Microscopic Mechanism and Road Performance Analysis of MgO Carbonation–Solidification of Dredged Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Testing Materials
2.2. Carbonation Device
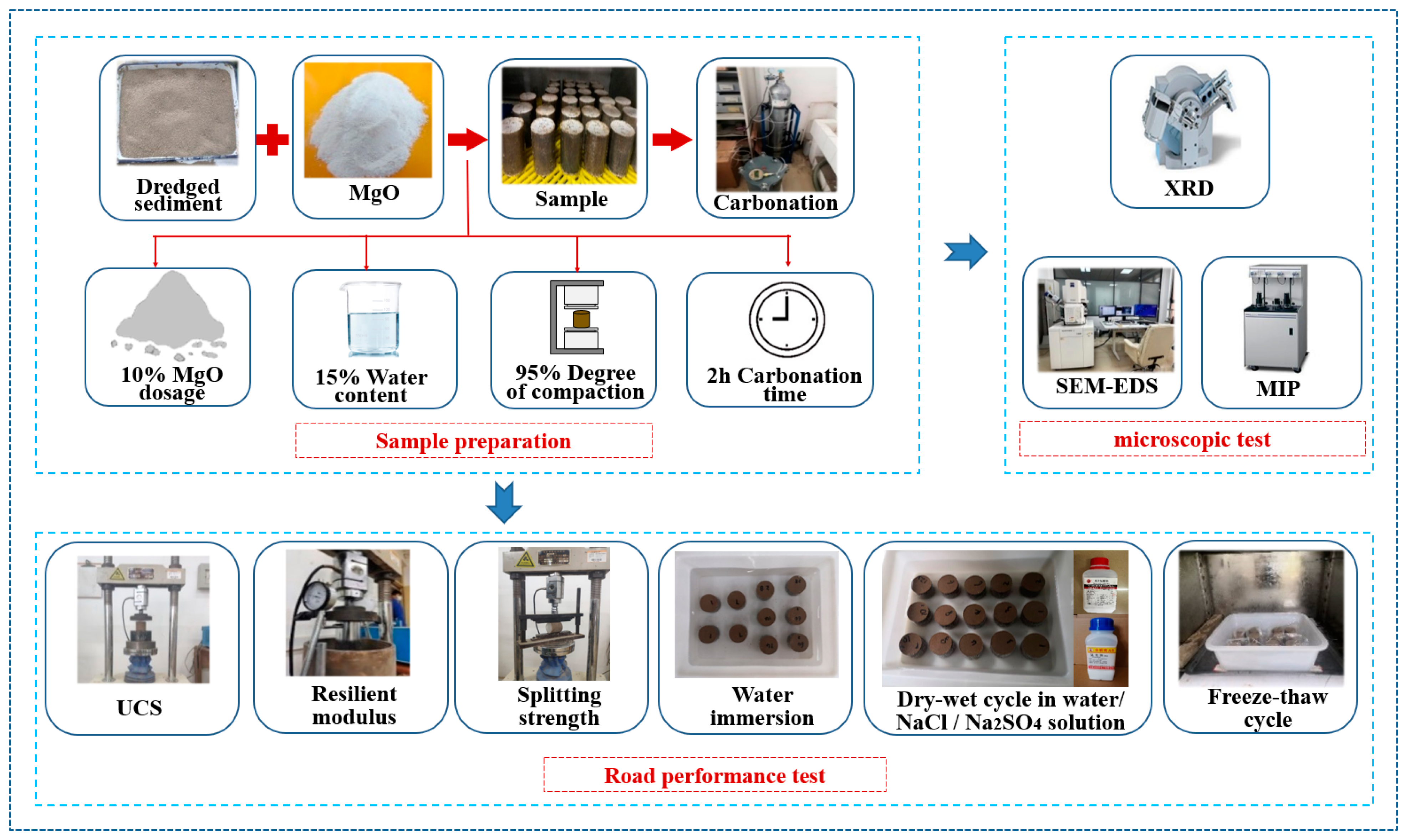
2.3. Testing Scheme and Methods
2.3.1. X-ray Diffraction (XRD) Test
2.3.2. Scanning Electron Microscope–Energy Dispersive Spectrometer (SEM-EDS) Test
2.3.3. Mercury Intrusion Porosimetry (MIP) Test
2.3.4. Unconfined Compressive Strength (UCS) Test
2.3.5. Resilient Modulus Test
2.3.6. Splitting Test
2.3.7. Immersion Test
2.3.8. Coupled Dry–Wet Cycle and Salt Solution Erosion Test
2.3.9. Freeze–Thaw Cycle Test
3. Microscopic Mechanism
3.1. Analysis of Microscopic Characteristics
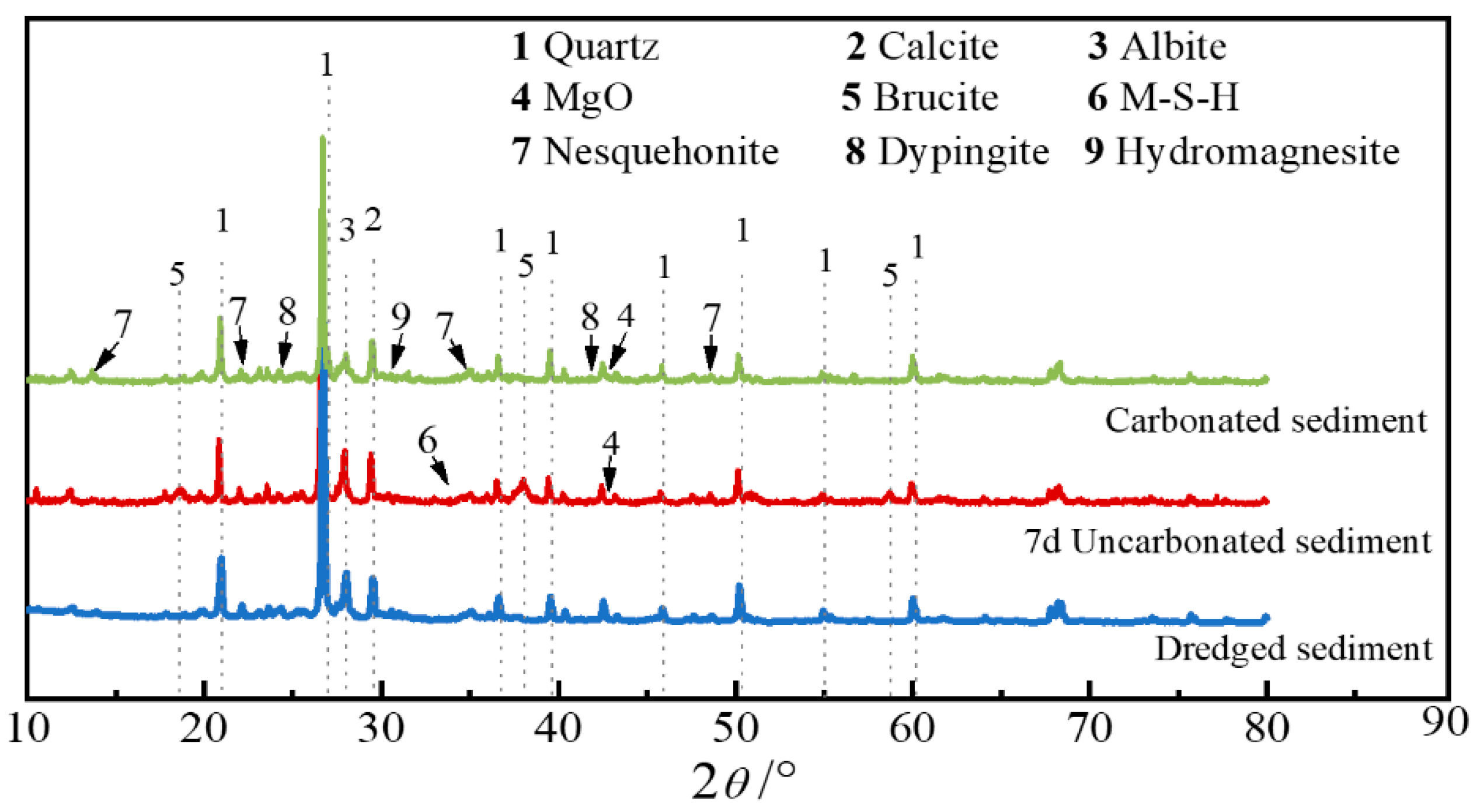
3.1.1. XRD Test Analysis
3.1.2. EDS Energy Spectrum Analysis
3.1.3. SEM Test Analysis
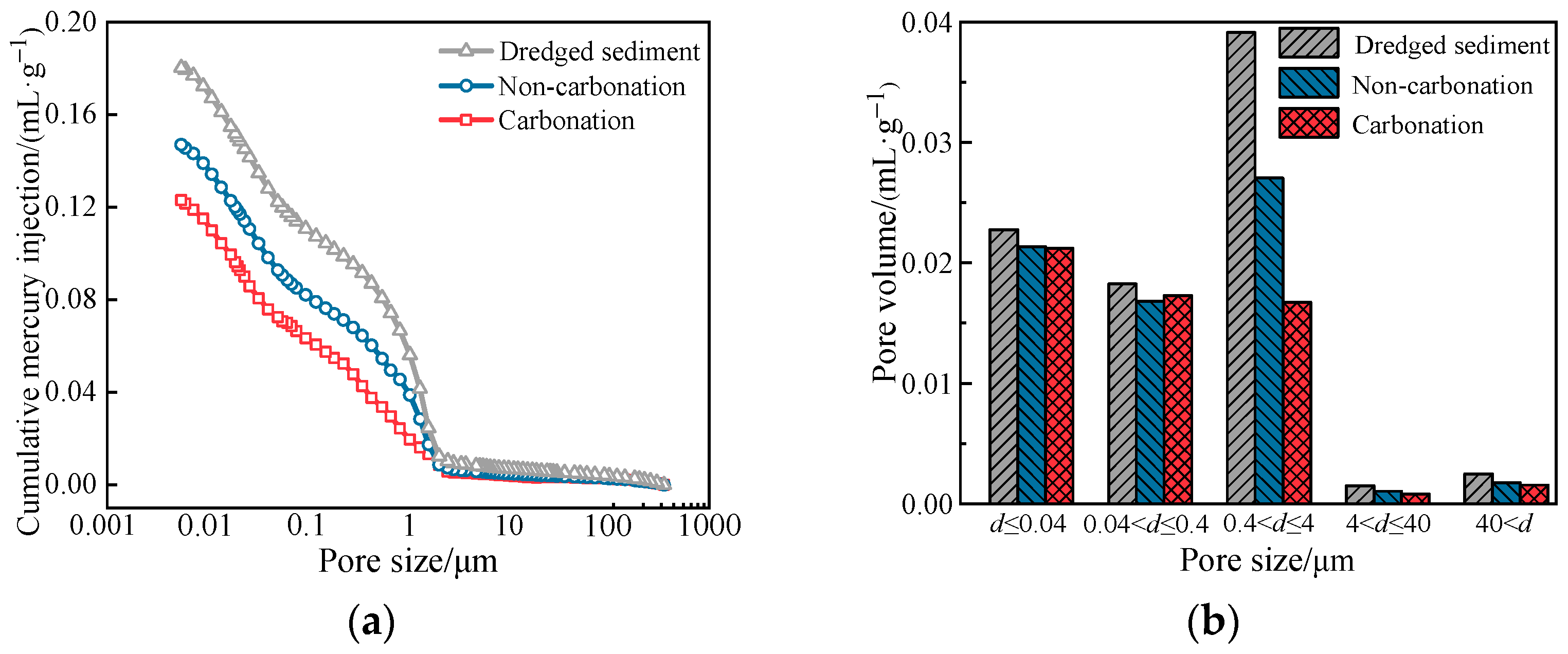
3.1.4. MIP Test Analysis
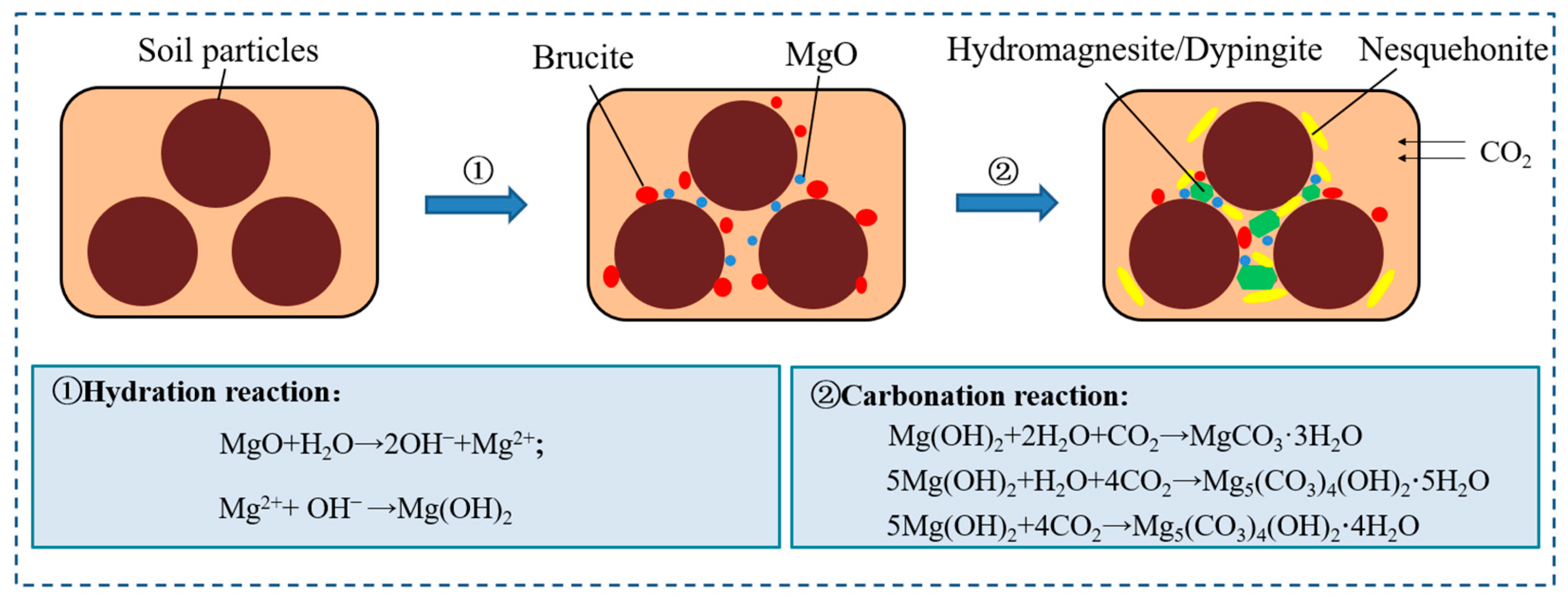
3.2. Mechanism Analysis
4. Road Performance
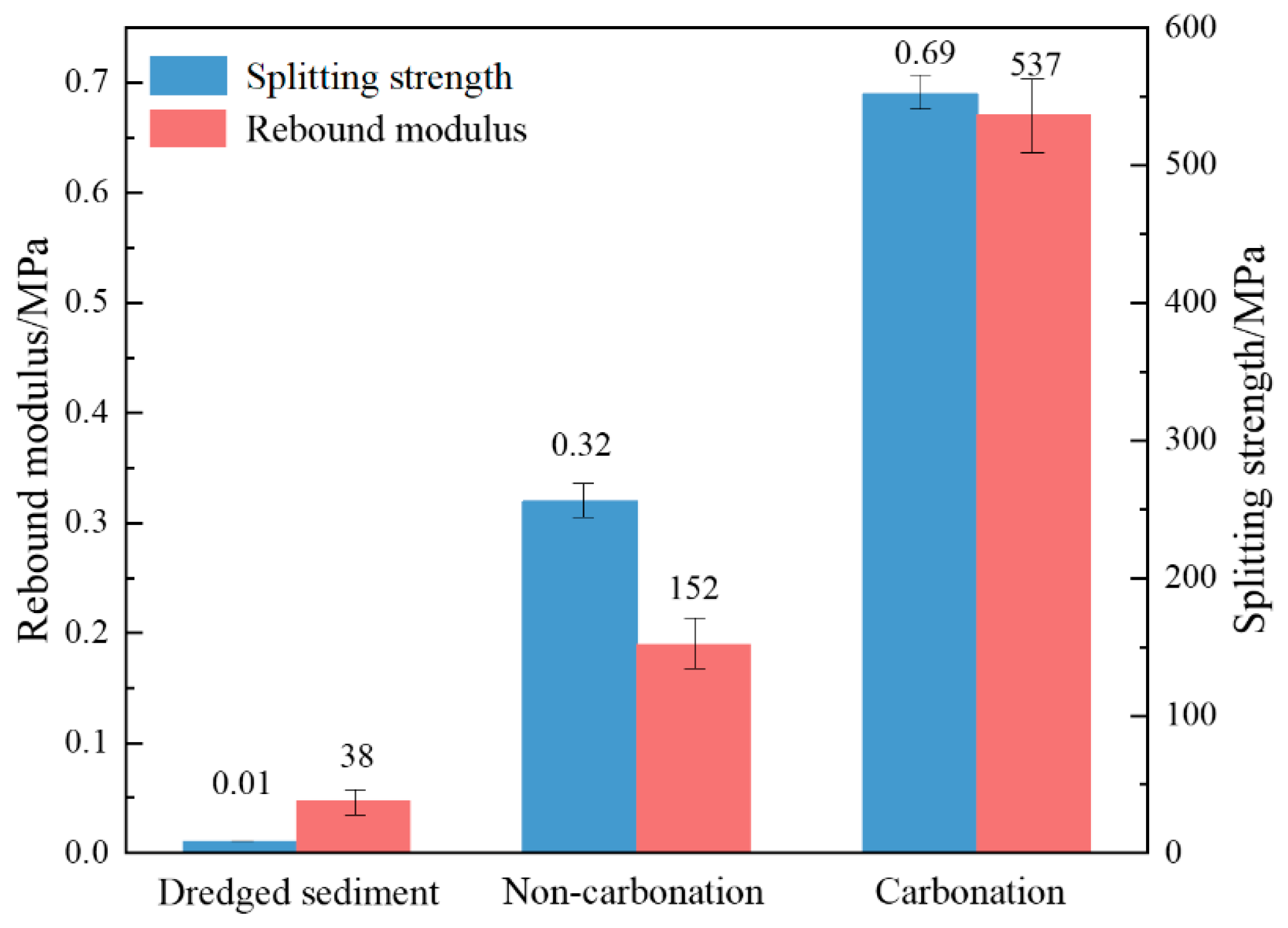
4.1. Mechanical Properties
4.2. Durability
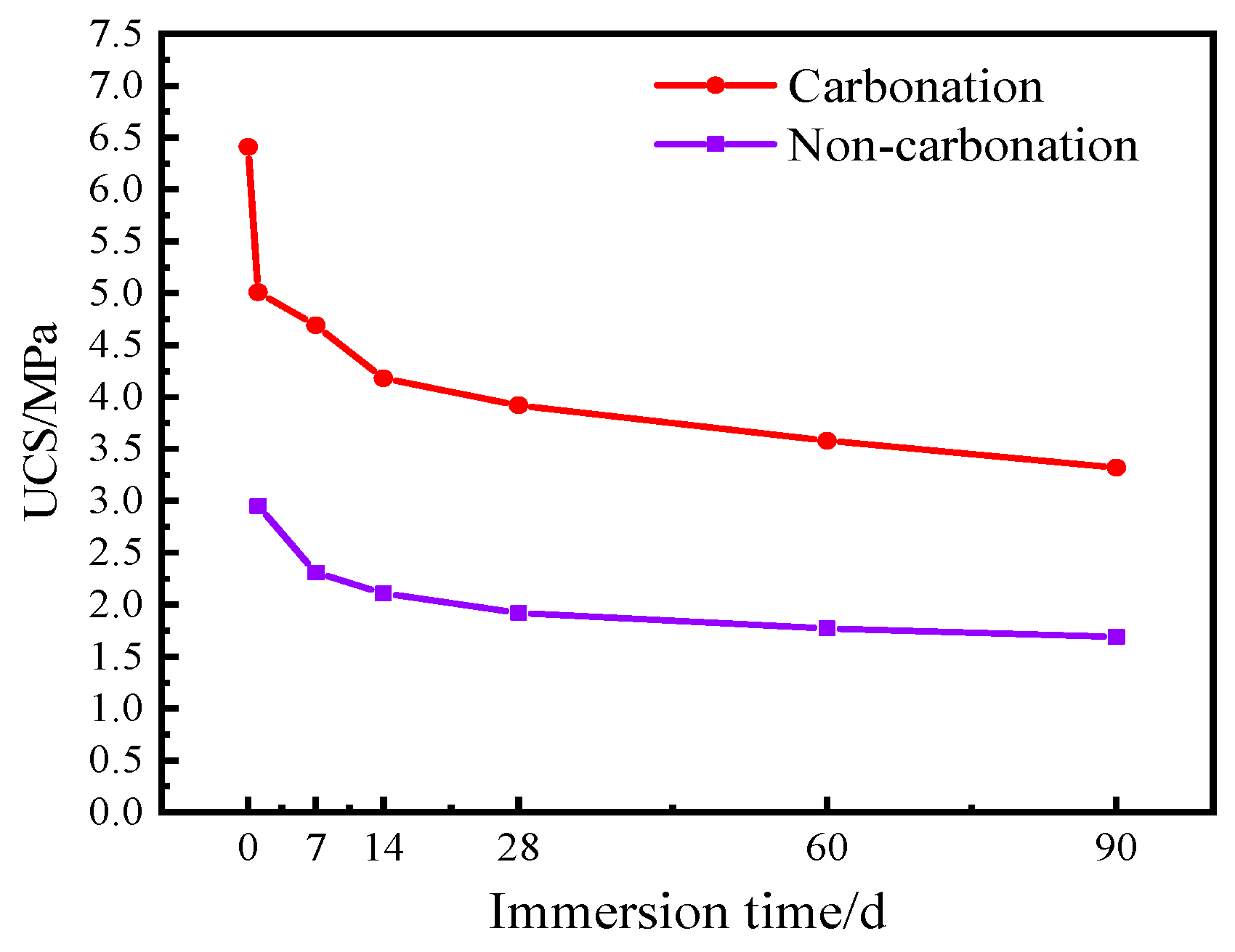
4.2.1. Immersion Test
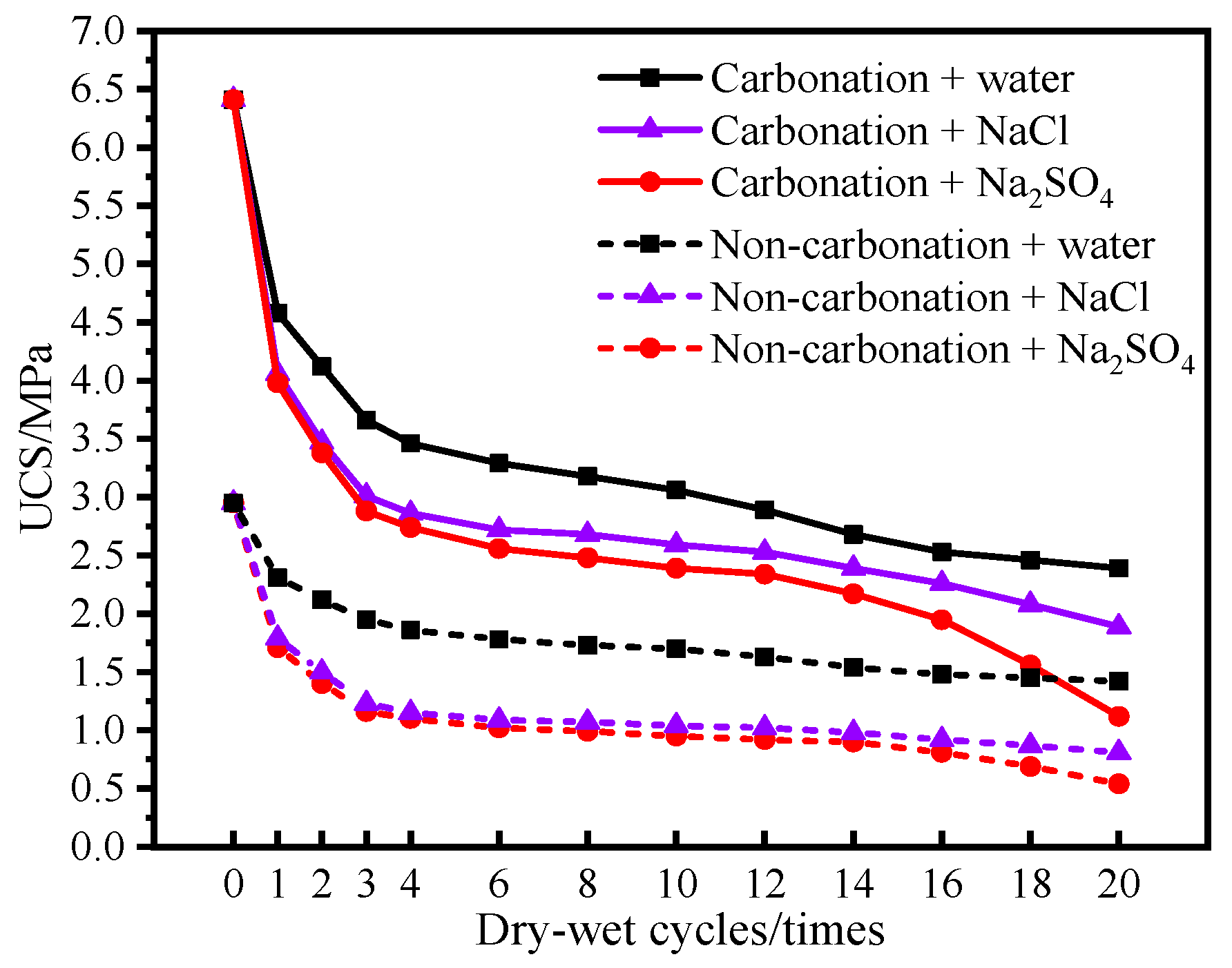
4.2.2. Coupled Dry–Wet Cycle and Salt Solution Erosion Test
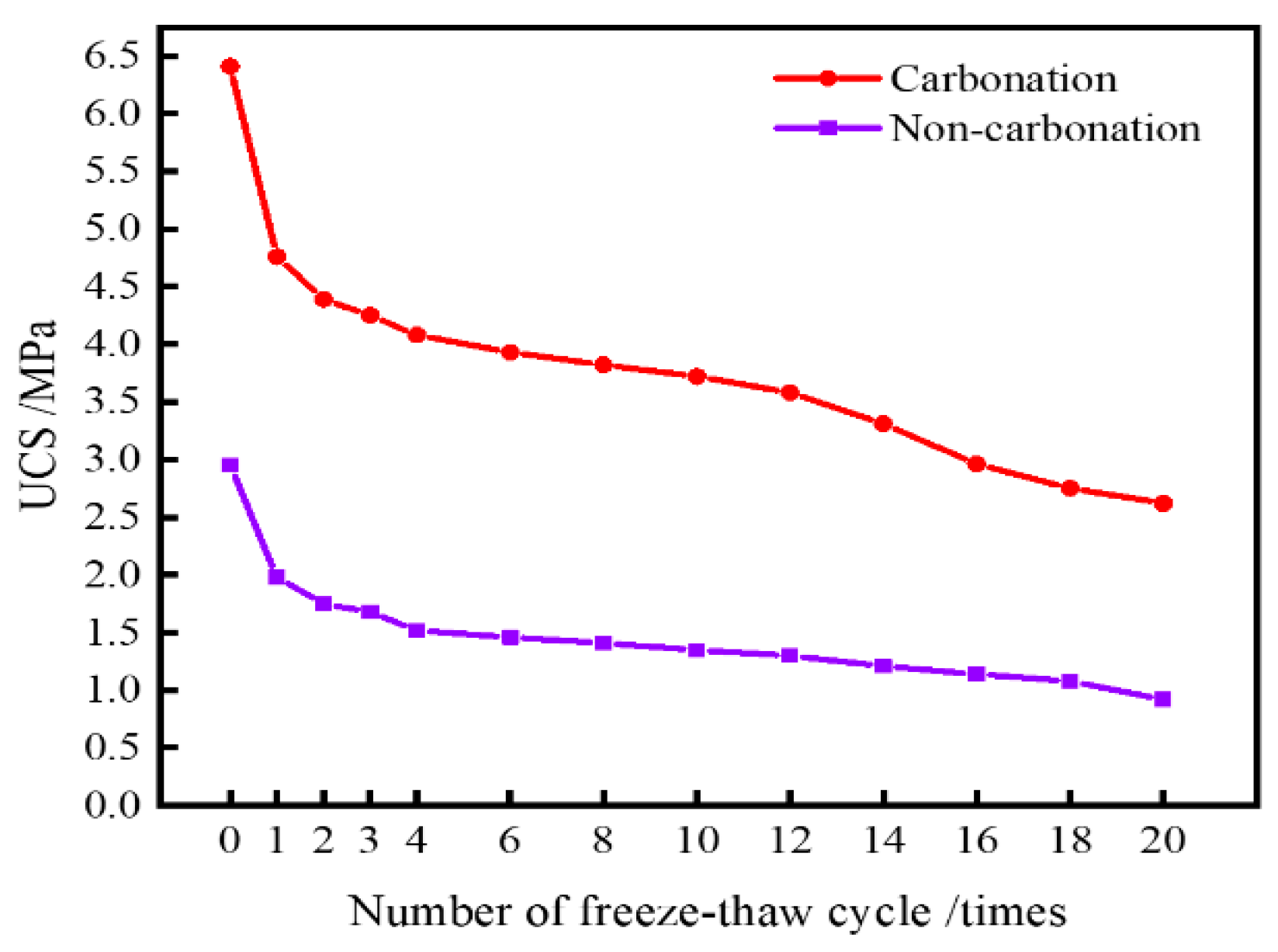
4.2.3. Freeze–Thaw Cycle Test
5. Conclusions
- (1)
- MgO carbonation has a significant improvement effect on sediment. The encapsulation, bonding, and filling effects of hydration and carbonation products can greatly reduce the pore volume in the sediment, leading to a more stable microstructural arrangement of the soil.
- (2)
- For non-carbonated sediment, the formation of hydrated products such as brucite (Mg(OH)2) and magnesium silicate hydrate (M-S-H) is the fundamental reason for strength improvement. However, for carbonated sediment, the strength improvement is mainly attributed to the formation of dypingite (Mg5(CO3)4(OH)2-5H2O), hydromagnesite (Mg5(CO3)4(OH)2-4H2O), and nesquehonite (MgCO3-3H2O) through the carbonation reaction of brucite.
- (3)
- The carbonation process has a positive impact on the mechanical properties and durability of dredged sediment. The rebound modulus and splitting strength of carbonated sediment were 3.53 times and 2.16 times that of non-carbonated sediment, respectively. For carbonized specimens, the strength was 3.32 MPa after 90 days of water saturation. The strength was 2.39 MPa, 1.89 MPa, and 1.12 MPa after 20 times of dry–wet cycles in water, NaCl, and Na2SO4 solutions, respectively. The strength was 2.62 MPa after 20 times of freeze–thaw cycles. It can be seen that carbonation is beneficial for improving the road performance of sediment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Svensson, N.; Noren, A.; Modin, O.; Fedje, K.; Rauch, S.; Stromvall, A.; Andersson-Skold, Y. Integrated cost and environmental impact assessment of management options for dredged sediment. Waste Manag. 2022, 138, 30–40. [Google Scholar] [CrossRef]
- Bianchini, A.; Cento, F.; Guzzini, A.; Pellegrini, M.; Saccani, C. Sediment management in coastal infrastructures: Techno-economic and environmental impact assessment of alternative technologies to dredging. J. Environ. Manag. 2019, 248, 109332. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Z.; Liang, Y.; Wang, X.; Liu, M. Experimental study on solidified dredged sediment with MgO and industrial waste residue. Constr. Build. Mater. 2023, 366, 130105. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N. From dredged sediment to supplementary cementitious material: Characterization, treatment, and reuse. Int. J. Sedim. Res. 2021, 36, 92–109. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Pandita, S.; Bhardwaj, R.; Thukral, A.; Cerda, A. A review of ecological risk assessment and associated health risks with heavy metals in sediment from India. Int. J. Sedim. Res. 2020, 35, 516–526. [Google Scholar] [CrossRef]
- Zhang, Y.; Labianca, C.; Chen, L.; Gisi, S.; Notarnicola, M.; Guo, B.; Sun, J.; Ding, S.; Wang, L. Sustainable ex-situ remediation of contaminated sediment: A review. Environ. Pollut. 2021, 287, 117333. [Google Scholar] [CrossRef]
- Wang, D.; Abriak, N.; Zentar, R. Dredged marine sediment used as novel supply of filling materials for road construction. Mar. Georesour. Geotechnol. 2017, 35, 472–480. [Google Scholar] [CrossRef]
- Chen, P.; Feng, B.; Lin, Y.; Lin, C. Solidification and stabilization of sewage sludge and MSWI bottom ash for beneficial use as construction materials. J. Mater. Civ. Eng. 2019, 31, 04018351. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Y.; Chen, L.; Cheng, X.; Chen, G. Experimental and field study on treatment waste mud by in situ solidification. Munic. Eng. 2020, 173, 237–245. [Google Scholar] [CrossRef]
- Lang, L.; Liu, N.; Chen, B. Strength development of solidified dredged sludge containing humic acid with cement, lime and nano-SiO2. Constr. Build. Mater. 2020, 230, 116971. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Cai, G.; Lang, L.; Ma, H.; Gong, S.; Niu, Z. Investigation on water transformation and pore structure of cement-stabilized dredged sediment based on NMR technology. Materials 2022, 15, 3178. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, C.; Yang, K.; Ni, J.; Hai, J.; Gao, S. Effects of fly ash and slag content on the solidification of river-dredged sludge. Mar. Georesour. Geotechnol. 2021, 39, 65–73. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, Y.; Chen, G.; Li, B. Characterization and prediction for the strength development of cement stabilized dredged sediment. Mar. Georesour. Geotechnol. 2021, 39, 1015–1024. [Google Scholar] [CrossRef]
- Zentar, R.; Wang, H.; Wang, D. Comparative study of stabilization/solidification of dredged sediments with ordinary Portland cement and calcium sulfo-aluminate cement in the framework of valorization in road construction material. Constr. Build. Mater. 2021, 279, 122447. [Google Scholar] [CrossRef]
- Singh, R.; Budarayavalasa, S. Solidification and stabilization of hazardous wastes using geopolymers as sustainable binders. J. Mater. Cycles Waste 2021, 23, 1699–1725. [Google Scholar] [CrossRef]
- Shah, V.; Scott, A. Hydration and microstructural characteristics of MgO in the presence of metakaolin and silica fume. Cem. Concr. Compos. 2021, 121, 104068. [Google Scholar] [CrossRef]
- Yi, Y.; Lu, K.; Liu, S.; Al-Tabbaa, A. Property changes of reactive magnesia–stabilized soil subjected to forced carbonation. Can. Geotech. J. 2016, 53, 314–325. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Alavi, M.; Morsali, A. Syntheses and characterization of Mg(OH)2 and MgO nanostructures by ultrasonic method. Ultrason. Sonochem. 2010, 17, 441–446. [Google Scholar] [CrossRef]
- Harrison, J. New cements based on the addition of reactive magnesia to Portland cement with or without added pozzolan. In Proceedings of the CIA Conference: Concrete in the Third Millennium, CIA, Brisbane, Australia, 17–19 July 2003. [Google Scholar]
- Harrison, J. Reactive Magnesium Oxide Cements. U.S. Patent US2003041785, 6 March 2003. [Google Scholar]
- Yao, K.; Wang, W.; Li, N.; Zhang, C.; Wang, L. Investigation on Strength and Microstructure Characteristics of Nano-MgO Admixed with Cemented Soft Soil. Constr. Build. Mater. 2019, 206, 160–168. [Google Scholar] [CrossRef]
- Cai, G.; Du, Y.; Liu, S.; Singh, D. Physical properties, electrical resistivity, and strength characteristics of carbonated silty soil admixed with reactive magnesia. Can. Geotech. J. 2015, 52, 1699–1713. [Google Scholar] [CrossRef]
- Seo, J.; Yoon, H.; Kim, S.; Wang, Z.; Kil, T.; Lee, H. Characterization of reactive MgO-modified calcium sulfoaluminate cements upon carbonation. Cem. Concr. Res. 2021, 146, 106484. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Hou, G. Strength and microstructure of CO2 cured low-calcium clinker. Constr. Build. Mater. 2018, 188, 417–423. [Google Scholar] [CrossRef]
- Olajire, A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Rahmani, O. Siderite precipitation using by-product red gypsum for CO2 sequestration. J. CO2 Util. 2018, 24, 321–327. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, T.; Guo, R. Sustainable building material from CO2 mineralization slag: Aggregate for concretes and effect of CO2 curing. J. CO2 Util. 2020, 40, 101196. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C.; Mo, K.H. CO2 sequestration of fresh concrete slurry waste: Optimization of CO2 uptake and feasible use as a potential cement binder. J. CO2 Util. 2020, 42, 101330. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y. Stabilization/solidification of lead- and zinc-contaminated soils using MgO and CO2. J. CO2 Util. 2019, 33, 215–221. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Kalinowska-Wichrowska, K.; Fernandez, J.; Jimenez, J. Accelerated carbonation of fresh cement-based products containing recycled masonry aggregates for CO2 sequestration. J. CO2 Util. 2021, 46, 101461. [Google Scholar] [CrossRef]
- Li, Z.; He, Z.; Chen, X. The performance of carbonation-cured concrete. Materials 2019, 12, 3729. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Naqi, A.; Jang, J. Influence of water to cement ratio on CO2 uptake capacity of belite-rich cement upon exposure to carbonation curing. Cem. Concr. Compos. 2020, 111, 103616. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Cao, Z.; Ling, T.; Zheng, J. Performance of mortar prepared with recycled concrete aggregate enhanced by CO2 and pozzolan slurry. Cem. Concr. Compos. 2018, 86, 130–138. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Li, J.; Tao, F.; Li, C.; Qian, B.; Jiang, P. Influence of Carbonation Process on the Mechanical Properties of Nano-MgO Modified Cement Soil. Sustainability 2021, 13, 3558. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Unluer, C.; Al-Tabbaa, A. Carbonating magnesia for soil stabilization. Can. Geotech. J. 2013, 50, 899–905. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, J.; He, F.; Zhou, Y. Durability evolution and associated micro-mechanisms of carbonated reactive MgO-fly ash solidified sludge from East Lake, China. Constr. Build. Mater. 2019, 208, 1–12. [Google Scholar] [CrossRef]
- Liu, S.; Cai, G.; Cao, J.; Wang, F. Influence of soil type on strength and microstructure of carbonated reactive magnesia-treated soil. Eur. J. Environ. Civ. Eng. 2020, 24, 248–266. [Google Scholar] [CrossRef]
- Dung, N.; Unluer, C. Performance of reactive MgO concrete under increased CO2 dissolution. Cem. Concr. Res. 2019, 118, 92–101. [Google Scholar] [CrossRef]
- Yang, Y.; Ruan, S.; Wu, S.; Chu, J.; Unluer, C.; Liu, H.; Cheng, L. Biocarbonation of reactive magnesia for soil improvement. Acta Geotech. 2020, 16, 1113–1125. [Google Scholar] [CrossRef]
- Xiao, X.; Goh, L.; Unluer, C.; Yang, E. Bacteria-induced internal carbonation of reactive magnesia cement. Constr. Build. Mater. 2021, 267, 121748. [Google Scholar] [CrossRef]
- Liska, M.; AI-Tabbaa, A. Performance of magnesia cements in porous blocks in acid and magnesium environments. Adv. Cem. Res. 2012, 24, 221–232. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Wu, K.; Yuan, Z. Experimental investigation into the self-carbonation mechanism of magnesium oxide carbon sequestration foamed concrete. Cem. Concr. Compos. 2024, 148, 105486. [Google Scholar] [CrossRef]





| Properties | Value |
|---|---|
| Gs | 2.62 |
| Liquid limit (%) | 29.3 |
| Plastic limit (%) | 22.9 |
| Plasticity index (%) | 6.4 |
| Fine-grained group (<0.075 mm) (%) | 23.6 |
| Sand group (0.075–2 mm) (%) | 62.7 |
| Fine gravel group (2–5 mm) (%) | 13.7 |
| Maximum dry density (g/cm³) | 1.93 |
| Optimum water content (%) | 14.4 |
| Unconfined compression strength (kPa) | 392.4 |
| CBR (%) | 9.6 |
| Resilient modulus (MPa) | 38 |
| Composition | MgO | CaO | Fe2O3 | Al2O3 | LOI |
|---|---|---|---|---|---|
| Content (%) | 96.87 | 1.50 | 0.09 | 0.08 | 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, X.; Wang, X.; Zhang, Z.; Sun, A.; Yang, L.; Zhang, F.; Xie, B.; Li, Y. Microscopic Mechanism and Road Performance Analysis of MgO Carbonation–Solidification of Dredged Sediment. Sustainability 2024, 16, 5097. https://doi.org/10.3390/su16125097
Kong X, Wang X, Zhang Z, Sun A, Yang L, Zhang F, Xie B, Li Y. Microscopic Mechanism and Road Performance Analysis of MgO Carbonation–Solidification of Dredged Sediment. Sustainability. 2024; 16(12):5097. https://doi.org/10.3390/su16125097
Chicago/Turabian StyleKong, Xianghui, Xiaokang Wang, Zhibin Zhang, Aoqi Sun, Lei Yang, Fengrong Zhang, Bingquan Xie, and Yutong Li. 2024. "Microscopic Mechanism and Road Performance Analysis of MgO Carbonation–Solidification of Dredged Sediment" Sustainability 16, no. 12: 5097. https://doi.org/10.3390/su16125097
APA StyleKong, X., Wang, X., Zhang, Z., Sun, A., Yang, L., Zhang, F., Xie, B., & Li, Y. (2024). Microscopic Mechanism and Road Performance Analysis of MgO Carbonation–Solidification of Dredged Sediment. Sustainability, 16(12), 5097. https://doi.org/10.3390/su16125097






