates may indicate alternative mechanisms employed by these bacteria to promote plant growth [41]. Antagonistic behavior, exhibited by MMH 101 and MMH 103 in inhibiting potential plant pathogens, is a valuable trait for protecting plants from diseases [42]. MMH 102 and MMH 104 did not exhibit such behavior.
The quantitative evaluation of plant growth-regulating traits, specifically indole-3-acetic acid (IAA) production and biofilm formation, in the PGPR isolates (MMH 101, MMH 102, MMH 103, and MMH 104) provides insights into their potential as agents for enhancing plant growth and stress resilience. IAA is a key phytohormone that plays a pivotal role in plant growth and development by regulating various physiological processes, including cell elongation, lateral root formation, and stress response [43]. In this study, MMH 101 exhibited the highest IAA production at 31.9 μg/mL, followed by MMH 102, MMH 103, and MMH 104 with progressively lower IAA production levels. This significant variation in IAA production among the isolates suggests their varying abilities to influence plant growth and stress tolerance. High IAA production, as demonstrated by MMH 101, can promote enhanced root and shoot growth, leading to improved overall plant vigor [44]. The capacity to produce IAA is a valuable trait in PGPR as it can stimulate root development and nutrient uptake, which are essential for plant health and productivity [43].
Biofilms are communities of microorganisms that adhere to surfaces and are encased in a matrix of extracellular polymeric substances (EPS). Biofilm formation by PGPR is an important trait, as it enhances their ability to colonize plant roots and exert beneficial effects on plants [45]. In this study, MMH 104 exhibited the highest biofilm production with an optical density (OD) of 0.826, followed by MMH 103, MMH 101, and MMH 102. The ability of MMH 104 to form a robust biofilm is noteworthy, as it can enhance the persistence of these PGPR on plant roots. Biofilm formation enables PGPR to resist environmental stresses, compete with pathogenic microorganisms, and establish a stable relationship with the plant [46]. This trait contributes to the long-term effectiveness of PGPR in promoting plant growth and health.
The 16S rRNA gene sequencing revealed that three of the PGPR isolates belong to the phylum Firmicutes, specifically within the genus Bacillus, with distinct species assignments: MMH101 (Bacillus rugosus), MMH102 (Bacillus licheniformis), and MMH103 (Bacillus halotolerans). The fourth isolate, MMH104 (Myroides sp.), was classified within the phylum Bacteroidota and identified as a member of the genus Myroides. Bacillus species are well-documented PGPRs known for their ability to enhance plant growth and suppress plant pathogens [47]. Myroides, on the other hand, while less explored in the context of plant-microbe interactions, may possess unique plant growth-promoting attributes that warrant further investigation. Taxonomic diversity within PGPR communities can provide a range of beneficial functions, contributing to comprehensive plant health and productivity [48]. The construction of a phylogenetic tree using the 16S rDNA data reveals the evolutionary relationships between the PGPR isolates and other related bacteria. Understanding the phylogenetic placement of these isolates can offer insights into their evolutionary history and potential functional traits.
The evaluation of wheat morphological traits under drought stress unequivocally demonstrates that inoculating with the four PGPRs results in substantial improvements in wheat growth and morphology. Specifically, Treatment 2 (T2), which utilized the PGPR strain Bacillus rugosus, resulted in dramatic increases in fresh biomass (61.8%), dry biomass (77.2%), shoot length (108.5%), and root length (134.9%) compared to the uninoculated control (T1). Other tested PGPR strains (used in Treatments T3, T4, and T5) also enhanced these growth parameters, though to a lesser degree than T2. These findings align with past research, which has illustrated the ability of PGPR to enhance drought tolerance and resilience in various crop species [49,50]. The exact mechanisms behind the observed growth-promoting effects are still being elucidated. Some hypothesized modes of action include altering plant hormone levels, increasing nutrient availability through nitrogen fixation and phosphorous solubilization, and modulating levels of osmoprotectants and antioxidants [51]. Further studies should explore the specific modes of action utilized by the PGPR strain in T2 and also assess whether the dramatic improvements in wheat morphology and biomass observed under controlled drought conditions in this study can translate to enhanced yields under field conditions.
The evaluation of wheat’s response to drought stress following inoculation with the four PGPR isolates has provided critical insights into their potential to enhance plant stress tolerance. Chlorophyll levels are a vital indicator of a plant’s photosynthetic capacity and overall health [52]. In this study, the chlorophyll levels in wheat samples were significantly affected by the inoculation of PGPR isolates. Notably, Bacillus rugosus-inoculated wheat exhibited the most substantial increase in chlorophyll content, surpassing the control group by a significant margin. Wheat inoculated with Bacillus licheniformis also demonstrated enhanced chlorophyll levels, with both wheats inoculated with Bacillus halotolerans and Myroides sp. recording noticeable improvements. The enhancement of chlorophyll levels is indicative of improved photosynthetic efficiency and stress tolerance in wheat plants. PGPR-induced increases in chlorophyll content have been previously reported [53]. Improved photosynthetic efficiency can help plants maintain growth and productivity under drought conditions [54,55].
The significant increase in chlorophyll content observed in wheat inoculated with Bacillus rugosus aligns with previous studies that have reported enhanced chlorophyll levels in drought-stressed wheat following inoculation with Bacillus species. For example, in a previous study, wheat inoculated with Bacillus licheniformis, Bacillus pumilus, and Bacillus amyloliquefaciens exhibited 12–25% higher chlorophyll content compared to uninoculated drought-stressed wheat [56]. The authors attributed this increase to the ability of Bacillus species to produce exopolysaccharides that help maintain leaf water status and gas exchange under water-deficit conditions [56]. Another study reported that wheat inoculated with a consortium of Bacillus spp., including B. pumilus, had up to 29% higher chlorophyll than non-inoculated wheat under drought stress [57]. They suggested that the Bacillus strains enhanced nutrient solubilization and uptake, stimulating chlorophyll synthesis [57]. The increases in chlorophyll content induced by Bacillus licheniformis inoculation are also supported by earlier work. For instance, drought-stressed wheat treated with B. licheniformis had 11–22% higher chlorophyll levels versus the control, which was linked to greater nutrient availability and antioxidant production by the bacterium [58]. The present study’s findings on Myroides sp. and B. halotolerans increasing wheat chlorophyll levels under drought are novel, as limited literature exists on their effects on chlorophyll in cereals.
Proline serves as an osmoprotectant and plays a crucial role in mitigating the effects of drought stress. In this study, the wheat plants inoculated with PGPR isolates exhibited varying proline levels. Wheat inoculated with Bacillus rugosus showed the highest proline content, followed by wheat inoculated with Bacillus licheniformis, Bacillus halotolerans, and Myroides sp. The elevation of proline levels in the inoculated wheat plants indicates their capacity to withstand drought stress more effectively. Proline accumulation is a common response of plants to water deficits, as it helps maintain cellular turgor and protect cellular structures. Enhanced proline levels are indicative of improved drought tolerance [59], and PGPR-induced proline accumulation has been associated with improved drought tolerance in various crops [60].
The significant increase in proline levels observed in wheat inoculated with Bacillus rugosus correlates with previous studies reporting enhanced proline accumulation in drought-stressed wheat following inoculation with Bacillus species. For instance, an earlier study found that wheat treated with a consortium of Bacillus strains including B. amyloliquefaciens, B. subtilis, and B. licheniformis exhibited a 1.5–2-fold increase in proline content compared to non-inoculated plants under drought [61]. The authors suggested that osmolyte production was stimulated in the Bacillus-inoculated plants, enhancing their osmotic adjustment and drought tolerance [61]. Additionally, another study showed that inoculating wheat with Bacillus spp. led to over 40% higher proline levels versus the control under water deficit conditions [57]. The proline increases induced by B. licheniformis align with an earlier study that reported up to 67% greater proline accumulation in drought-stressed wheat inoculated with this species [62]. The findings on proline enhancement by B. halotolerans and Myroides sp. are novel, as limited studies have examined their effects on osmolyte production in cereals under drought.
Malondialdehyde (MDA) is a marker of lipid peroxidation and oxidative stress in plant cells. In this study, the wheat samples inoculated with PGPR isolates exhibited varying degrees of reduction in MDA levels compared to the non-inoculated control group. Wheat inoculated with Bacillus rugosus demonstrated the most substantial reduction in MDA levels, followed by wheat inoculated with Bacillus halotolerans, Bacillus licheniformis, and Myroides sp. Reduced MDA levels signify a lower degree of oxidative damage and lipid peroxidation in plant cells [63]. PGPR-mediated reductions in MDA levels reflect their role in mitigating oxidative stress under drought conditions [64].
The considerable decrease in MDA content observed in wheat inoculated with Bacillus rugosus is consistent with previous studies showing reduced lipid peroxidation in drought-stressed wheat treated with Bacillus species. For instance, an earlier study reported a 20–30% reduction in MDA levels in wheat inoculated with a mix of Bacillus strains, including B. amyloliquefaciens and B. subtilis, compared to the uninoculated control under water deficit conditions [61]. The authors attributed this antioxidant effect to the ability of the Bacillus inoculants to stimulate the production of antioxidant enzymes in the plants [61]. Additionally, another study demonstrated that wheat inoculated with a consortium of Bacillus spp. had up to 45% lower MDA content relative to non-inoculated wheat under drought stress [58]. The reductions in lipid peroxidation induced by B. licheniformis align with earlier work, which showed drought-stressed wheat treated with this species exhibited 24% lower MDA levels versus the control [65]. The findings on MDA decreases by B. halotolerans and Myroides sp. inoculation are novel.
While the current study demonstrates the potential of PGPR isolates like Bacillus spp. to improve drought tolerance in wheat over a single growing season, evaluating their long-term impact can provide greater insight into their sustainability in agricultural systems. Repeated PGPR inoculation has been found to produce cumulative benefits in soil health and crop yields over successive seasons. For instance, long-term Bacillus inoculation has been shown to enhance soil organic carbon, microbial activity, and nutrient availability in cereal cropping systems [66,67]. The build-up of beneficial microbial populations can also lead to positive “legacy effects” that persist even after inoculation is ceased [68]. From an economic standpoint, meta-analyses have found PGPR inoculation to be highly cost-effective, improving crop yields and farm profits over time through both direct growth promotion and soil enhancement [69]. However, factors like inoculant shelf-life, application logistics, and potential interactions with agrochemicals need consideration for large-scale implementation [45]. Overall, integrating PGPR inoculants into management practices could provide sustainable, long-term productivity gains but requires holistic agronomic and economic assessments under real farm conditions.
5. Conclusions
This study successfully isolated and characterized native PGPR from Egyptian wheat rhizosphere soil. Comprehensive in vitro assays revealed an array of plant growth-promoting attributes among isolates, including IAA synthesis, nitrogen fixation, siderophore production, and ACC deaminase activity. The greenhouse assessment of isolated PGPR strains as inoculants for drought-challenged wheat showed pronounced improvements in morphological and physiological parameters. In particular, Bacillus rugosus strain MMH101 elicited dramatic increases of 60–135% in growth metrics coupled with augmented chlorophyll and proline levels compared to non-inoculated plants under a 10-day drought regime. Findings highlight the potential of native PGPR as bio-inoculants to bolster wheat productivity and water stress resilience. Further investigation should explore translating enhanced drought tolerance from controlled environments to field conditions. In summary, this research makes valuable progress in developing microbiome-driven solutions to intensify cereal cultivation in Egypt’s arid climate while reducing reliance on agrochemicals.
Author Contributions
Conceptualization: H.M.A.-E.; methodology, M.A.S., M.A.I. and H.M.A.-E.; experiment, M.A.S., M.A.I., H.M.A.-E. and K.H.R.; writing—original draft preparation, H.M.A.-E., M.A.S. and M.A.I.; writing—review and editing, H.M.A.-E. and M.A.S.; supervision, H.M.A.-E. and K.H.R.; funding acquisition, H.M.A.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science, Technology & Innovation Funding Authority, Egypt, grant number ID: 38239.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Byregowda, R.; Prasad, S.R.; Oelmüller, R.; Nataraja, K.N.; Prasanna Kumar, M.K. Is endophytic colonization of host plants a method of alleviating drought stress? Conceptualizing the hidden world of endophytes. Int. J. Mol. Sci. 2022, 23, 9194. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- Omar, S.A.; Fetyan, N.A.; Eldenary, M.E.; Abdelfattah, M.H.; Abd-Elhalim, H.M.; Wrobel, J.; Kalaji, H.M. Alteration in expression level of some growth and stress-related genes after rhizobacteria inoculation to alleviate drought tolerance in sensitive rice genotype. Chem. Biol. Technol. Agric. 2021, 8, 41. [Google Scholar] [CrossRef]
- Bueno-Ramos, N.; González-Hernández, A.I.; Marcos-Barbero, E.L.; Miranda-Apodaca, J.; Bendou, O.; Gutiérrez-Fernández, I.; Arellano, J.B.; Morcuende, R. Impact of Water Deficit on Primary Metabolism at the Whole Plant Level in Bread Wheat Grown under Elevated CO2 and High Temperature at Different Developmental Stages. Chem. Proc. 2022, 10, 6. [Google Scholar]
- Ferioun, M.; Srhiouar, N.; Tirry, N.; Belahcen, D.; Siang, T.C.; Louahlia, S.; El Ghachtouli, N. Optimized drought tolerance in barley (Hordeum vulgare L.) using plant growth-promoting rhizobacteria (PGPR). Biocatal. Agric. Biotechnol. 2023, 50, 102691. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with biofilm-forming ability: A multifaceted agent for sustainable agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Wang, S.; Ouyang, L.; Ju, X.; Zhang, L.; Zhang, Q.; Li, Y. Survey of plant drought-resistance promoting bacteria from Populus euphratica tree living in arid area. Indian J. Microbiol. 2014, 54, 419–426. [Google Scholar] [CrossRef]
- Khan, M.R.; Ahmad, F.; Shah, A.A.; Ali, S.M. Role of plant growth-promoting rhizobacteria (PGPR) in the biocontrol of plant diseases. In Plant Growth-Promoting Rhizobacteria (PGPR) for Sustainable Agriculture; Springer: Cham, Switzerland, 2014; pp. 195–221. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Abd-Elhalim, H.M.; El Allali, A. A large-scale assessment of the quality of plant genome assemblies using the LTR assembly index. AoB Plants 2023, 15, plad015. [Google Scholar] [CrossRef]
- Abd El-Halim, H.M.; Ismail, I.M.; Al Aboud, N.M.; Elghareeb, D.; Metry, E.A.; Hossien, A.F.; Fahmy, E.M. Evaluation of two promoters for generating transgenic potato plants as salicylic acid biosensors. Biol. Plant. 2020, 64, 535–540. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Synergistic effects of biofilm-producing PGPR strains on wheat plant colonization, growth and soil resilience under drought stress. Saudi J. Biol. Sci. 2023, 30, 103664. [Google Scholar] [CrossRef]
- Furlan, F.; Saatkamp, K.; Volpiano, C.G.; Franco, F.D.A.; dos Santos, M.F.; Vendruscolo, E.C.G.; Guimarães, V.F.; da Costa, A.C.T. Plant growth-promoting bacteria effect in withstanding drought in wheat cultivars. Sci. Agrar. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Ramasamy, K.P.; Mahawar, L. Coping with salt stress-interaction of halotolerant bacteria in crop plants: A mini review. Front. Microbiol. 2023, 14, 1077561. [Google Scholar] [CrossRef]
- Niu, X.; Song, L.; Xiao, Y.; Ge, W. Drought-tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front. Microbiol. 2018, 8, 2580. [Google Scholar] [CrossRef]
- Sati, D.; Pande, V.; Samant, M. Plant-beneficial Bacillus, Pseudomonas, and Staphylococcus spp. from Kumaon Himalayas and their drought tolerance response. Front. Sustain. Food Syst. 2023, 7, 1085223. [Google Scholar] [CrossRef]
- Gusmiaty Restu, M.; Bachtiar, B.; Larekeng, S.H. Gibberellin and IAA production by rhizobacteria from various private forest. IOP Conf. Ser. Earth Environ. Sci. 2019, 270, 012018. [Google Scholar] [CrossRef]
- Melo, L.D.; Ferreira, R.; Costa, A.R.; Oliveira, H.; Azeredo, J. Efficacy and safety assessment of two enterococci phages in an in vitro biofilm wound model. Sci. Rep. 2019, 9, 6643. [Google Scholar] [CrossRef]
- Sultana, S.; Alam, S.; Karim, M.M. Screening of siderophore-producing salt-tolerant rhizobacteria suitable for supporting plant growth in saline soils with iron limitation. J. Agric. Food Res. 2021, 4, 100150. [Google Scholar] [CrossRef]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Penrose, D.M.; Moffatt, B.A.; Glick, B.R. Determination of 1-aminocycopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Can. J. Microbiol. 2001, 47, 77–80. [Google Scholar] [CrossRef]
- Gupta, S.; Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya 1948, 17, 362–370. [Google Scholar]
- Khoury, C.K.; Heider, B.; Castañeda-Álvarez, N.P.; Achicanoy, H.A.; Sosa, C.C.; Miller, R.E.; Scotland, R.W.; Wood, J.R.; Rossel, G.; Eserman, L.A.; et al. Distributions, ex situ conservation priorities, and genetic resource potential of crop wild relatives of sweetpotato [Ipomoea batatas (L.) Lam., I. series Batatas]. Front. Plant Sci. 2015, 6, 251. [Google Scholar] [CrossRef]
- Calvo, P.; Ormeño-Orrillo, E.; Martínez-Romero, E.; Zúñiga, D. Characterization of Bacillus isolates of potato rhizosphere from andean soils of Peru and their potential PGPR characteristics. Braz. J. Microbiol. 2010, 41, 899–906. [Google Scholar] [CrossRef]
- Abdelatty, A.; Mandouh, M.; Mohamed, S.; Busato, S.; Badr, O.; Bionaz, M.; Elolimy, A.; Moustafa, M.; Farid, O.; Al-Mokaddem, A. Azolla leaf meal at 5% of the diet improves growth performance, intestinal morphology and p70S6K1 activation, and affects cecal microbiota in broiler chicken. Animal 2021, 15, 100362. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Khan, M.Y.; Nadeem, S.M.; Sohaib, M.; Waqas, M.R.; Alotaibi, F.; Ali, L.; Zahir, Z.A.; Al-Barakah, F.N.I. Potential of plant growth promoting bacterial consortium for improving the growth and yield of wheat under saline conditions. Front. Microbiol. 2022, 13, 958522. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Plant-Microbe Interactions; Springer: New York, NY, USA, 2021. [Google Scholar]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; de-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum sp. B515CD-1 improves plant resistance to abiotic stress. World J. Microbiol. Biotechnol. 2010, 26, 719–733. [Google Scholar]
- Singh, R.P.; Yadav, V.B.; Yadav, N.S.; Dhyani, D. Plant-growth-promoting rhizobacteria: A potential tool for sustainable agriculture in drought-prone areas. Front. Microbiol. 2021, 12, 739395. [Google Scholar]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Yavuz, D.; Baştaş, K.K.; Seymen, M.; Yavuz, N.; Kurtar, E.S.; Süheri, S.; Türkmen, Ö.; Gür, A.; Kıymacı, G. Role of ACC deaminase-producing rhizobacteria in alleviation of water stress in watermelon. Sci. Hortic. 2023, 321, 112288. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a001438. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Santoyo, G.; Equihua, A.; Flores, A.; Sepulveda, E.; Valencia-Cantero, E.; Sanchez-Yañez, J.M.; Morales, L.R.; Govindappa, M.; de los Santos-Villalobos, S. Plant growth promotion by ACC deaminase-producing bacilli under salt stress conditions. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Springer: Cham, Switzerland, 2019; Volume 2, pp. 81–95. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Wang, L.; Yue, Y.; Wang, L.; Yang, X. The effects of plant growth-promoting rhizobacteria on plants under temperature stress: A meta-analysis. Rhizosphere 2023, 28, 100788. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashraf, M. Microbial ACC-deaminase: Prospects and applications for inducing salt tolerance in plants. Crit. Rev. Plant Sci. 2010, 29, 360–393. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, D.; Nezarat, S.; Shafiee, R. The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Res. J. Biol. Sci. 2009, 4, 670–677. [Google Scholar]
- Bharti, N.; Barnawal, D.; Awasthi, A.; Kalra, A. Plant growth promoting rhizobacteria alleviate salinity induced negative effects on growth, oil content and physiological status in Jatropha curcas L. Bioresour. Technol. 2016, 215, 155–164. [Google Scholar]
- Ahanger, M.A.; Agarwal, R.M.; Tomar, N.S. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2014, 252, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, T.; Handayani, D.; Soedarsono, J. The potential of consortium Bacillus sp. as biofertilizer to increase nutrients uptake and yield of wheat (Triticum aestivum L.) under drought stress. Sustainability 2020, 12, 7952. [Google Scholar]
- Armada, E.; Probanza, A.; Roldán, A.; Azcón, R. Native plant growth promoting bacteria Bacillus thuringiensis and mixed or individual mycorrhizal species improved drought tolerance and oxidative metabolism in Lavandula dentata plants. J. Plant Physiol. 2015, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase-containing rhizobacteria protect Ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 2014, 81, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gusain, Y.S.; Singh, U.S.; Sharma, A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryza sativa L.). Afr. J. Biotechnol. 2015, 14, 918–928. [Google Scholar]
- Kohler, J.; Hernández, J.A.; Caravaca, F.; Roldán, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Bailly, C.; Benamar, A.; Corbineau, F.; Come, D. Free radical scavenging as affected by accelerated aging and subsequent priming in sunflower seeds. Physiol. Plant. 2001, 111, 223–231. [Google Scholar]
- Barnawal, D.; Ojha, A.; Singh, R.; Garg, S.K. Combinatorial inoculation of plant growth-promoting bacteria and earthworms in the restoration of mine spoil: A field experiment. Ecol. Eng. 2017, 98, 183–191. [Google Scholar]
- Yadav, K.; Singh, J.; Giri, S.; Singh, G.; Tiwari, K.L.; Singh, K. Bio-priming with plant growth promoting rhizobacteria isolates ameliorates negative impact of drought in wheat (Triticum aestivum L.). Biocatal. Agric. Biotechnol. 2020, 28, 101703. [Google Scholar]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef] [PubMed]
- Mrkovacki, N.; Milic, V.; Bjelic, D.; Marinkovic, J. Quantitative effects of Zea mays L. inoculation with Bacillus amyloliquefaciens FZB42 on microbial community structure in rhizosphere soil under field conditions. Eur. J. Soil Biol. 2014, 60, 15–19. [Google Scholar]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).