Experimental Combustion of Different Biomass Wastes, Coals and Two Fuel Mixtures on a Fire Bench
Abstract
1. Introduction
- Reduction of SOx and partly NOx emissions depending on the biomass type;
- Reduction of coal consumption;
- Low cost of biomass waste, depending only on the distance of its delivery to the boiler house;
- Utilization of biomass waste;
- Reduction of carbon dioxide emissions due to the fact that biomass is carbon neutral;
- No need for high costs for reconstruction of boiler equipment;
- Possibility of switching quickly back to coal combustion in case of interruptions or lack of supply of biomass waste;
- Biomass is a highly reactive fuel.
2. Materials and Methods
2.1. Fuels
2.2. Methodology of Experiments on the Firing Bench
2.3. Analysis of Fuel Particle Surface
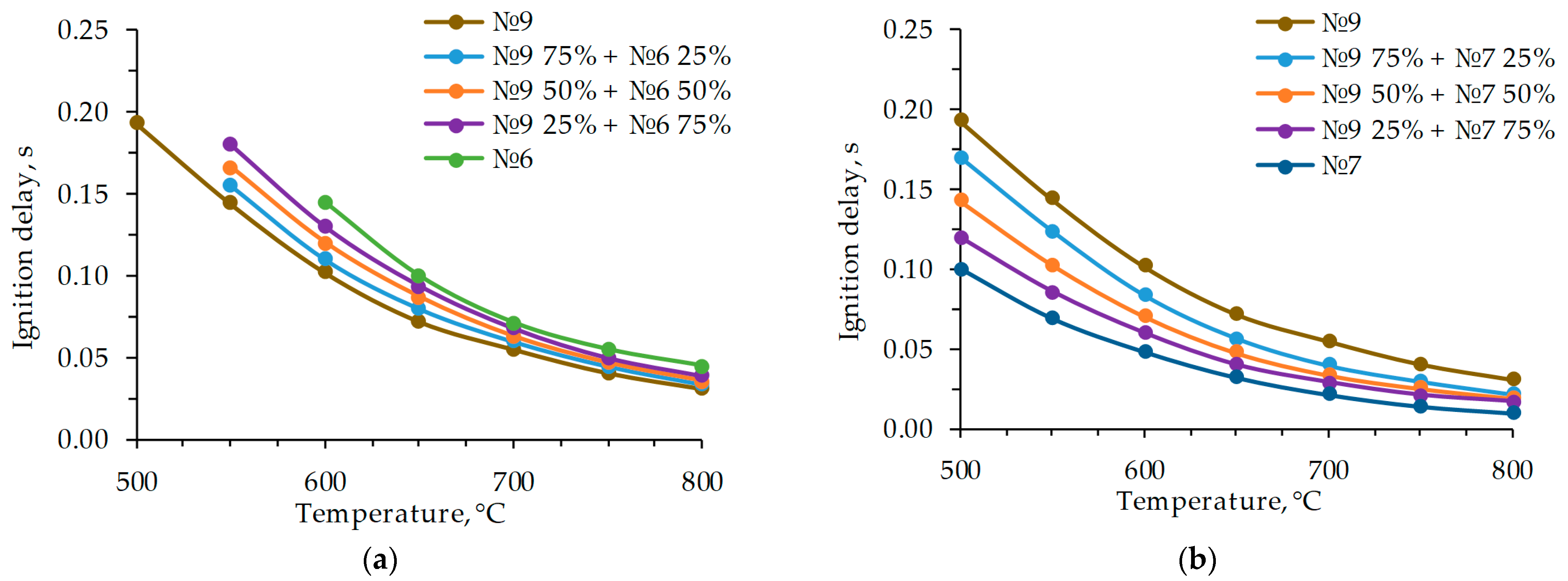
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Ad | ash in a dry state (%) |
| Cdaf, Hdaf, Ndaf, Odaf, Sdaf | fraction of carbon, hydrogen, nitrogen, oxygen and sulfur converted to a dry, ash-free state (%) |
| Dp | particle diameter (micron) |
| Ga | gas-air flow velocity (m/s) |
| Gp | fuel particle velocity (m/s) |
| HHV | higher heating value (MJ/kg) |
| L | length (m) |
| MC | moisture content (%) |
| Re | Reynolds number |
| Tg | heated air temperature (°C) |
| t | time (s) |
| td | ignition delay time (s) |
| Δt | time step (s) |
| VCdaf | volatile content converted to a dry, ash-free state (%) |
| ρa | air density (kg/m3) |
| ρp | particle density (kg/m3) |
| µa | dynamic viscosity of air (Pa·s) |
| ν | dimensionless velocity |
| ν0 | initial dimensionless velocity |
| τ | non-dimensional time |
References
- Qiang, G.; Tang, S.; Hao, J.; Di Sarno, L.; Wu, G.; Ren, S. Building automation systems for energy and comfort management in green buildings: A critical review and future directions. Renew. Sustain. Energy Rev. 2023, 179, 113301. [Google Scholar] [CrossRef]
- Wang, W.; Yang, H.; Xiang, C. Green roofs and facades with integrated photovoltaic system for zero energy eco-friendly building—A review. Sustain. Energy Technol. Assess. 2023, 60, 103426. [Google Scholar] [CrossRef]
- Chicherin, S.; Zhuikov, A.; Junussova, L. Factors Affecting Indoor Temperature in the Case of District Heating. Sustainability 2023, 15, 15603. [Google Scholar] [CrossRef]
- Dang, L.M.; Nguyen, L.Q.; Nam, J.; Nguyen, T.N.; Lee, S.; Song, H.-K.; Moon, H. Fifth generation district heating and cooling: A comprehensive survey. Energy Rep. 2024, 11, 1723–1741. [Google Scholar] [CrossRef]
- Chicherin, S.; Zhuikov, A.; Junussova, L. Integrating a heat pump into a 4th generation district heating (4GDH) system—Two-mode configuration inputting operational data. Energy Build. 2022, 275, 112445. [Google Scholar] [CrossRef]
- Sorknæs, P.; Østergaard, P.A.; Thellufsen, J.Z.; Lund, H.; Nielsen, S.; Djørup, S.; Sperling, K. The benefits of 4th generation district heating in a 100% renewable energy system. Energy 2020, 213, 119030. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal solid waste management and waste-to-energy in the context of a circular economy and energy recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Thomsen, T.P.; Henriksen, U.; Clausen, L.R. Biomass gasification cogeneration—A review of state of the art technology and near future perspectives. Appl. Therm. Eng. 2013, 50, 1407–1417. [Google Scholar] [CrossRef]
- Shah, A.V.; Srivastava, V.K.; Mohanty, S.S.; Varjani, S. Municipal solid waste as a sustainable resource for energy production: State-of-the-art review. J. Environ. Chem. Eng. 2021, 9, 105717. [Google Scholar] [CrossRef]
- Biancini, G.; Cioccolanti, L.; Moradi, R.; Moglie, M. Comparative study of steam, organic Rankine cycle and supercritical CO2 power plants integrated with residual municipal solid waste gasification for district heating and cooling. Appl. Therm. Eng. 2024, 241, 122437. [Google Scholar] [CrossRef]
- Tang, C.; Pan, W.; Zhang, J.; Wang, W.; Sun, X. A comprehensive review on efficient utilization methods of High-alkali coals combustion in boilers. Fuel 2022, 316, 123269. [Google Scholar] [CrossRef]
- Kumar, V.; Saxena, V.K.; Kumar, R.; Kumar, S. Energy, exergy, sustainability and environmental emission analysis of coal-fired thermal power plant. Ain Shams Eng. J. 2024, 15, 102416. [Google Scholar] [CrossRef]
- Vig, N.; Ravindra, K.; Mor, S. Environmental impacts of Indian coal thermal power plants and associated human health risk to the nearby residential communities: A potential review. Chemosphere 2023, 341, 140103. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Goldfarb, J.L.; Song, J.; Chang, C.; Ma, Q. Enhancing cleaner biomass-coal co-combustion by pretreatment of wheat straw via washing versus hydrothermal carbonization. J. Clean. Prod. 2022, 366, 132991. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Ling, P.; An, X.; Han, H.; Chen, Y.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; et al. A study on the release characteristics and formation mechanism of SO2 during co-combustion of sewage sludge and coal slime. Fuel 2023, 333, 126511. [Google Scholar] [CrossRef]
- Sahu, S.G.; Chakraborty, N.; Sarkar, P. Coal-biomass co-combustion: An overview. Renew. Sustain. Energy Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Vassilev, S.V.; He, C.; Vassileva, C.G.; Wei, Y. Migration behavior of chlorine and sulfur during gasification and combustion of biomass and coal. Biomass Bioenergy 2024, 182, 107080. [Google Scholar] [CrossRef]
- Liu, L.; Memon, M.Z.; Xie, Y.; Gao, S.; Guo, Y.; Dong, J.; Gao, Y.; Li, A.; Ji, G. Recent advances of research in coal and biomass co-firing for electricity and heat generation. Circ. Econ. 2023, 2, 100063. [Google Scholar] [CrossRef]
- Yang, W.; Pudasainee, D.; Gupta, R.; Li, W.; Wang, B.; Sun, L. An overview of inorganic particulate matter emission from coal/biomass/MSW combustion: Sampling and measurement, formation, distribution, inorganic composition and influencing factors. Fuel Process. Technol. 2021, 213, 106657. [Google Scholar] [CrossRef]
- Verma, M.; Loha, C.; Sinha, A.N.; Chatterjee, P.K. Drying of biomass for utilising in co-firing with coal and its impact on environment—A review. Renew. Sustain. Energy Rev. 2017, 71, 732–741. [Google Scholar] [CrossRef]
- Sami, M.; Annamalai, K.; Wooldridge, M. Co-firing of coal and biomass fuel blends. Prog. Energy Combust. Sci. 2001, 27, 171–214. [Google Scholar] [CrossRef]
- Agbor, E.; Zhang, X.; Kumar, A. A review of biomass co-firing in North America. Renew. Sustain. Energy Rev. 2014, 40, 930–943. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Matiushenko, A.I.; Nurpeiis, A.E.; Zhuikov, A.V. An experimental investigation into the fuel oil-free start-up of a coal-fired boiler by the main solid fossil fuel with additives of brown coal, biomass and charcoal for ignition enhancement. Fuel Process. Technol. 2021, 223, 106986. [Google Scholar] [CrossRef]
- Dubinin, Y.V.; Yazykov, N.A.; Yeletsky, P.M.; Tabakaev, R.B.; Belyanovskaya, A.I.; Yakovlev, V.A. Catalytic co-combustion of biomass and brown coal in a fluidized bed: Economic and environmental benefits. China Environ. Sci. 2024, 140, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, J.-S.; Lee, Y.-E.; Shin, D.-C.; Ahn, K.-H.; Jung, J.; Kim, K.-H.; Ku, M.-J.; Kim, S.-M.; Jeon, C.-H.; et al. Investigation and Optimization of Co-Combustion Efficiency of Food Waste Biochar and Coal. Sustainability 2023, 15, 14596. [Google Scholar] [CrossRef]
- Jaworski, T.J.; Kajda-Szcześniak, M. Study on the Similarity of the Parameters of Biomass and Solid Waste Fuel Combustion for the Needs of Thermal Power Engineering. Sustainability 2020, 12, 7894. [Google Scholar] [CrossRef]
- Ashraf, A.; Sattar, H.; Munir, S. A comparative performance evaluation of co-combustion of coal and biomass in drop tube furnace. J. Energy Inst. 2022, 100, 55–65. [Google Scholar] [CrossRef]
- Schönnenbeck, C.; Maryandyshev, P.; Trouvé, G.; Brillard, A.; Lyubov, V.; Brilhac, J.-F. Combustion of hydrolysis lignin in a drop tube furnace and subsequent gaseous and particulate emissions. Bioresour. Technol. 2019, 288, 121498. [Google Scholar] [CrossRef] [PubMed]
- Celaya, A.M.; Lade, A.T.; Goldfarb, J.L. Co-combustion of brewer’s spent grains and Illinois No. 6 coal: Impact of blend ratio on pyrolysis and oxidation behavior. Fuel Process. Technol. 2015, 129, 39–51. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Matheus, L.R.; Mançano, R.R.; Ferreira, V.C.; Barroso, T.L.C.T.; da Rosa, R.G.; Colpini, L.M.S. Application of brewers’ spent grains as an alternative biomass for renewable energy generation in a boiler combustion process. Sustain. Chem. Environ. 2023, 4, 100039. [Google Scholar] [CrossRef]
- Vasileiadou, A. Energy recovery from brewers’ spent grain combustion/co-combustion with lignite. Int. J. Environ. Sci. Technol. 2024, 21, 5335–5350. [Google Scholar] [CrossRef]
- Batistella, L.; Silva, V.; Suzin, R.C.; Virmond, E.; Althoff, C.A.; Moreira, R.F.P.M.; José, H.J. Gaseous emissions from sewage sludge combustion in a moving bed combustor. Waste Manag. 2015, 46, 430–439. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, D.; Feng, P.; Hao, B.; Guo, Y.; Wang, S. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 2021, 295, 126456. [Google Scholar] [CrossRef]
- Strandberg, A.; Thyrel, M.; Falk, J.; Öhman, M.; Skoglund, N. Morphology and phosphate distribution in bottom ash particles from fixed-bed co-combustion of sewage sludge and two agricultural residues. Waste Manag. 2024, 177, 56–65. [Google Scholar] [CrossRef]
- Sever Akdağ, A.; Atak, O.; Atimtay, A.T.; Sanin, F.D. Co-combustion of sewage sludge from different treatment processes and a lignite coal in a laboratory scale combustor. Energy 2018, 158, 417–426. [Google Scholar] [CrossRef]
- ISO 3310-1:2016; Test Sieves—Technical Requirements and Testing. ISO: Geneva, Switzerland, 2016.
- ISO 562:2010; Hard Coal and Coke—Determination of Volatile Matter. ISO: Geneva, Switzerland, 2010.
- ISO 11722:2013; Solid Mineral Fuels—Hard Coal—Determination of Moisture in the General Analysis Test Sample by Drying in Nitrogen. ISO: Geneva, Switzerland, 2013.
- ISO 1171:2024; Solid Mineral Fuels—Determination of Ash. ISO: Geneva, Switzerland, 2024.
- ISO 1928:2020; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. ISO: Geneva, Switzerland, 2024.
- ASTM D5373-14e1; Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke Significance and Use. ASTM: West Conshohocken, PA, USA, 2016.
- Hannl, T.K.; Skoglund, N.; Priščák, J.; Öhman, M.; Kuba, M. Bubbling fluidized bed co-combustion and co-gasification of sewage sludge with agricultural residues with a focus on the fate of phosphorus. Fuel 2024, 357, 129822. [Google Scholar] [CrossRef]
- Gu, T.; Ma, W.; Guo, Z.; Berning, T.; Yin, C. Stable and clean co-combustion of municipal sewage sludge with solid wastes in a grate boiler: A modeling-based feasibility study. Fuel 2022, 328, 125237. [Google Scholar] [CrossRef]
- Glushkov, D.; Zhuikov, A.; Zemlyansky, N.; Pleshko, A.; Fetisova, O.; Kuznetsov, P. Influence of the Composition and Particle Sizes of the Fuel Mixture of Coal and Biomass on the Ignition and Combustion Characteristics. Appl. Sci. 2023, 13, 11060. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Boiko, V.M.; Goldin, V.D.; Maslov, E.A.; Orlov, S.E.; Poplavskiy, S.V.; Usanina, A.S.; Zharova, I.K. Mathematical modelling of the liquid atomization process by cocurrent gas flow. IOP Conf. Ser. Mater. Sci. Eng. 2016, 124, 012076. [Google Scholar] [CrossRef]

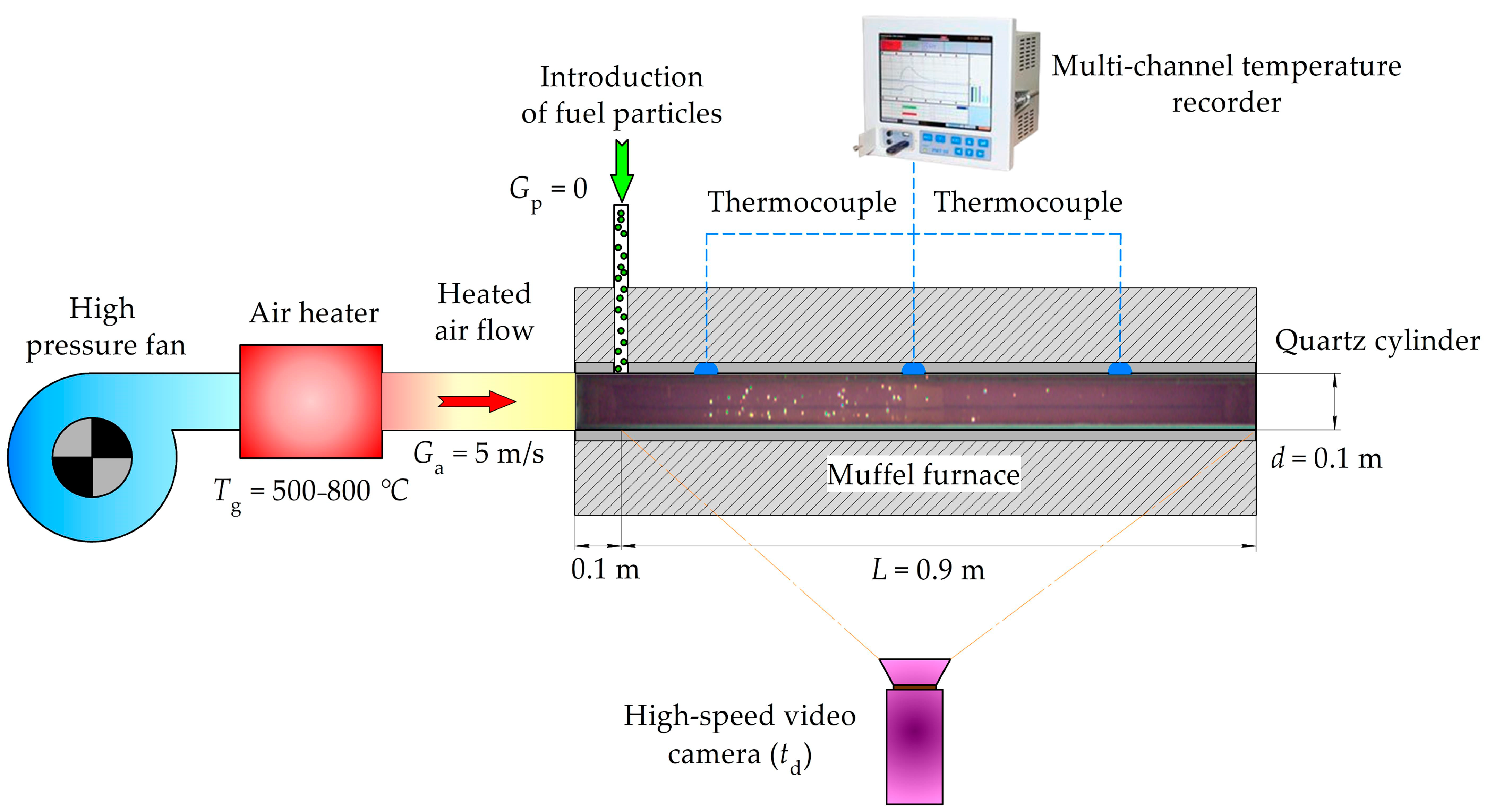

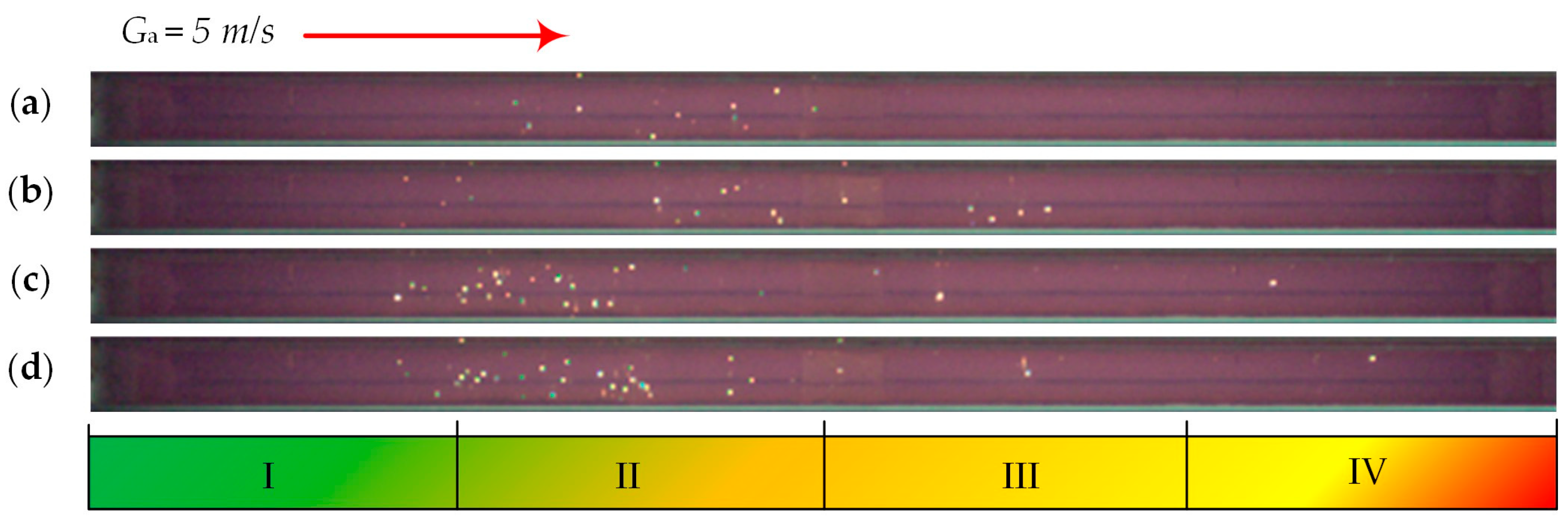
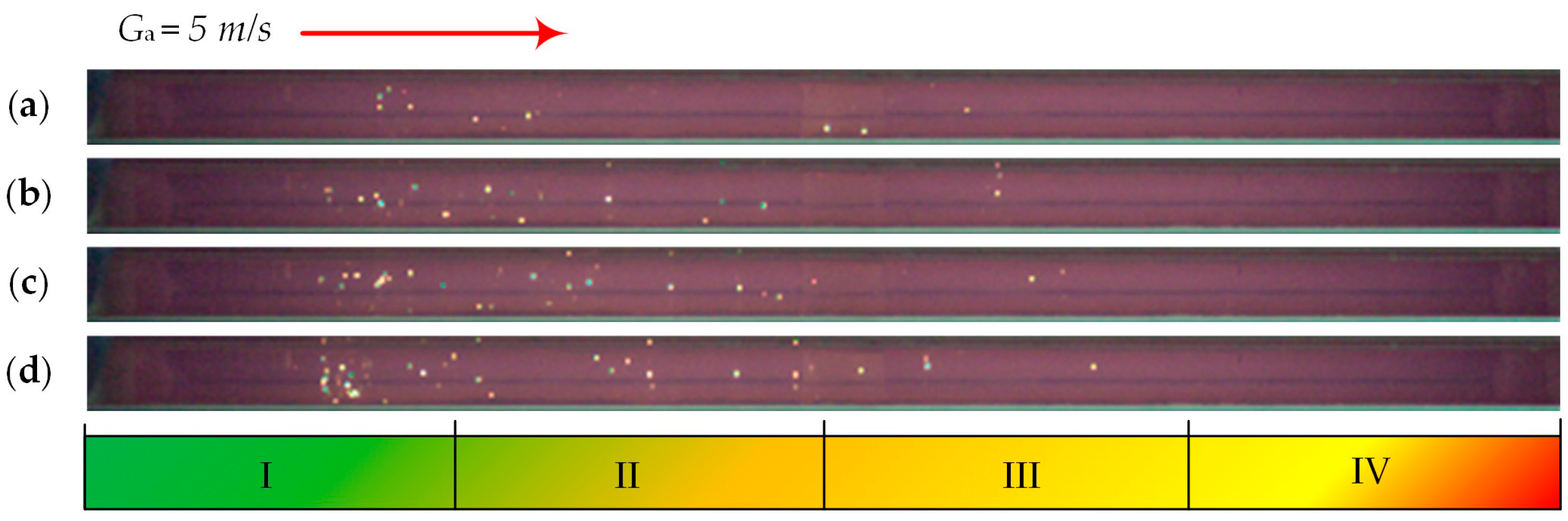
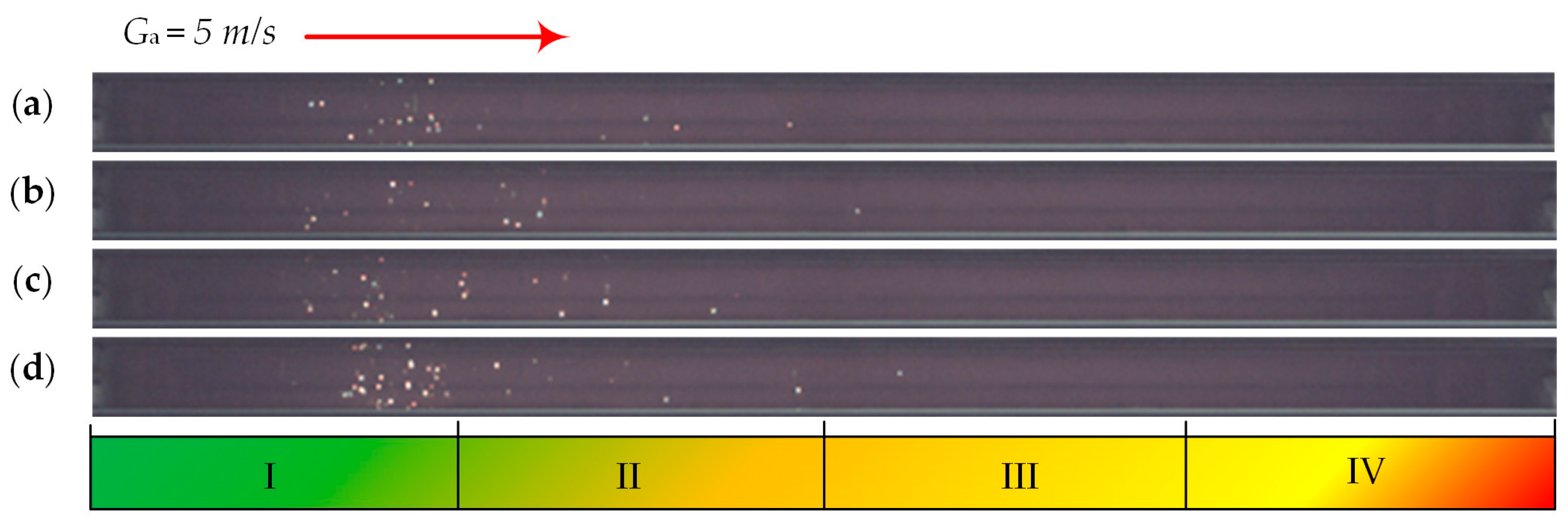

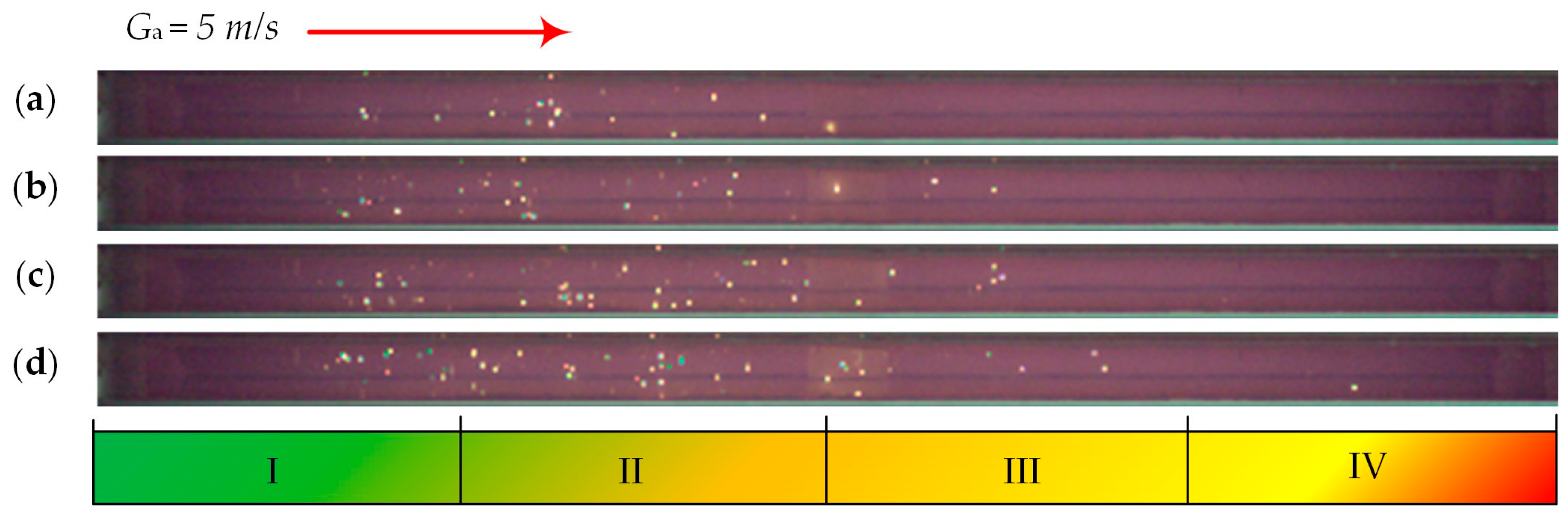
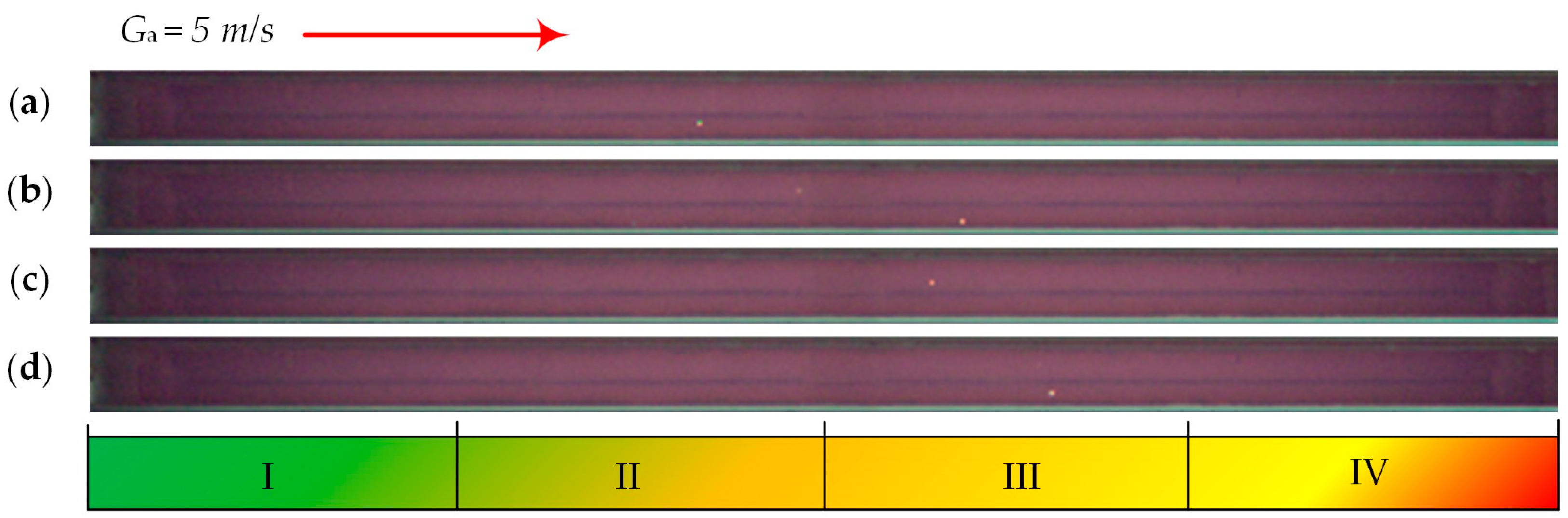
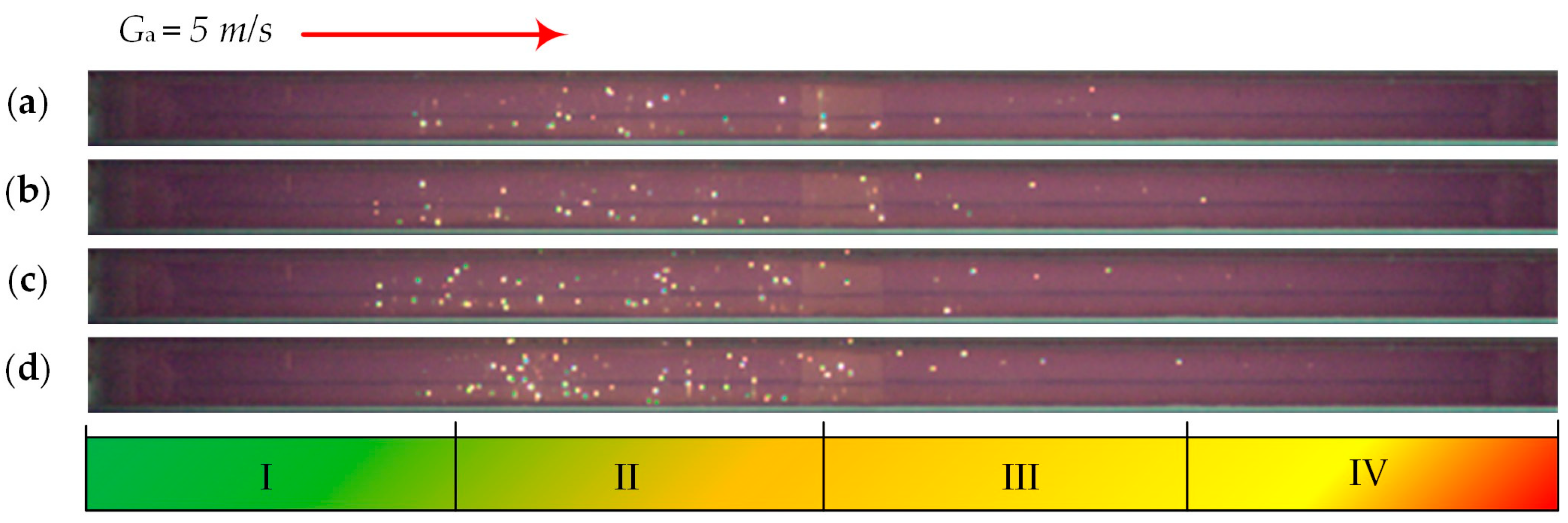
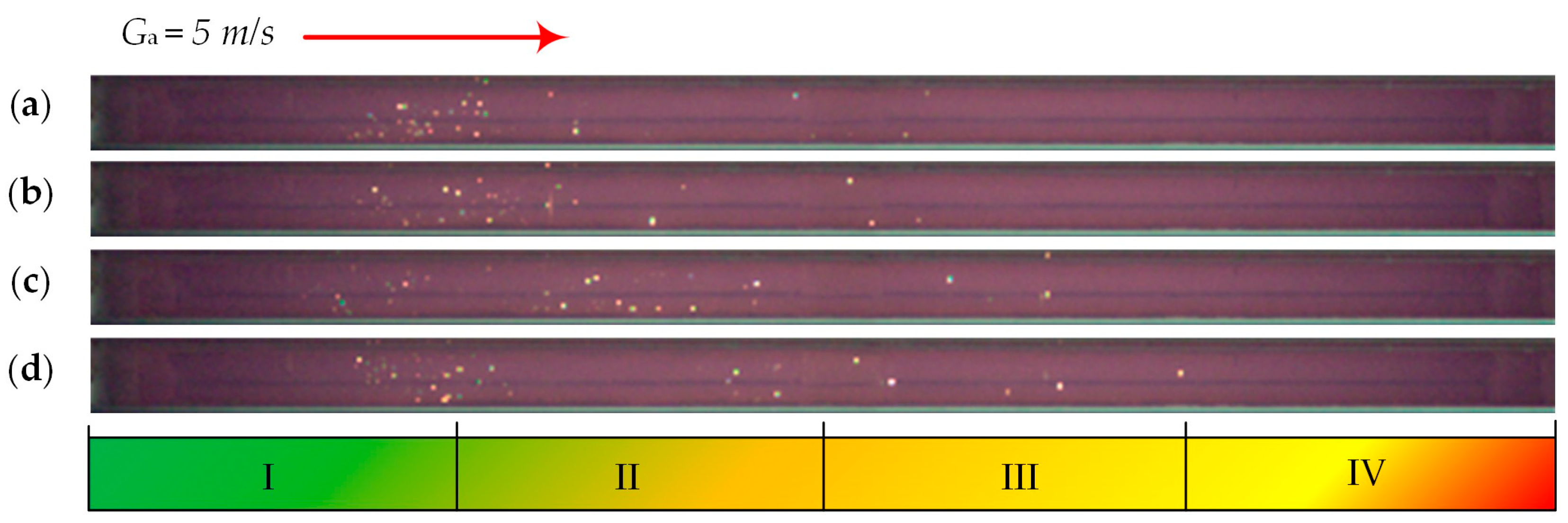
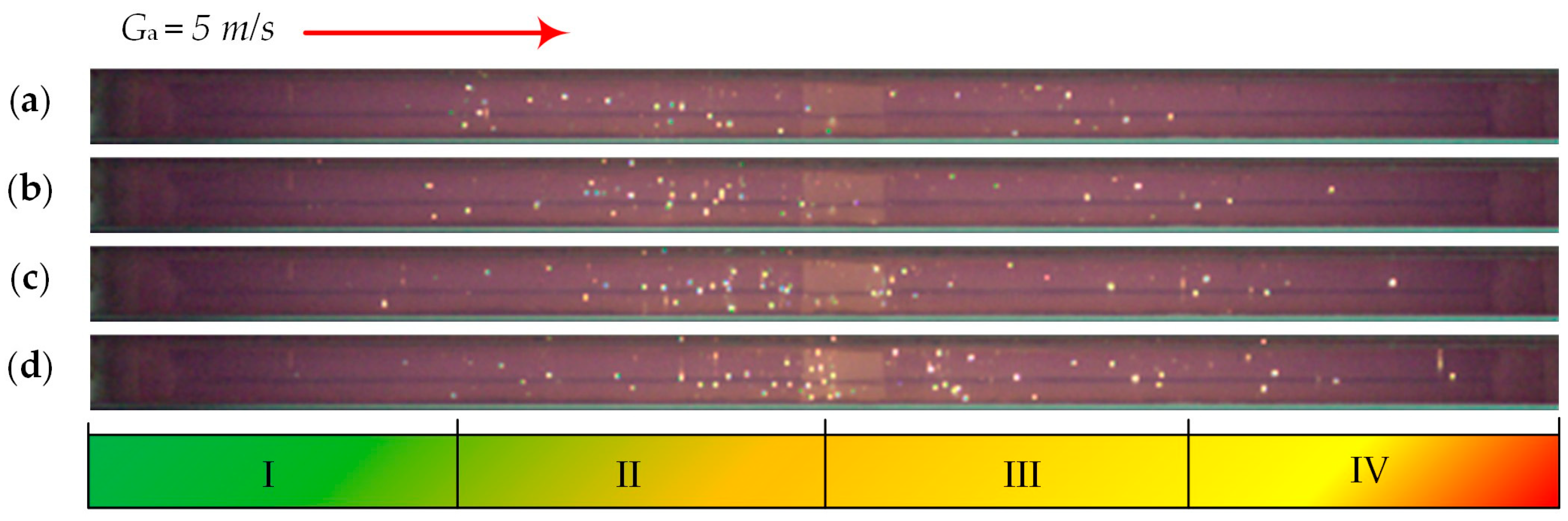
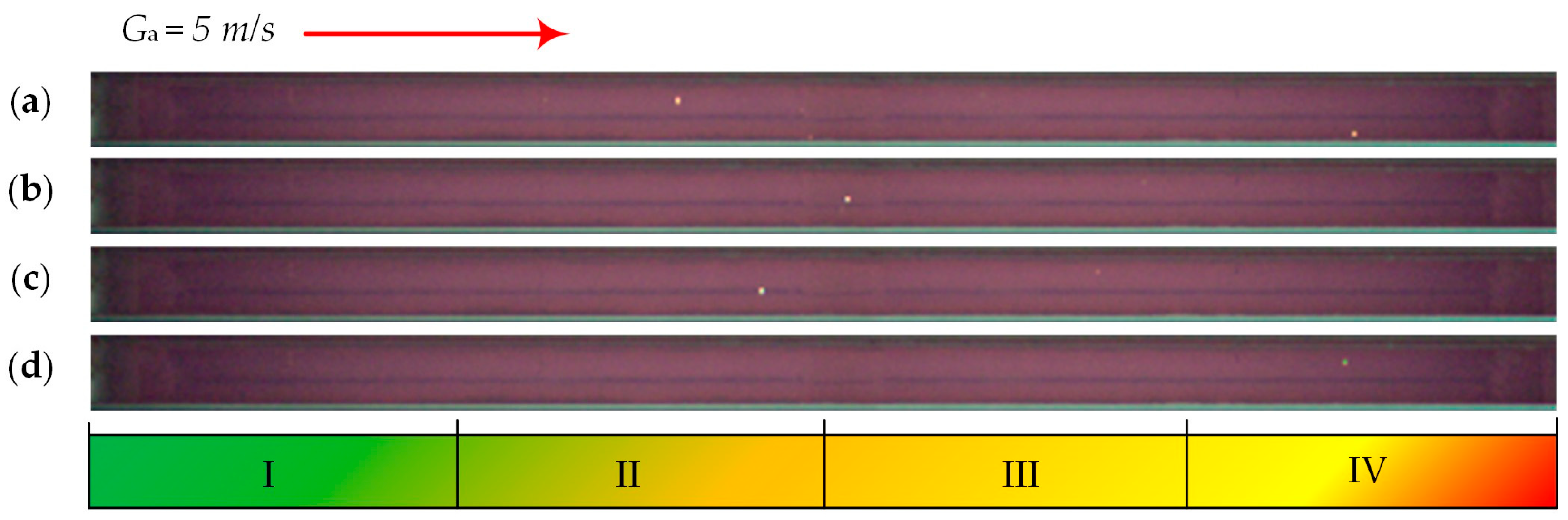
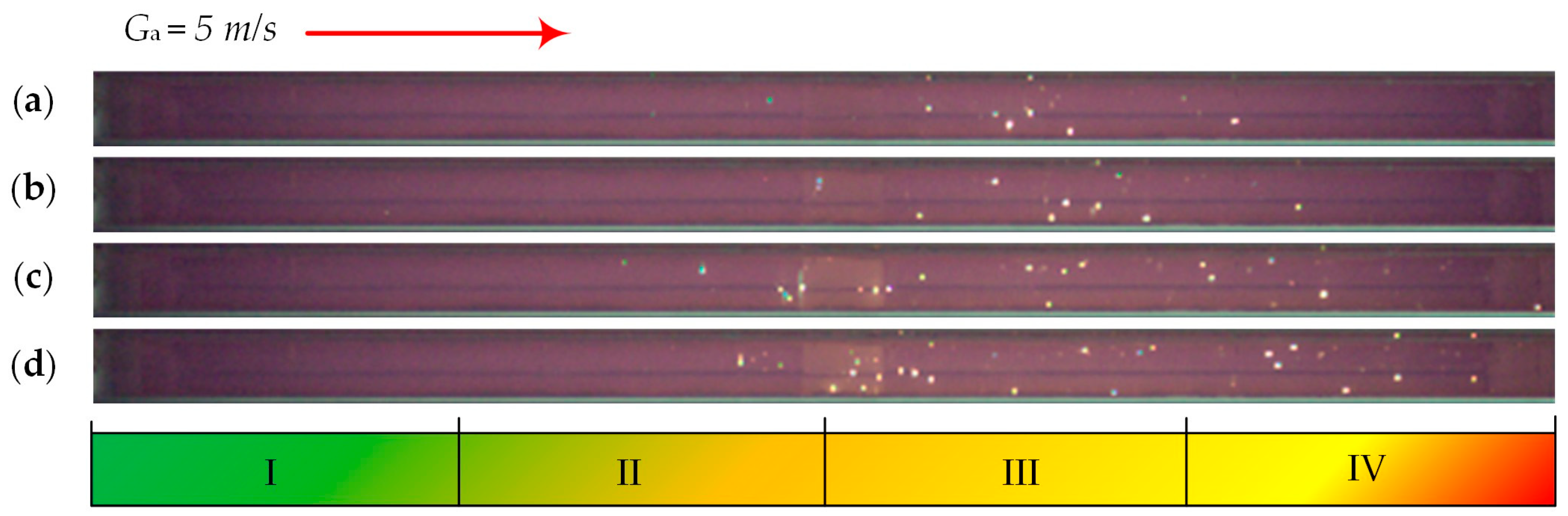
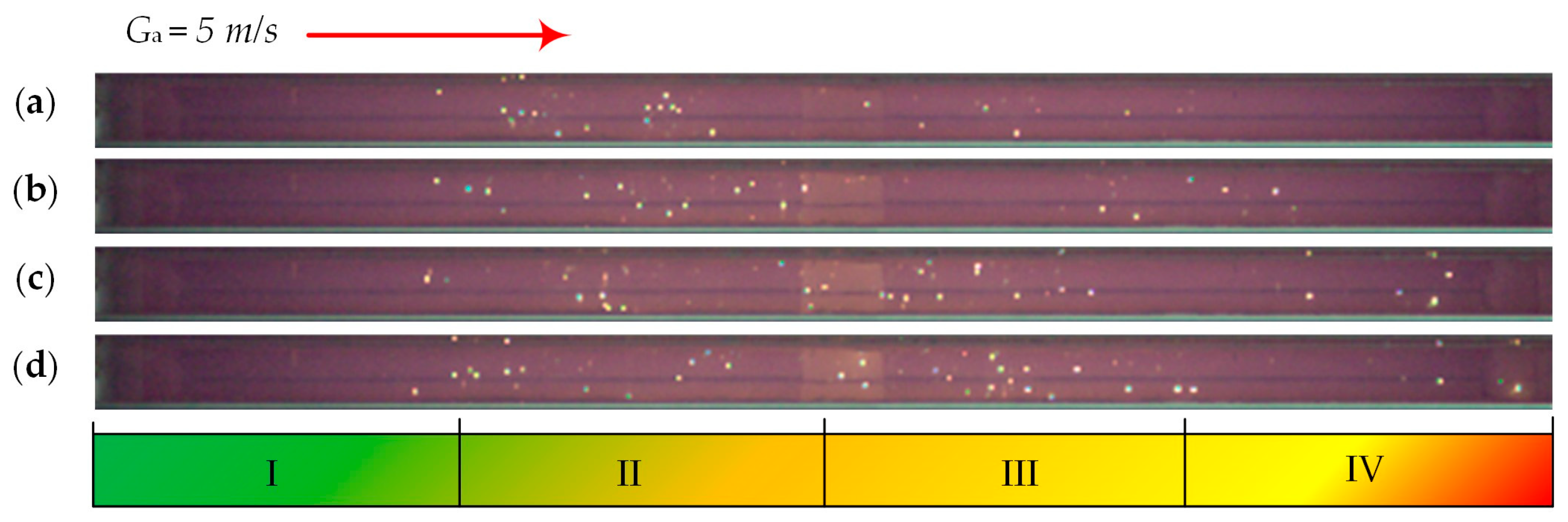

| Individual Fuels | |
| No. 1 | larch sawdust |
| No. 2 | larch bark |
| No. 3 | birch sawdust |
| No. 4 | pine cones |
| No. 5 | cedar needles |
| No. 6 | brewer’s spent grain (BSG) |
| No. 7 | hydrolysis lignin |
| No. 8 | sewage sludge |
| No. 9 | lignite coal |
| No. 10 | bituminous coal |
| Fuel mixtures | |
| No. 11 | No. 9 75% + No. 6 25% |
| No. 12 | No. 9 50% + No. 6 50% |
| No. 13 | No. 9 25% + No. 6 75% |
| No. 14 | No. 9 75% + No. 7 25% |
| No. 15 | No. 9 50% + No. 7 50% |
| No. 16 | No. 9 25% + No. 7 75% |
| Fuels | MC | A d | VC daf | C daf | H daf | N daf | S daf | O daf | HHV |
|---|---|---|---|---|---|---|---|---|---|
| % | MJ/kg | ||||||||
| № 1 | 9.9 | 0.2 | 80.0 | 50.4 | 5.86 | 0.3 | - | 43.4 | 19.57 |
| № 2 | 1.8 | 3.1 | 74.5 | 52.9 | 5.58 | - | - | 41.5 | 20.53 |
| № 3 | 9.0 | 0.3 | 81.1 | 51.2 | 5.78 | - | - | 43.0 | 19.65 |
| № 4 | 10.5 | 1.0 | 77.1 | 52.4 | 5.7 | 0.2 | - | 41.7 | 20.74 |
| № 5 | 14.2 | 3.4 | 79.5 | 56.3 | 6.7 | 0.3 | 0.1 | 36.6 | 23.39 |
| № 6 | 59.4 | 5.2 | 78.7 | 52.7 | 6.6 | 3.2 | 0.3 | 37.2 | 21.66 |
| № 7 | 47.5 | 6.2 | 65.9 | 63.2 | 5.4 | - | 0.5 | 30.9 | 24.45 |
| № 8 | 35.7 | 62.3 | 80.3 | 54.3 | 5.8 | 3.4 | 0.7 | 35.8 | 21.45 |
| № 9 | 20.8 | 6.1 | 44.5 | 74.1 | 5.0 | 0.9 | 0.3 | 19.7 | 29.1 |
| № 10 | 3.3 | 6.8 | 47.5 | 82.0 | 5.8 | 1.5 | 0.3 | 10.4 | 32.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuikov, A.; Zemlyanskiy, N.; Grishina, I.; Chicherin, S. Experimental Combustion of Different Biomass Wastes, Coals and Two Fuel Mixtures on a Fire Bench. Sustainability 2024, 16, 5227. https://doi.org/10.3390/su16125227
Zhuikov A, Zemlyanskiy N, Grishina I, Chicherin S. Experimental Combustion of Different Biomass Wastes, Coals and Two Fuel Mixtures on a Fire Bench. Sustainability. 2024; 16(12):5227. https://doi.org/10.3390/su16125227
Chicago/Turabian StyleZhuikov, Andrey, Nikolay Zemlyanskiy, Irina Grishina, and Stanislav Chicherin. 2024. "Experimental Combustion of Different Biomass Wastes, Coals and Two Fuel Mixtures on a Fire Bench" Sustainability 16, no. 12: 5227. https://doi.org/10.3390/su16125227
APA StyleZhuikov, A., Zemlyanskiy, N., Grishina, I., & Chicherin, S. (2024). Experimental Combustion of Different Biomass Wastes, Coals and Two Fuel Mixtures on a Fire Bench. Sustainability, 16(12), 5227. https://doi.org/10.3390/su16125227







