Novel Copper Alginate Microspheres as Ecological Fungicides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Copper Alginate Microsphere Preparation
2.1.2. Preparation of Pathogens
2.2. Methods
2.2.1. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.2.2. Physicochemical Characterization of Copper Alginate Microspheres
Encapsulation Efficiency, Loading Capacity and Swelling Degree
In Vitro Release of Cu Ions from Copper Alginate Microspheres
Rheological Measurements
2.2.3. Microscopic Observations
2.2.4. The Electrostatic Charge and Size of Fungal Spores Suspended in Water and Copper Ions Solutions
2.2.5. Testing the Antifungal Effect of Copper Alginate Microspheres
Effect of Copper Alginate Microspheres on B. cinerea Conidia Germination
Antifungal Effect of Alginate Copper Microspheres on Mycelium Growth of C. beticola
Antifungal Effect of Copper Alginate Microspheres on Infestations of Rhododendron sp. Leaves by P. ramorum
Data Analysis
3. Results and Discussion
3.1. Copper Alginate Microsphere Physicochemical Properties
3.1.1. Identification of Interactions between Microsphere Constituents
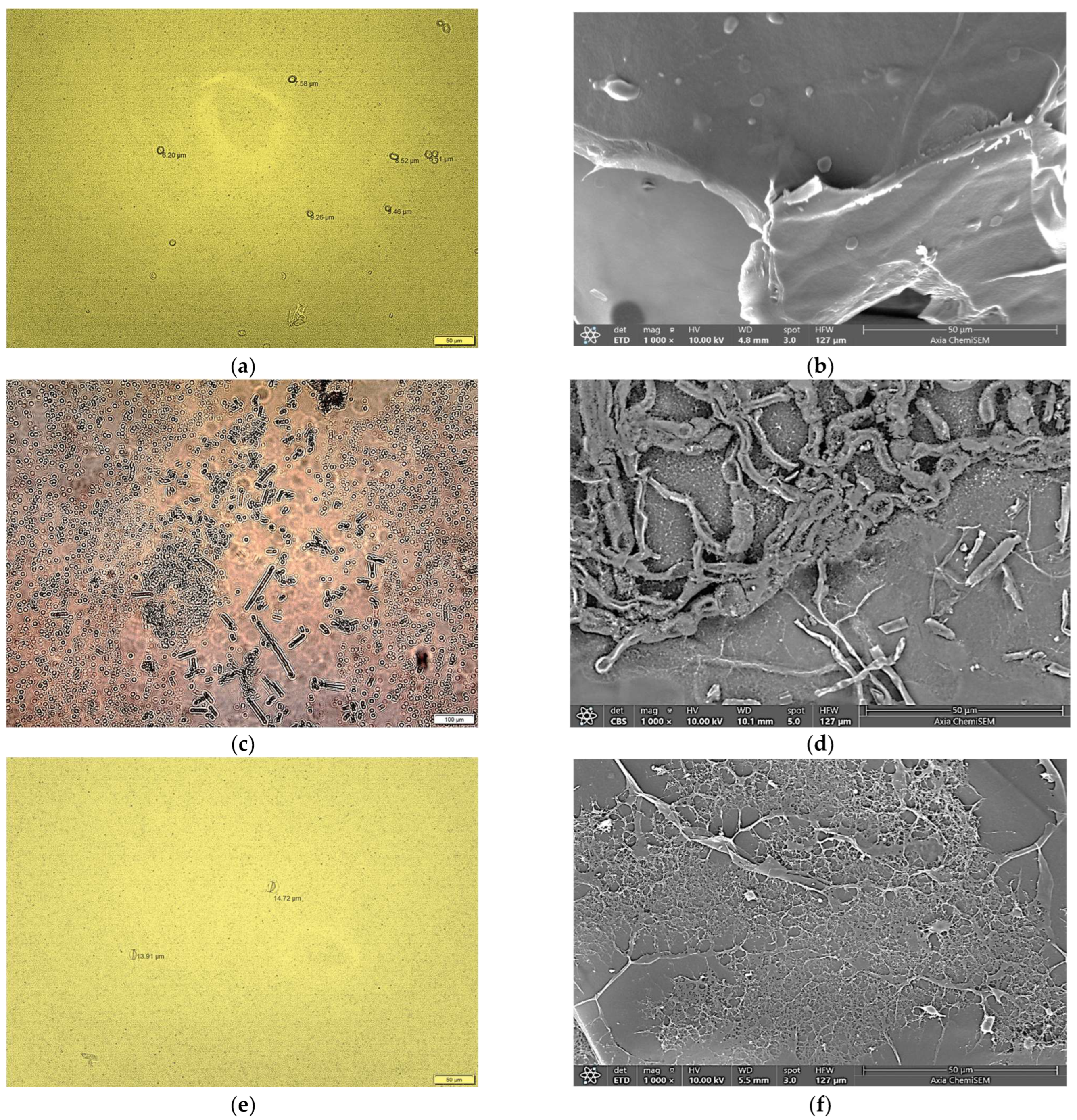
3.1.2. Morphology of Copper Alginate Microspheres
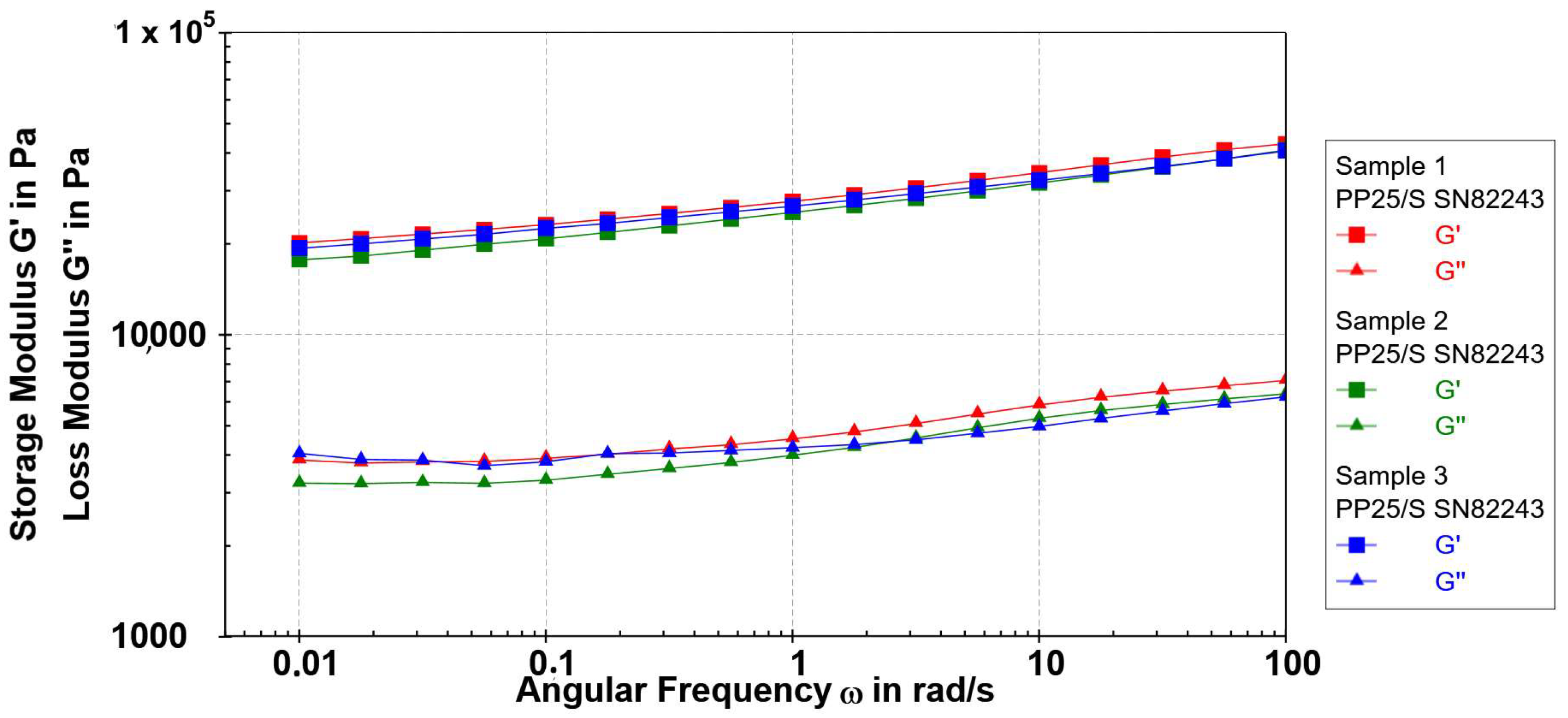
3.1.3. Rheological Properties of Copper Alginate Microspheres
Amplitude Sweep
Frequency Sweep
Temperature Sweep
3.1.4. In Vitro Copper Ions Release from Copper Alginate Microspheres
3.2. Antifungal Effect of Copper Alginate Microspheres on B. cinerea C. beticola and P. ramorum
3.2.1. Fourier Transformed Infrared (FTIR-ATR) Spectroscopy of B. cinerea, C. beticola and P. ramorum Mycelium
3.2.2. Morphological Properties of B. cinerea, C. beticola and P. ramorum Spores and Mycelium
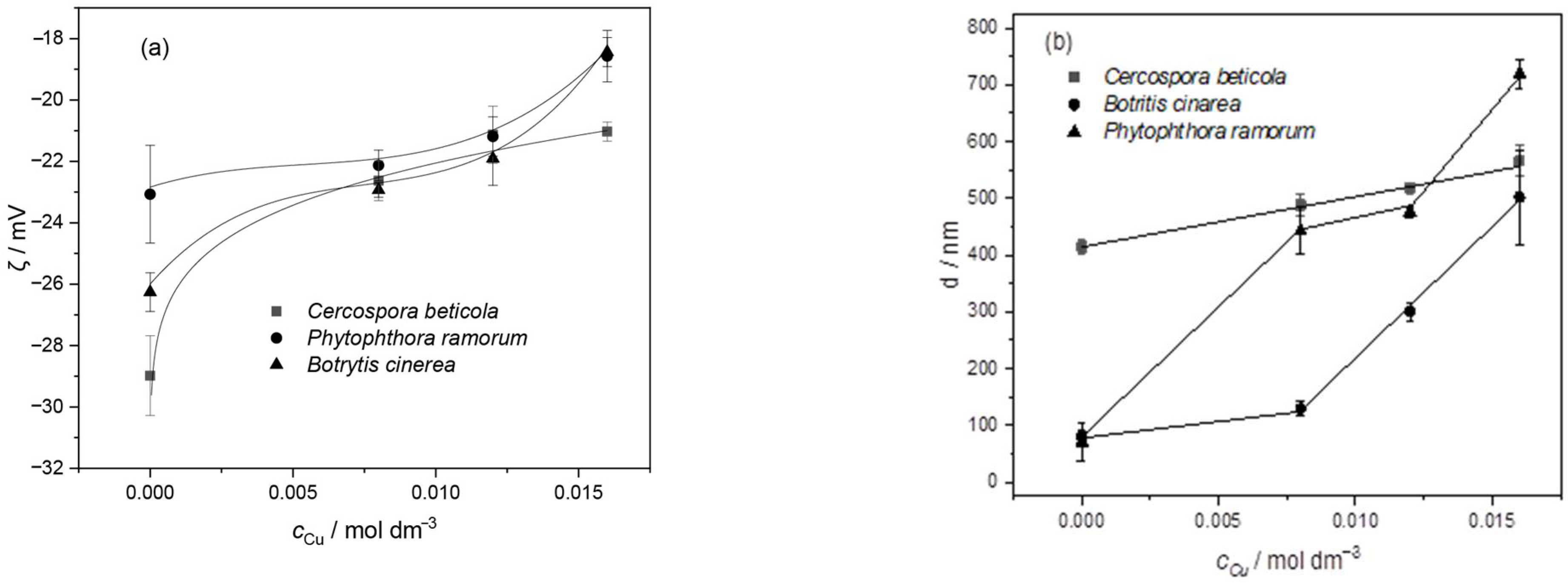
3.2.3. Influence of Copper Ion Concentrations on the Electrostatic Charge and Size of Fungal Spores
3.2.4. Antifungal Effect of Copper Alginate Microspheres on B. cinerea, C. beticola and P. ramorum
Antifungal Effect of Copper Alginate Microspheres on B. cinerea
Antifungal Effect of Copper Alginate Microspheres on C. beticola
Antifungal Effect of Copper Alginate Microspheres on P. ramorum
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 2017, 5, 703–726. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Li, H.; Li, H.; Wang, L.; Lyu, J.; Yang, L.; Tong, S.; Yu, Q.J.; Ruan, H.D.; Atabila, A.; et al. Exposure Routes and Health Risks Associated with Pesticide Application. Toxics 2022, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Pariona, N.; Mtz-Enriquez, A.; Sanchez-Rangel, D.; Carrion, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D. Copper in Horticulture. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Thajuddin, N., Panneerselvam, A., Eds.; Intec Open: London, UK, 2012; Chapter 13; pp. 257–278. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Kohl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- La, T.A.; Iovinom, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Plante, S.; Normant, V.; Ramos-Torres, K.M.; Labbé, S. Cell-surface copper transporters and superoxide dismutase 1 are essential for outgrowth during fungal spore germination. J. Biol. Chem. 2017, 292, 11896–11914. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflugers Arch. Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Wang, Q.; Saleem, M.H.; Bashir, S.; Ullah, S.; Peng, D. Copper environmental toxicology, recent advances, and future outlook: A review. Environ. Sci. Pollut. Res. 2019, 26, 18003–18016. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application. A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jalšenjak, N.; Topolovec-Pintarić, S.; Đermić, E.; Bujan, M.; Jurić, S. Encapsulation of Biological and Chemical Agents for Plant Nutrition and Protection: Chitosan/Alginate Microcapsules Loaded with Copper Cations and Trichoderma viride. J. Agric. Food Chem. 2016, 64, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jurić, S.; Vlahovićek-Kahlina, K.; Martinko, K.; Šegota, S.; Marijan, M.; Krčelić, A.; Svečnjak, L.; Majdak, M.; Nemet, I.; et al. Novel Zinc/Silver Ions-Loaded Alginate/Chitosan Microparticles Antifungal Activity against Botrytis cinerea. Polymers 2023, 15, 4359. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Parkin, I.O.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Vivier, M.; Fillinger, S. Botrytis, the Good, the Bad and the Ugly. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–15. [Google Scholar] [CrossRef]
- De Miccolis, A.; Pollastro, S.; Faretra, F. Genetics of Botrytis cinerea. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 433–455. [Google Scholar] [CrossRef]
- Notte, A.M.; Plaza, V.; Marambio-Alvarado, B.; Olivares-Urbina, L.; Poblete-Morales, M.; Silva-Moreno, E.; Castillo, L. Molecular identification and characterization of Botrytis cinerea associated to the endemic flora of semi-desert climate in Chile. Curr. Res. Microb. Sci. 2021, 2, 100049. [Google Scholar] [CrossRef] [PubMed]
- CABI. Cercospora beticola (Cercospora Leaf Spot of Beets). Invasive Species, Compendium. 2022. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.12191 (accessed on 16 July 2022).
- Rangel, L.I.; Spanner, R.E.; Ebert, M.K.; Pethybridge, S.J.; Stukenbrock, E.H.; de Jonge, R.; Secor, G.A.; Bolton, M.D. Cercospora beticola: The intoxicating lifestyle of the leaf spot pathogen of sugar beet. Mol. Plant Pathol. 2020, 21, 1020–1041. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mazakova, J.; Ali, A.; Sur, V.P.; Sen, M.K.; Bolton, M.D.; Manasova, M.; Rysanek, P.; Zouhar, M. Characterization of the Molecular Mechanisms of Resistance against DMI Fungicides in Cercospora beticola Populations from the Czech Republic. J. Fungi 2021, 7, 1062. [Google Scholar] [CrossRef] [PubMed]
- Rolando, C.; Somchit, C.; Bader, M.K.-F.; Fraser, S.; Williams, N. Can Copper Be Used to Treat Foliar Phytophthora Infections in Pinus radiata? Plant Dis. 2019, 103, 1809–2147. [Google Scholar] [CrossRef] [PubMed]
- Coque, J.J.R.; Álvarez-Pérez, J.M.; Cobos, R.; González-García, S.; Ibáñez, A.M.; Diez Galán, A.; Calvo-Peña, C. Advances in the control of phytopathogenic fungi that infect crops through their root system. Adv. Appl. Microbiol. 2020, 111, 123–170. [Google Scholar] [CrossRef] [PubMed]
- Werres, S.; Marwitz, R.; Man In’t veld, W.A.; De Cock, A.W.A.M.; Bonants, P.J.M.; De Weerdt, M.; Baayen, R.P. Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycool. Res. 2001, 105, 1155–1165. [Google Scholar] [CrossRef]
- European Food Safety Authority. Peer review of the pesticide risk assessment of the active substance copper compounds copper(I), copper(II) variants namely copper hydroxide, copper oxychloride, tribasic copper sulfate, copper(I) oxide, Bordeaux mixture. EFSA J. 2018, 16, e05152. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Centr. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Vinceković, M.; Topolovec Pintarić, S.; Jurić, S.; Viskić, M.; Jalšenjak, N.; Bujan, M.; Đermić, E.; Žutić, I.; Fabek, S. Release of Trichoderma viride Spores from Microcapsules Simultaneously Loaded with Chemical and Biological Agents. Agric. Consp. Sci. 2017, 82, 395–401. [Google Scholar]
- Gamo, I. Infrared Absorption Spectra of Water of Crystallization in Copper Sulfate Penta- and Monohydrate Crystals. Bull. Chem. Soc. Jpn. 1961, 34, 764–766. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric. 2019, 18, 2–16. [Google Scholar] [CrossRef]
- Haug, A.; Smidsrod, O.A.; Högdahl, B.; Øye, H.A.; Rasmussen, S.E.; Sunde, E.; Sörensen, N.A. Selectivity of Some Anionic Polymers for Divalent Metal Ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef]
- De Souza, J.F.; Aquino, T.F.; Nascimento, J.E.; Jacob, R.G.; Fajardo, A.R. Alginate-copper microspheres as efficient and reusable heterogeneous catalysts for the one-pot synthesis of 4-organylselanyl-1H-pyrazoles. Catal. Sci. Technol. 2020, 10, 3918–3930. [Google Scholar] [CrossRef]
- Rashedy, S.H.; Abd El Hafez, M.S.M.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and Characterization of Alginate Extracted from Brown Seaweed Collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Lu, L.; Liu, X.; Tong, Z. Critical exponents for sol–gel transition in aqueous alginate solutions induced. Carbohydr. Polym. 2006, 65, 544–551. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Šegota, S.; Vinceković, M. Influence of surface morphology and structure of alginate microparticles on the bioactive agents release behavior. Carbohydr. Polym. 2019, 218, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.G.; Zheng, J.N.; Li, X.X.; Liu, X.D.; Zhu, J.; Wang, F.; Xie, W.Y.; Ma, X.J. Effect of surface morphology and charge on the amount and conformation of fibrinogen adsorbed onto alginate/chitosan microcapsules. Langmuir 2010, 26, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Singh, S.K.; Bajpai, J.; Bajpai, J.K. Controlled pesticide release from biodegradable Polymers. Cent. Eur. J. Chem. 2014, 12, 453–469. [Google Scholar] [CrossRef]
- Gray, A.; Egan, S.J.; Bakalis, S.; Zhang, Z. Determination of microcapsule physicochemical, structural, and mechanical properties. Particuology 2016, 24, 32–43. [Google Scholar] [CrossRef]
- Cheng, W.; Dunn, P.F.; Brach, R.M. Surface roughness effects on microparticle adhesion. J. Adhesion. 2002, 78, 929–965. [Google Scholar] [CrossRef]
- Leick, S.; Kott, M.; Degen, P.; Henning, S.; Päsler, T.; Suter, D.; Rehage, H. Mechanical properties of liquid-filled shellac composite capsules. Phys. Chem. Chem. Phys. PCCP 2011, 13, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Malektaj, H.; Drozdov, A.D.; de Claville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jurić, S.; Đermić, E.; Topolovec Pintarić, S. Kinetics and Mechanisms of Chemical and Biological Agents Release from Biopolymeric Microcapsules. J. Agric. Food Chem. 2017, 65, 9608–9617. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Ruiz, M.E. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of Drug Release. In The ADME Encyclopedia; Springer: Cham, Switzerland, 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Mularczyk-Oliwa, M.; Bombalska, A.; Kaliszewski, M.; Włodarski, M.; Kwaśny, M.; Kopczyński, K.; Szpakowska, M.; Trafny, E.A. Rapid discrimination of several fungus species with FTIR spectroscopy and statistical analysis. Biul. Wojsk. Akad. Tech. 2013, 62, 71–80. [Google Scholar]
- Salman, A.; Pomerantz, A.; Tsror, L.; Lapidot, I.; Moreh, R.; Mordechai, S.; Huleihel, M. Utilizing FTIR-ATR spectroscopy for classification and relative spectral similarity evaluation of different Colletotrichum coccodes isolates. Analyst 2012, 137, 3558–3564. [Google Scholar] [CrossRef] [PubMed]
- Skolik, P.; Morais, C.L.M.; Martin, F.L.; McAinsh, M.R. Attenuated total reflection Fourier-transform infrared spectroscopy coupled with chemometrics directly detects pre- and post-symptomatic changes in tomato plants infected with Botrytis cinerea. Vib. Spectrosc. 2020, 111, 103171. [Google Scholar] [CrossRef]
- Wittayapipath, K.; Laolit, S.; Yenjai, C.; Chio-Srichan, S.; Pakarasang, M.; Tavichakorntrakool, R.; Prariyachatigul, C. Analysis of xanthyletin and secondary metabolites from Pseudomonas stutzeri ST1302 and Klebsiella pneumoniae ST2501 against Pythium insidiosum. BMC Microbiol. 2019, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Lapidot, I.; Pomerantz, A.; Tsror, L.; Shufan, E.; Moreh, R.; Mordechai, S.; Huleihel, M. Identification of fungal phytopathogens using Fourier transform infrared-attenuated total reflection spectroscopy and advanced statistical methods. J. Biomed. Opt. 2012, 17, 017002. [Google Scholar] [CrossRef]
- Szeghalmi, A.; Kaminskyj, S.; Gough, K.M. A synchrotron FTIR microspectroscopy investigation of fungal hyphae grown under optimal and stressed conditions. Anal. Bioanal. Chem. 2007, 387, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Tao, Q.J.; Zheng, X.; Li, Y.; Sun, X.; Li, Z.; Gong, G. Phenotypic and Genetic Characterization of Botrytis cinerea Population from Kiwifruit in Sichuan Province, China. Plant Dis. 2019, 103, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C.; Leucker, M.; Steiner, U. Sensory assessment of Cercospora beticola sporulation for phenotyping the partial disease resistance of sugar beet genotypes. Plant Methods 2019, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, N.J.; Goss, E.M.; Press, C.M. Phytophthora ramorum: A pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Mol. Plant Pathol. 2008, 9, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Lenardon, M.D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2023, 21, 248–259. [Google Scholar] [CrossRef]
- McGowan, J.; Fitzpatrick, D.A. Chapter Five—Recent advances in comycete genomics. Adv. Genet. 2020, 105, 175–228. [Google Scholar] [CrossRef] [PubMed]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Ouma, K.O. Recent Advances in Biosorption of Copper and Cobalt by Filamentous Fungi. Front. Microbiol. 2020, 11, 582016. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Tanuwidjaja, I.; Fuka, M.M.; Vlahoviček-Kahlina, K.; Marijan, M.; Boras, A.; Kolić, N.U.; Vinceković, M. Encapsulation of two fermentation agents, Lactobacillus sakei and calcium ions in microspheres. Colloids Surf. B 2021, 197, 111387. [Google Scholar] [CrossRef] [PubMed]
- Wargenau, A.; Fleissner, A.; Bolten, C.J.; Rohde, M.; Kampen, I.; Kwade, A. On the origin of the electrostatic surface potential of Aspergillus niger spores in acidic environments. Res. Microbiol. 2011, 162, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, C.A.; Biresaw, G.; Jackson, M.A. Hydrophobic and electrostatic cell surface properties of blastospores of the entomopathogenic fungus Paecilomyces fumosoroseus. Colloids Surf. B Biointerfaces 2005, 46, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, J.; Liu, Z.; Gong, G.; Zha, J. Microbial cell surface engineering for high-level synthesis of bio-products. Biotechnol. Adv. 2022, 55, 107912. [Google Scholar] [CrossRef] [PubMed]
- Vasina, D.V.; Antonova, N.P.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Grishin, A.V.; Akoulina, E.A.; Trusova, E.A.; Lendel, A.M.; Mazunina, E.P.; Kozlova, S.R.; et al. Alginate Gel Encapsulated with Enzybiotics Cocktail Is Effective against Multispecies Biofilms. Gels 2024, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Vicedo, B.; de la O Leyva, M.; Flors, V.; Finiti, I.; Del Amo, G.; Walters, D.; Real, M.D.; García-Agustín, P.; González-Bosch, C. Control of the phytopathogen Botrytis cinerea using adipic acid monoethyl ester. Arch. Microbiol. 2006, 184, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramos, F.; Briones-Labarca, V.; Plaza, V.; Castillo, L. Iron and copper on Botrytis cinerea: New inputs in the cellular characterization of their inhibitory effect. PeerJ 2023, 11, e15994. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Xue, X.J.; Yu, Y.; Xu, M.M.; Lu, C.C.; Meng, X.L.; Zhang, B.G.; Ding, X.H.; Chu, Z.H. Copper ions suppress abscisic acid biosynthesis to enhance defence against Phytophthora infestans in potato. Mol. Plant Pathol. 2020, 21, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.M.; Yu, W.T.; Liu, X.D.; He, X.; Wang, W.X.; Ma, J. Chemical method of breaking the cell-loaded sodium alginate/chitosan microcapsules. Chem. J. Chin. Univ. 2004, 25, 1342–1346. Available online: http://www.cjcu.jlu.edu.cn/EN/Y2004/V25/I7/1342 (accessed on 16 July 2022).
- Mokale, V.; Jitendra, N.; Yogesh, S.; Gokul, K. Chitosan reinforced alginate controlled release beads of losartan potassium: Design, formulation and in vitro evaluation. J. Pharm. Investig. 2014, 44, 243–252. [Google Scholar] [CrossRef]


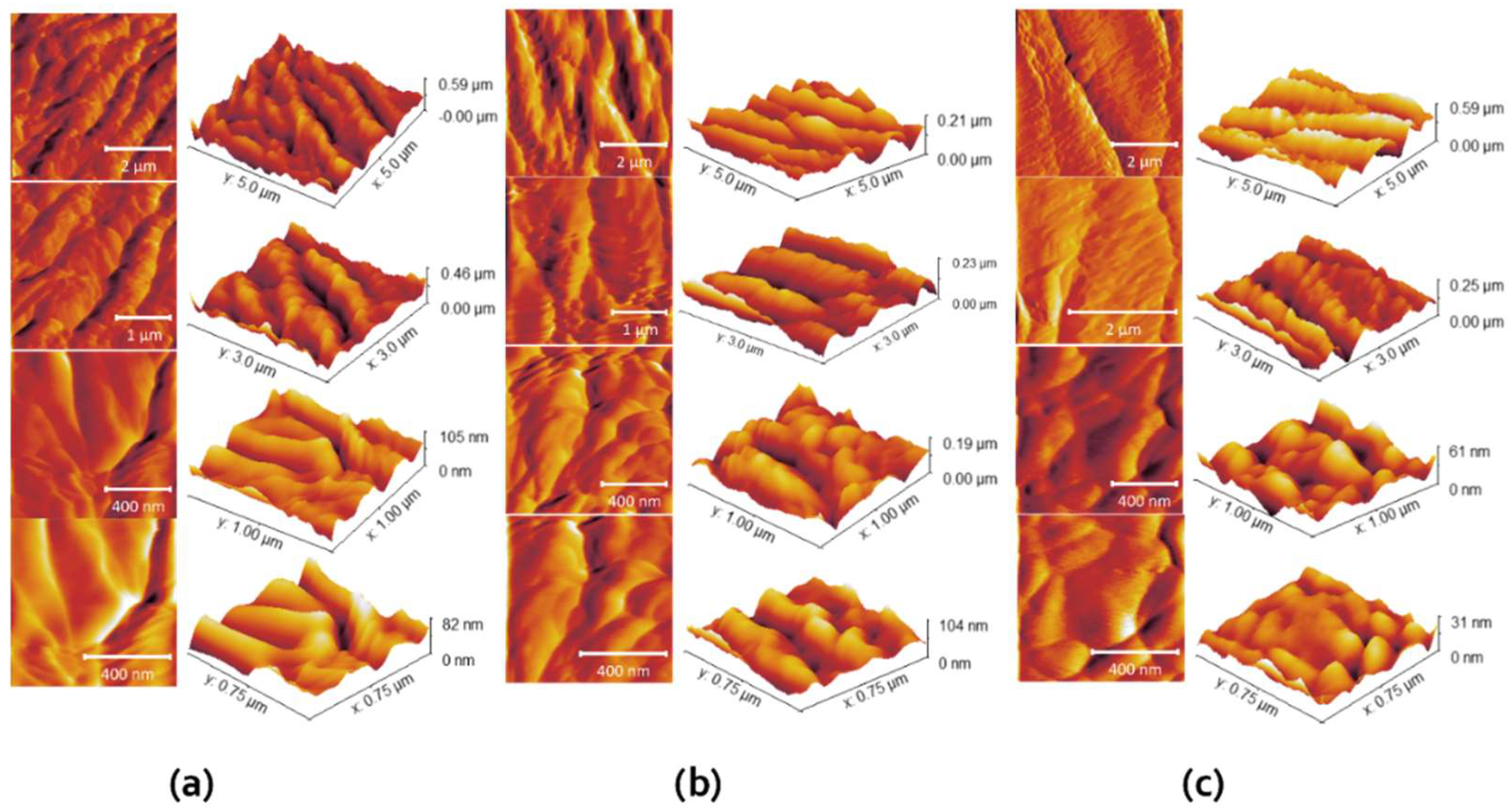



| ALG/Cu | Ra/nm | Rq/nm |
|---|---|---|
| Sample 1 | 23.89 | 29.45 |
| Sample 2 | 18.04 | 20.68 |
| Sample 3 | 3.99 | 5.44 |
| ALG/Cu | G′ (max)/Pa | Yield Point Stress/Pa | Yield Point Strain/% | Flow Point/Pa | Loss Factor | Flow Transition Index |
|---|---|---|---|---|---|---|
| Sample 1 | 39,075 | 1334 | 3.5 | 2170 | 0.17 | 1.63 |
| Sample 2 | 35,700 | 1249 | 4.8 | 2015 | 0.18 | 1.61 |
| Sample 3 | 31,117 | 1173 | 5.2 | 1721 | 0.17 | 1.47 |
| ALG/Cu | k | n | R2 |
|---|---|---|---|
| Sample 1 | 0.164 ± 0.018 | 0.13 ± 0.012 | 0.9975 |
| Sample 2 | 0.180 ± 0.009 | 0.13 ± 0.010 | 0.9931 |
| Sample 3 | 0.233 ± 0.025 | 0.14 ± 0.015 | 0.9987 |
| n | Control | Sample 1 | Sample 2 | Sample 3 | Neoram |
|---|---|---|---|---|---|
| 1 | 32.70 | 16.32 | 54.55 | 41.21 | 40.81 |
| 2 | 56.20 | 13.64 | 24.39 | 24.96 | 39.78 |
| 3 | 27.36 | 40.99 | 64.84 | 30.29 | 76.43 |
| 4 | 35.85 | 18.81 | 25.84 | 8.25 | 6.80 |
| 5 | 27.61 | 3.40 | 33.66 | 55.42 | 53.57 |
| 6 | 42.81 | 54.56 | 21.08 | 9.89 | 27.31 |
| 7 | 16.88 | 19.78 | 26.33 | 8.16 | 45.10 |
| 8 | 38.99 | 30.33 | 51.07 | 17.88 | 26.62 |
| 9 | 31.15 | 19.72 | 30.92 | 23.80 | 23.94 |
| 10 | 66.73 | 8.88 | 32.33 | 14.49 | 26.81 |
| 11 | 54.25 | 11.37 | 55.29 | 52.46 | 31.25 |
| 12 | 15.04 | 38.08 | 33.26 | 16.25 | 21.17 |
| 13 | 33.01 | 38.15 | 67.43 | 6.00 | 42.59 |
| 14 | 77.46 | 32.68 | 46.33 | 25.86 | 19.49 |
| 15 | 16.88 | 24.70 | 15.12 | 22.37 | 45.90 |
| 16 | 50.36 | 11.44 | 35.85 | 15.73 | 59.02 |
| 17 | 24.46 | 82.32 | 50.10 | 10.20 | 16.93 |
| 18 | 32.97 | 63.17 | 46.83 | 18.54 | 23.41 |
| 19 | 33.20 | 48.29 | 31.79 | 23.65 | 50.34 |
| 20 | 39.49 | 108.87 | 4.96 | 16.29 | 26.72 |
| 21 | 43.68 | 48.03 | 66.35 | 9.40 | 16.88 |
| 22 | 77.77 | 36.13 | 37.77 | 29.89 | 52.15 |
| 23 | 73.12 | 19.52 | 20.44 | 26.65 | 38.75 |
| 24 | 50.13 | 22.18 | 19.24 | 24.46 | 37.10 |
| 25 | 45.73 | 31.25 | 42.06 | 38.77 | 30.22 |
| 26 | 41.71 | 55.40 | 62.78 | 18.52 | 31.47 |
| 27 | 59.30 | 11.44 | 41.11 | 18.60 | 19.69 |
| 28 | 42.68 | 21.34 | 79.34 | 37.09 | 38.73 |
| 29 | 91.29 | 42.08 | 44.75 | 19.88 | 29.34 |
| 30 | 61.76 | 48.30 | 21.40 | 28.97 | 44.36 |
| Inhibition | 23% | 12% | 48% | 22% |
| Sample | Number of Colonies | Average | |||
|---|---|---|---|---|---|
| Control | 2 | 2 | 1 | 0 | 1.25 |
| Control | 2 | 2 | 0 | 2 | 1.50 |
| Control | 3 | 3 | 1 | 0 | 1.75 |
| Sample 1 | 1 | 1 | 0 | 0 | 0.50 |
| Sample 1 | 1 | 2 | 0 | 0 | 0.75 |
| Sample 1 | 0 | 0 | 0 | 0 | 0.00 |
| Sample 2 | 0 | 0 | 0 | 0 | 0.00 |
| Sample 2 | 1 | 0 | 0 | 0 | 0.25 |
| Sample 2 | 1 | 1 | 1 | 1 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinceković, M.; Jurić, S.; Vlahoviček-Kahlina, K.; Novak, A.; Ivić, D.; Hazler, L.; Jurkin, T.; Bafti, A.; Šijaković Vujičić, N. Novel Copper Alginate Microspheres as Ecological Fungicides. Sustainability 2024, 16, 5637. https://doi.org/10.3390/su16135637
Vinceković M, Jurić S, Vlahoviček-Kahlina K, Novak A, Ivić D, Hazler L, Jurkin T, Bafti A, Šijaković Vujičić N. Novel Copper Alginate Microspheres as Ecological Fungicides. Sustainability. 2024; 16(13):5637. https://doi.org/10.3390/su16135637
Chicago/Turabian StyleVinceković, Marko, Slaven Jurić, Kristina Vlahoviček-Kahlina, Adrijana Novak, Dario Ivić, Laura Hazler, Tanja Jurkin, Arijeta Bafti, and Nataša Šijaković Vujičić. 2024. "Novel Copper Alginate Microspheres as Ecological Fungicides" Sustainability 16, no. 13: 5637. https://doi.org/10.3390/su16135637
APA StyleVinceković, M., Jurić, S., Vlahoviček-Kahlina, K., Novak, A., Ivić, D., Hazler, L., Jurkin, T., Bafti, A., & Šijaković Vujičić, N. (2024). Novel Copper Alginate Microspheres as Ecological Fungicides. Sustainability, 16(13), 5637. https://doi.org/10.3390/su16135637










