Integrated Investigations to Study the Materials and Degradation Issues of the Urban Mural Painting Ama Il Tuo Sogno by Jorit Agoch
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Characterization of the Mural Painting Materials
- PLM in transmitted and reflected light.
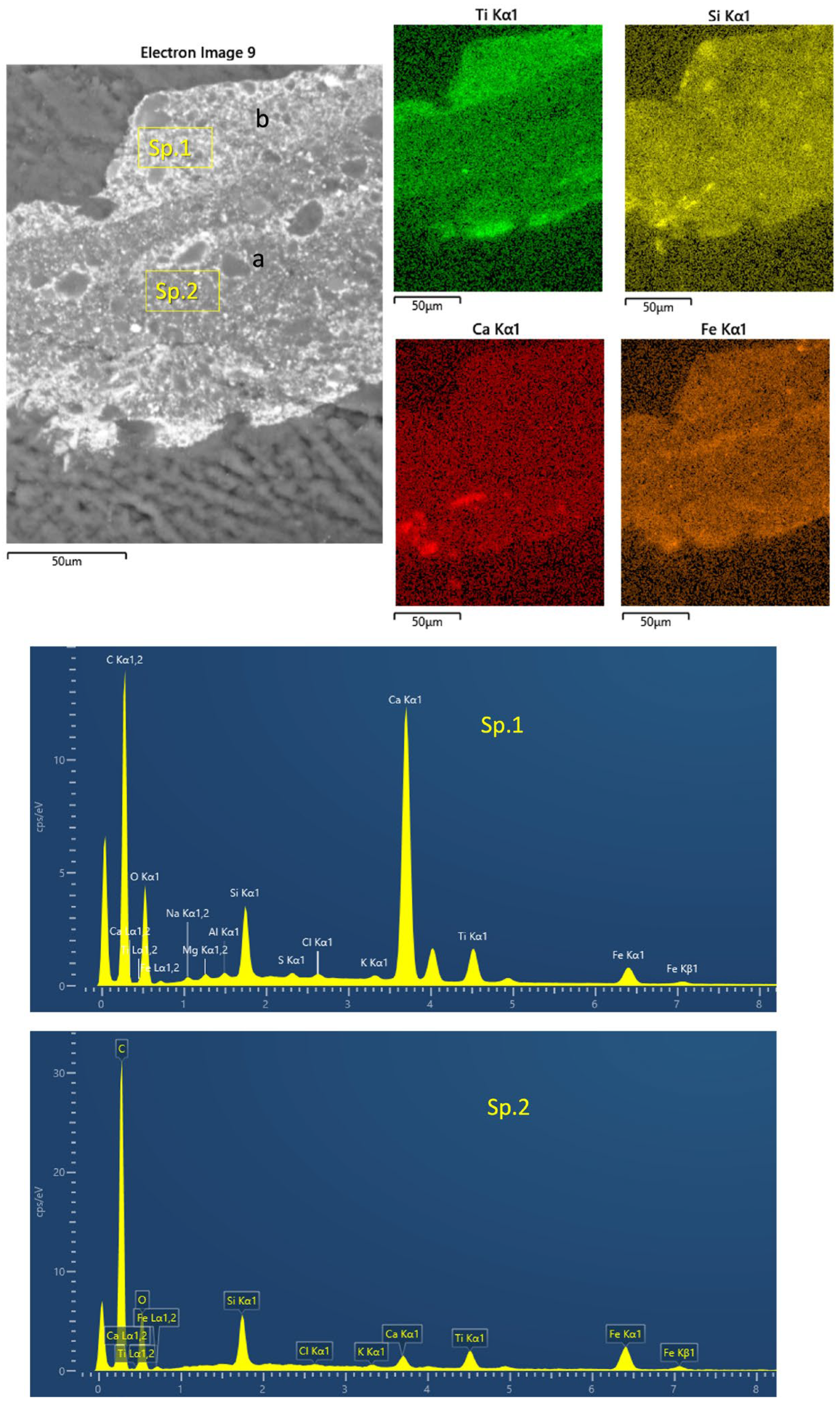
- SEM-EDS
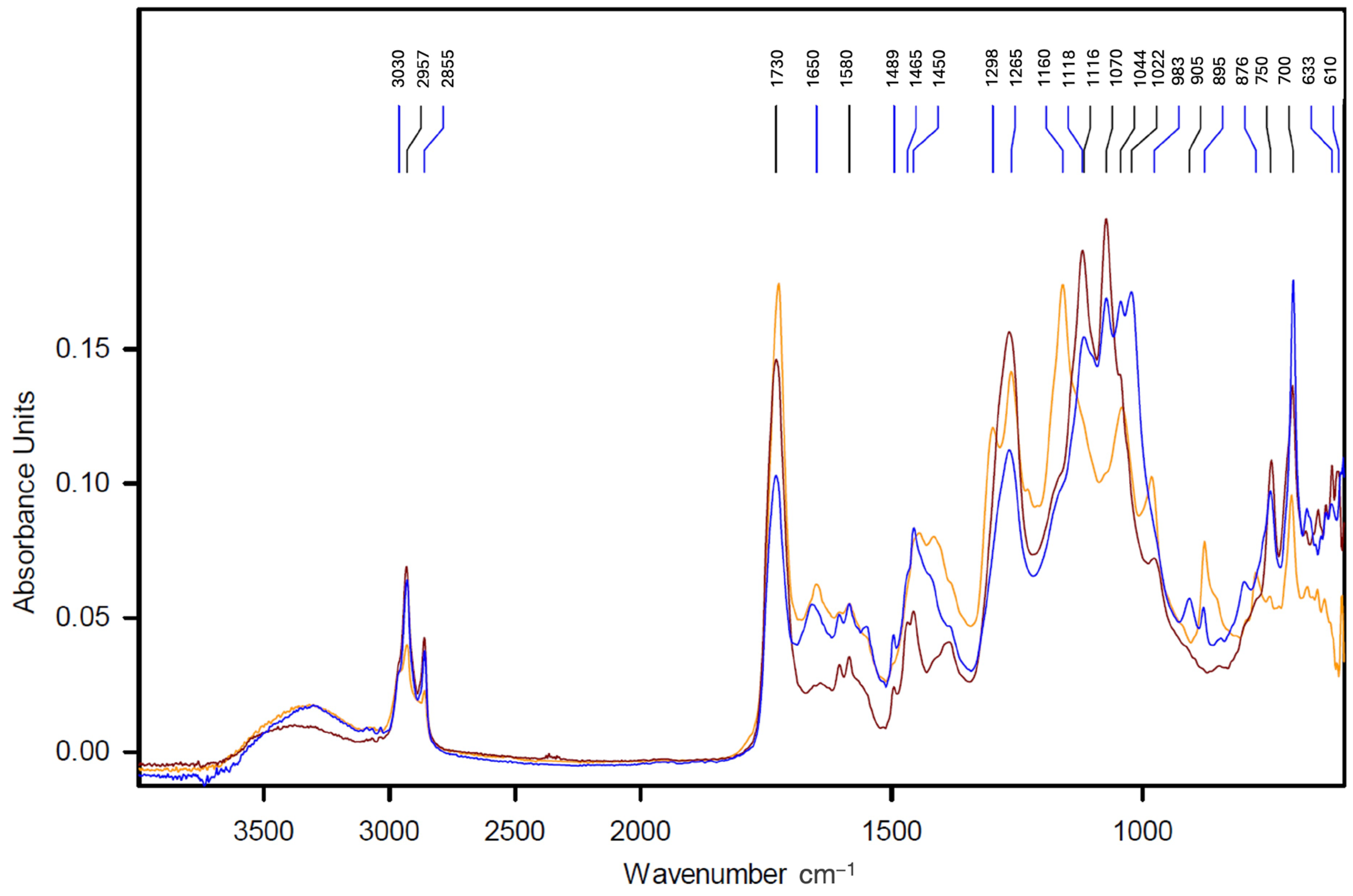
- Micro-FTIR spectroscopy
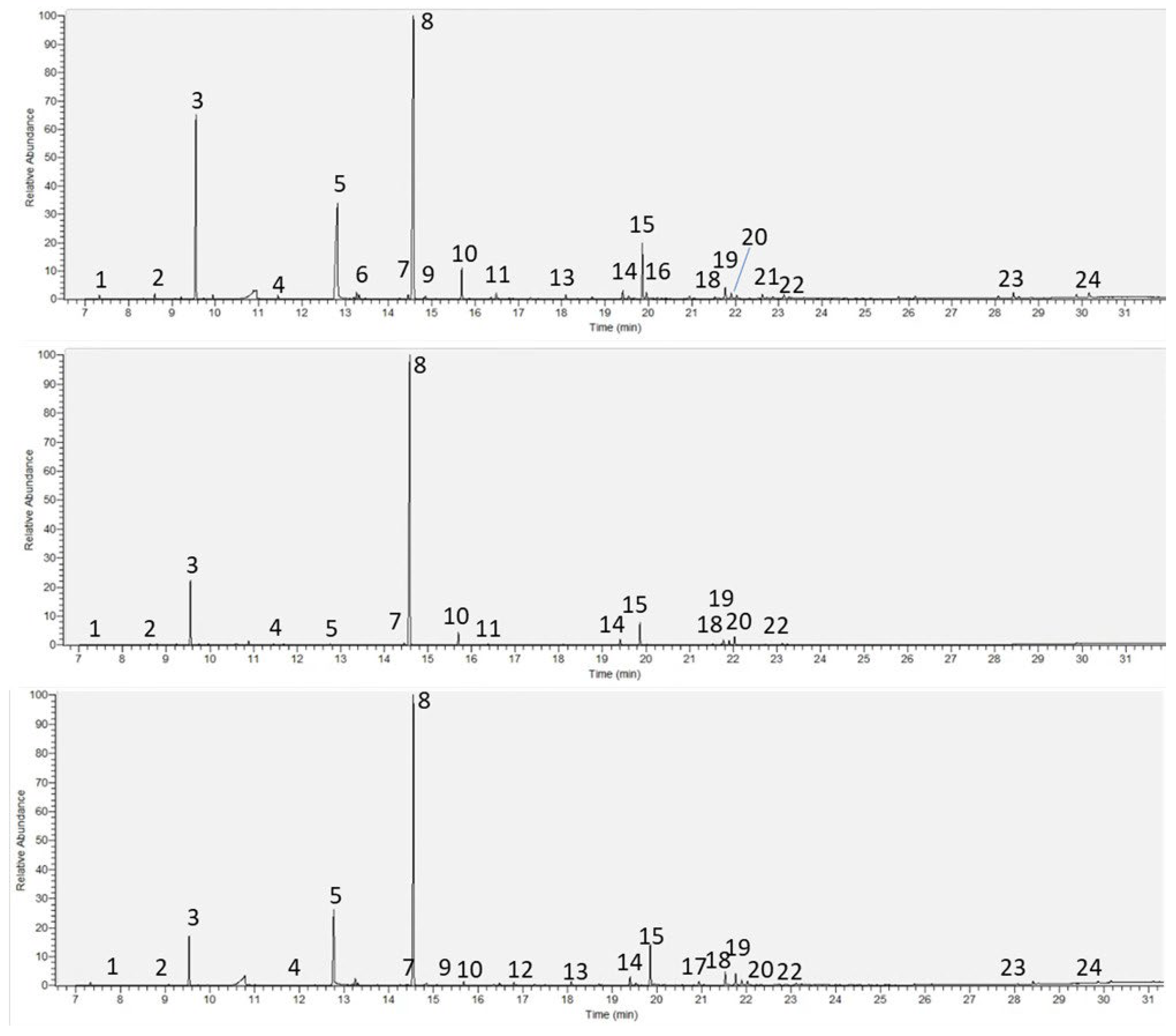
- Py-GC-HRAMS
2.3. Investigation of Decay Issues
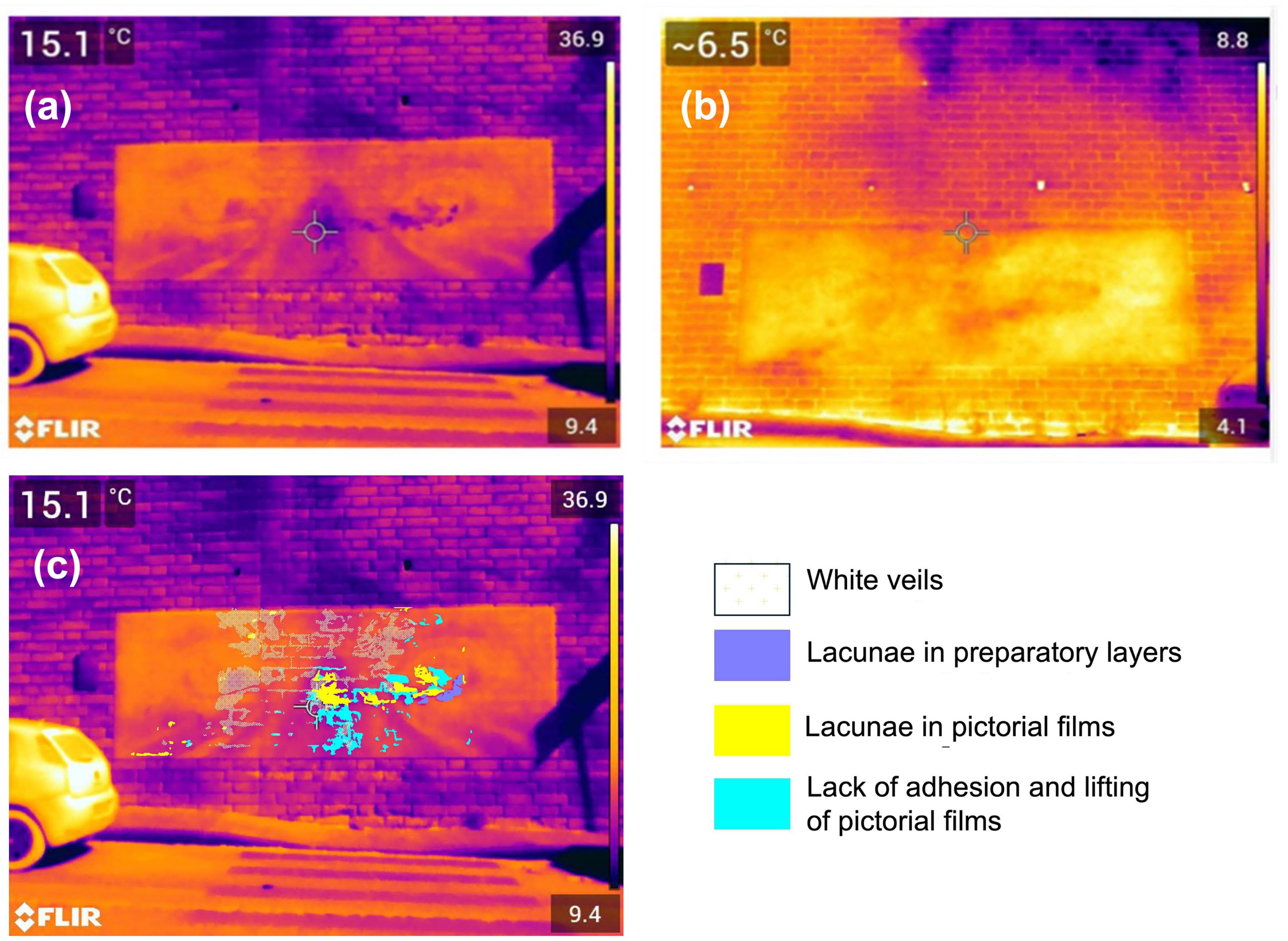
- In situ IRT
- Infrared thermography was used to evaluate, through a non-invasive methodology [35], the thermal behavior of the mural painting over almost one year. A long-wave infrared camera (FLIR 396 produced by Teledyne Flir, Wilsonville, OR, USA) with a resolution of 640 × 480 pixels and an absorption band of 7.5–14 µm was employed. The calibration was performed through an NUC operation, which is an image correction performed by the thermal imaging camera software to compensate for any differences in the sensitivity of the elements of the detector and other optical and geometric disturbances (EN 16714-3). During this operation (offset update), a shutter is placed in the optical path and all detector elements are exposed to the same amount of radiation originating from the shutter.
- The painting was investigated through passive methodology using surface heating generated by solar radiation. Thermographs were acquired and images were processed in false color with a reference scale with shades from violet to black for the areas with low temperatures and to yellow/white for those with high ones. All acquisitions were performed when the painting was entirely in shadow.
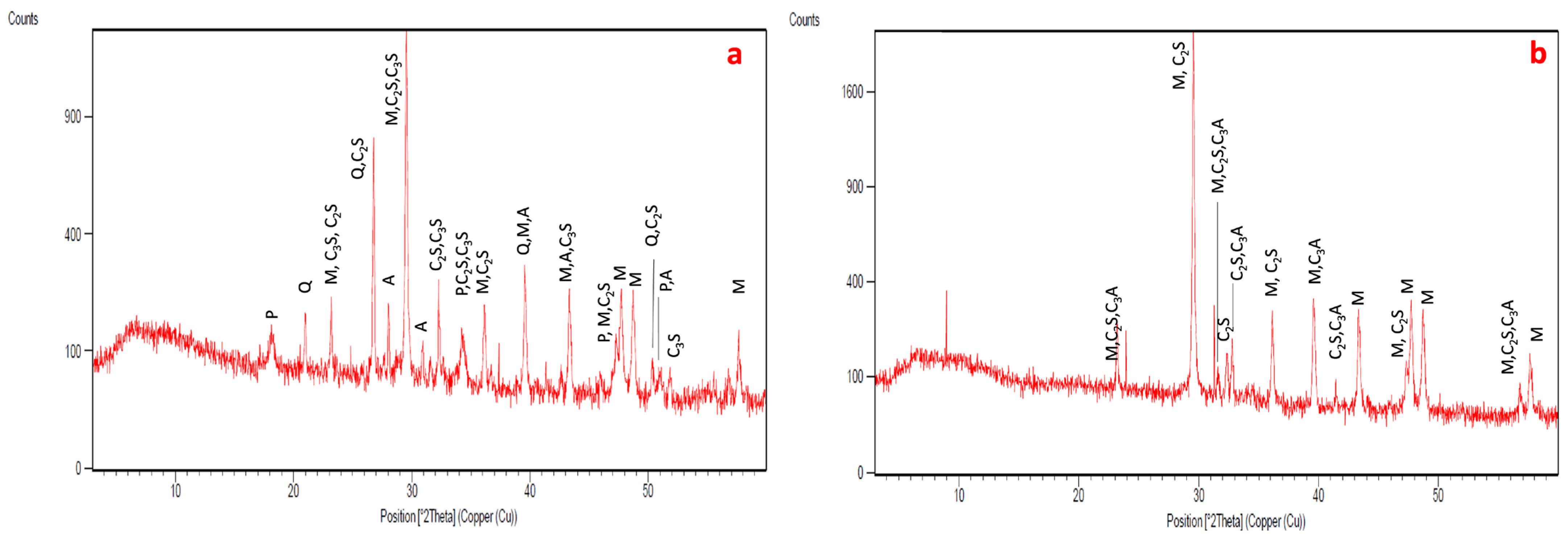
- XRD
- XRD analyses were carried out on powdered samples to identify the mineralogical salt phases. The samples were ground in an agate mortar until an impalpable powder was obtained. A Philips (Philips, Amsterdam, the Netherlands) diffractometer (Mod. PW1729, APD—3.6j version) was used (CuKα radiation at 40 kV/20 mA, 0.02° step size, 1.25 s counting time, 2θ scan interval between 3° and 60°). The diffractometer was provided with manual divergence slit and graphite monochromator. Diffraction data were processed with X’Pert software 2.2e—Philips Analytical.
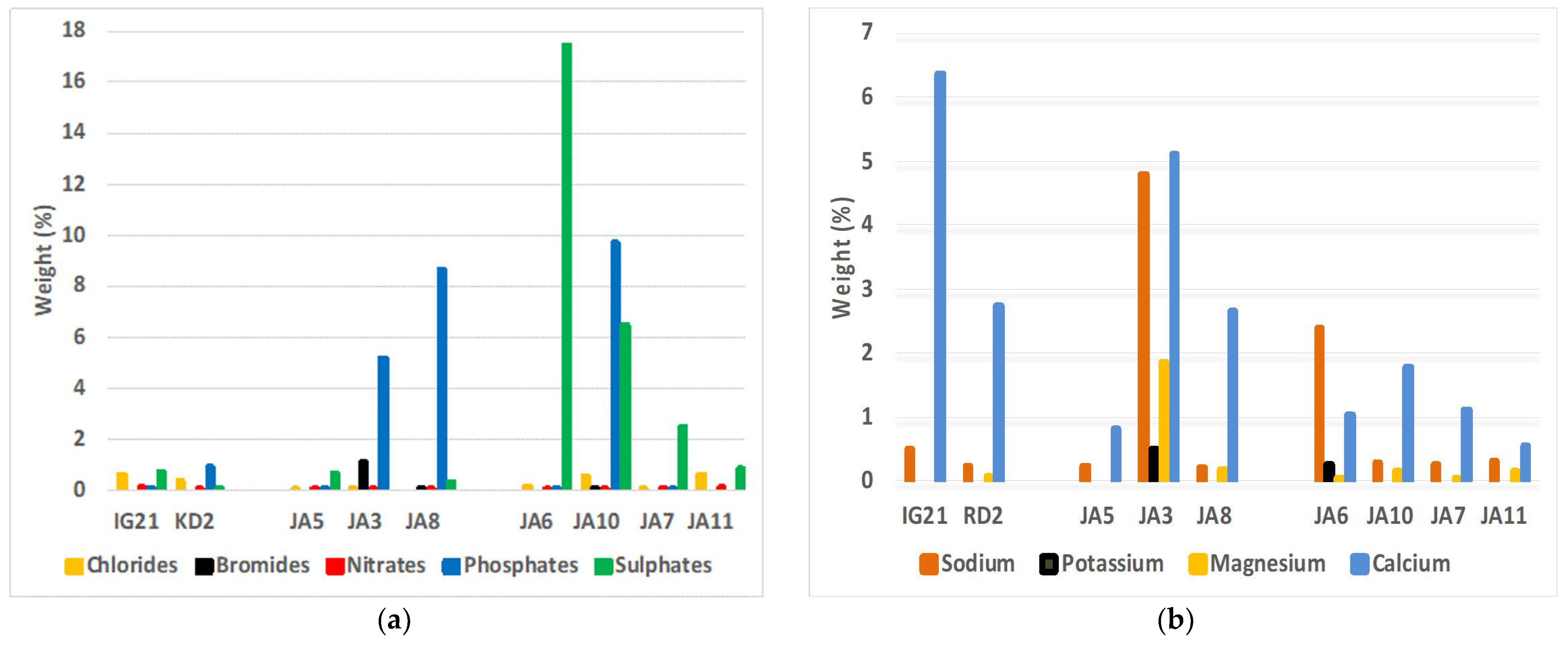
- IC
3. Results and Discussion
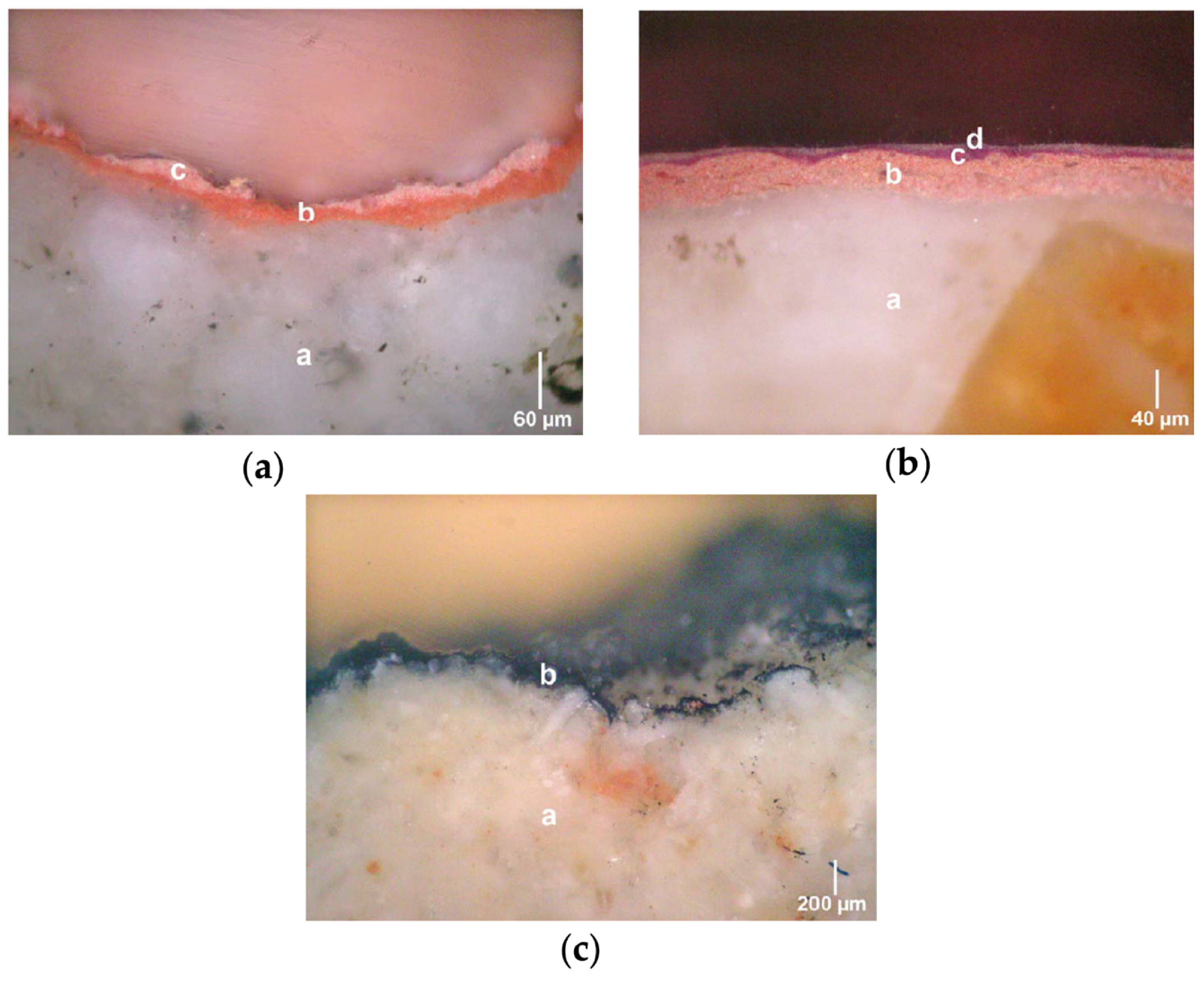
3.1. Stratigraphy and Composition of the Pictorial Films
3.2. Microscopic Characteristics and Mineralogical Composition of the Mortar of the Preparation Layers
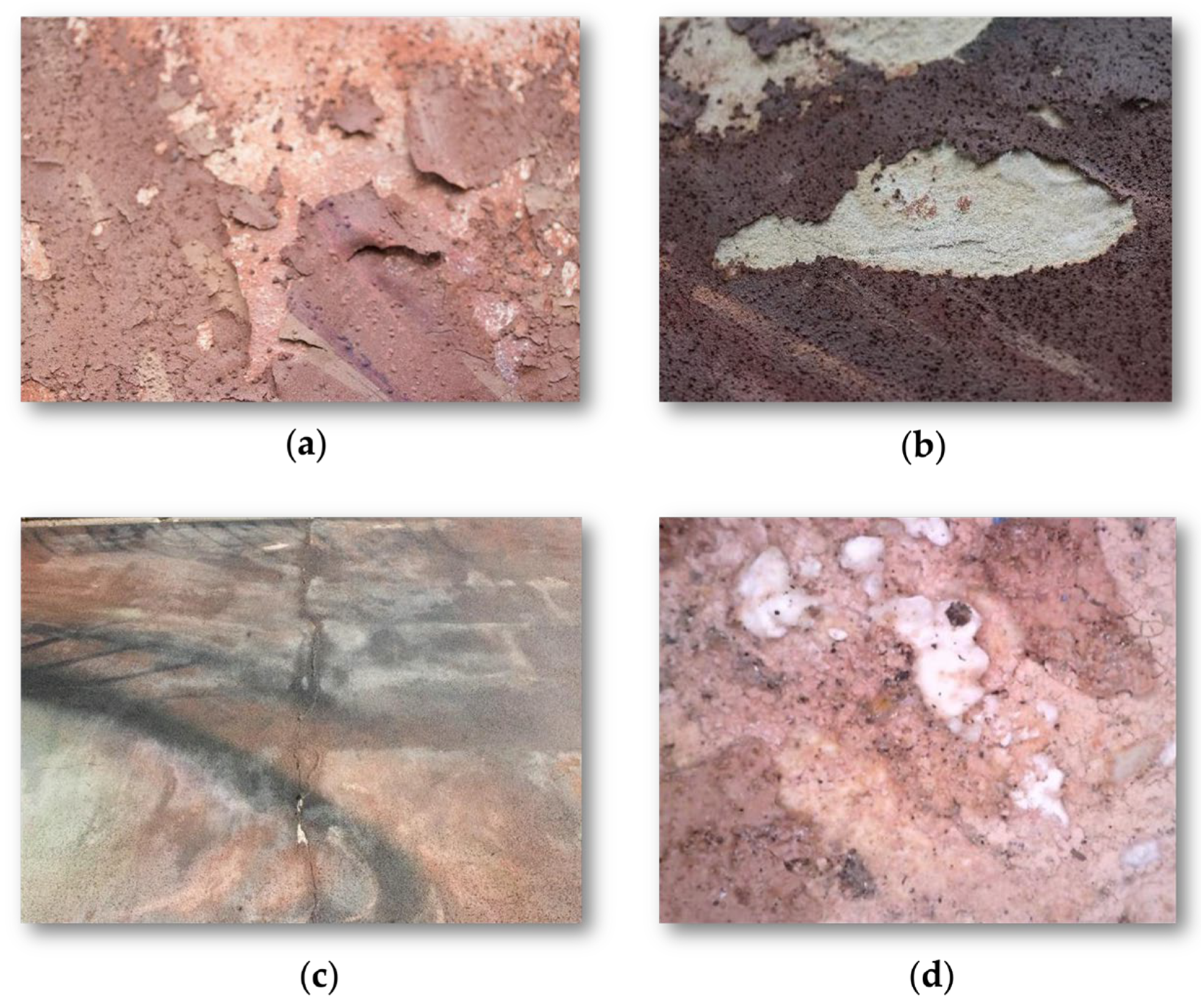
3.3. Decay Features
3.3.1. Humidity Presence and Distribution
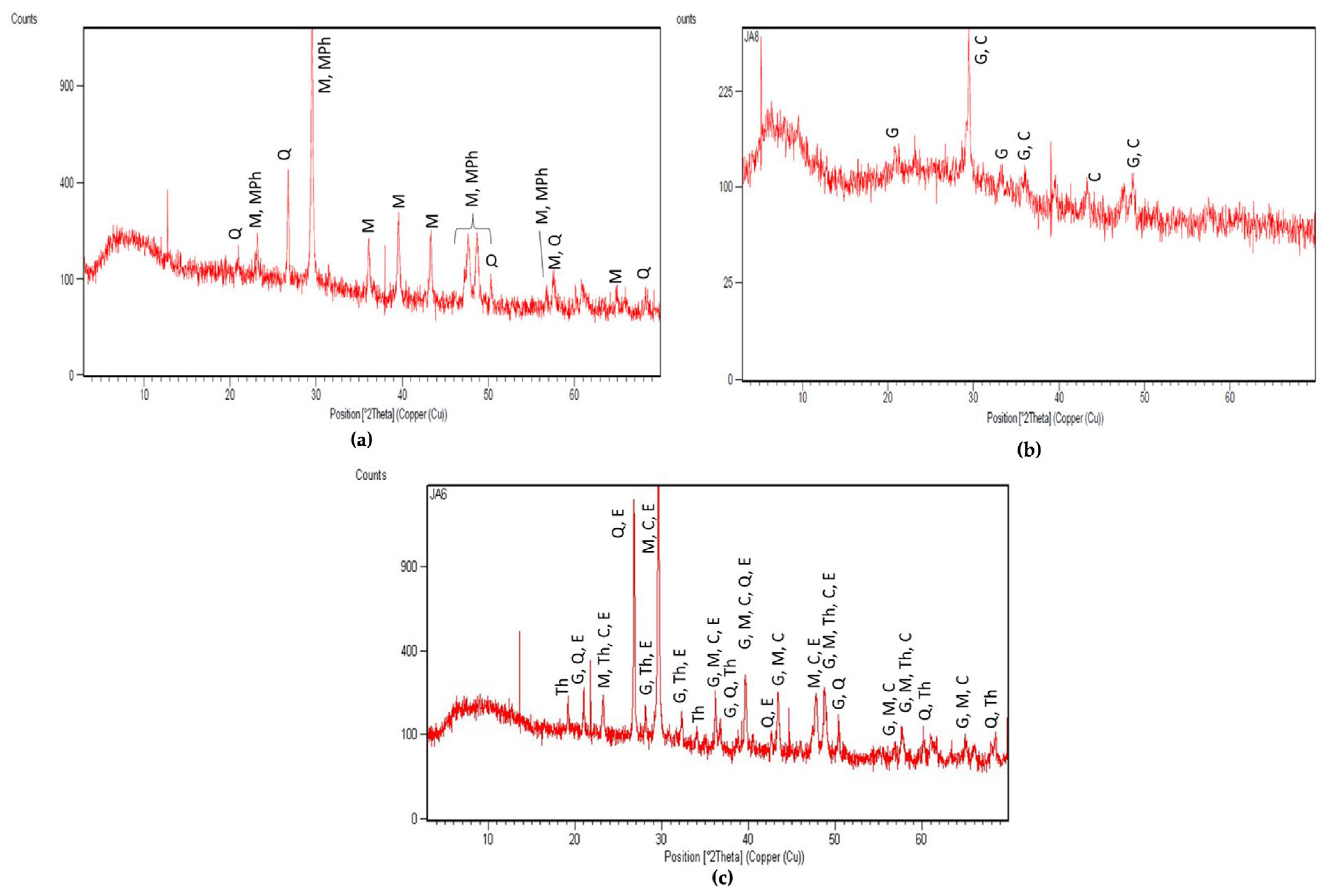
3.3.2. Salt Identification and Quantification
| IG21 | KD2 | JA5 | JA3 | JA8 | JA6 | JA10 | JA7 | JA11 | |
|---|---|---|---|---|---|---|---|---|---|
| Chloride | 0.60 | 0.37 | 0.08 | 0.04 | - | 0.16 | 0.56 | 0.09 | 0.61 |
| Bromide | - | - | - | 1.10 | 0.03 | - | 0.06 | - | - |
| Nitrate | 0.11 | 0.06 | 0.03 | 0.07 | 0.05 | 0.09 | 0.06 | 0.05 | 0.12 |
| Phosphate | 0.03 | 0.93 | 0.01 | 5.20 | 8.66 | 0.02 | 9.74 | 0.02 | - |
| Sulfate | 0.74 | 0.08 | 0.65 | - | 0.31 | 17.45 | 6.46 | 2.53 | 0.88 |
| Total anions | 1.48 | 1.44 | 0.78 | 6.41 | 9.05 | 17.71 | 16.87 | 2.69 | 1.61 |
| Sodium | 0.52 | 0.24 | 0.23 | 4.80 | 0.22 | 2.40 | 0.30 | 0.27 | 0.33 |
| Potassium | - | - | - | 0.49 | - | 0.27 | - | - | - |
| Magnesium | - | 0.07 | 1.86 | 0.20 | 0.06 | 0.16 | 0.06 | 0.16 | |
| Calcium | 6.36 | 2.75 | 0.83 | 5.11 | 2.65 | 1.05 | 1.79 | 1.12 | 0.57 |
| Total cations | 6.88 | 3.06 | 1.06 | 12.26 | 3.07 | 3.78 | 2.25 | 1.44 | 1.06 |
| Total ions | 8.35 | 4.50 | 1.83 | 18.67 | 12.11 | 21.49 | 15.49 | 4.12 | 2.67 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poon, S.T. International Journal of Cultural and Creative Industries Street Murals as a Unique Tangible Cultural Heritage: A Case Study of Artifact Value Preservation. Int. J. Cult. Creat. Ind. 2016, 4, 48–61. [Google Scholar]
- Germinario, G.; van der Werf, I.D.; Sabbatini, L. Chemical Characterisation of Spray Paints by a Multi-Analytical (Py/GC–MS, FTIR, μ-Raman) Approach. Microchem. J. 2016, 124, 929–939. [Google Scholar] [CrossRef]
- Germinario, G.; Van Der Werf, I.D.; Sabbatini, L. Pyrolysis Gas Chromatography Mass Spectrometry of Two Green Phthalocyanine Pigments and Their Identification in Paint Systems. J. Anal Appl. Pyrolysis 2015, 115, 175–183. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV Ageing Studies of Acrylic, Alkyd, and Polyvinyl Acetate Paints: Influence of Inorganic Pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Schreiner, M. A Comparison Study of Alkyd Resin Used in Art Works by Py-GC/MS and GC/MS: The Influence of Aging. J. Anal. Appl. Pyrolysis 2013, 104, 441–447. [Google Scholar] [CrossRef]
- Ploeger, R.; Scalarone, D.; Chiantore, O. The Characterization of Commercial Artists’ Alkyd Paints. J. Cult. Herit. 2008, 9, 412–419. [Google Scholar] [CrossRef]
- Marazioti, V.; Douvas, A.M.; Katsaros, F.; Koralli, P.; Chochos, C.; Gregoriou, V.G.; Boyatzis, S.; Facorellis, Y. Chemical Characterisation of Artists’ Spray-Paints: A Diagnostic Tool for Urban Art Conservation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122375. [Google Scholar] [CrossRef] [PubMed]
- Rigante, E.C.L.; Calvano, C.D.; Picca, R.A.; Modugno, F.; Cataldi, T.R.I. An Insight into Spray Paints for Street Art: Chemical Characterization of Two Yellow Varnishes by Spectroscopic and MS-Based Spectrometric Techniques. Vacuum 2023, 215, 112350. [Google Scholar] [CrossRef]
- Pintus, V.; Viana, C.; Angelin, E.M.; De Sá, S.F.; Wienland, K.; Sterflinger, K.; Ferreira, J.L. Applicability of Single-Shot and Double-Shot Py-GC/MS for the Detection of Components in Vinyl Acetate-Based Emulsions Used in Modern-Contemporary Art. J. Anal. Appl. Pyrolysis 2022, 168, 105782. [Google Scholar] [CrossRef]
- Rava, A.; Chiantore, O. Outdoor Painted Surfaces. In Contemporary Science and Art: The Painted Surface; Sgamellotti, A., Brunetti, B.G., Miliani, C., Eds.; Royal Society of Chemistry: London, UK, 2014; pp. 542–561. [Google Scholar]
- La Nasa, J.; Orsini, S.; Degano, I.; Rava, A.; Modugno, F.; Colombini, M.P. A Chemical Study of Organic Materials in Three Murals by Keith Haring: A Comparison of Painting Techniques. Microchem. J. 2016, 124, 940–948. [Google Scholar] [CrossRef]
- Magrini, D.; Bracci, S.; Cantisani, E.; Conti, C.; Rava, A.; Sansonetti, A.; Shank, W.; Colombini, M.P. A Multi-Analytical Approach for the Characterization of Wall Painting Materials on Contemporary Buildings. Spectrochim Acta A Mol. Biomol. Spectrosc. 2017, 173, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Cucci, C.; Bartolozzi, G.; De Vita, M.; Marchiafava, V.; Picollo, M.; Casadio, F. The Colors of Keith Haring: A Spectroscopic Study on the Materials of the Mural Painting Tuttomondo and on Reference Contemporary Outdoor Paints. Appl. Spectrosc. 2016, 70, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Marazioti, V.; Douvas, A.M.; Gregoriou, V.G.; Facorellis, Y. Contemporary Murals: Chemical Characterisation of Artists’ Spray-Paints. 2022. Available online: https://www.researchsquare.com/article/rs-1519449/v1 (accessed on 6 March 2024).
- La Nasa, J.; Campanella, B.; Sabatini, F.; Rava, A.; Shank, W.; Lucero-Gomez, P.; De Luca, D.; Legnaioli, S.; Palleschi, V.; Colombini, M.P.; et al. 60 Years of Street Art: A Comparative Study of the Artists’ Materials through Spectroscopic and Mass Spectrometric Approaches. J. Cult. Herit. 2021, 48, 129–140. [Google Scholar] [CrossRef]
- Bosi, A.; Ciccola, A.; Serafini, I.; Guiso, M.; Ripanti, F.; Postorino, P.; Curini, R.; Bianco, A. Street Art Graffiti: Discovering Their Composition and Alteration by FTIR and Micro-Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 225, 117474. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Antonio, J.S.; Rivas, T.; González, N.; Alonso-Villar, E.M. Deterioration of Graffiti Spray Paints Applied on Granite after a Decade of Natural Environment. Sci. Total Environ. 2022, 826, 154169. [Google Scholar] [CrossRef] [PubMed]
- Cimino, D.; Lamuraglia, R.; Saccani, I.; Berzioli, M.; Izzo, F.C. Assessing the (In)Stability of Urban Art Paints: From Real Case Studies to Laboratory Investigations of Degradation Processes and Preservation Possibilities. Heritage 2022, 5, 581–609. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Silva, M.F.; Aura-Castro, E.; Fuster-López, L.; Kröner, S.; Martínez-Bazán, M.L.; Más-Barberá, X.; Mecklenburg, M.F.; Osete-Cortina, L.; Doménech, A.; et al. Study of Behaviour on Simulated Daylight Ageing of Artists’ Acrylic and Poly(Vinyl Acetate) Paint Films. Anal. Bioanal. Chem. 2011, 399, 2921–2937. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.X.; Irigoyen, M.; Aragon, E.; Vernet, J.L. Artificial Aging of Acrylurethane and Alkyd Paints: A Micro-ATR Spectroscopic Study. Polym. Degrad. Stab. 2000, 70, 469–475. [Google Scholar] [CrossRef]
- Ploeger, R.; Scalarone, D.; Chiantore, O. Thermal Analytical Study of the Oxidative Stability of Artists’ Alkyd Paints. Polym. Degrad. Stab. 2009, 94, 2036–2041. [Google Scholar] [CrossRef]
- Sanmartín, P.; Pozo-Antonio, J.S. Weathering of Graffiti Spray Paint on Building Stones Exposed to Different Types of UV Radiation. Constr. Build. Mater. 2020, 236, 117736. [Google Scholar] [CrossRef]
- Rivas, T.; Alonso-Villar, E.M.; Pozo-Antonio, J.S. Forms and Factors of Deterioration of Urban Art Murals under Humid Temperate Climate; Influence of Environment and Material Properties. Eur. Phys. J. Plus 2022, 137, 1257. [Google Scholar] [CrossRef]
- Rousaki, A.; Vandenabeele, P.; Berzioli, M.; Saccani, I.; Fornasini, L.; Bersani, D. An In-and-out-the-Lab Raman Spectroscopy Study on Street Art Murals from Reggio Emilia in Italy. Eur. Phys. J. Plus 2022, 137, 252. [Google Scholar] [CrossRef]
- Pellis, G.; Bertasa, M.; Ricci, C.; Scarcella, A.; Croveri, P.; Poli, T.; Scalarone, D. A Multi-Analytical Approach for Precise Identification of Alkyd Spray Paints and for a Better Understanding of Their Ageing Behaviour in Graffiti and Urban Artworks. J. Anal. Appl. Pyrolysis 2022, 165, 105576. [Google Scholar] [CrossRef]
- Learner, T.J.S. Analysis of Modern Paints-Getty Publications—Series Research in Conservation; Getty Publications: Los Angeles, CA, USA, 2004. [Google Scholar]
- Pagnin, L.; Calvini, R.; Wiesinger, R.; Weber, J.; Schreiner, M. Photodegradation Kinetics of Alkyd Paints: The Influence of Varying Amounts of Inorganic Pigments on the Stability of the Synthetic Binder. Front. Mater. 2020, 7, 423. [Google Scholar] [CrossRef]
- Martinho, E.; Dionísio, A. Main Geophysical Techniques Used for Non-Destructive Evaluation in Cultural Built Heritage: A Review. J. Geophys. Eng. 2014, 11, 053001. [Google Scholar] [CrossRef]
- Brunetti, B.; Miliani, C.; Rosi, F.; Doherty, B.; Monico, L.; Romani, A.; Sgamellotti, A. Non-Invasive Investigations of Paintings by Portable Instrumentation: The MOLAB Experience. Top. Curr. Chem. 2016, 374, 10. [Google Scholar] [CrossRef] [PubMed]
- Maldague, X.P.V. Nondestructive Evaluation of Materials by Infrared Thermography; Springer: London, UK, 1993; ISBN 978-1-4471-1997-5. [Google Scholar]
- Barreira, E.; Almeida, R.M.S.F.; Delgado, J.M.P.Q. Infrared Thermography for Assessing Moisture Related Phenomena in Building Components. Constr. Build. Mater. 2016, 110, 251–269. [Google Scholar] [CrossRef]
- Terry, R.D.; Chilingar, G.V. Summary of “Concerning Some Additional Aids in Studying Sedimentary Formations,” by M. S. Shvetsov. J. Sediment. Res. 1955, 25, 229–234. [Google Scholar] [CrossRef]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1992, 30, 377–392. [Google Scholar] [CrossRef]
- Powers, M.C. A New Roundness Scale for Sedimentary Particles. J. Sediment. Res. 1953, 23, 117–119. [Google Scholar] [CrossRef]
- Rahrig, M.; Lerma, J.L. Multispectral Imaging for the Documentation of Graffiti in an Urban Environment. In Proceedings of the Proceedings of the 5th Joint International Symposium on Deformation Monitoring—JISDM 2022, Valencia, Spain, 6–8 April 2022; Editorial de la Universitat Politècnica de València: Valencia, Spain, 2022. [Google Scholar]
- Duce, C.; Della Porta, V.; Tiné, M.R.; Spepi, A.; Ghezzi, L.; Colombini, M.P.; Bramanti, E. FTIR Study of Ageing of Fast Drying Oil Colour (FDOC) Alkyd Paint Replicas. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Pagnin, L.; Zendri, E.; Izzo, F.C. How Can Ozone and Relative Humidity Affect Artists’ Alkyd Paints? A FT-IR and Py-GC/MS Systematic Study. Polymers 2022, 14, 1831. [Google Scholar] [CrossRef] [PubMed]
- Dhoke, S.K.; Khanna, A.S. Effect of Nano-Fe2O3 Particles on the Corrosion Behavior of Alkyd Based Waterborne Coatings. Corros. Sci. 2009, 51, 6–20. [Google Scholar] [CrossRef]
- Charola, A.E. Salts in the Deterioration of Porous Materials: An Overview. J. Am. Inst. Conserv. 2000, 39, 327–343. [Google Scholar] [CrossRef]
- Hewlett, P.; Liska, M. (Eds.) Lea’s Chemistry of Cement and Concrete; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780750662567. [Google Scholar]
- Zimdahl, R.L. Phosphorous. In Six Chemicals That Changed Agriculture; Academic Press: Cambridge, MA, USA, 2015; pp. 73–88. [Google Scholar]
- Benavente, D.; De Jongh, M.; Cañaveras, J.C. Weathering Processes and Mechanisms Caused by Capillary Waters and Pigeon Droppings on Porous Limestones. Minerals 2020, 11, 18. [Google Scholar] [CrossRef]
- Christmann, O.; Naarmann, H. Bromine-Containing Pigment Dyes of the Perinone Series. U.S. Patent 3,538,095, 3 November 1970. [Google Scholar]
- Tsui, N.; Flatt, R.J.; Scherer, G.W. Crystallization Damage by Sodium Sulfate. J. Cult. Herit. 2003, 4, 109–115. [Google Scholar] [CrossRef]
- Lindström, N.; Talreja, T.; Linnow, K.; Stahlbuhk, A.; Steiger, M. Crystallization Behavior of Na2SO4–MgSO4 Salt Mixtures in Sandstone and Comparison to Single Salt Behavior. Appl. Geochem. 2016, 69, 50–70. [Google Scholar] [CrossRef]
- Houston, J.R.; Maxwell, R.S.; Carroll, S.A. Transformation of Meta-Stable Calcium Silicate Hydrates to Tobermorite: Reaction Kinetics and Molecular Structure from XRD and NMR Spectroscopy. Geochem. Trans. 2009, 10, 1. [Google Scholar] [CrossRef]
- Biagioni, C.; Merlino, S.; Bonaccorsi, E. The Tobermorite Supergroup: A New Nomenclature. Miner. Mag 2015, 79, 485–495. [Google Scholar] [CrossRef]
- Collepardi, M. Degradation and Restoration of Masonry Walls of Historical Buildings. Mater. Struct./Matrriaux Constr. 1990, 23, 81–102. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.R.; Lindgård, J.; De Weerdt, K. Determining the Free Alkali Metal Content in Concrete—Case Study of an ASR-Affected Dam. Cem. Concr. Res. 2018, 105, 111–125. [Google Scholar] [CrossRef]
- Vidal, F.; Vicente, R.; Mendes Silva, J. Review of Environmental and Air Pollution Impacts on Built Heritage: 10 Questions on Corrosion and Soiling Effects for Urban Intervention. J. Cult. Herit. 2019, 37, 273–295. [Google Scholar] [CrossRef]
- Ángeles García-del-Cura, M.; Spairani, Y.; Louls, M.; Garcia, M.A. The Civil Palaces in Gravina Street, Alicante: Building Stones and Salt Weathering. Mater. Construcciòn 2001, 51, 15–21. [Google Scholar]
- Cuttano, M.; Mastronardi, P.; Rossi-Manaresi, R. The Conservation of Stone II. In Proceedings of the Alveolar Weathering of the “tuff” of Matera. Mechanism of Deterioration and Effectiveness of Preservation Treatments; Rossi-Manaresi, R., Ed.; Centro “Cesare Gnudi” per la Conservazione delle Sculture All’aperto: Bologna, Italy, 1981; pp. 355–377. [Google Scholar]
- Magrini, D.; Bartolozzi, G.; Bracci, S.; Carlesi, S.; Cucci, C.; Picollo, M. Evaluation of the Efficacy and Durability of the “Barium Hydroxide Method” after 40 Years. Multi-Analytical Survey on the Crocifissione by Beato Angelico. J. Cult. Herit. 2020, 45, 362–369. [Google Scholar] [CrossRef]
- Matteini, M. Inorganic Treatments for the Consolidation and Protection of Stone Artefacts. Conserv. Sci. Cult. Herit. 2008, 8, 13–27. [Google Scholar]




| Sample Name | Sample Type | Analytical Techniques |
|---|---|---|
| JA1 | Fragment of mortar from the bottom layer of the painted panel | PLM (t.l.), FT-IR |
| JA2 | Fragment of mortar from the top layer with red pictorial film from the painted panel | PLM (t.l.; r.l.) |
| JA3 | Salt efflorescence powder on the pictorial layer of the painted panel | XRD, IC |
| JA4 | Pictorial layer consisting of brushstrokes with red, pink and purple colors and affected by lifting from the painted panel | Py-GC-MS, FT-IR, PLM (r.l.) |
| A5 | Powder from the top mortar layer just under the pictorial film of the painted panel | XRD, IC |
| JA6 | Salt efflorescence from the mortar joint from the masonry just at the foot of the painted panel | XRD, IC |
| JA7 | Mortar fragment from the joint from the masonry on the right of the painted panel | XRD, IC |
| JA8 | Powder from the white veil on the pictorial layer of the painted panel | XRD, IC |
| JA9 | Black pictorial layer from the masonry ashlar surface on the right of the painted panel | PLM (t.l.; r.l.), FT-IR, XRD, IC |
| JA10 | Powder from the white veil from the masonry ashlar surface on the right of the painted panel | XRD, IC |
| JA11 | Stone fragment from the masonry ashlar surface on the right of the painted panel | PLM (t.l.), XRD, IC |
| IG21: | Raw mortar powder used in the top layer of the paint support | XRD, IC |
| KD2 | Raw mortar powder used in the bottom layer of the paint support | XRD, IC |
| RV-35 | Montana Colors 94 spray paint—Chocolate Brown | Py-GC-HRAMS |
| RV-205 | Montana Colors 94 spray paint—Warrion Brown | Py-GC-HRAMS |
| RV-136 | Montana Colors 94 spray paint—Inca Brown | Py-GC-HRAMS |
| RV-100 | Montana Colors 94 spray paint—Coffee Brown | Py-GC-HRAMS |
| RV-97 | Montana Colors 94 spray paint—Chiapas Brown | Py-GC-HRAMS |
| RT | Compound | RV100 | RV136 | RV205 | RV35 | RV97 | JA4 |
|---|---|---|---|---|---|---|---|
| 7.35 | Benzaldehyde | X | X | X | X | X | X |
| 7.61 | Phenol | X | X | X | X | X | X |
| 7.67 | α-methyl styrene | X | X | X | X | ||
| 7.74 | Benzonitrile | X | X | X | X | X | X |
| 8.49 | Butanedioic acid, ME | X | X | X | X | X | X |
| 8.63 | Octanoic acid, ME | X | X | X | X | X | X |
| 9.56 | Benzoic acid, ME | X | X | X | X | X | |
| 9.75 | 1-propanol, 3-methoxy-2,2-bis-(methoxymethyl) | X | X | X | X | X | X |
| 9.96 | Nonanoic acid, ME | X | X | X | X | ||
| 10.63 | Benzoic acid, ME | X | X | X | |||
| 10.88 | Pentaerithrol, tetramethyl ether | X | X | X | X | X | X |
| 11.46 | Decanoic acid, ME | X | X | X | X | ||
| 11.57 | Styrene | X | X | X | X | ||
| 12.76 | Phthalic anhydride | X | X | X | X | X | X |
| 12.87 | Undecanoic acid, ME | X | X | X | X | X | X |
| 13.48 | Trimethoxy benzene | X | X | X | |||
| 13.54 | Dodecanoic acid, ME | X | X | ||||
| 13.75 | Biphenyl | X | X | X | X | X | X |
| 13.87 | Heptanedioic acid, 2ME | X | X | X | X | ||
| 14.27 | N-methyl.phthalamide | X | X | X | X | X | X |
| 14.45 | Octanedioic acid, 2ME | X | X | X | X | X | X |
| 14.59 | Dimethyl phthalate | X | X | X | X | X | X |
| 14.87 | N-propyl benzamide | X | X | X | X | X | X |
| 15.70 | Nonanedioic acid, ME | X | X | X | X | X | X |
| 16.25 | 2,3-dimethoxy benzoic acid, ME | X | X | ||||
| 16.38 | Decenedioic acid, diethyl ester | X | X | X | X | X | |
| 16.87 | Decanedioic acid, DME | X | X | X | X | X | X |
| 17.28 | N-4-(2-aminoethyl) -N4 ethyl-2-methyl-1,4 benzenediamine | X | X | ||||
| 17.76 | Tetradecanoic acid, ME | X | X | X | X | X | X |
| 18.10 | 1,4-benzene dicarboxylic acid, ME | X | X | X | X | X | X |
| 18.83 | Pentadecanoic acid, ME | X | X | X | X | X | X |
| 19.41 | Dimethyl phthalate | X | X | X | X | ||
| 19.86 | Hexadecanoic acid, ME | X | X | X | X | X | X |
| 19.94 | Isopropyl phthalate | X | X | X | X | X | X |
| 20.83 | Heptadecanoic acid, ME | X | X | X | X | X | X |
| 20.95 | Dibutyl pththalate | X | X | X | X | X | X |
| 21.54 | (Z)-octadec-9-enoic acid, ME | X | X | X | X | X | X |
| 21.73 | Phthalic acid, cyclobutyl hexyl ester | X | X | X | X | X | X |
| 21.77 | Octadecanoic acid, ME | X | X | X | X | X | X |
| 21.90 | Hexyl methyl phthalate | X | X | X | X | X | X |
| 22.02 | Phthalate | X | X | X | X | X | X |
| 22.14 | Docosanoic acid, ME | X | X | X | X | X | X |
| 22.33 | (9Z,12Z)-octadeca-9,12-dienoic acid, ME | X | X | X | X | X | X |
| 22.62 | Methyl propyl phthalate | X | X | X | X | X | X |
| 23.13 | oxiraneoctanoic acid, 3-octyl, ME | X | X | X | X | X | X |
| 23.50 | Tetracosanoic acid, ME | X | X | X | X | X | X |
| 23.52 | Eicosanoic acid, ME | X | X | X | X | X | X |
| 23.70 | Isobutyl methyl phthalate | X | X | X | X | X | X |
| 28.42 | Phthalic acid, methyl phenyl ester | X | X | X | X | X | |
| 30.17 | Phthalic acid, methyl phenyl ester | X |
| ID | % BA | % PBA | % PE | % FA | % PhT | A/P | P/S | PhA/A |
|---|---|---|---|---|---|---|---|---|
| Montana 94 spray paints | ||||||||
| RV100 | 16.31 | 81.74 | 0.14 | 0.92 | 0.89 | 0.21 | 7.70 | 3.50 |
| RV136 | 17.10 | 76.22 | 0.10 | 2.98 | 3.61 | 0.38 | 4.92 | 44.85 |
| RV205 | 9.60 | 83.34 | 0.08 | 1.83 | 5.15 | 0.46 | 3.57 | 56.11 |
| RV35 | 7.21 | 88.78 | 0.43 | 2.66 | 0.91 | 0.32 | 4.16 | 0.27 |
| RV97 | 12.78 | 82.61 | 0.11 | 2.13 | 2.37 | 0.48 | 3.94 | 0.67 |
| Paint sample | ||||||||
| JA4 | 7.05 | 85.53 | 0.13 | 2.95 | 4.35 | 0.08 | 3.29 | 132.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germinario, G.; Logiodice, A.L.; Mezzadri, P.; Di Fusco, G.; Ciabattoni, R.; Melica, D.; Calia, A. Integrated Investigations to Study the Materials and Degradation Issues of the Urban Mural Painting Ama Il Tuo Sogno by Jorit Agoch. Sustainability 2024, 16, 5069. https://doi.org/10.3390/su16125069
Germinario G, Logiodice AL, Mezzadri P, Di Fusco G, Ciabattoni R, Melica D, Calia A. Integrated Investigations to Study the Materials and Degradation Issues of the Urban Mural Painting Ama Il Tuo Sogno by Jorit Agoch. Sustainability. 2024; 16(12):5069. https://doi.org/10.3390/su16125069
Chicago/Turabian StyleGerminario, Giulia, Andrea Luigia Logiodice, Paola Mezzadri, Giorgia Di Fusco, Roberto Ciabattoni, Davide Melica, and Angela Calia. 2024. "Integrated Investigations to Study the Materials and Degradation Issues of the Urban Mural Painting Ama Il Tuo Sogno by Jorit Agoch" Sustainability 16, no. 12: 5069. https://doi.org/10.3390/su16125069
APA StyleGerminario, G., Logiodice, A. L., Mezzadri, P., Di Fusco, G., Ciabattoni, R., Melica, D., & Calia, A. (2024). Integrated Investigations to Study the Materials and Degradation Issues of the Urban Mural Painting Ama Il Tuo Sogno by Jorit Agoch. Sustainability, 16(12), 5069. https://doi.org/10.3390/su16125069








