The Impact of Microorganisms Transported in Ships’ Ballast Water on the Fish of the Estuarine Waters and Environmental Sustainability in the Southern Baltic Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analysis of Samples
2.3. Sequencing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gollasch, S.; David, M. Chapter 13—Ballast Water: Problems and Management. In World Seas: An Environmental Evaluation, Vol. III: Ecological Issues and Environmental Impacts, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–250. [Google Scholar]
- Stuer–Lauridsen, F.; Drillet, G.; Hansen, F.; Saunders, J. Same Risk Area: An area-based approach for the management of bioinvasion risks from ships’ ballast water. Mar. Policy 2018, 97, 147–155. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti–Ambrogi, A.; Minchin, D.; Narščius, A.; Ojaveer, H.; Olenin, S. International arrivals: Widespread bioinvasions in European seas. Ethol. Ecol. Evol. 2014, 26, 152–171. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, P.; Rokicka–Praxmayer, J.; Cupak, J.; Radziejewska, T.; Wolska, M. Unintended “biological cargo” of ships entering the River Odra estuary: Assemblages of organisms in ballast tanks. Sci. J. Marit. Univ. Szczec. 2013, 33, 22–29. [Google Scholar]
- Hernandez, M.R.; Ismail, N.; Drouillard, K.G.; MacIsaac, H.J. Ships’ Ballast Water Treatment by Chlorination Can Generate Toxic Trihalomethanes. Bull. Environ. Contam. Toxicol. 2017, 99, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Maier, R.M.; Pepper, I.L.; Gerba, C.P. Environmental Microbiology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780080919409. [Google Scholar]
- Shah, A.; Liu, Z.; Salhi, E.; Höfer, T.; Werschkund, B.; Von Gunten, U. Formation of disinfection by-products during ballast water treatment with ozone, chlorine and peracetic acid: Influence of water quality parameters. Environ. Sci. Water Res. Technol. 2015, 1, 465–480. [Google Scholar] [CrossRef]
- Hess–Erga, O.; Moreno–Andrés, J.; Enger, Ø.; Vadstein, O. Microorganisms in ballastwater: Disinfection, community dynamics, and implications for management. Sci. Total Environ. 2019, 657, 704–716. [Google Scholar] [CrossRef]
- Rojas–Tirado, P.; Pedersen, P.; Vadstein, O.; Pedersen, L. Microbial dynamics in RAS water: Effects of adding acetate as a biodegradable carbon-source. Aquac. Eng. 2019, 84, 106–116. [Google Scholar] [CrossRef]
- Khandeparker, L.; Kuchi, N.; Desai, D.V.; Anil, A.C. Changes in the ballast water tank bacterial community during a trans-sea voyage: Elucidation through next generation DNA sequencing. J. Environ. Manag. 2020, 273, 111018. [Google Scholar] [CrossRef] [PubMed]
- Maglić, L.; Frančić, V.; Zec, D.; David, M. Ballast water sediment management in ports. Mar. Pollut. Bull. 2019, 147, 237–244. [Google Scholar] [CrossRef]
- Shang, L.; Hu, Z.; Deng, Y.; Liu, Y.; Zhai, X.; Chai, Z.; Liu, X.; Zhan, Z.; Dobbs, F.C.; Tang, Y.Z. Metagenomic sequencing identifies highly diverse assemblages of dinoflagellate cysts in sediments from ships’ ballast tanks. Microorganisms 2019, 7, 250. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Q.; Wu, H. Study on the dinoflagellate cysts in ballast tank sediments of international vessels in Chinese shipyards. Mar. Environ. Res. 2021, 169, 105348. [Google Scholar] [CrossRef] [PubMed]
- Briski, E.; Allinger, L.E.; Balcer, M.; Cangelosi, A.; Fanberg, L.; Markee, T.P.; Mays, N.; Polkinghorne, C.N.; Prihoda, K.R.; Reavie, E.D.; et al. Combining Ballast Water Exchange and Treatment To Maximize Prevention of Species Introductions to Freshwater Ecosystems. Environ. Sci. Technol. 2015, 49, 9566–9573. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.A. An overview of thirty years of research on ballast water as a vector for aquatic invasive species to freshwater and marine environments. Aquat. Ecosyst. Health Manag. 2015, 18, 261–268. [Google Scholar] [CrossRef]
- Paolucci, E.M.; Ron, L.; MacIsaac, H.J. Combining ballast water treatment and ballast water exchange: Reducing colonization pressure and propagule pressure of phytoplankton organisms. Aquat. Ecosyst. Health Manag. 2017, 20, 369–377. [Google Scholar] [CrossRef]
- Altug, G.; Gurun, S.; Cardak, M.; Ciftci, P.S.; Kalkan, S. The occurrence of pathogenic bacteria in some ships’ ballast water incoming from various marine regions to the Sea of Marmara, Turkey. Mar. Environ. Res. 2012, 81, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Brinkmeyer, R. Diversity of bacteria in ships ballast water as revealed by next generation DNA sequencing. Mar. Pollut. Bull. 2016, 107, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ojaveer, H.; Olenin, S.; Narščius, A.; Florin, A.B.; Ezhova, E.; Gollasch, S.; Jensen, K.; Lehtiniemi, M.; Minchin, D.; Normant-Saremba, M.; et al. Dynamics of biological invasions and pathways overtime: A case study of a temperate coastal sea. Biol. Invasions 2016, 5, 799–813. [Google Scholar] [CrossRef]
- Normant–Saremba, M.; Marszewska, L.; Kerckhof, F. First record of the North American amphipod Melita nitida Smith, 1873 in Polish coastal waters. Oceanol. Hydrobiol. Stud. 2017, 46, 108–115. [Google Scholar] [CrossRef]
- Lv, B.; Cui, Y.; Tian, W.; Li, J.; Xie, B.; Yin, F. Abundances and profiles of antibiotic resistance genes as well as co-occurrences with human bacterial pathogens in ship ballast tank sediments from a shipyard in Jiangsu Province, China. Ecotoxicol. Environ. Saf. 2018, 157, 169–175. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G. Detection and various environmental factors of antibiotic resistance gene horizontal transfer. Environ. Res. 2022, 212, 113267. [Google Scholar] [CrossRef]
- IMO. International Convention for the Control and Management of Ships’ Ballast Water and Sediments; International Maritime Organization: London, UK, 2004. [Google Scholar] [CrossRef]
- Drake, L.A.; Doblin, M.A.; Dobbs, F.C. Potential microbial bioinvasions via ships’ ballast water, sediment, and biofilm. Mar. Pollut. Bull. 2007, 55, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Han, Y.; Liu, B.; Gu, Y.; Tian, W.; Whiting-Wagner, N.; Zhao, H.; Zhang, W. Bacterial diversity in ballast water and sediments revealed by 2b-RAD sequencing. Mar. Pollut. Bull. 2021, 169, 112523. [Google Scholar] [CrossRef] [PubMed]
- Jurelevicius, D.; Cotta, S.R.; Montezzi, L.F.; Dias, A.C.F.; Mason, O.U.; Picão, R.C.; Jansson, J.K.; Seldin, L. Enrichment of potential pathogens in marine microbiomes with different degrees of anthropogenic activity. Environ. Pollut. 2021, 268 Pt A, 115757. [Google Scholar] [CrossRef] [PubMed]
- Kongprajug, A.; Chyerochana, N.; Rattanakul, S.; Denpetkul, T.; Sangkaew, W.; Somnark, P.; Patarapongsant, Y.; Tomyim, K.; Sresung, M.; Mongkolsuk, S.; et al. Integrated analyses of fecal indicator bacteria, microbial source tracking markers, and pathogens for Southeast Asian beach water quality assessment. Water Res. 2021, 203, 117479. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulou, D.S.; Dobbs, F. Bacterial Diversity in Ships’ Ballast Water, Ballast-Water Exchange, and Implications for Ship-Mediated Dispersal of Microorganisms. Environ. Sci. Technol. 2017, 51, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Burbianka, M.; Pliszka, A.; Burzyńska, H. Mikrobiologia Żywności; Wydanie V; PZWL: Warszawa, Poland, 1983. [Google Scholar]
- Muyzer, G.; De Waal, E.C.; Ultterlinden, A.G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef]
- Hesham, A.E.L.; Qi, R.; Yang, M. Comparison of bacterial community structures in two systems of a sewage treatment plant using PCR-DGGE analysis. J. Environ. Sci. 2011, 23, 2049–2054. [Google Scholar] [CrossRef] [PubMed]
- Dobaradaran, S.; Soleimani, F.; Nabipour, I.; Saeedi, R.; Mohammadi, M.J. Heavy metal levels of ballast waters in commercial ships entering Bushehr port along the Persian Gulf. Mar. Pollut. Bull. 2018, 126, 74–76. [Google Scholar] [CrossRef]
- Kadriu, S.; Sadiku, M.; Kelmendi, M.; Aliu, M.; Mulliqi, I.; Hyseni, A. Impact of Kishnica mines on pollution of the Graçanka River and water wells nearby, Kosovo. J. Water Land Dev. 2021, I–III, 16–21. [Google Scholar] [CrossRef]
- Jørgensen, L.v.G. Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens 2020, 9, 609. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, Prebiotics and Synbiotics Improved the Functionality of Aquafeed: Upgrading Growth, Reproduction, Immunity and Disease Resistance in Fish. Fish Shellfish Immunol. 2020, 120, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.B.; Ranzani-Paiva, M.J.T.; Tachibana, L.; de Carla Dias, D.; Ishikawa, C.M.; Esteban, M.A. Fish Pathogen Bacteria: Adhesion, Parameters Influencing Virulence and Interaction with Host Cells. Fish Shellfish Immunol. 2018, 80, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Kozińska, A. Atypical cases of disorders in cyprinid wintering caused by Pseudomonas fluorescens infections. Bull. Eur. Assoc. Fish Pathol. 1999, 19, 216–220. [Google Scholar]
- Pękala–Safińska, A. Contemporary Threats of Bacterial Infections in Freshwater Fish. J. Vet. Res. 2018, 62, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, D.; Souissi, S. Antibiotic Resistance and Virulence Traits of Bacterial Pathogens from Infected Freshwater Fish, Labeo rohita. Microb. Pathog. 2018, 116, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Menanteau-Ledouble, S.; Kumar, G.; Saleh, M.; El-Matbouli, M. Aeromonas salmonicida: Updates on an Old Acquaintance. Dis. Aquat. Org. 2016, 120, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Alghabshi, A.; Austin, B.; Crumlish, M. Aeromonas salmonicida Isolated from Wild and Farmed Fish and Invertebrates in Oman. Int. Aquat. Res. 2018, 10, 145–152. [Google Scholar] [CrossRef]
- Mzula, A.; Wambura, P.N.; Mdegela, R.H.; Shirima, G.M. Current State of Modern Biotechnological-Based Aeromonas hydrophila Vaccines for Aquaculture: A Systematic Review. BioMed Res. Int. 2019, 2019, 3768948. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, E.; Lelin, C.; Sathishkumar, R.; Vimal, S.; Anand, S.B.; Babu, M.M.; Citarasu, T. Oral Delivery of PVAX-OMP and PVAX-Hly DNA Vaccine Using Chitosan-Tripolyphosphate (Cs-TPP) Nanoparticles in Rohu, (Labeo rohita) for Protection against Aeromonas Hydrophila Infection. Fish Shellfish Immunol. 2021, 115, 189–197. [Google Scholar] [CrossRef]
- Gross, R.; Guzman, C.A.; Sebaihia, M.; Martins dos Santos, V.; Pieper, D.H.; Koebnik, R.; Lechner, M.; Bartels, D.; Buhrmester, J.; Choudhuri, J.V.; et al. The missing link: Bordetella petrii is endowed with both the metabolic versatility of environmental bacteria and virulence traits of pathogenic Bordetellae. BMC Genom. 2008, 9, 449. [Google Scholar] [CrossRef]
- Wang, F.; Grundmann, S.; Schmid, M.; Dörfler, U.; Roherer, S.; Munch, J.C.; Hartmann, A.; Jiang, X.; Schroll, R. Isolation and characterization of 1,2,4-trichlorobenzene mineralizing Bordetella sp. and its bioremediation potential in soil. Chemosphere 2007, 67, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Spilker, T.; Liwienski, A.A.; LiPuma, J.J. Identification of Bordetella spp. in respiratory specimens from individuals with cystic fibrosis. Clin. Microbiol. Infect. 2008, 14, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Sfanos, K.; Harmody, D.; Dang, P.; Ledger, A.; Pomponi, S.; McCarthy, P.; Lopez, J. A molecular systematic survey of cultured microbial associates of deep-water marine invertebrates. Syst. Appl. Microbiol. 2005, 28, 242–264. [Google Scholar] [CrossRef] [PubMed]
- Sellyei, B.; Varga, Z.; Cech, G.; Varga, Á.; Székely, C. Mycoplasma infections in freshwater carnivorous fishes in Hungary. J. Fish Dis. 2020, 44, 297–304. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El Hady, M.A. Antigenic variations and pathogenicity between Mycoplasma spp. isolated from fishes and shellfishes. In Proceedings of the Conference: 8th International Symposium on Tilapia Aquaculture, Cairo, Egypt, 12–14 October 2008; Available online: https://www.researchgate.net/publication/235673743_ANTIGENIC_VARIATIONS_AND_PATHOGENICITY_BETWEEN_MYCOPLASMA_SPP_ISOLATED_FROM_FISHES_AND_SHELLFISHES (accessed on 24 March 2024).
- Brown, D.R. Mycoplasmosis and immunity of fish and reptiles. Front. Biosci. 2002, 7, 1338–1346. [Google Scholar] [CrossRef]

| Season | Sampling Date | Origin of the Sample | Sample Designation | Type of Ship | Installed BWTS |
|---|---|---|---|---|---|
| Spring | 20 April 2019 | Port water | P1 | ||
| Short-range ship | S1 | general cargo ship | filtration + UV | ||
| Long-range ship | L1 | bulk carrier | filtration + EC | ||
| Summer | 19 July 2019 | Short-range ship | S2 | cargo | filtration + UV |
| Long-range ship | L2 | bulk carrier | filtration + EC | ||
| Autumn | 16 October 2020 | Port water | P2 | ||
| Short-range ship | S3 | cargo | filtration + UV | ||
| Short-range ship | S4 | general cargo ship | filtration + UV | ||
| Long-range ship | L3 | bulk carrier | filtration + UV | ||
| Long-range ship | L4 | cargo | filtration + EC | ||
| Winter | 9 December 2020 | Port water | P3 | ||
| Short-range ship | S5 | general cargo ship | filtration + UV | ||
| Short-range ship | S6 | cargo | filtration + EC | ||
| Long-range ship | L5 | bulk carrier | filtration + EC | ||
| Long-range ship | L6 | cargo | filtration + EC |
| Collection Time | Microbiological Fractions (log10/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAMC | TYMC | TMMC | TCPs | TCPsf | THC | TLC | TAC | TPC | Average of Total | ||
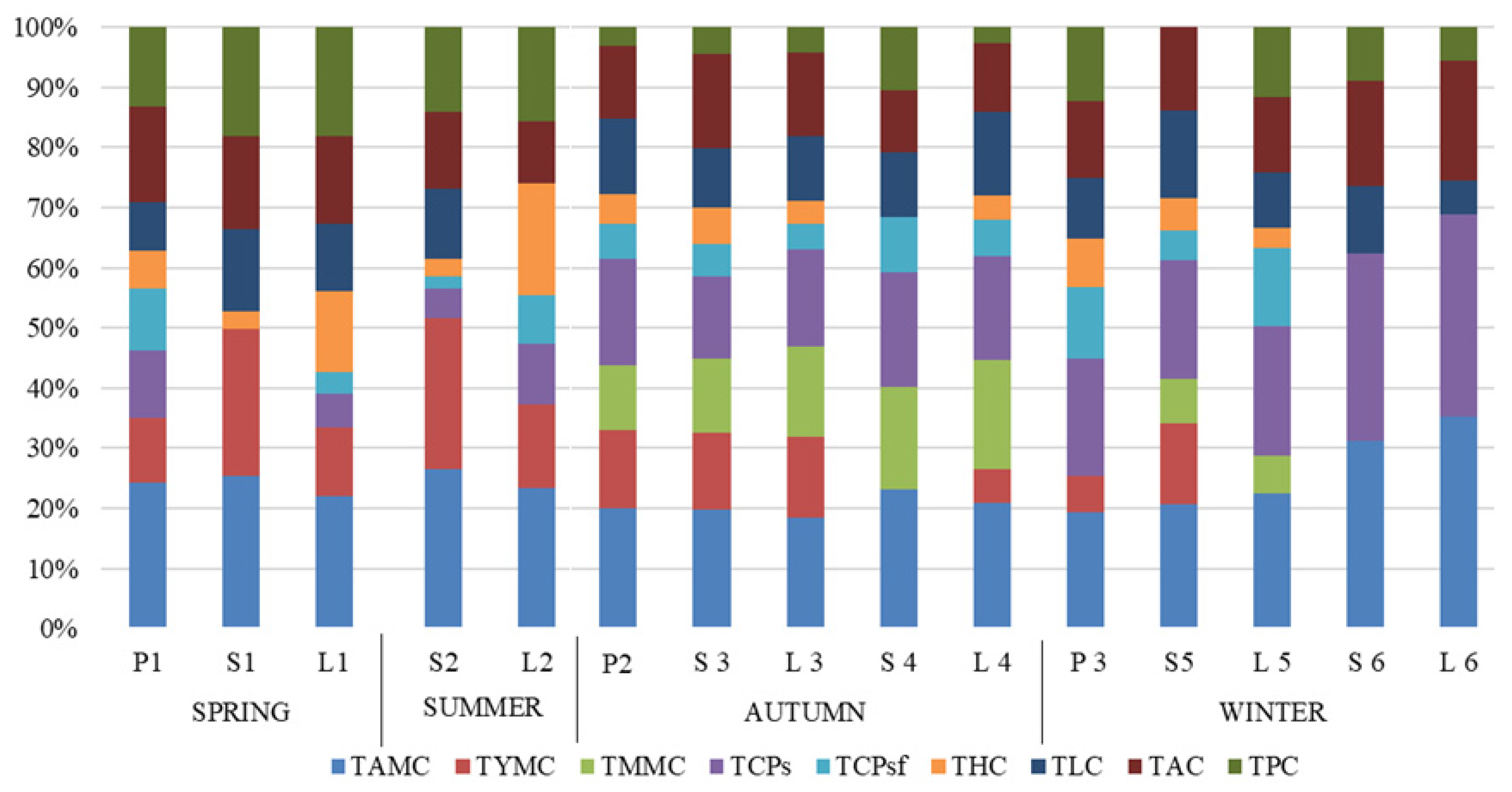
| spring | P1 | 2.33 | 1.04 | 0 | 1.08 | 1.00 | 0.60 | 0.78 | 1.53 | 1.28 | 1.20 ± (0.08) de |
| S1 | 2.59 | 2.48 | 0 | 0 | 0 | 0.30 | 1.40 | 1.56 | 1.85 | 1.15 ± (0.06) e | |
| L1 | 2.27 | 1.18 | 0 | 0.60 | 0.36 | 1.38 | 1.18 | 1.49 | 1.88 | 1.29 ± (0.11) de | |
| summer | S2 | 2.63 | 2.48 | 0 | 0.48 | 0.20 | 0.30 | 1.15 | 1.26 | 1.40 | 1.29 ± (0.11) de |
| L2 | 2.18 | 1.28 | 0 | 0.95 | 0.75 | 1.73 | 0 | 0.95 | 1.46 | 1.16 ± (0.07) e | |
| autumn | P2 | 4.61 | 2.98 | 2.45 | 4.11 | 1.30 | 1.15 | 2.90 | 2.78 | 0.70 | 2.55 ± (0.44) a |
| S3 | 4.45 | 2.84 | 2.78 | 3.08 | 1.18 | 1.40 | 2.18 | 3.53 | 1.00 | 2.49 ± (0.38) | |
| L3 | 3.98 | 2.90 | 3.26 | 3.49 | 0.90 | 0.85 | 2.30 | 3.02 | 0.90 | 2.40 ± (0.41) b | |
| S4 | 4.19 | 0.00 | 3.09 | 3.41 | 1.70 | 0.00 | 1.93 | 1.88 | 1.88 | 2.01 ± (0.40) | |
| L4 | 3.70 | 1.00 | 3.19 | 3.08 | 1.08 | 0.70 | 2.48 | 2.00 | 0.48 | 1.97 ± (0.40) ab | |
| winter | P3 | 3.29 | 1.00 | 0.00 | 3.32 | 2.00 | 1.40 | 1.70 | 2.15 | 2.10 | 1.77 ± (0.47) c |
| S5 | 4.19 | 2.71 | 1.48 | 4.03 | 1.00 | 1.08 | 2.93 | 2.81 | 0.00 | 2.25 ± (0.48) d | |
| L5 | 4.57 | 0.00 | 1.30 | 4.40 | 2.65 | 0.70 | 1.85 | 2.57 | 2.39 | 2.27 ± (0.51) c | |
| S6 | 4.18 | 0.00 | 0.00 | 4.18 | 0.00 | 0.00 | 1.48 | 2.36 | 1.18 | 1.49 ± (0.58) d | |
| L6 | 4.42 | 0.00 | 0.00 | 4.23 | 0.00 | 0.00 | 0.70 | 2.49 | 0.70 | 1.39 ± (0.61) cd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zatoń-Sieczka, K.; Czerniejewski, P. The Impact of Microorganisms Transported in Ships’ Ballast Water on the Fish of the Estuarine Waters and Environmental Sustainability in the Southern Baltic Sea. Sustainability 2024, 16, 5229. https://doi.org/10.3390/su16125229
Zatoń-Sieczka K, Czerniejewski P. The Impact of Microorganisms Transported in Ships’ Ballast Water on the Fish of the Estuarine Waters and Environmental Sustainability in the Southern Baltic Sea. Sustainability. 2024; 16(12):5229. https://doi.org/10.3390/su16125229
Chicago/Turabian StyleZatoń-Sieczka, Kinga, and Przemysław Czerniejewski. 2024. "The Impact of Microorganisms Transported in Ships’ Ballast Water on the Fish of the Estuarine Waters and Environmental Sustainability in the Southern Baltic Sea" Sustainability 16, no. 12: 5229. https://doi.org/10.3390/su16125229





