The Development of Sustainable Biocomposite Materials Based on Poly(lactic acid) and Silverskin, a Coffee Industry By-Product, for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Treatments of Coffee Silverskin
2.3. Preparation of PLA Composite Films
2.4. Material Characterization
2.4.1. Physicochemical Properties
2.4.2. Chemical Structure and Morphological Characteristics Assessed by Fourier-Transform Infra-Red (FTIR) Spectroscopy and Scanning Electron Microscopy (SEM)
2.4.3. Thermal Properties Determined by Differential Scanning Calorimetry (DSC)
2.4.4. Oxygen and Water Vapor Permeability
2.4.5. Color Measurements
2.4.6. Antioxidant Activity
2.4.7. Mechanical Properties
3. Results and Discussion
3.1. Chemical Structure and Morphological Characteristics of Coffee Silverskin and PLA/Silverskin Composites
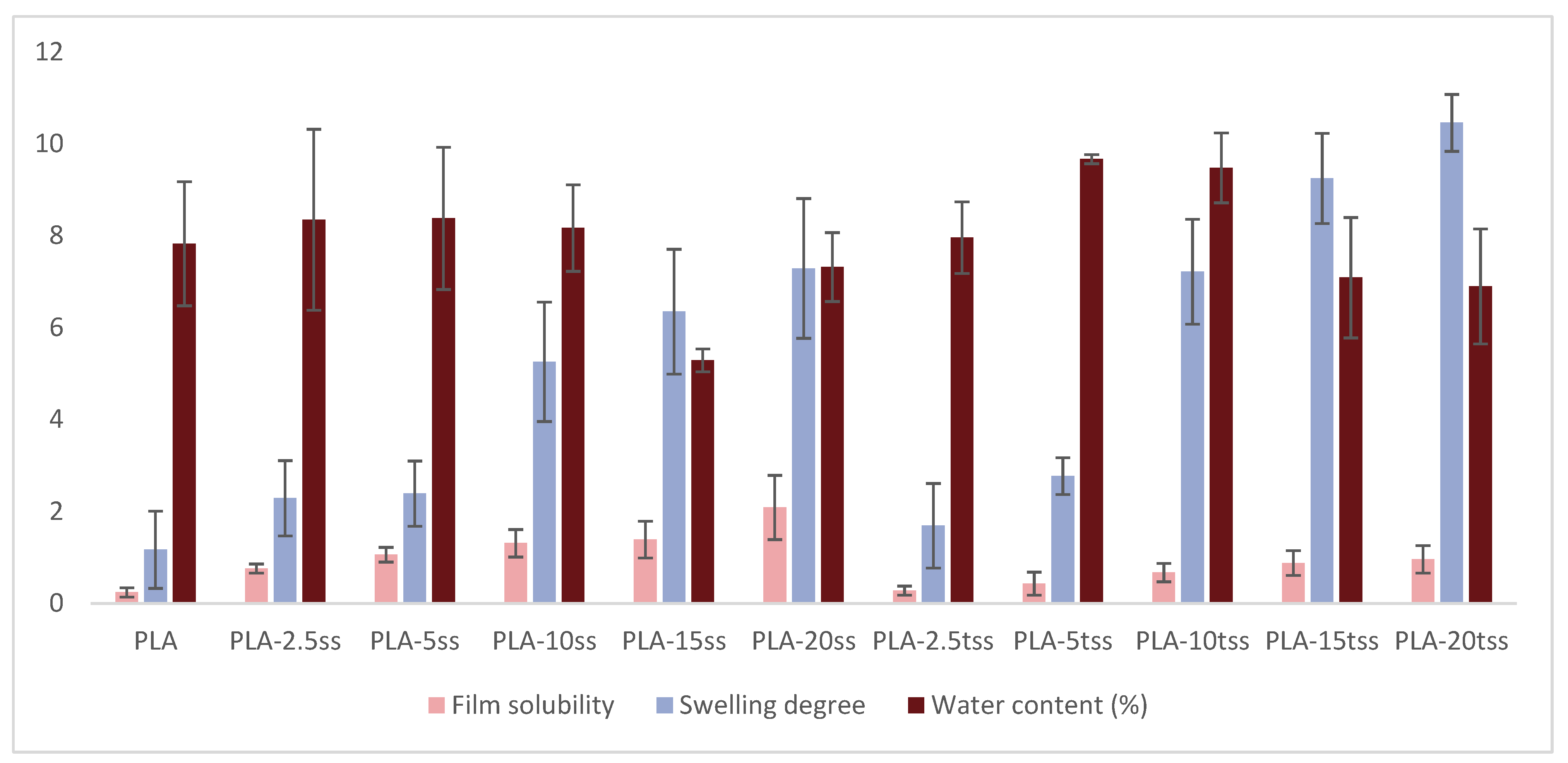
3.2. Physico-Chemical Characterization
3.3. Oxygen and Water Vapor Permeability
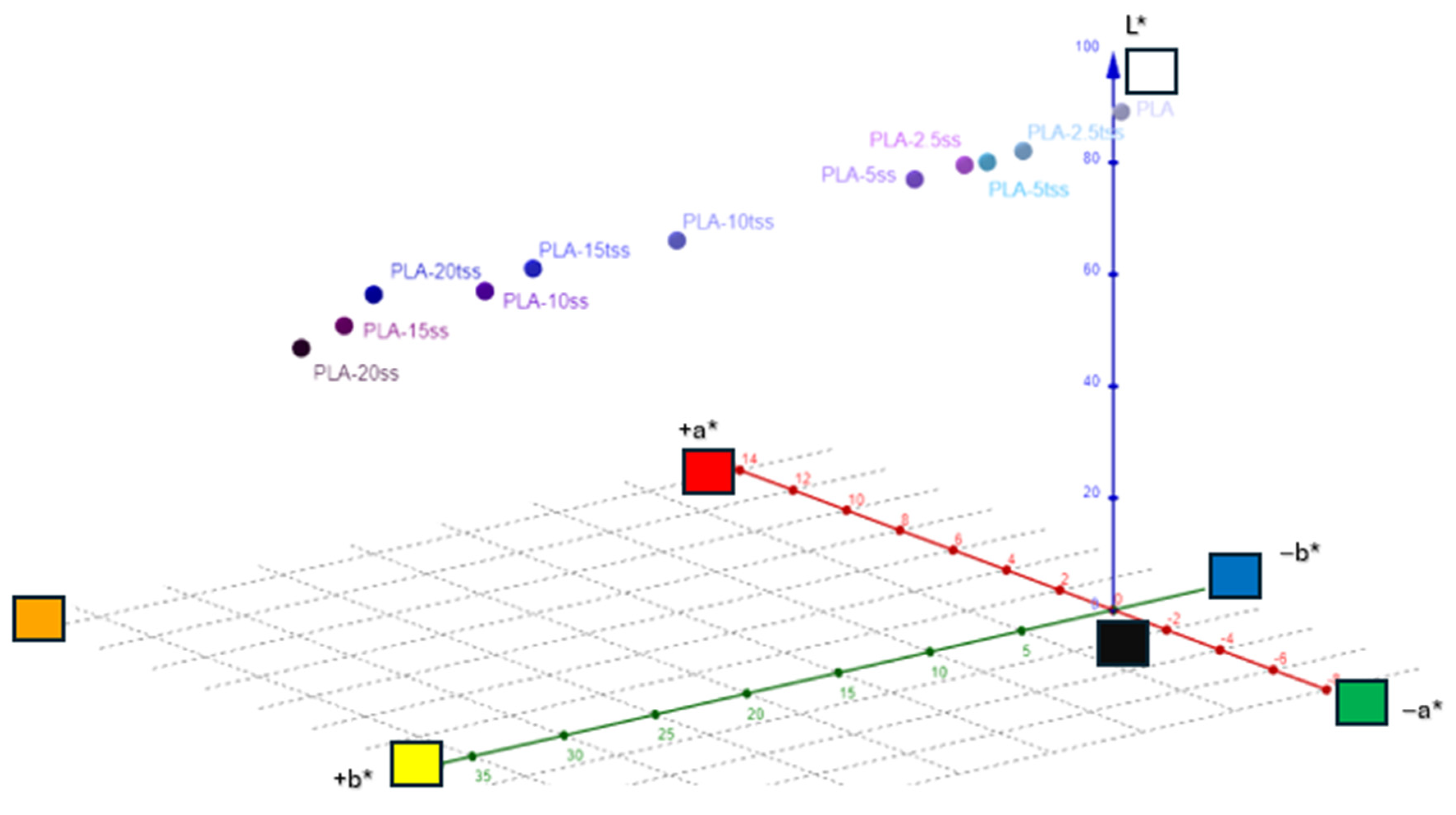
3.4. Color Measurement
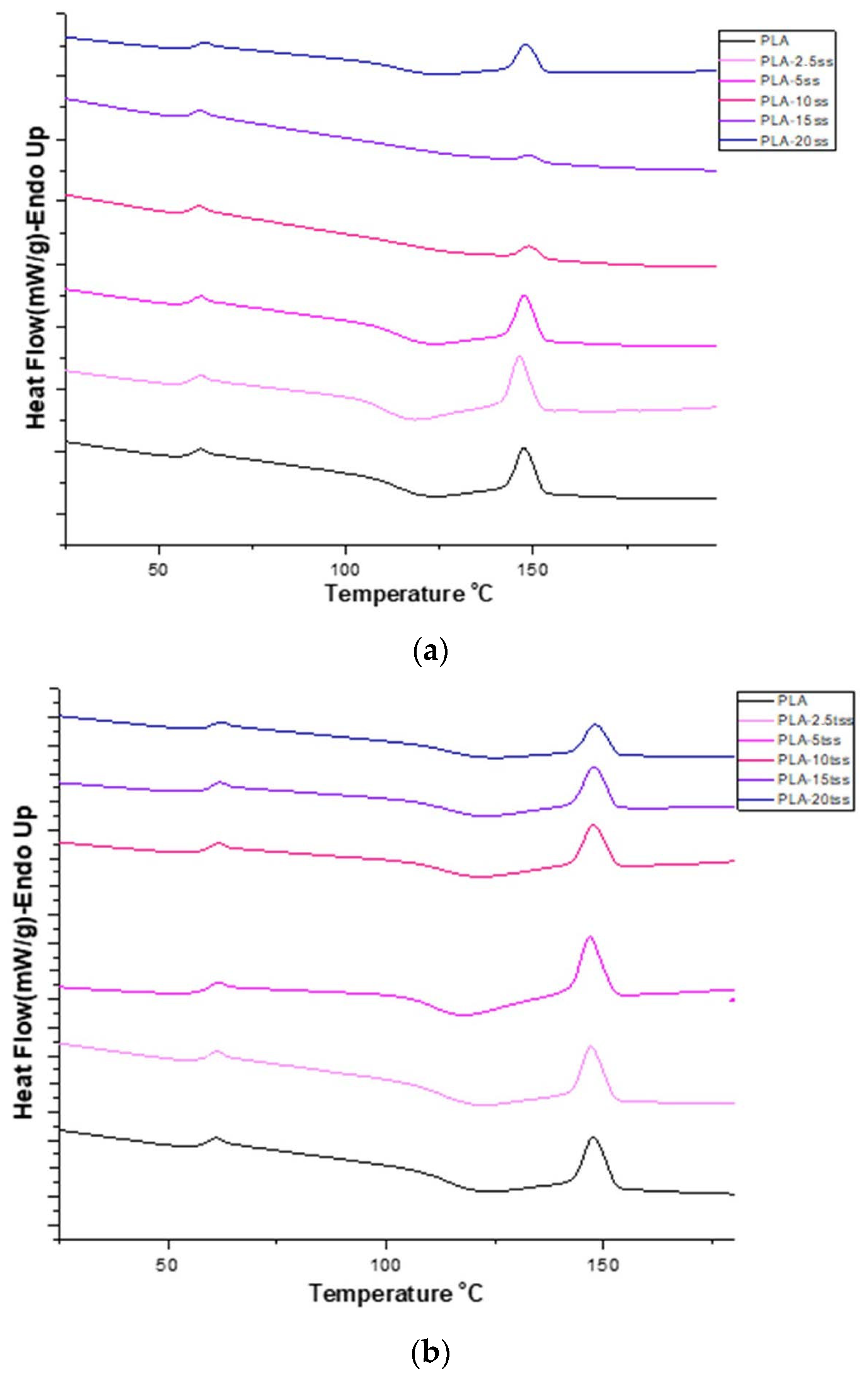
3.5. Thermodynamic Properties
3.6. Antioxidant Activity
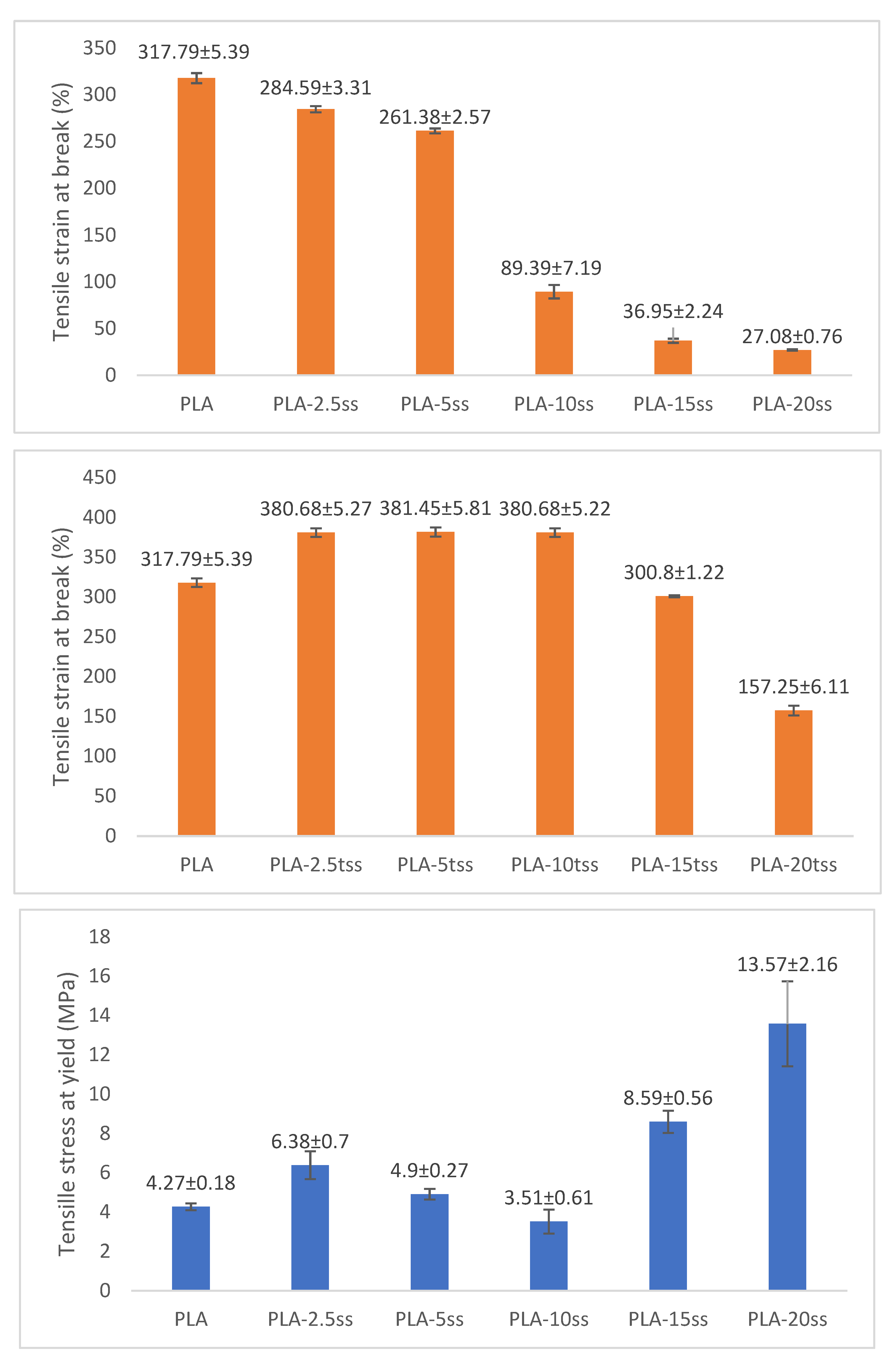
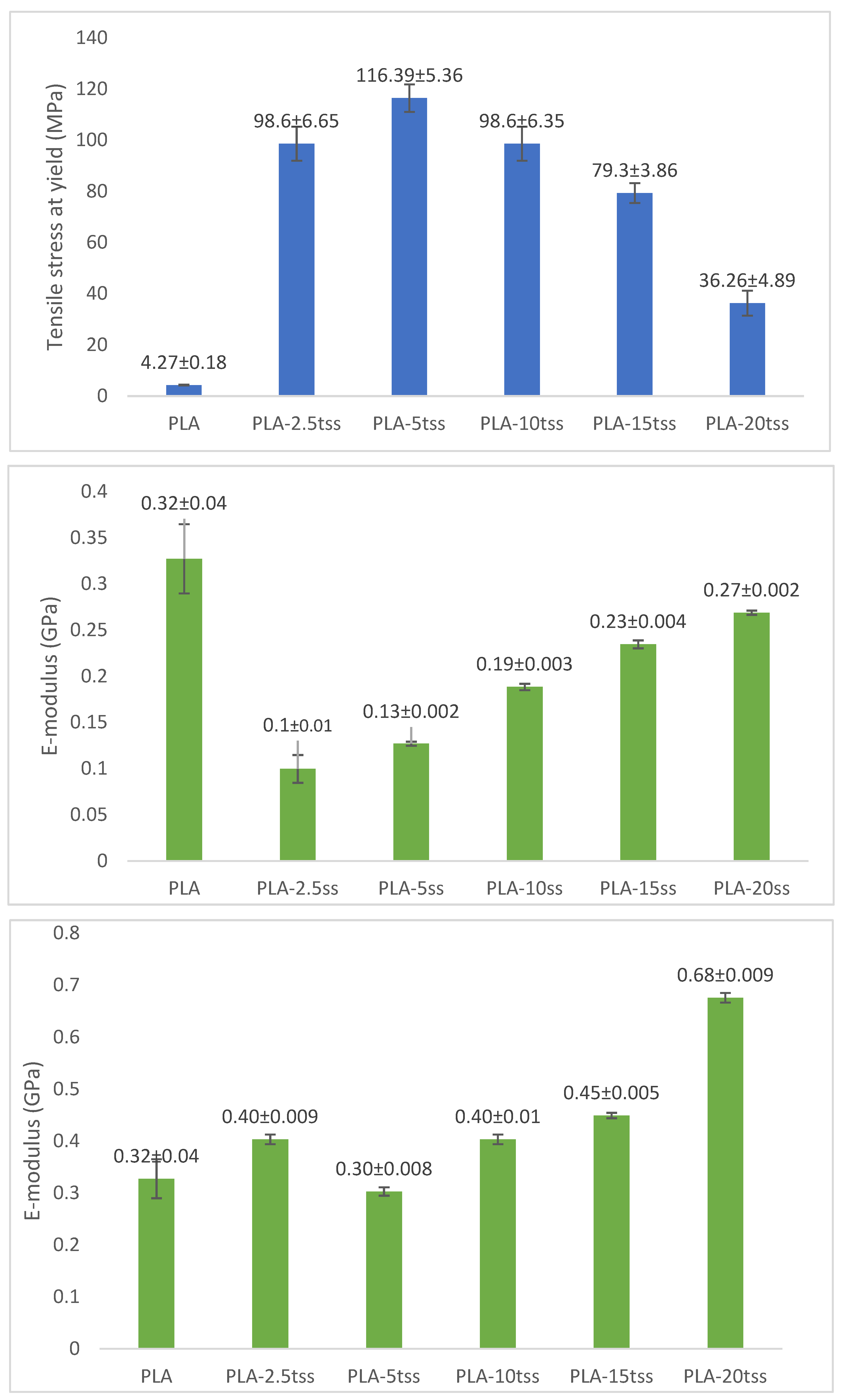
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, C.V.; Kim, Y.T. Spent Coffee Grounds and Coffee Silverskin as Potential Materials for Packaging: A Review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Pagliarini, E.; Totaro, G.; Saccani, A.; Gaggìa, F.; Lancellotti, I.; Di Gioia, D.; Sisti, L. Valorization of coffee wastes as plant growth promoter in mulching film production: A contribution to a circular economy. Sci. Total Environ. 2023, 871, 162093. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Ottedijk, R.; Meybeck, A. Global food losses and food waste: Extent, causes and prevention. In Proceedings of the Save Food, Interpack 2011, Düsseldorf, Germany, 12–18 May 2011; FAO: Rome, Italy, 2011. [Google Scholar]
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S.I. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- Sisti, L.; Celli, A.; Totaro, G.; Cinelli, P.; Signori, F.; Lazzeri, A.; Bikaki, M.; Corvini, P.; Ferri, M.; Tassoni, A.; et al. Monomers, materials and energy from coffee by-products: A review. Sustainability 2021, 13, 6921. [Google Scholar] [CrossRef]
- Besset, C.J.; Lonnecker, A.T.; Streff, J.M.; Wooley, K.L. Polycarbonates from the polyhydroxy natural product quinic acid. Biomacromolecules 2011, 12, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Vannini, M.; Sisti, L.; Marchese, P.; Ehrnell, M.; Xanthakis, E.; Celli, A.; Tassoni, A. Cascade strategies for the full valorisation of Garganega white grape pomace towards bioactive extracts and bio-based materials. PLoS ONE 2020, 15, e0239629. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential uses and applications of treated wine waste: A review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Sisti, L.; Marchese, P.; Celli, A.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silverskin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Garcia-Serna, E.; Martinez-Saez, N.; Mesias, M.; Morales, F.J.; Del Castillo, M.D. Use of coffee silverskin and stevia to improve the formulation of biscuits. Pol. J. Food Nutr. Sci. 2014, 64, 243–251. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. In Resources, Conservation and Recycling; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 128, pp. 110–117. [Google Scholar] [CrossRef]
- Sarasini, F.; Luzi, F.; Dominici, F.; Maffei, G.; Iannone, A.; Zuorro, A.; Lavecchia, R.; Torre, L.; Carbonell-Verdu, A.; Balart, R.; et al. Effect of different compatibilizers on sustainable composites based on a PHBV/PBAT matrix filled with coffee silverskin. Polymers 2018, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Totaro, G.; Sisti, L.; Fiorini, M.; Lancellotti, I.; Andreola, F.N.; Saccani, A. Formulation of Green Particulate Composites from PLA and PBS Matrix and Wastes Deriving from the Coffee Production. J. Polym. Environ. 2019, 27, 1488–1496. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Review on utilization and composition of coffee silverskin. In Food Research International; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 61, pp. 16–22. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Tech. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Conde, T.; Mussatto, S.I. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 2016, 46, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Laaziz, S.A.; Raji, M.; Hilali, E.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int. J. Biol. Macromol. 2017, 104, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.M.A.; Shams, M.S.A.; Kabir, M.R.; Gafur, M.A.; Terano, M.; Alam, M.S. Influence of chemical treatment on the properties of banana stem fiber and banana stem fiber/coir hybrid fiber reinforced maleic anhydride grafted polypropylene/low-density polyethylene composites. J. Appl. Polym. Sci. 2013, 128, 1020–1029. [Google Scholar] [CrossRef]
- Kaewkuk, S.; Sutapun, W.; Jarukumjorn, K. Effects of interfacial modification and fiber content on physical properties of sisal fiber/polypropylene composites. Compos. B Eng. 2013, 45, 544–549. [Google Scholar] [CrossRef]
- Narendar, R.; Dasan, K.P. Chemical treatments of coir pith: Morphology, chemical composition, thermal and water retention behavior. Compos. B Eng. 2014, 56, 770–779. [Google Scholar] [CrossRef]
- Senthamaraikannan, P.; Kathiresan, M. Characterization of raw and alkali treated new natural cellulosic fiber from Coccinia grandis.L. Carbohydr. Polym. 2018, 186, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S.; Al-Deyab, S.S. One-step bleaching process for cotton fabrics using activated hydrogen peroxide. Carbohydr. Polym. 2013, 92, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Essabir, H.; Raji, M.; Laaziz, S.A.; Rodrique, D.; Bouhfid, R.; el kacem Qaiss, A. Thermo-mechanical performances of polypropylene biocomposites based on untreated, treated and compatibilized spent coffee grounds. Compos. B Eng. 2018, 149, 1–11. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Ayaseh, A.; Hamishehkar, H.; Kafil, H.S.; Moghaddam, T.N.; Haghi, P.B.; Tavassoli, M.; Amjadi, S.; Lorenzo, J.M. Designing a multifunctional packaging system based on gelatin/alove vera gel film containing of rosemary essential oil and common poppy anthocyanins. Food Control 2023, 154, 110017. [Google Scholar] [CrossRef]
- Zhong, Y.; Song, X.; Li, Y. Antimicrobial, physical and mechanical properties of kudzu starch-chitosan composite films as a function of acid solvent types. Carbohydr. Polym. 2011, 84, 335–342. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA. Available online: https://cdn.standards.iteh.ai/samples/17902/67564640b6714daab5d03f5f1bc868f4/ASTM-D882-00.pdf (accessed on 8 June 2024).
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology—Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef]
- Dominici, F.; García, D.G.; Fombuena, V.; Luzi, F.; Puglia, D.; Torre, L.; Balart, R. Bio-polyethylene-based composites reinforced with alkali and palmitoyl chloride-treated coffee silverskin. Molecules 2019, 24, 3113. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Kosmela, P.; Kuzmin, A.; Piasecki, A.; Kulawik, A.; Chmielnicki, B. By-products from food industry as a promising alternative for the conventional fillers for wood–polymer composites. Polymers 2021, 13, 893. [Google Scholar] [CrossRef]
- Chattopadhyay, P.K.; Das, N.C.; Chattopadhyay, S. Influence of interfacial roughness and the hybrid filler microstructures on the properties of ternary elastomeric composites. Compos. Part. A Appl. Sci. Manuf. 2011, 42, 1049–1059. [Google Scholar] [CrossRef]
- Marghalani, H.Y. Effect of filler particles on surface roughness of experimental composite series. J. Appl. Oral Sci. 2010, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. Coffee Silverskin as a Potential Bio-Based Antioxidant for Polymer Materials: Brief Review. Proceedings 2021, 69, 20. [Google Scholar] [CrossRef]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2009, 13, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Selmin, F.; Franceschini, I.; Cupone, I.E.; Minghetti, P.; Cilurzo, F. Aminoacids as non-traditional plasticizers of maltodextrins fast-dissolving films. Carbohydr. Polym. 2015, 115, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Sarasini, F.; Tirillò, J.; Zuorro, A.; Maffei, G.; Lavecchia, R.; Puglia, D.; Dominici, F.; Luzi, F.; Valente, T.; Torre, L. Recycling coffee silverskin in sustainable composites based on a poly(butylene adipate-co-terephthalate)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) matrix. Ind. Crops Prod. 2018, 118, 311–320. [Google Scholar] [CrossRef]
- Kruszelnicka, I.; Michałkiewicz, M.; Ginter-Kramarczyk, D.; Muszyński, P.; Materna, K.; Wojcieszak, M.; Mizera, K.; Ryszkowska, J. Spent Coffee as a Composite Filler for Wastewater Treatment. Materials 2023, 16, 1181. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Damiao, A.E.; Masi, P.; Porta, R. Chitosan-whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Amena, B.T.; Altenbach, H.; Tibba, G.S.; Hossain, N. Investigation of Mechanical Properties of Coffee Husk-HDPE-ABS Polymer Composite Using Injection-Molding Method. J. Compos. Sci. 2022, 6, 354. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef]
- Escobal, A.; Iriondo, C.; Katime, I. Organic solvents adsorbed in polymeric films used in food packaging: Determination by head-space gas chromatography. Polym. Test. 1999, 18, 249–255. [Google Scholar] [CrossRef]
- Suaduang, N.; Ross, S.; Ross, G.M.; Pratumshat, S.; Mahasaranon, S. Effect of Spent Coffee Grounds Filler on the Physical and Mechanical Properties of Poly(Lactic Acid) Bio-Composite Films. Mater. Today: Proc. 2019, 17, 2104–2110. [Google Scholar] [CrossRef]
- Yang, J.; Pan, H.; Li, X.; Sun, S.; Zhang, H.; Dong, L. A study on the mechanical, thermal properties and crystallization behavior of poly(lactic acid)/thermoplastic poly(propylene carbonate) polyurethane blends. RSC Adv. 2017, 7, 46183–46194. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Kuzmin, A. Coffee silverskin as a multifunctional waste filler for high-density polyethylene green composites. J. Compos. Sci. 2021, 5, 44. [Google Scholar] [CrossRef]
- Giannakas, A. Na-montmorillonite vs. organically modified montmorillonite as essential oil nanocarriers for melt-extruded low-density poly-ethylene nanocomposite active packaging films with a controllable and long-life antioxidant activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
- De Hond, A.I.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; Del Castillo, M.D. Coffee silverskin extract protects against accelerated aging caused by oxidative agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. In Food and Function; Royal Society of Chemistry: London, UK, 2012; pp. 903–915. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef]
- Yorseng, K.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Rajulu, V. Mechanical and thermal properties of spent coffee bean filler/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biocomposites: Effect of recycling. Process Saf. Environ. Prot. 2019, 124, 187–195. [Google Scholar] [CrossRef]

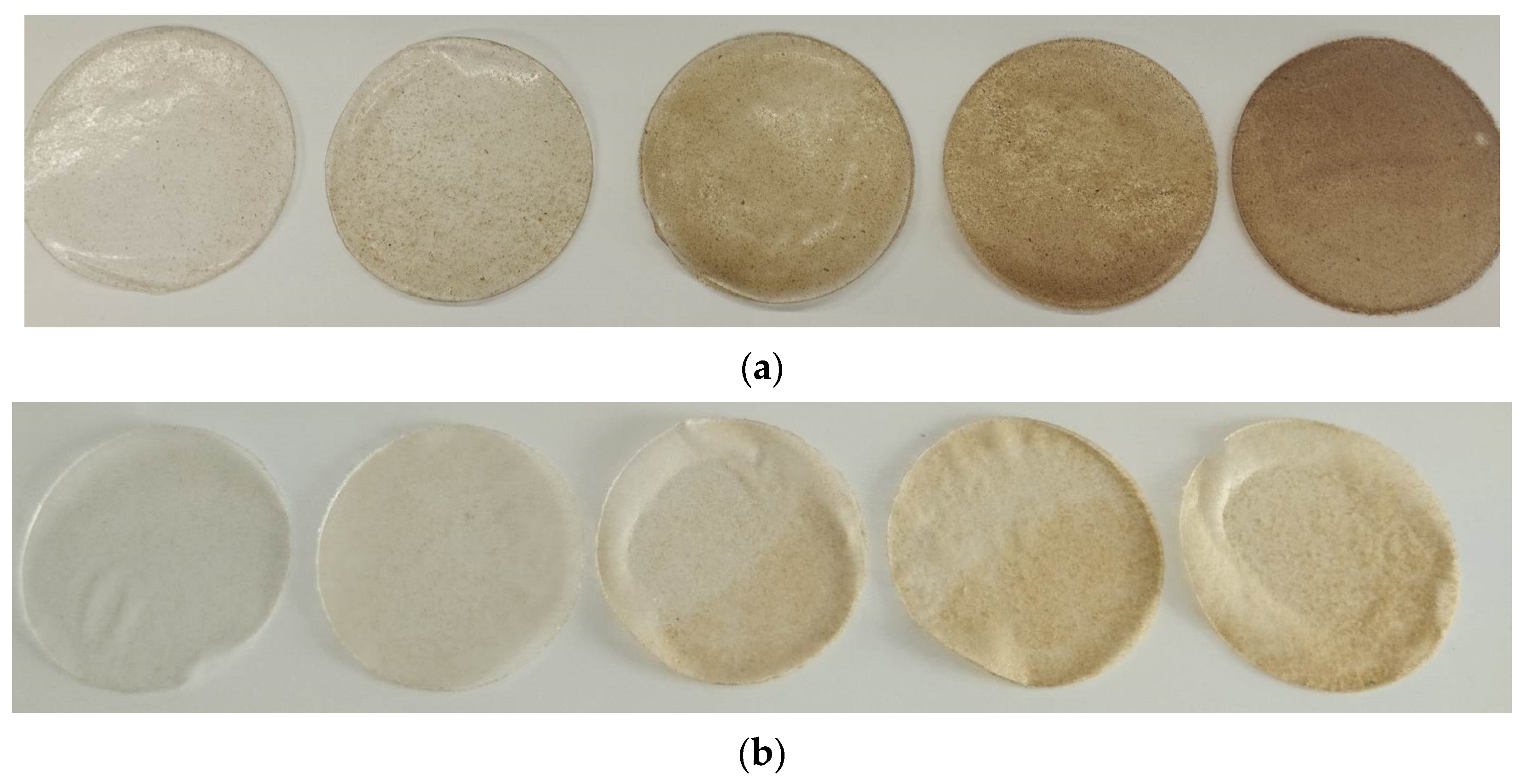
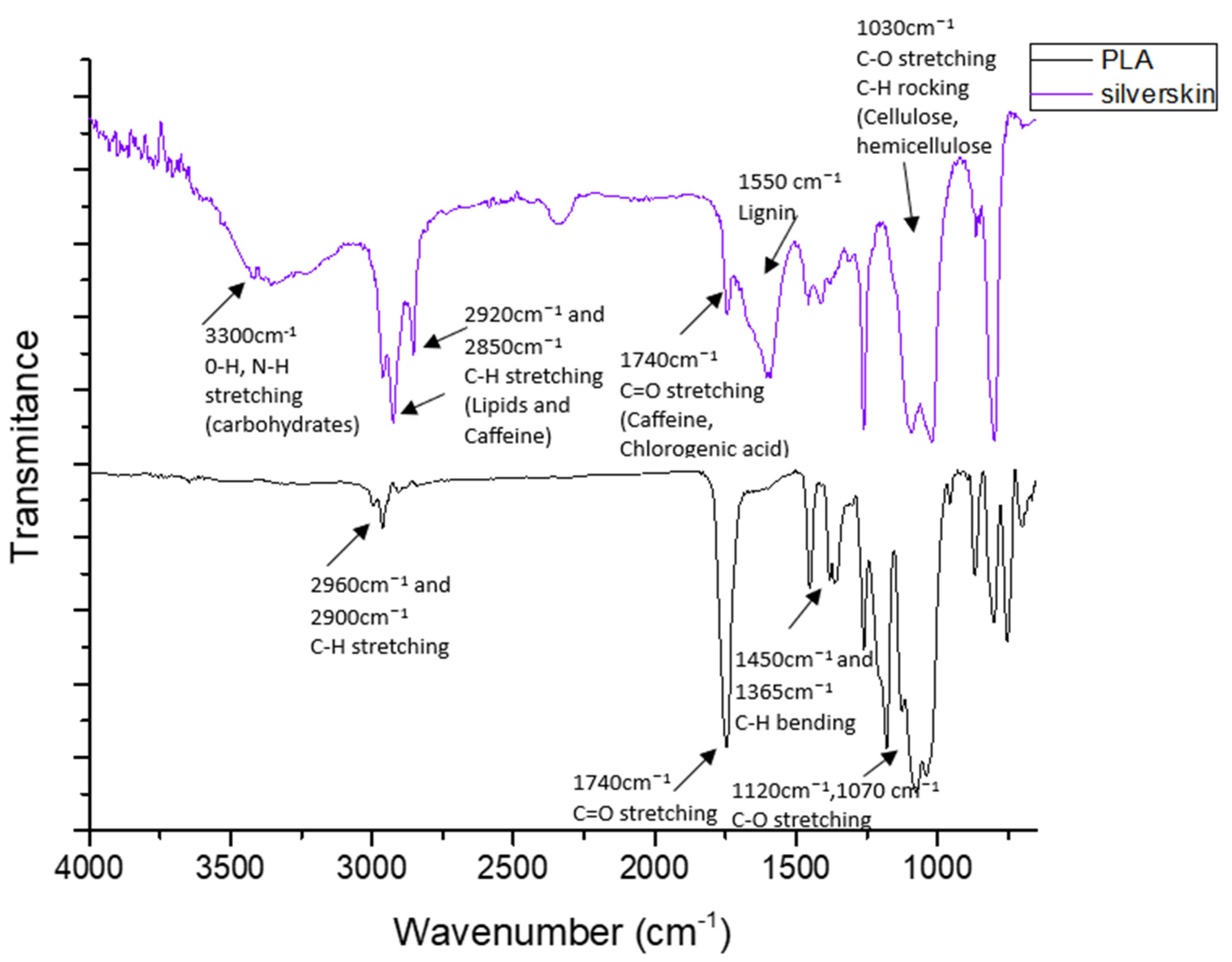


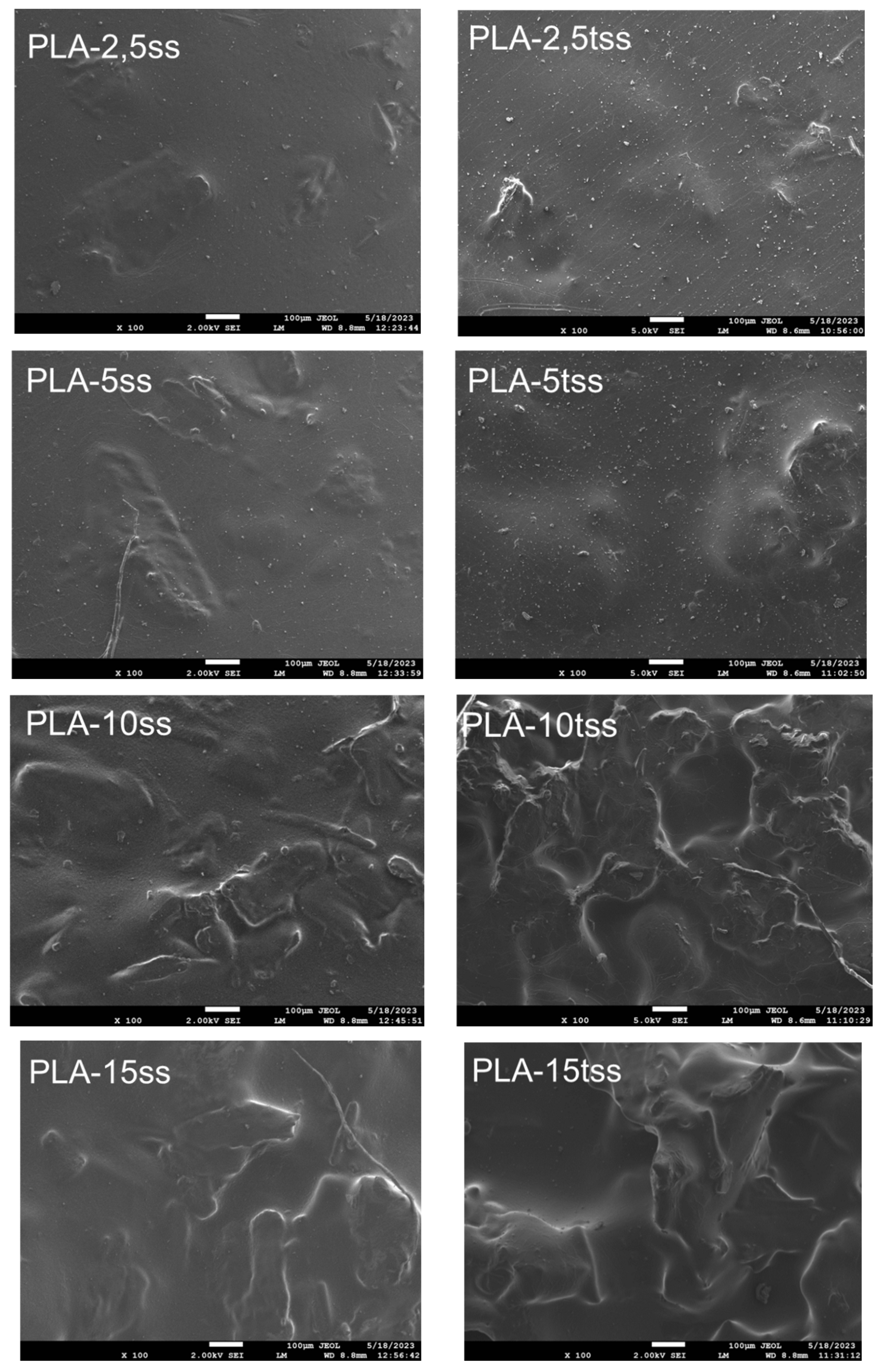
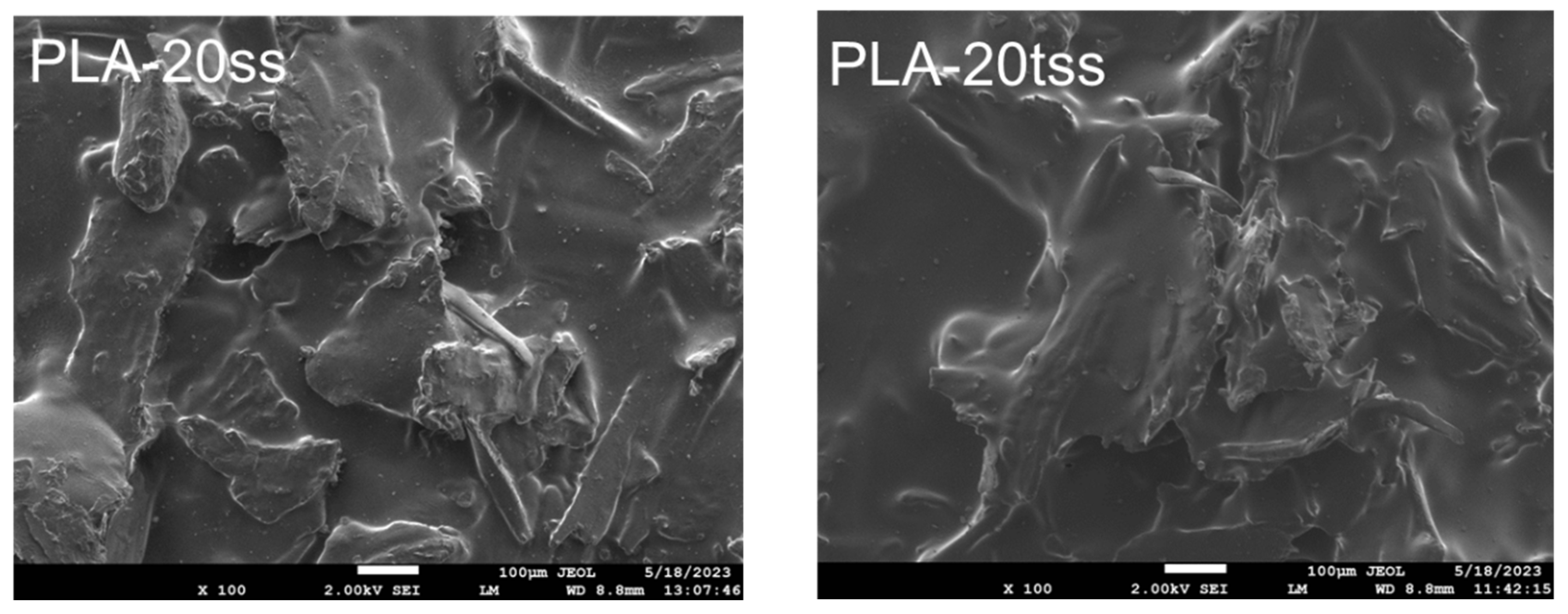

| Component | Coffee Silverskin (Content, % Dry Mass) |
|---|---|
| Cellulose | 17.9–23.8 |
| Hemicellulose | 7.5–16.9 |
| Holocellulose | 28.6–40.5 |
| Lignin | 28.6–31.0 |
| Ash | 4.5–7.6 |
| Protein | 11.8–18.7 |
| Lipids | 2.1–5.8 |
| Τhickness (μm) | Water Content (%) | Film Solubility | Swelling Degree | |
|---|---|---|---|---|
| PLA | 125 ± 10 | 7.83 ± 1.35 | 0.24 ± 0.1 | 1.17 ± 0.84 |
| PLA-2.5ss | 136 ± 15 | 8.35 ± 1.97 | 0.76 ± 0.1 | 2.29 ± 0.82 |
| PLA-5ss | 140 ± 9 | 8.38 ± 1.55 | 1.06 ± 0.16 | 2.39 ± 0.71 |
| PLA-10ss | 146 ± 15 | 8.17 ± 0.94 | 1.31 ± 0.3 | 5.26 ± 1.3 |
| PLA-15ss | 178 ± 18 | 5.29 ± 0.25 | 1.39 ± 0.4 | 6.35 ± 1.36 |
| PLA-20ss | 200 ± 14 | 7.32 ± 0.75 | 2.09 ± 0.7 | 7.29 ± 1.52 |
| PLA-2.5tss | 133 ± 12 | 7.96 ± 0.78 | 0.28 ± 0.1 | 1.69 ± 0.92 |
| PLA-5tss | 146 ± 12 | 9.67 ± 0.1 | 0.43 ± 0.25 | 2.77 ± 0.4 |
| PLA-10tss | 167 ± 13 | 9.48 ± 0.76 | 0.67 ± 0.2 | 7.22 ± 1.14 |
| PLA-15tss | 173 ± 15 | 7.09 ± 1.31 | 0.88 ± 0.27 | 9.25 ± 0.98 |
| PLA-20tss | 192 ± 19 | 6.90 ± 1.25 | 0.96 ± 0.3 | 10.46 ± 0.62 |
| WVTR (g/m2∙d) | WVP (10−7∙g/(m∙d∙Pa) | OTR [cm3/(m2∙d∙0.1 Mpa)] | |
|---|---|---|---|
| PLA | 4.35 | 2.41 | 0.0013 |
| PLA-2.5ss | 3.68 | 2.31 | 0.0015 |
| PLA-5ss | 3.86 | 2.77 | 0.0014 |
| PLA-10ss | 4.07 | 2.45 | 0.0017 |
| PLA-15ss | 4.14 | 2.60 | 0.0020 |
| PLA-20ss | 4.38 | 3.00 | 0.0012 |
| PLA-2.5tss | 4.31 | 2.21 | 0.0017 |
| PLA-5tss | 4.46 | 2.42 | 0.0015 |
| PLA-10tss | 4.99 | 2.56 | 0.0015 |
| PLA-15tss | 5.52 | 3.00 | 0.0010 |
| PLA-20tss | 6.44 | 3.49 | 0.0020 |
| L* | a* | b* | c* | h | R% (400 nm) | K/S | |
|---|---|---|---|---|---|---|---|
| PLA | 91.59 | −0.98 | 1 | 1.39 | 136.44 | 61.33 | 0.12 |
| PLA-2.5ss | 86.66 | −0.36 | 8.64 | 8.6 | 92.41 | 43.03 | 0.38 |
| PLA-5ss | 85.21 | −0.03 | 10.89 | 11.47 | 89.88 | 27.01 | 0.99 |
| PLA-10ss | 69.61 | 4.54 | 27.7 | 28.63 | 80.26 | 12.68 | 3.01 |
| PLA-15ss | 56.96 | 8.79 | 29.2 | 30.97 | 72.96 | 5.8 | 7.65 |
| PLA-20ss | 52.52 | 9.55 | 30.44 | 31.52 | 73.15 | 5.15 | 8.73 |
| PLA-2.5tss | 89.4 | −1.27 | 6.77 | 6.81 | 101.33 | 48.76 | 0.27 |
| PLA-5tss | 88.77 | −1.23 | 8.67 | 9.25 | 97.75 | 42.73 | 0.38 |
| PLA-10tss | 82.22 | 0.58 | 22.98 | 23.26 | 88.47 | 19.05 | 1.72 |
| PLA-15tss | 79.41 | 1.85 | 28.99 | 29.11 | 86.36 | 14.45 | 2.53 |
| PLA-20tss | 75.41 | 3.89 | 34.73 | 35.05 | 83.58 | 9.59 | 4.26 |
| Tg (°C) | Tm (°C) | ΔH (J/g) | |
|---|---|---|---|
| PLA | 56.29 | 146.08 | 13.720 |
| PLA-2.5ss | 57.84 | 146.41 | 17.274 |
| PLA-5ss | 58.29 | 147.60 | 13.666 |
| PLA-10ss | 57.77 | 148.96 | 3.271 |
| PLA-15ss | 58.69 | 148.96 | 1.496 |
| PLA-20ss | 58.77 | 149.13 | 0.552 |
| PLA-2.5tss | 58.89 | 146.93 | 15.151 |
| PLA-5tss | 59.29 | 146.76 | 17.315 |
| PLA-10tss | 59.23 | 147.44 | 11.243 |
| PLA-15tss | 59.59 | 147.61 | 11.801 |
| PLA-20tss | 59.91 | 148.23 | 0.561 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petaloti, A.-I.; Achilias, D.S. The Development of Sustainable Biocomposite Materials Based on Poly(lactic acid) and Silverskin, a Coffee Industry By-Product, for Food Packaging Applications. Sustainability 2024, 16, 5075. https://doi.org/10.3390/su16125075
Petaloti A-I, Achilias DS. The Development of Sustainable Biocomposite Materials Based on Poly(lactic acid) and Silverskin, a Coffee Industry By-Product, for Food Packaging Applications. Sustainability. 2024; 16(12):5075. https://doi.org/10.3390/su16125075
Chicago/Turabian StylePetaloti, Argyri-Ioanna, and Dimitris S. Achilias. 2024. "The Development of Sustainable Biocomposite Materials Based on Poly(lactic acid) and Silverskin, a Coffee Industry By-Product, for Food Packaging Applications" Sustainability 16, no. 12: 5075. https://doi.org/10.3390/su16125075






