Notifications on Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF) Reported in 2001–2023
Abstract
1. Introduction
1.1. Determinants Associated with Anisakis spp.
1.2. Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF)
1.3. Goal of the Study
2. Data and Methods
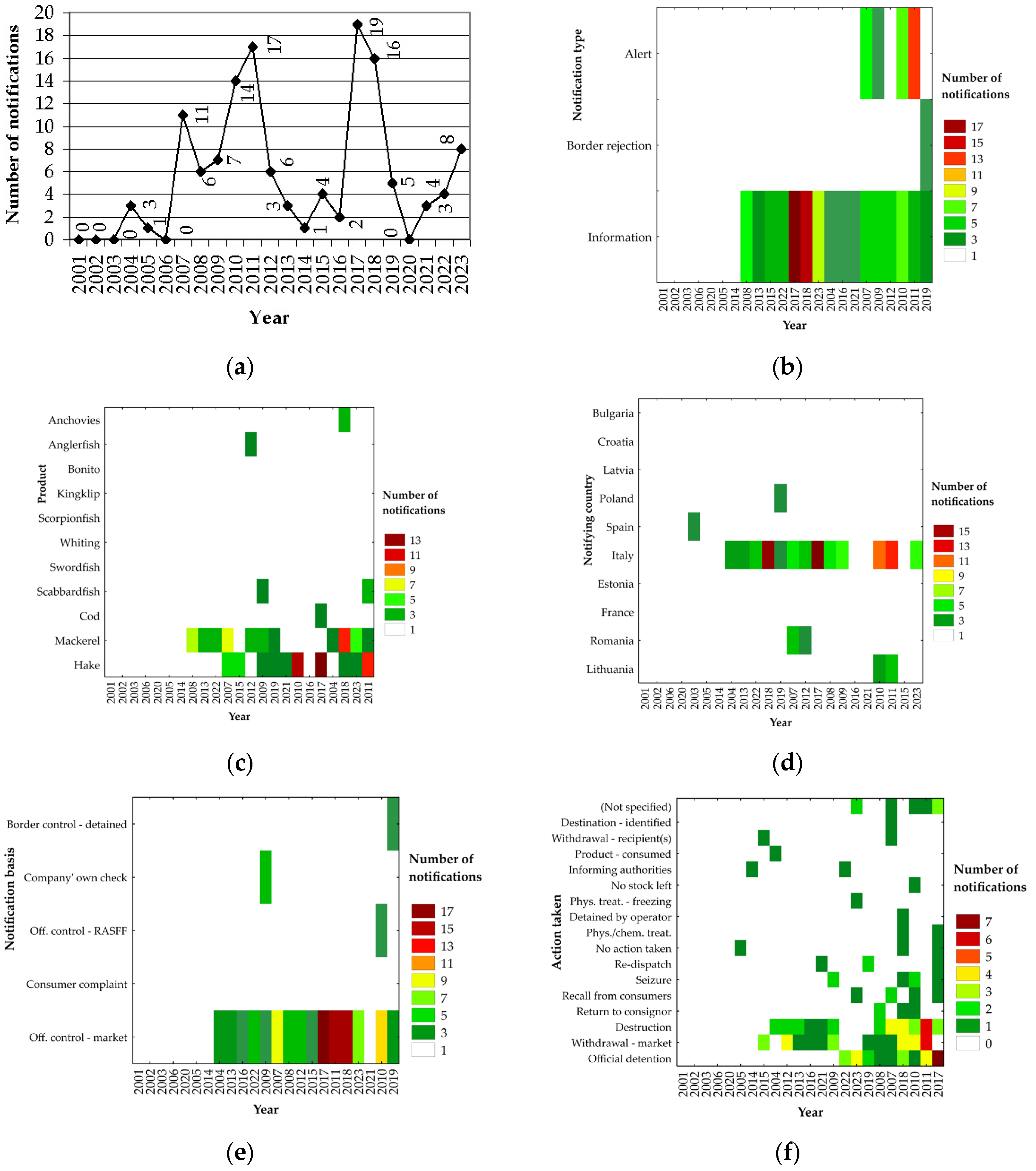
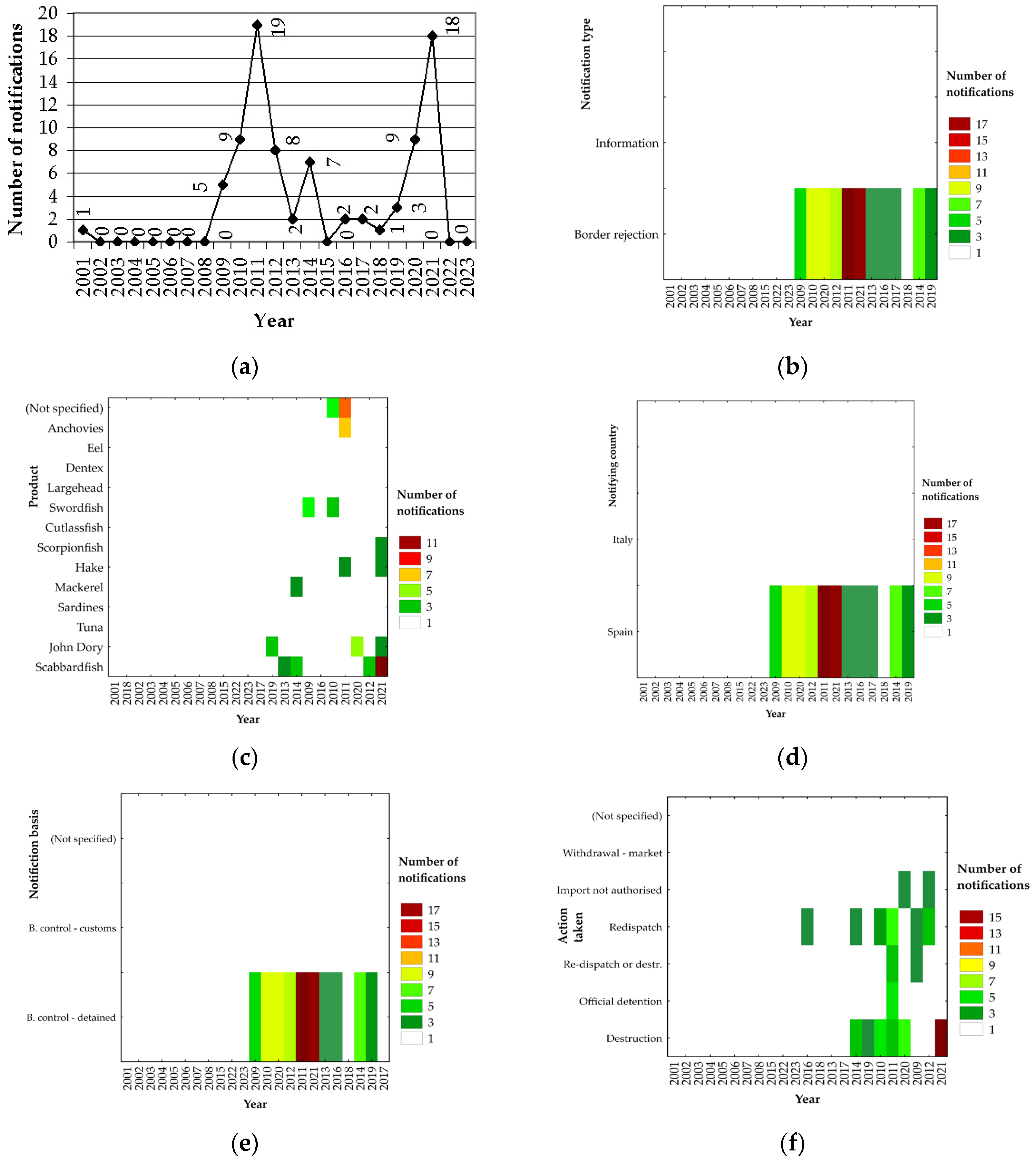
3. Results
3.1. General Analysis
3.2. Most Frequently Reported Countries of Origin
4. Discussion
4.1. Notifications in the RASFF in Studies to Date
4.2. Consumers from Italy and Spain Facing the Risks of Anisakis spp. Infection
4.3. Exposure to Anisakis spp. Infection and Ways of Prevention
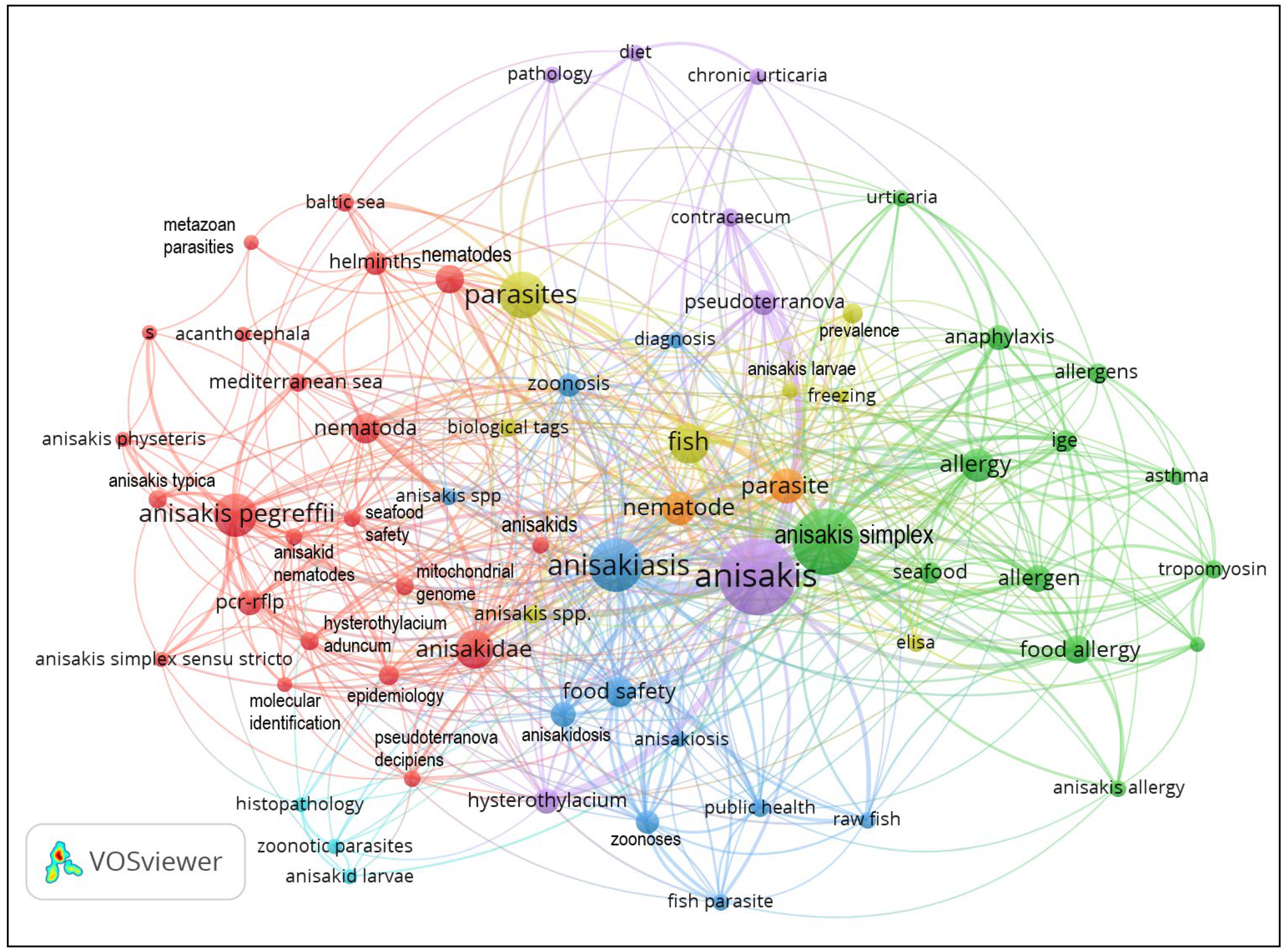
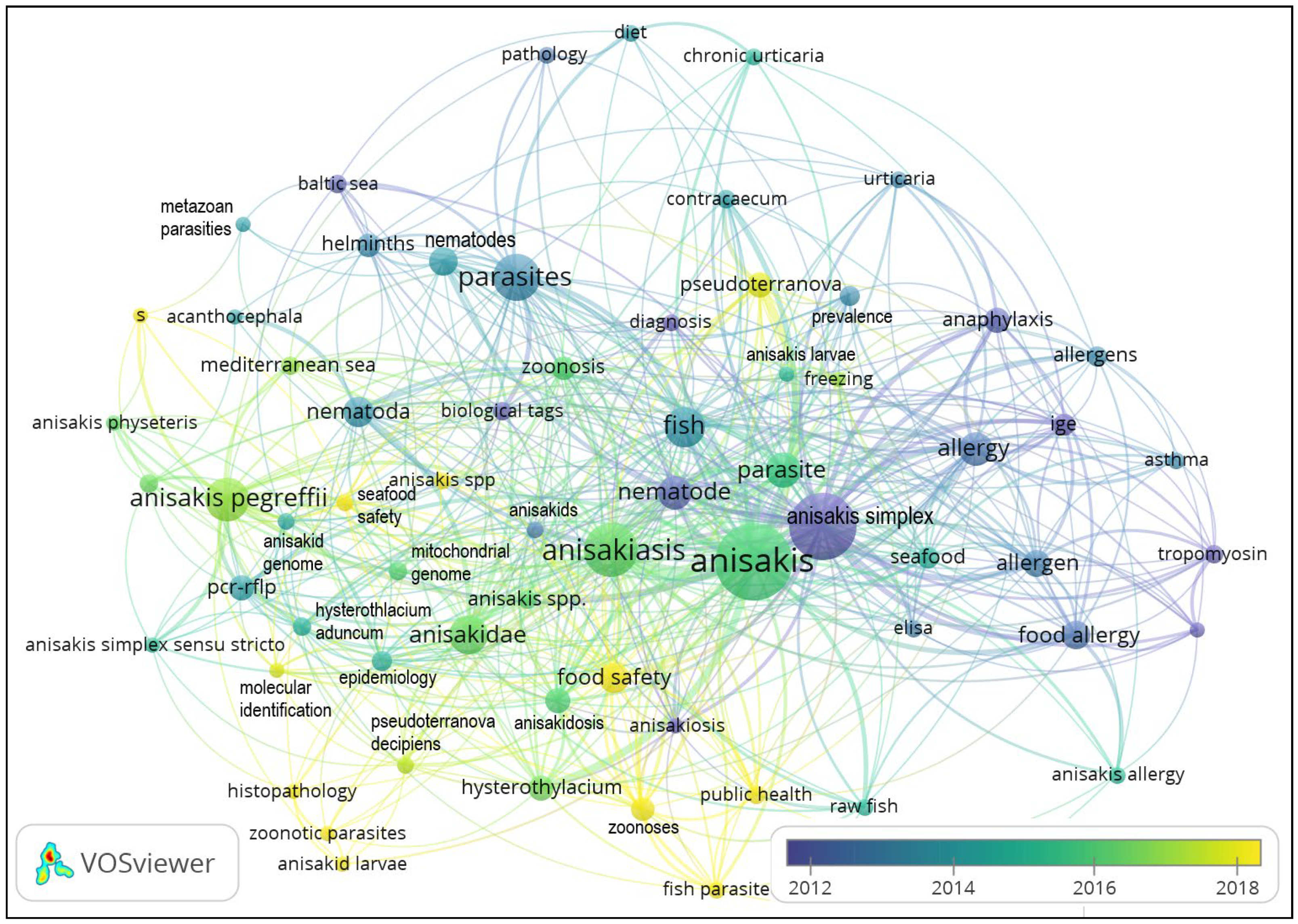
4.4. Maps of Links between the Keyword “Anisakis” and Other Words
4.5. Limitations Related to Obtaining and Processing of Data
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farm to Fork Strategy. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 30 April 2024).
- European Parliament, Council of the European Union. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying down Procedures in Matters of Food Safety. OJ L 31, 1.2.2002, 1–24. Available online: https://eur-lex.europa.eu/eli/reg/2002/178/oj (accessed on 30 April 2024).
- EFSA Panel on Biological Hazards (BIOHAZ); Ricci, A.; Chemaly, M.; Davies, R.H.; Fernández Escámez, P.S.; Girones, R.; Herman, L.M.; Lindqvist, R.; Nørrung, B.; Robertson, L.J.; et al. Hazard analysis approaches for certain small retail establishments in view of the application of their food safety management systems. EFSA J. 2017, 15, 4697. [Google Scholar]
- Maggiore, A.; Afonso, A.; Barrucci, F.; Sanctis, G.D. Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar]
- Ramos, P. Parasites in fishery products-Laboratorial and educational strategies to control. Exp. Parasitol. 2020, 211, 107865. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, G.; Abollo, E.; Marrone, R.; Bernardi, C.E.; Chirollo, C.; Anastasio, A.; del Hierro, S. Risk-based scoring and genetic identification for anisakids in frozen fish products from Atlantic FAO areas. BMC Vet. Res. 2020, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.L.; Quiazon, K.M.; Kotake, M.; Itoh, N.; Yoshinaga, T. Anisakis spp. in fishery products from Japanese waters: Updated insights on host prevalence and human infection risk factors. Parasitol. Int. 2020, 78, 102137. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar]
- EFSA; ECDC. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar]
- EFSA; ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- Fong, I.W. Animals and Mechanisms of Disease Transmission. In Emerging Zoonoses. Emerging Infectious Diseases of the 21st Century; Fong, I.W., Ed.; Springer: Cham, Germany, 2017; pp. 15–38. [Google Scholar]
- Chalmers, R.M.; Robertson, L.J.; Dorny, P.; Jordan, S.; Kärssin, A.; Katzer, F.; La Carbona, S.; Lalle, M.; Lassen, B.; Mladineo, I.; et al. Parasite detection in food: Current status and future needs for validation. Trends Food Sci. Technol. 2020, 99, 337–350. [Google Scholar] [CrossRef]
- EFSA. The use of the so-called ‘tubs’ for transporting and storing fresh fishery products. EFSA J. 2020, 18, 6091. [Google Scholar]
- Robertson, L.J. Parasites in Food: From a Neglected Position to an Emerging Issue. Adv. Food Nutr. Res. 2018, 86, 71–113. [Google Scholar] [PubMed]
- Aibinu, I.E.; Smooker, P.M.; Lopata, A.L. Anisakis Nematodes in Fish and Shellfish- from infection to allergies. Int. J. Parasitol. Parasites Wildl. 2019, 9, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Golden, O.; Caldeira, A.J.; Santos, M.J. Raw fish consumption in Portugal: A survey on trends in consumption and consumer characteristics. Food Control 2022, 135, 108810. [Google Scholar] [CrossRef]
- Yemmen, C.; Gargouri, M. Potential hazards associated with the consumption of Scombridae fish: Infection and toxicity from raw material and processing. J. Appl. Microbiol. 2022, 132, 4077–4096. [Google Scholar] [CrossRef] [PubMed]
- López, I.A.; Pardo, M.A. A phage display system for the identification of novel Anisakis simplex antigens. J. Immunol. Methods 2011, 373, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Mazzucco, W.; Lacca, G.; Cusimano, R.; Provenzani, A.; Costa, A.; Di Noto, A.M.; Massenti, M.F.; Leto-Barone, M.S.; Lorenzo, G.D.; Vitale, F. Prevalence of Sensitization to Anisakis simplex Among Professionally Exposed Populations in Sicily. Arch. Environ. Occup. Health 2012, 67, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.E.; Ebisawa, M.; Ferreira, F.; Sampson, H.A.; van Ree, R.; Vieths, S.; Baumert, J.L.; Bohle, B.; Lalithambika, S.; Wise, J.; et al. AllergenOnline: A peer-reviewed, curated allergen database to assess novel food proteins for potential cross-reactivity. Mol. Nutr. Food Res. 2016, 60, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, M.T.; Rodríguez-Bobada, C.; Zuloaga, J.; Gómez-Aguado, F.; Rodríguez-Pérez, R.; Mendizabal, Á.; González, P.; Arias-Díaz, J.; Caballero, M.L. Exploring tumourigenic potential of the parasite Anisakis: A pilot study. Parasitol. Res. 2018, 117, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.C.; Rodríguez-Pérez, R.; Ballestero, A.; Zuloaga, J.; Fernández-Puntero, B.; Arias-Díaz, J.; Caballero, M.L. Previous Exposure to the Fish Parasite Anisakis as a Potential Risk Factor for Gastric or Colon Adenocarcinoma. Medicine 2015, 94, e1699. [Google Scholar] [CrossRef] [PubMed]
- Ruethers, T.; Taki, A.C.; Johnston, E.B.; Nugraha, R.; Le, T.T.; Kalic, T.; McLean, T.R.; Kamath, S.D.; Lopata, A.L. Seafood allergy: A comprehensive review of fish and shellfish allergens. Mol. Immunol. 2018, 100, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, E.; Pekmezci, G.Z.; Yıldırım, A.; Duzlu, O.; Onder, Z.; Çiloğlu, A.; Sursal, N.; Yılmaz, E.; Gonulalan, Z.; Inci, A. Investigation of Anisakis larvae in different products of ready-to-eat fish meat and imported frozen fish in Turkey. Int. J. Food Microbiol. 2020, 333, 108829. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Fernandes, T.; Rodrigues, M.; Castro, I.; Paixão, P.; Pinto-Marques, P.; Roque, L.; Belo, S.; Ferreira, P.M.; Mansinho, K.; Toscano, C. Human gastric hyperinfection by Anisakis simplex: A severe and unusual presentation and a brief review. Int. J. Infect. Dis. 2017, 64, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Golden, O.; Caldeira, A.J.; Rangel, L.F.; Santos, M.J. Seafood safety and food-borne zoonoses from fish: Examining the risk of Anisakis in the Portuguese Population and Consumer Risk Perceptions of Fish Consumption. EFSA J. 2022, 20 (Suppl. S1), e200409. [Google Scholar] [CrossRef] [PubMed]
- Asatryan, A.; Mladineo, I.; Santos, M.J. Assessing Portuguese health risks: Anisakis parasite in Atlantic chub mackerel (Scomber colias) sold in Portuguese markets. EFSA J. 2023, 21 (Suppl. S1), e211004. [Google Scholar] [CrossRef] [PubMed]
- Yeak, K.Y.C.; Dank, A.; den Besten, H.M.; Zwietering, M.H. A web-based microbiological hazard identification tool for infant foods. Food Res. Int. 2024, 178, 113940. [Google Scholar] [CrossRef] [PubMed]
- Vidaček, S. Seafood. In Food Safety Management; Motarjemi, Y., Lelieveld, H., Eds.; Academic Press: Waltham, MA, USA; Elsevier: London, UK, 2014; pp. 189–212. [Google Scholar]
- EFSA. Drivers of emerging risks and their interactions in the domain of biological risks to animal, plant and public health: A pilot study. EFSA Support. Publ. 2014, 11, 588E. [Google Scholar]
- Rapid Alert System for Food and Feed (RASFF). Available online: https://food.ec.europa.eu/safety/rasff_en (accessed on 13 February 2024).
- RASFF–Rapid Alert System for Food and Feed. Available online: https://data.europa.eu/data/datasets/restored_rasff?locale=en (accessed on 25 March 2022).
- RASFF Window. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 9 February 2024).
- TIBCO Statistica® User’s Guide. Available online: https://docs.tibco.com/pub/stat/14.0.0/doc/html/UsersGuide/ (accessed on 13 January 2024).
- Web of Science Search. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 3 June 2024).
- Fiorenza, E.; Wendt, C.A.; Dobkowski, K.A.; King, T.L.; Pappaionou, M.; Rabinowitz, P.M.; Samhouri, J.F.; Wood, C.L. It’s a wormy world: Meta-analysis reveals several decades of change in the global abundance of the parasitic nematodes Anisakis spp. and Pseudoterranova spp. in marine fishes and invertebrates. Glob. Chang. Biol. 2020, 26, 2854–2866. [Google Scholar] [CrossRef] [PubMed]
- Gregori, M.; Roura, Á.; Abollo, E.; González, Á.F.; Pascual, S. Anisakis simplex complex (Nematoda: Anisakidae) in zooplankton communities from temperate NE Atlantic waters. J. Nat. Hist. 2015, 49, 755–773. [Google Scholar] [CrossRef]
- Díez, G.; Chust, G.; Andonegi, E.; Santurtún, M.; Abaroa, C.; Bilbao, E.; Maceira, A.; Mendibil, I. Analysis of potential drivers of spatial and temporal changes in anisakid larvae infection levels in European hake, Merluccius merluccius (L.), from the North-East Atlantic fishing grounds. Parasitol. Res. 2022, 121, 1903–1920. [Google Scholar] [CrossRef]
- Mercken, E.; van Damme, I.; Serradell, A.; Gabriël, S. Presence of Anisakidae in commercial fish species imported into the Belgian food markets: A systematic review and meta-analyses. Int. J. Food Microbiol. 2019, 318, 108456. [Google Scholar] [CrossRef]
- Díez, G.; Santos, M.; Boyra, G.; Chust, G.; Santurtún, M.; Maceira, A.; Mendibil, I.; Bilbao, E.; Abaroa, C. Variation in the levels of anisakid infection in the European anchovy Engraulis encrasicolus (Linnaeus) from the Bay of Biscay during the period 2000-2023 (ICES Subarea 8). Parasitol. Res. 2024, 123, 95. [Google Scholar] [CrossRef]
- Mladineo, I.; Šimat, V.; Miletić, J.; Beck, R.; Poljak, V. Molecular identification and population dynamic of Anisakis pegreffii (Nematoda: Anisakidae Dujardin, 1845) isolated from the European anchovy (Engraulis encrasicolus L.) in the Adriatic Sea. Int. J. Food Microbiol. 2012, 157, 224–229. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on assessment of epidemiological data in relation to the health risks resulting from the presence of parasites in wild caught fish from fishing grounds in the Baltic Sea. EFSA J. 2011, 9, 2320. [Google Scholar]
- Eissa, A.E.; Showehdi, M.L.; Ismail, M.M.; El-Naas, A.S.; Abu Mhara, A.A.; Abolghait, S.K. Identification and prevalence of Anisakis pegreffii and A. pegreffii × A. Simplex (s.s.) hybrid genotype larvae in Atlantic horse Mackerel (Trachurus trachurus) from some North African Mediterranean coasts. Egypt. J. Aquat. Res. 2018, 44, 21–27. [Google Scholar] [CrossRef]
- Levsen, A.; Svanevik, C.S.; Cipriani, P.; Mattiucci, S.; Hastie, L.C.; Bušelić, I.; Mladineo, I.; Karl, H.; Ostermeyer, U.; Buchmann, K.; et al. A survey of zoonotic nematodes of commercial key fish species from major European fishing grounds—Introducing the FP7 PARASITE exposure assessment study. Fish. Res. 2018, 202, 4–21. [Google Scholar] [CrossRef]
- Marvin, H.J.; Bouzembrak, Y.; Asselt, E.V.; Meijer, N.; Kleter, G.A.; Lorentzen, G.; Johansen, L.H. Applicability of a food chain analysis on aquaculture of Atlantic salmon to identify and monitor vulnerabilities and drivers of change for the identification of emerging risks. EFSA Support. Publ. 2019, 16, 1619E. [Google Scholar] [CrossRef]
- Rigos, G.; Katharios, P.; Kogiannou, D.; Cascarano, C.M. Infectious diseases and treatment solutions of farmed greater amberjack Seriola dumerili with particular emphasis in Mediterranean region. Rev. Aquacult. 2021, 13, 301–323. [Google Scholar] [CrossRef]
- EFSA. Collection and routine analysis of import surveillance data with a view to identification of emerging risks. EFSA J. 2010, 8, 1531. [Google Scholar]
- EFSA. Development and implementation of a system for the early identification of emerging risks in food and feed. EFSA J. 2010, 8, 1888. [Google Scholar] [CrossRef]
- D’Amico, P.; Malandra, R.; Costanzo, F.; Castigliego, L.; Guidi, A.; Gianfaldoni, D.; Armani, A. Evolution of the Anisakis risk management in the European and Italian context. Food Res. Int. 2014, 64, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Tiozzo, B.; Pinto, A.; Neresini, F.; Sbalchiero, S.; Parise, N.; Ruzza, M.; Ravarotto, L. Food risk communication: Analysis of the media coverage of food risk on Italian online daily newspapers. Qual. Quant. 2019, 53, 2843–2866. [Google Scholar] [CrossRef]
- D’Amico, P.; Nucera, D.M.; Guardone, L.; Mariotti, M.; Nuvoloni, R.; Armani, A. Seafood products notifications in the EU Rapid Alert System for Food and Feed (RASFF) database: Data analysis during the period 2011–2015. Food Control 2018, 93, 241–250. [Google Scholar] [CrossRef]
- Caldeira, A.J.; Alves, C.P.; Santos, M.J. Anisakis notification in fish: An assessment of the cases reported in the European Union rapid alert system for food and feed (RASFF) database. Food Control 2021, 124, 107913. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024; Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- EU Trade Since 1999 by SITC. Available online: https://ec.europa.eu/eurostat/web/main/data/database (accessed on 1 May 2024).
- Guardone, L.; Nucera, D.M.; Lodola, L.; Tinacci, L.; Acutis, P.L.; Guidi, A.; Armani, A. Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int. J. Food Microbiol. 2018, 268, 10–18. [Google Scholar] [PubMed]
- Bao, M.; Pierce, G.J.; Strachan, N.J.; Martínez, C.; Fernández, R.; Theodossiou, I. Consumers’ attitudes and willingness to pay for Anisakis-free fish in Spain. Fish. Res. 2018, 202, 149–160. [Google Scholar] [CrossRef]
- Pekmezci, G.Z. Occurrence of Anisakis simplex sensu stricto in imported Atlantic mackerel (Scomber scombrus) represents a risk for Turkish consumers. Int. J. Food Microbiol. 2014, 185, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, G.Z. Occurrence of Anisakis pegreffii (Nematoda: Anisakidae) Larvae in Imported John Dory (Zeus faber) from Senegalese Coast Sold in Turkish Supermarkets. Acta Parasitol. 2019, 64, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Suzuki, J.; Kodo, Y.; Kobayashi, K.; Sadamasu, K.; Takano, T.; Iwaki, T.; Waki, T.; Ogawa, K. Probable association between Anisakis infection in the muscle of skipjack tuna (Katsuwonus pelamis) and human anisakiasis in Tokyo, Japan. Int. J. Food Microbiol. 2021, 337, 108930. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.; Vilares, A.; Caçador, T.; Martins, S.; Ferreira, I.; Carvalho, L.M.; Gargaté, M.J. Occurrence of larval anisakids in horse mackerel (Trachurus trachurus) caught in Portuguese waters. Parasitol. Res. 2020, 119, 2799–2811. [Google Scholar] [CrossRef] [PubMed]
- De Castelli, L.; Arioli, F.; Bianchi, D.M.; Barbaro, A.; Nobile, M.; Panseri, S.; Chiesa, L.M. An Italian survey of undeclared allergens in food over the years 2014–2018. Food Addit. Contam. B 2020, 13, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Hernández, A.M.; Morales-Yuste, M.; Buzo-Domínguez, S.; Adroher, F.J.; Benítez, R. Anisakis infection in anchovies (Engraulis encrasicolus) from Iberian waters, southwestern Europe: Post-mortem larval migration. Res. Vet. Sci. 2023, 157, 26–34. [Google Scholar] [PubMed]
- Shamsi, S.; Barton, D.P. A critical review of anisakidosis cases occurring globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonso, I.; Carballeda-Sangiao, N.; Rodríguez, S.; Tejada, M.; Navas, A.; Arcos, S.C.; González-Muñoz, M.; Careche, M. Anisakis simplex (s.l.) resistance to the action of gastric enzymes depends upon previous treatments applied to infected fish mince and affects antigen release. J. Sci. Food Agric. 2020, 101, 3908–3916. [Google Scholar] [CrossRef] [PubMed]
- European Parliament, Council of the European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin. OJ L 139, 30.4.2004, 55–205. Available online: https://eur-lex.europa.eu/eli/reg/2004/853/oj (accessed on 25 February 2024).
- Bao, M.; Cipriani, P.; Giulietti, L.; Roiha, I.S.; Paoletti, M.; Palomba, M.; Levsen, A. Air-dried stockfish of Northeast Arctic cod do not carry viable anisakid nematodes. Food Control 2020, 116, 107322. [Google Scholar] [CrossRef]
- Gómez-Mateos Pérez, M.; Navarro Moll, C.; Merino Espinosa, G.; Valero López, A. Evaluation of different Mediterranean essential oils as prophylactic agents in anisakidosis. Pharm. Biol. 2016, 55, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Klapper, R.; Kuhn, T.; Münster, J.; Levsen, A.; Karl, H.; Klimpel, S. Anisakid nematodes in beaked redfish (Sebastes mentella) from three fishing grounds in the North Atlantic, with special notes on distribution in the fish musculature. Vet. Parasitol. 2015, 207, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Franssen, F.; Gérard, C.; Cozma-Petruț, A.; Vieira-Pinto, M.; Jambrak, A.R.; Rowan, N.J.; Paulsen, P.; Różycki, M.; Tysnes, K.R.; Rodríguez-Lázaro, D.; et al. Inactivation of parasite transmission stages: Efficacy of treatments on food of animal origin. Trends Food Sci. Technol. 2019, 83, 114–128. [Google Scholar] [CrossRef]
- Mattiucci, S.; Cipriani, P.; Levsen, A.; Paoletti, M.; Nascetti, G. Molecular Epidemiology of Anisakis and Anisakiasis: An Ecological and Evolutionary Road Map. In Advances in Parasitology; Rollinson, D., Stothard, J.R., Eds.; Elsevier: London, UK, 2018; Volume 99, pp. 93–263. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2018; pp. 53–107. [Google Scholar]
- Bao, M.; Pierce, G.J.; Strachan, N.J.; Pascual, S.; González-Muñoz, M.; Levsen, A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: Challenges in risk assessment. Trends Food Sci. Technol. 2019, 86, 298–310. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Zhu, Y.; Wang, Y. Seafood allergy: Occurrence, mechanisms and measures. Trends Food Sci. Technol. 2019, 88, 80–92. [Google Scholar] [CrossRef]
- Trevisan, C.; Torgerson, P.R.; Robertson, L.J. Foodborne Parasites in Europe: Present Status and Future Trends. Trends Parasitol. 2019, 35, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Deksne, G.; Lalle, M.; Elwin, K.; Schares, G.; Troell, K. Why do we need training?—A “Training school on molecular methods used for foodborne parasite diagnostics in different matrices” is a example of knowledge transfer to foster research quality in EU. Exp. Parasitol. 2020, 211, 107863. [Google Scholar] [CrossRef] [PubMed]
- World Register on Marine Species. Available online: https://www.marinespecies.org/ (accessed on 18 February 2024).



| Parasite | Notifications | Parasite | Notifications |
|---|---|---|---|
| Anisakis spp. | 651 | Sarcocystis spp. | 3 |
| Nematodes | 41 | Acanthocephalus spp. | 2 |
| Pseudoterranova spp. | 14 | Kudoa spp. | 2 |
| Trichinella spp. | 10 | Coccidia | 1 |
| Tapeworms | 9 | Echinococcus granulosus | 1 |
| Microsporidia | 7 | Insects | 1 |
| Cysticercus bovis | 4 | Myxobolus spp. | 1 |
| Trematodes | 4 | Trypanosoma spp. | 1 |
| Hysterothylacium spp. | 3 | Not specified | 127 |
| Plerocercoids | 3 | Total | 885 |
| Product | Species (Notifications) |
|---|---|
| Mackerel | Atlantic mackerel (Scomber scombrus) (109), Pacific mackerel (Scomber japonicus) (9), Atlantic horse mackerel (Trachurus trachurus) (3), Mediterranean horse mackerel (Trachurus mediterraneus) (1), Chilean jack mackerel (Trachurus murphyi) (1) and species not specified (46) |
| Hake | European hake (Merluccius merluccius) (56), Argentine hake (Merluccius hubbsi) (16), Southern hake (Merluccius australis) (2), Atlantic hake (Merluccius bilinearis) (2), South African hake (Merluccius capensis) (1) and species not specified (51) |
| Anglerfish | European anglerfish (Lophius piscatorius) (81), American anglerfish (Lophius americanus) (2), blackbellied anglerfish (Lophius budegassa) (1) and species not specified (22) |
| Scabbardfish | Silver scabbardfish (Lepidopus caudatus) (35) and species not specified (2) |
| Cod | Atlantic cod (Gadus morhua) (13), Pacific cod (Gadus macrocephalus) (1) and species not specified (21) |
| Anchovies | European anchovy (Engraulis encrasicolus) (14) and species not specified (19) |
| Squid | Nototodarus spp. (not specified) (20) and other species not specified (1) |
| John Dory | John Dory or also Peter’s fish (Zeus faber) (17) and species not specified (2) |
| Salmon | Pink salmon (Oncorhynchus gorbuscha) (2) and species not specified (8) |
| Herring | Atlantic herring (Clupea harengus) (2) and species not specified (7) |
| Swordfish | Swordfish (Xiphias gladius) (3) and species not specified (6) |
| Other | 79 |
| Total | 651 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigłowski, M. Notifications on Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF) Reported in 2001–2023. Sustainability 2024, 16, 5453. https://doi.org/10.3390/su16135453
Pigłowski M. Notifications on Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF) Reported in 2001–2023. Sustainability. 2024; 16(13):5453. https://doi.org/10.3390/su16135453
Chicago/Turabian StylePigłowski, Marcin. 2024. "Notifications on Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF) Reported in 2001–2023" Sustainability 16, no. 13: 5453. https://doi.org/10.3390/su16135453
APA StylePigłowski, M. (2024). Notifications on Anisakis spp. in the Rapid Alert System for Food and Feed (RASFF) Reported in 2001–2023. Sustainability, 16(13), 5453. https://doi.org/10.3390/su16135453






