Glycerol-Free Biodiesel via Catalytic Interesterification: A Pathway to a NetZero Biodiesel Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
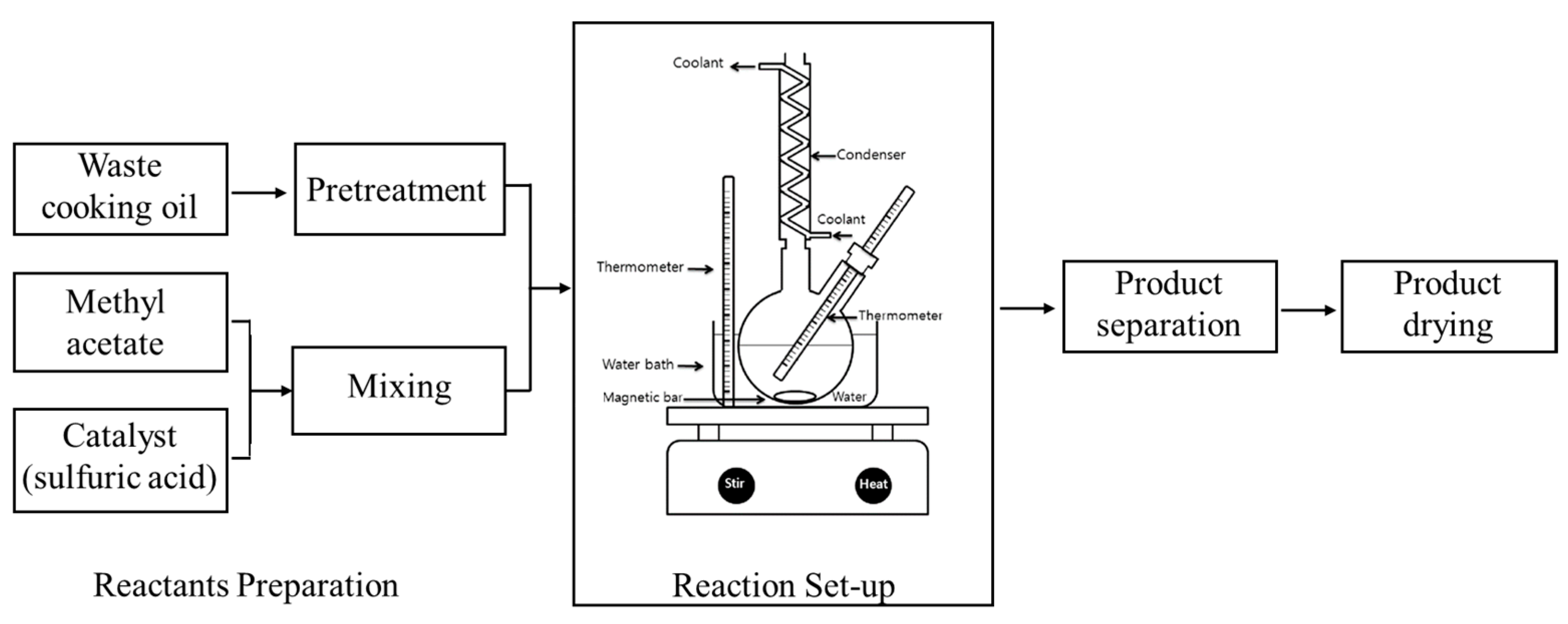
2.2. Experimental Setup and Procedure
2.2.1. Pretreatment
2.2.2. Operational Upstream Procedures
2.2.3. Operational Downstream Procedures
2.3. Experimental Design
2.4. Statistical Analysis
2.5. Chromatographic Analysis
3. Results
3.1. Model Development and Statistical Analysis
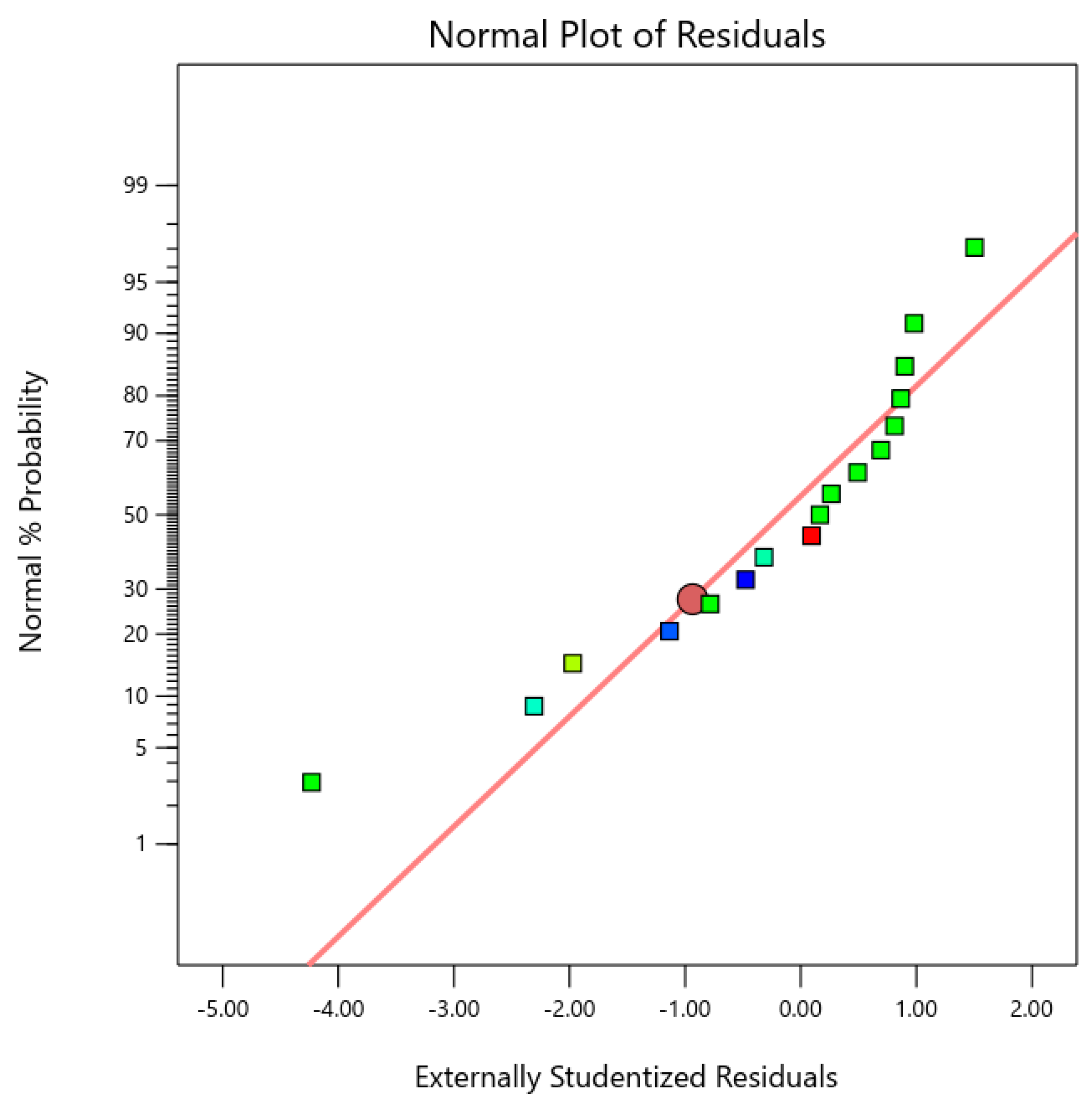
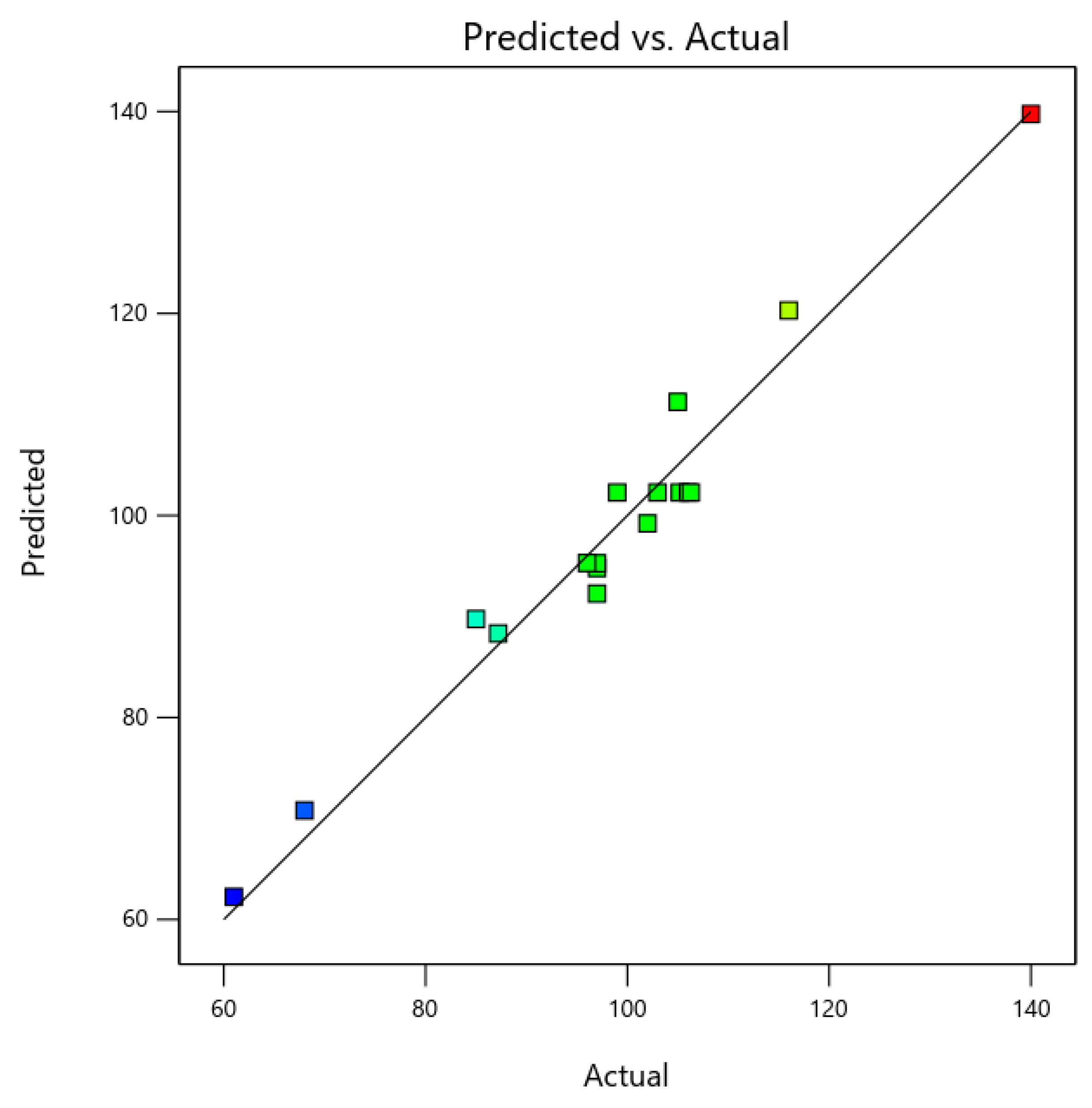
3.2. Statistical Validation
3.3. Effect of Process Parameters
3.4. Product Analysis
3.5. Process Optimisation
4. Discussion
4.1. Reaction Time
4.2. Methyl Acetate to Oil (MA:O) Molar Ratio
4.3. Catalyst Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esan, A.O.; Adeyemi, A.D.; Ganesan, S. A Review on the Recent Application of Dimethyl Carbonate in Sustainable Biodiesel Production. J. Clean. Prod. 2020, 257, 120561. [Google Scholar] [CrossRef]
- Farrag, N.M.; Gadalla, M.A.; Fouad, M.K. Reaction Parameters and Energy Optimisation for Biodiesel Production Using a Supercritical Process. Chem. Eng. Trans. 2016, 52, 1207–1212. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farrag, N.M. Statistical Optimization and Experimental Assesment for Biodiesel Production from Waste Cooking Oil. J. Southwest Jiaotong Univ. 2022, 57, 388–396. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Syazwina, N.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. A Sustainability Study of the Processing of Kitchen Waste as a Potential Source of Biofuel: Biodiesel Production from Waste Cooking Oil (WCO). Mater. Today Proc. 2022, 63, S484–S489. [Google Scholar] [CrossRef]
- Niza, N.M.; Tan, K.T.; Lee, K.T.; Ahmad, Z. Influence of Impurities on Biodiesel Production from Jatropha curcas L. by Supercritical Methyl Acetate Process. J. Supercrit. Fluids 2013, 79, 73–75. [Google Scholar] [CrossRef]
- Singh, N.; Saluja, R.K.; Rao, H.J.; Kaushal, R.; Gahlot, N.K.; Suyambulingam, I.; Sanjay, M.R.; Divakaran, D.; Siengchin, S. Progress and Facts on Biodiesel Generations, Production Methods, Influencing Factors, and Reactors: A Comprehensive Review from 2000 to 2023. Energy Convers. Manag. 2024, 302, 118157. [Google Scholar] [CrossRef]
- Nikhom, R.; Mueanmas, C.; Suppalakpanya, K. Enhancement of Biodiesel Production from Palm Fatty Acid Distillate Using Methyl T-Butyl Ether Co-Solvent: Process Optimization. Int. J. Renew. Energy Res. 2019, 9, 1319–1327. [Google Scholar] [CrossRef]
- Akinbami, O.M.; Oke, S.R.; Bodunrin, M.O. The State of Renewable Energy Development in South Africa: An Overview. Alex. Eng. J. 2021, 60, 5077–5093. [Google Scholar] [CrossRef]
- Al-Dawody, M.F.; Jazie, A.A.; Abdulkadhim Abbas, H. Experimental and Simulation Study for the Effect of Waste Cooking Oil Methyl Ester Blended with Diesel Fuel on the Performance and Emissions of Diesel Engine. Alex. Eng. J. 2019, 58, 9–17. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Kazmi, M.; Fayaz, H.; Mujtaba, M.A.; Ali, C.H.; Kalam, M.A.; Soudagar, M.E.M.; et al. Production and Utilization Aspects of Waste Cooking Oil Based Biodiesel in Pakistan. Alex. Eng. J. 2021, 60, 5831–5849. [Google Scholar] [CrossRef]
- Esan, A.O.; Olabemiwo, O.M.; Smith, S.M.; Ganesan, S. A Concise Review on Alternative Route of Biodiesel Production via Interesterification of Different Feedstocks. Int. J. Energy Res. 2021, 45, 12614–12637. [Google Scholar] [CrossRef]
- Wong, W.Y.; Lim, S.; Pang, Y.L.; Shuit, S.H.; Lam, M.K.; Tan, I.S.; Chen, W.H. A Comprehensive Review of the Production Methods and Effect of Parameters for Glycerol-Free Biodiesel Production. Renew. Sustain. Energy Rev. 2023, 182, 113397. [Google Scholar] [CrossRef]
- Umar, Y.; Velasco, O.; Abdelaziz, O.Y.; Aboelazayem, O.; Gadalla, M.A.; Hulteberg, C.P.; Saha, B. A Renewable Lignin-Derived Bio-Oil for Boosting the Oxidation Stability of Biodiesel. Renew. Energy 2022, 182, 867–878. [Google Scholar] [CrossRef]
- Chuepeng, S.; Komintarachat, C. Interesterification Optimization of Waste Cooking Oil and Ethyl Acetate over Homogeneous Catalyst for Biofuel Production with Engine Validation. Appl. Energy 2018, 232, 728–739. [Google Scholar] [CrossRef]
- Khodadadi, M.R.; Malpartida, I.; Tsang, C.W.; Lin, C.S.K.; Len, C. Recent Advances on the Catalytic Conversion of Waste Cooking Oil. Mol. Catal. 2020, 494, 111128. [Google Scholar] [CrossRef]
- dos Santos Ribeiro, J.; Celante, D.; Simões, S.S.; Bassaco, M.M.; da Silva, C.; de Castilhos, F. Efficiency of Heterogeneous Catalysts in Interesterification Reaction from Macaw Oil (Acrocomia aculeata) and Methyl Acetate. Fuel 2017, 200, 499–505. [Google Scholar] [CrossRef]
- Kampars, V.; Kampare, R.; Sile, E.; Roze, M. Harmonisation of Biodiesel Composition via Competitive Interesterification-Transesterification. Chem. Eng. Trans. 2020, 80, 91–96. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Pristiyani, R.; Dewajani, H. A New Route of Biodiesel Production through Chemical Interesterification of Jatropha Oil Using Ethyl Acetate. Int. J. Chemtech Res. 2016, 9, 627–634. [Google Scholar]
- Visioli, L.J.; Nunes, A.L.B.; Wancura, J.H.C.; Enzweiler, H.; Vernier, L.J.; de Castilhos, F. Batch and Continuous γ-Alumina-Catalyzed FAME Production from Soybean Oil Deodorizer Distillate by Interesterification. Fuel 2023, 351, 128954. [Google Scholar] [CrossRef]
- Casas, A.; Ramos, M.J.; Pérez, Á. New Trends in Biodiesel Production: Chemical Interesterification of Sunflower Oil with Methyl Acetate. Biomass Bioenergy 2011, 35, 1702–1709. [Google Scholar] [CrossRef]
- Dhawan, M.S.; Barton, S.C.; Yadav, G.D. Interesterification of Triglycerides with Methyl Acetate for the Co-Production Biodiesel and Triacetin Using Hydrotalcite as a Heterogenous Base Catalyst. Catal. Today 2021, 375, 101–111. [Google Scholar] [CrossRef]
- Portilho Trentini, C.; de Mello, B.T.F.; Ferreira Cabral, V.; da Silva, C. Crambe Seed Oil: Extraction and Reaction with Dimethyl Carbonate under Pressurized Conditions. J. Supercrit. Fluids 2020, 159, 104780. [Google Scholar] [CrossRef]
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. Emerging Technologies for Biodiesel Production: Processes, Challenges, and Opportunities. Biomass Bioenergy 2022, 163, 106521. [Google Scholar] [CrossRef]
- Brondani, L.N.; Ribeiro, J.S.; Castilhos, F. A New Kinetic Model for Simultaneous Interesterification and Esterification Reactions from Methyl Acetate and Highly Acidic Oil. Renew. Energy 2020, 156, 579–590. [Google Scholar] [CrossRef]
- Prestigiacomo, C.; Biondo, M.; Galia, A.; Monflier, E.; Ponchel, A.; Prevost, D.; Scialdone, O.; Tilloy, S.; Bleta, R. Interesterification of Triglycerides with Methyl Acetate for Biodiesel Production Using a Cyclodextrin-Derived SnO@γ-Al2O3 Composite as Heterogeneous Catalyst. Fuel 2022, 321, 124026. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Pandit, A.B.; Gogate, P.R. Ultrasound Assisted Interesterification of Waste Cooking Oil and Methyl Acetate for Biodiesel and Triacetin Production. Fuel Process. Technol. 2013, 116, 241–249. [Google Scholar] [CrossRef]
- Santoso, A.; Sumari; Urfa Zakiyya, U.; Tiara Nur, A. Methyl Ester Synthesis of Crude Palm Oil off Grade Using the K2O/Al2O3 Catalyst and Its Potential as Biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2019, 515, 012042. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Gogate, P.R.; Pakhale, V.D. Intensification of Interesterification of Sustainable Feedstock as Mahua Oil for Biodiesel Production. Int. J. Green. Energy 2023, 20, 1514–1523. [Google Scholar] [CrossRef]
- Priebe, J.M.; Dall’Oglio, E.; de Vasconcelos, L.; de Sousa, P., Jr.; Ramos, A.; Rodrigues, E.; Kuhnen, C.A. Dielectric Properties During Microwave-Induced Interesterification Reactions for Biodiesel and Triacetin Production. J. Braz. Chem. Soc. 2024, 35, e20240057. [Google Scholar] [CrossRef]
- Dougher, M.; Soh, L.; Bala, A.M. Techno-Economic Analysis of Interesterification for Biodiesel Production. Energy Fuels 2023, 37, 2912–2925. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V. Synthesis of Biodiesel by Interesterification of Triglycerides with Methyl Formate. Appl. Sci. 2022, 12, 9912. [Google Scholar] [CrossRef]
- Casas, A.; Pérez, Á.; Ramos, M.J. Effects of Diacetinmonoglycerides and Triacetin on Biodiesel Quality. Energies 2023, 16, 6146. [Google Scholar] [CrossRef]
- EN 14214:2013 V2+A2:2019; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2019.






| Independent Variable | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Time | A | 1 | 2.50 | 4 |
| MA:Oil (Molar ratio) | B | 6 | 9 | 12 |
| H2SO4 Concentration (vol%) | C | 0.10 | 0.30 | 0.50 |
| Run | Time (h) | MeAc: Oil Molar Ratio | Catalyst Loading (%) | Actual Yield (%) | Predicted Yield (%) |
|---|---|---|---|---|---|
| 1 | 4 | 9 | 0.5 | 87.2 | 89.15 |
| 2 | 4 | 6 | 0.3 | 97 | 92.78 |
| 3 | 2.5 | 12 | 0.1 | 140 | 137.72 |
| 4 | 1 | 12 | 0.3 | 105 | 109.22 |
| 5 | 4 | 9 | 0.1 | 97 | 98.50 |
| 6 | 1 | 6 | 0.3 | 61 | 60.23 |
| 7 | 2.5 | 6 | 0.1 | 85 | 87.73 |
| 8 | 2.5 | 12 | 0.5 | 96 | 93.28 |
| 9 | 1 | 9 | 0.5 | 97 | 95.50 |
| 10 | 2.5 | 9 | 0.3 | 99 | 103.90 |
| 11 | 2.5 | 9 | 0.3 | 106 | 103.90 |
| 12 | 2.5 | 6 | 0.5 | 116 | 118.28 |
| 13 | 2.5 | 9 | 0.3 | 103 | 103.90 |
| 14 | 2.5 | 9 | 0.3 | 106.3 | 103.90 |
| 15 | 2.5 | 9 | 0.3 | 105.2 | 103.90 |
| 16 | 4 | 12 | 0.3 | 68 | 68.78 |
| 17 | 1 | 9 | 0.1 | 102 | 100.05 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4882.98 | 9 | 542.55 | 46.84 | <0.0001 |
| A-Time | 49.00 | 1 | 49.00 | 4.23 | 0.0787 |
| B-Methyl Ac:Oil | 364.50 | 1 | 364.50 | 31.47 | 0.0008 |
| C-H2SO4:Oil | 96.60 | 1 | 96.60 | 8.34 | 0.0234 |
| AB | 1332.25 | 1 | 1332.25 | 115.00 | <0.0001 |
| AC | 5.76 | 1 | 5.76 | 0.4972 | 0.5035 |
| BC | 1406.25 | 1 | 1406.25 | 121.39 | <0.0001 |
| A2 | 1260.17 | 1 | 1260.17 | 108.78 | <0.0001 |
| B2 | 62.41 | 1 | 62.41 | 5.39 | 0.0533 |
| C2 | 356.38 | 1 | 356.38 | 30.76 | 0.0009 |
| Residual | 81.09 | 7 | 11.58 | ||
| Lack of Fit | 44.41 | 3 | 14.80 | 1.61 | 0.3197 |
| Pure Error | 36.68 | 4 | 9.17 | ||
| Cor Total | 4964.07 | 16 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 4745 | 7 | 677.86 | 34.07 | <0.0001 |
| A-Time | 31.20 | 1 | 31.20 | 1.57 | 0.242 |
| B-Methyl Ac:Oil | 312.50 | 1 | 312.50 | 15.71 | 0.0033 |
| C-H2SO4:Oil | 96.61 | 1 | 96.60 | 4.86 | 0.045 |
| AB | 1332.25 | 1 | 1332.25 | 66.96 | <0.0001 |
| BC | 1406.25 | 1 | 1406.25 | 70.68 | <0.0001 |
| A2 | 1293.44 | 1 | 1293.44 | 65.01 | <0.0001 |
| C2 | 341.80 | 1 | 341.80 | 17.18 | 0.0025 |
| Residual | 179.06 | 9 | 19.90 | ||
| Lack of Fit | 142.38 | 5 | 28.48 | 3.11 | 0.147 |
| Pure Error | 36.68 | 4 | 9.17 | ||
| Cor Total | 4924.07 | 16 |
| RT | Area % | Compound Name |
|---|---|---|
| 5.40 | 0.69 | 4,4-Dimethyl-2-pentanol |
| 24.41 | 13.84 | Hexadecanoic acid, methyl ester |
| 25.85 | 2.17 | Hexadecanoic acid, ethyl ester |
| 27.21 | 55.61 | Octadecadienoic acid, methyl ester |
| 27.60 | 2.46 | Methyl stearate |
| 28.63 | 18.87 | 9,13-Octadecadienoic acid methyl ester |
| 36.74 | 6.37 | 2-hydroxy-1-(hydroxymethyl)ethyl ester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, O.; Khaled, E.; Aboelazayem, O.; Farrag, N. Glycerol-Free Biodiesel via Catalytic Interesterification: A Pathway to a NetZero Biodiesel Industry. Sustainability 2024, 16, 4994. https://doi.org/10.3390/su16124994
Youssef O, Khaled E, Aboelazayem O, Farrag N. Glycerol-Free Biodiesel via Catalytic Interesterification: A Pathway to a NetZero Biodiesel Industry. Sustainability. 2024; 16(12):4994. https://doi.org/10.3390/su16124994
Chicago/Turabian StyleYoussef, Omar, Esraa Khaled, Omar Aboelazayem, and Nessren Farrag. 2024. "Glycerol-Free Biodiesel via Catalytic Interesterification: A Pathway to a NetZero Biodiesel Industry" Sustainability 16, no. 12: 4994. https://doi.org/10.3390/su16124994
APA StyleYoussef, O., Khaled, E., Aboelazayem, O., & Farrag, N. (2024). Glycerol-Free Biodiesel via Catalytic Interesterification: A Pathway to a NetZero Biodiesel Industry. Sustainability, 16(12), 4994. https://doi.org/10.3390/su16124994







