Valorization of Hibiscus Flower (Hibiscus sabdariffa L.) Anthocyanins to Produce Sustainable Spray-Dried Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hibiscus-Flower Flour (HFF)
2.3. Preliminary Evaluation of Anthocyanins Extraction
2.3.1. Anthocyanin (ANC) Quantification
2.4. Optimization of Anthocyanins Extraction
2.5. Optimization of Production by Spray Drying
Solids Recovery (%) and ANC Retention (%)
2.6. Oxidative Stability Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Evaluation of ANC Extraction
3.2. Anthocyanin Recovery from HFF
3.3. Production of Spray-Dried Anthocyanin-Rich Components
3.4. Rancimat Test—Assessment of Oxidative Stability Index
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johansson, L.; Jonasson, I.; Reim, W. Sustainable Expansion: Capabilities for “New Food Source”—Companies. Future Foods 2023, 8, 100259. [Google Scholar] [CrossRef]
- Sivaraman, C.M.; Saju, F. Medicinal Value of Hibiscus Rosa Sinensis: A Review. Int. J. Pharmacogn. Chem. 2021, 2, 1–11. [Google Scholar] [CrossRef]
- Solangaarachchi, M.; Subasinghe, D.; Suraweera, M.; Suraweera, N.; Dilshani, N.; Senadheera, S. Home-made Hibiscus rosasinensis tea on post-prandial blood glucose level and blood pressure: An interventional study. Anuradhapura Med. J. 2022, 16, 4. [Google Scholar] [CrossRef]
- Dhobale, A.D.; Wakale, N.S. A Pharmacological Review on Hibiscus Rosa-Sinensis. Int. J. Adv. Res. Sci. Commun. Technol. 2022, 6, 1046–1053. [Google Scholar]
- Dini, C.; Zaro, M.J.; Viña, S.Z. Bioactivity and Functionality of Anthocyanins: A Review. Curr. Bioact. Compd. 2019, 15, 507–523. [Google Scholar] [CrossRef]
- Yücetepe, M.; Tuğba Özaslan, Z.; Karakuş, M.Ş.; Akalan, M.; Karaaslan, A.; Karaaslan, M.; Başyiğit, B. Unveiling the Multifaceted World of Anthocyanins: Biosynthesis Pathway, Natural Sources, Extraction Methods, Copigmentation, Encapsulation Techniques, and Future Food Applications. Food Res. Int. 2024, 187, 114437. [Google Scholar] [CrossRef] [PubMed]
- Geeganage, J.R.; Gunathilaka, M.D.T.L. Mechanistic Insight into Anti-Inflammatory Potential of Hibiscus Rosa-Sinensis Flower Extract as a Herbal Remedy: A Systematic Review. J. Herb. Med. 2024, 45, 100884. [Google Scholar] [CrossRef]
- Oliveira, M.F.S.; de Figueiredo, J.A.; Norcino, L.B.; Botrel, D.A.; Borges, S.V. Potential Use of Red Hibiscus Flower Extract for the Production of Spray-Chilled Microparticles: Characterization, Stability, and Bioaccessibility in Vitro of Anthocyanins. Food Res. Int. 2023, 174, 113570. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Polekkad, A.; Franklin, M.E.E.; Pushpadass, H.A.; Battula, S.N.; Rao, S.B.N.; Pal, D.T. Microencapsulation of Zinc by Spray-Drying: Characterisation and Fortification. Powder Technol. 2021, 381, 1–16. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Lila, M.A. Comparison of Berry Juice Concentrates and Pomaces and Alternative Plant Proteins to Produce Spray Dried Protein-Polyphenol Food Ingredients. Food Funct. 2019, 10, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, R.T.; Plundrich, N.; Vargochik, A.; Lila, M.A. Continuous Flow Microwave-Assisted Aqueous Extraction of Pomace Phytoactives for Production of Protein-Polyphenol Particles and a Protein-Enriched Ready-to-Drink Beverage. Future Foods 2022, 5, 100137. [Google Scholar] [CrossRef]
- Grace, M.H.; Hoskin, R.; Xiong, J.; Lila, M.A. Whey and Soy Proteins as Wall Materials for Spray Drying Rosemary: Effects on Polyphenol Composition, Antioxidant Activity, Bioaccessibility after in Vitro Gastrointestinal Digestion and Stability during Storage. LWT 2021, 149, 111901. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green Biopolymers from By-Products as Wall Materials for Spray Drying Microencapsulation of Phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Shen, J.; Li, Z.; Tan, S.; Liu, W.; Cheng, Z. Choosing the Appropriate Wall Materials for Spray-Drying Microencapsulation of Natural Bioactive Ingredients: Taking Phenolic Compounds as Examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Ferraz, M.C.; Procopio, F.R.; de Furtado, G.F.; Hubinger, M.D. Co-Encapsulation of Paprika and Cinnamon Oleoresin by Spray Drying Using Whey Protein Isolate and Maltodextrin as Wall Material: Development, Characterization and Storage Stability. Food Res. Int. 2022, 162, 112164. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Sanches Silva, A. Natural Pigments from Food Wastes: New Approaches for the Extraction and Encapsulation. Curr. Opin. Green Sustain. Chem. 2024, 47, 100929. [Google Scholar] [CrossRef]
- Eijkelboom, N.M.; van Boven, A.P.; Siemons, I.; Wilms, P.F.C.; Boom, R.M.; Kohlus, R.; Schutyser, M.A.I. Particle Structure Development during Spray Drying from a Single Droplet to Pilot-Scale Perspective. J. Food Eng. 2023, 337, 111222. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Pinela, J.; Prieto, M.A.; Pereira, E.; Jabeur, I.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Optimization of Heat- and Ultrasound-Assisted Extraction of Anthocyanins from Hibiscus Sabdariffa Calyces for Natural Food Colorants. Food Chem. 2019, 275, 309–321. [Google Scholar] [CrossRef]
- Escobar-Ortiz, A.; Castaño-Tostado, E.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Reynoso-Camacho, R. Anthocyanins extraction from Hibiscus sabdariffa and identification of phenolic compounds associated with their stability. J. Sci. Food Agric. 2021, 101, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Navidad-Murrieta, M.S.; Pérez-Larios, A.; Sanchéz-Burgos, J.A.; Ragazzo-Sánchez, J.A.; Luna-Bárcenas, G.; Sáyago-Ayerdi, S.G. Use of a Taguchi Design in Hibiscus Sabdariffa Extracts Encapsulated by Spray-Drying. Foods 2020, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.; Wrolsted, R. Anthocyanins: Characterization and Measurement with UV-Visible Sprectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 1–13. [Google Scholar]
- Li, X.; Guo, M.; Xue, Y.; Duan, Z. Effect of Extraction Methods on the Physicochemical Properties, Chemical Composition, and Antioxidant Activities of Samara Oil. Foods 2023, 12, 3163. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin Extraction from Plant Tissues: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.; Pinela, J.; Abreu, R.M.V.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Saldanha, A.L.; Alves, M.J.; Nogueira, A.; Ferreira, I.C.F.R.; Barros, L. Extraction of Anthocyanins from Red Raspberry for Natural Food Colorants Development: Processes Optimization and In Vitro Bioactivity. Processes 2020, 8, 1447. [Google Scholar] [CrossRef]
- Johnson, J.; Collins, T.; Walsh, K.; Naiker, M. Solvent Extractions and Spectrophotometric Protocols for Measuring the Total Anthocyanin, Phenols and Antioxidant Content in Plums. Chem. Pap. 2020, 74, 4481–4492. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Basílio, N.; Pina, F. Chemistry and Photochemistry of Anthocyanins and Related Compounds: A Thermodynamic and Kinetic Approach. Molecules 2016, 21, 1502. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, I.B.; Freitas, A.; Maçanita, A.L.; Lima, J.C. Effect of Water Content on the Acid–Base Equilibrium of Cyanidin-3-Glucoside. Food Chem. 2015, 172, 476–480. [Google Scholar] [CrossRef]
- Dos Santos, S.S.; de Magalhães, F.S.; Paraíso, C.M.; Ogawa, C.Y.L.; Sato, F.; de Santos Junior, O.O.; Visentainer, J.V.; Madrona, G.S.; Reis, M.H.M. Enhanced Conditions for Anthocyanin Extraction from Blackberry Pomace under Ultrasound Irradiation. J. Food Process. Eng. 2023, 46, e14077. [Google Scholar] [CrossRef]
- Samborska, K.; Poozesh, S.; Barańska, A.; Sobulska, M.; Jedlińska, A.; Arpagaus, C.; Malekjani, N.; Jafari, S.M. Innovations in Spray Drying Process for Food and Pharma Industries. J. Food Eng. 2022, 321, 110960. [Google Scholar] [CrossRef]
- Gawałek, J. Effect of Spray Dryer Scale Size on the Properties of Dried Beetroot Juice. Molecules 2021, 26, 6700. [Google Scholar] [CrossRef] [PubMed]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-Known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Strobel, S.A.; Scher, H.B.; Nitin, N.; Jeoh, T. Control of Physicochemical and Cargo Release Properties of Cross-Linked Alginate Microcapsules Formed by Spray-Drying. J. Drug Deliv. Sci. Technol. 2019, 49, 440–447. [Google Scholar] [CrossRef]
- Schlindweinn, E.B.; Chacon, W.D.C.; Koop, B.L.; de Matos Fonseca, J.; Monteiro, A.R.; Valencia, G.A. Starch-Based Materials Encapsulating Anthocyanins: A Review. J. Polym. Environ. 2022, 30, 3547–3565. [Google Scholar] [CrossRef]
- Baltrusch, K.L.; Torres, M.D.; Domínguez, H.; Flórez-Fernández, N. Spray-Drying Microencapsulation of Tea Extracts Using Green Starch, Alginate or Carrageenan as Carrier Materials. Int. J. Biol. Macromol. 2022, 203, 417–429. [Google Scholar] [CrossRef]
- Vergara, C.; Pino, M.T.; Zamora, O.; Parada, J.; Pérez, R.; Uribe, M.; Kalazich, J. Microencapsulation of Anthocyanin Extracted from Purple Flesh Cultivated Potatoes by Spray Drying and Its Effects on In Vitro Gastrointestinal Digestion. Molecules 2020, 25, 722. [Google Scholar] [CrossRef] [PubMed]
- Dos Martins, D.R.S.; Sanjinez-Argandoña, E.J.; de Ortega, N.F.; dos Garcia, V.A.S.; Oliveira, V.S.; Cardoso, C.A.L. Production and Characterization of Hibiscus sabdariffa by Spray Dryer Using Different Sprinkler Nozzles and Carrier Agents. J. Food Process. Preserv. 2020, 44, e14493. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Grace, M.H.; Xiong, J.; Lila, M.A. Spray-drying Microencapsulation of Blackcurrant and Cocoa Polyphenols Using Underexplored Plant-based Protein Sources. J. Food Sci. 2023, 88, 2665–2678. [Google Scholar] [CrossRef]
- Nguyen, Q.-D.; Dang, T.-T.; Nguyen, T.-V.-L.; Nguyen, T.-T.-D.; Nguyen, N.-N. Microencapsulation of Roselle (Hibiscus sabdariffa L.) Anthocyanins: Effects of Different Carriers on Selected Physicochemical Properties and Antioxidant Activities of Spray-Dried and Freeze-Dried Powder. Int. J. Food Prop. 2022, 25, 359–374. [Google Scholar] [CrossRef]
- De Medeiros, F.G.M.; Pereira, G.B.C.; da Pedrini, M.R.S.; Hoskin, R.T.; Nunes, A.O. Evaluation of the Environmental Performance of the Production of Polyphenol-Rich Fruit Powders: A Case Study on Acerola. J. Food Eng. 2024, 372, 112010. [Google Scholar] [CrossRef]
- de Souza Correa, M.; Boschen, N.L.; Rodrigues, P.R.P.; Corazza, M.L.; de Paula Scheer, A.; Ribani, R.H. Supercritical CO2 with Co-Solvent Extraction of Blackberry (Rubus spp. Xavante Cultivar) Seeds. J. Supercrit. Fluids 2022, 189, 105702. [Google Scholar] [CrossRef]
- de Souza, D.R.; Willems, J.L.; Low, N.H. Phenolic Composition and Antioxidant Activities of Saskatoon Berry Fruit and Pomace. Food Chem. 2019, 290, 168–177. [Google Scholar] [CrossRef]
- Serrano-Díaz, J.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the Applications of Crocus sativus Flowers as Natural Antioxidants. J. Food Sci. 2012, 77, C1162–C1168. [Google Scholar] [CrossRef]

| Experimental Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Temperature (°C) | 31 | 35 | 39 |
| pH | 1.5 | 2.3 | 3.2 |
| EtOH concentration (% v/v) | 44 | 50 | 56 |
| Extraction time (h) | 1.6 | 4.0 | 6.4 |
| Solid-to-liquid ratio (% w/v) | 19 | 23 | 27 |
| Experimental Factors | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| EtOH concentration (% v/v) | 39.9 | 44 | 50 | 56 | 60.1 |
| Extraction time (h) | 0.1 | 1.6 | 4.0 | 6.4 | 8 |
| Experimental Factors | Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| Carrier ratio (% w/v) | 0.6 | 1.0 | 2.0 | 3.0 | 3.4 |
| Inlet temperature (°C) | 153.4 | 160 | 176 | 192 | 198.6 |
| Factor | df | SS | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 5 | 1.424 × 107 | 2.849 × 106 | 4.39 | 0.0146 * |
| A: Temperature (°C) | 1 | 1.568 × 105 | 1.568 × 105 | 0.2416 | 0.6312 |
| B: pH | 1 | 4.442 × 105 | 4.442 × 105 | 0.6844 | 0.4230 |
| C: EtOH concentration (% v/v) | 1 | 3.907 × 106 | 3.907 × 106 | 6.02 | 0.0290 * |
| D: Extraction time (h) | 1 | 8.034 × 106 | 8.034 × 106 | 12.38 | 0.0038 ** |
| E: S/L ratio (% w/v) | 1 | 1.702 × 106 | 1.702 × 106 | 2.62 | 0.1294 |
| Residual | 13 | 8.438 × 106 | 6.491 × 105 | ||
| Lack of Fit | 11 | 8.217 × 106 | 7.470 × 105 | 6.75 | 0.1360 |
| Pure Error | 2 | 2.213 × 105 | 1.106 × 105 | ||
| Cor Total | 18 | 2.268 × 107 |
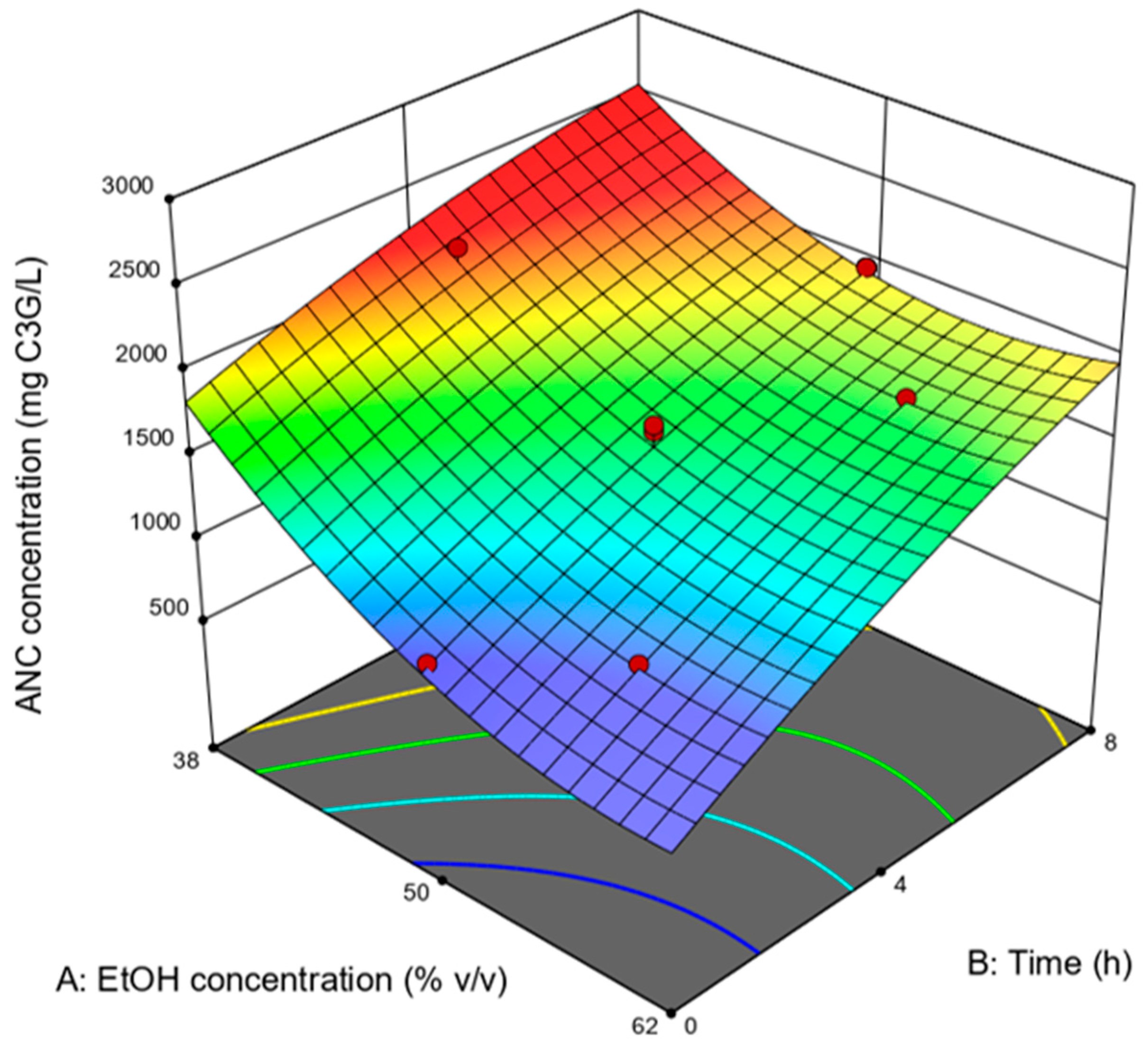
| EtOH Concentration (% v/v) | Time (h) | ANC Concentration (mg C3G/L) |
|---|---|---|
| 50 † | 4 † | 1453 |
| 50 | 8 | 1956 |
| 50 † | 4 † | 1415 |
| 44 | 6.4 | 1963 |
| 50 † | 4 † | 1654 |
| 50 † | 4 † | 1500 |
| 50 | 0.1 | 951 |
| 60.1 | 4 | 1337 |
| 44 | 1.6 | 1445 |
| 56 | 6.4 | 1724 |
| 56 | 1.6 | 1020 |
| 39.9 | 4 | 2211 |
| 50 † | 4 † | 1616 |
| Carrier Ratio (% w/v) | Inlet Temperature (°C) | Solids Recovery (%) | ANC Retention (%) |
|---|---|---|---|
| 1 | 160 | 50.0 | 88.7 |
| 2 | 198.6 | 72.5 | 92.7 |
| 0.6 | 176 | 59.3 | 85.7 |
| 3 | 192 | 59.3 | 94.8 |
| 2 † | 176 † | 65.3 | 93.5 |
| 2 † | 176 † | 63.0 | 93.7 |
| 3.4 | 176 | 57.4 | 96.0 |
| 2 † | 176 † | 60.6 | 94.3 |
| 1 | 192 | 58.0 | 88.4 |
| 3 | 160 | 56.7 | 95.1 |
| 2 | 153.4 | 46.5 | 94.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, D.A.; Vargas, N.; Osorio-Doblado, A.M.; Ruano-Ortiz, J.A.; Medeiros, F.G.M.d.; Hoskin, R.T.; Moncada, M. Valorization of Hibiscus Flower (Hibiscus sabdariffa L.) Anthocyanins to Produce Sustainable Spray-Dried Ingredients. Sustainability 2024, 16, 5523. https://doi.org/10.3390/su16135523
Vargas DA, Vargas N, Osorio-Doblado AM, Ruano-Ortiz JA, Medeiros FGMd, Hoskin RT, Moncada M. Valorization of Hibiscus Flower (Hibiscus sabdariffa L.) Anthocyanins to Produce Sustainable Spray-Dried Ingredients. Sustainability. 2024; 16(13):5523. https://doi.org/10.3390/su16135523
Chicago/Turabian StyleVargas, David A., Nathaly Vargas, Andrea M. Osorio-Doblado, Juan A. Ruano-Ortiz, Fábio G. M. de Medeiros, Roberta T. Hoskin, and Marvin Moncada. 2024. "Valorization of Hibiscus Flower (Hibiscus sabdariffa L.) Anthocyanins to Produce Sustainable Spray-Dried Ingredients" Sustainability 16, no. 13: 5523. https://doi.org/10.3390/su16135523






