Recycling of Blended Fabrics for a Circular Economy of Textiles: Separation of Cotton, Polyester, and Elastane Fibers
Abstract
:1. Introduction
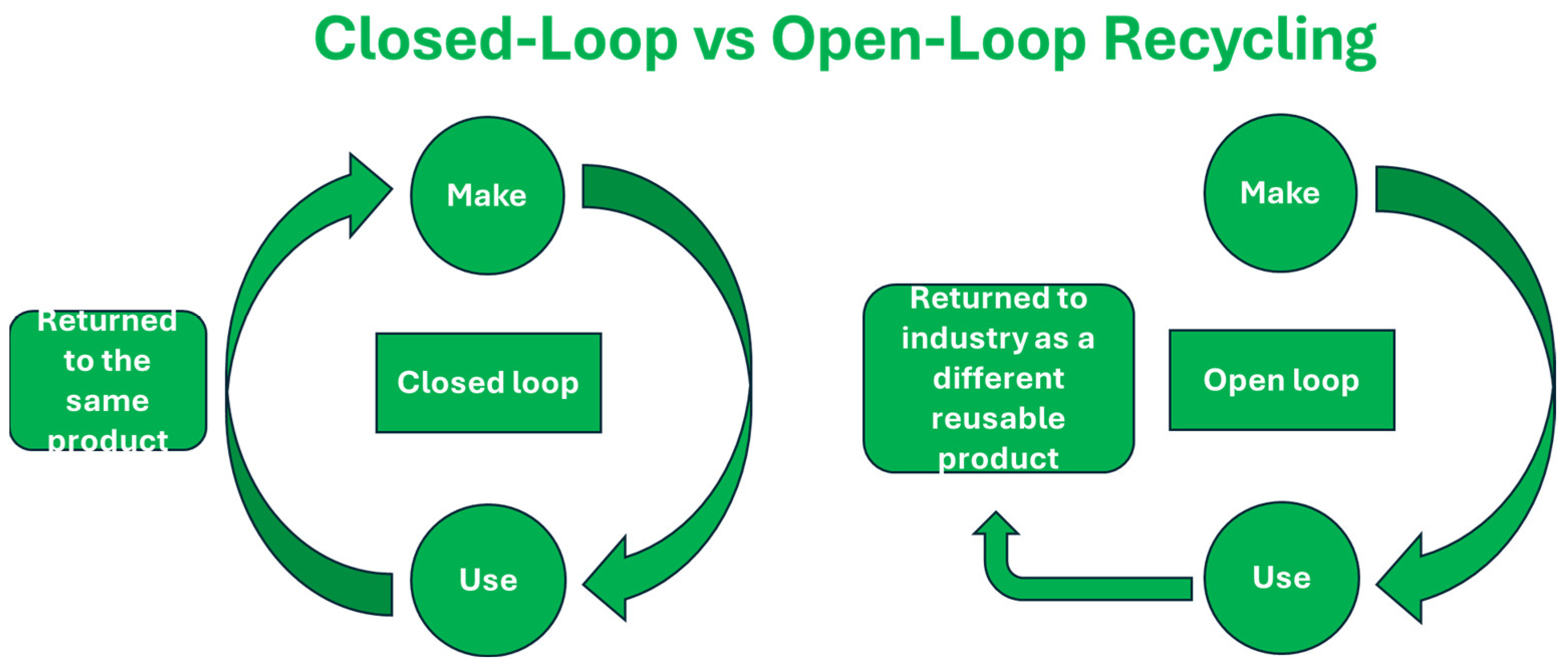
- Closed-loop recycling: This involves recycling the material into a nearly identical product.
- Open-loop recycling: This involves recycling the material in a different product category. Figure 4 illustrates the closed-loop and open-loop recycling methods.
- Upcycling: Upcycling is the process of creating a product out of recycled resources that is more valuable than the original.
- Downcycling: In downcycling, the recycled material is less valuable than what was used to make the original item.
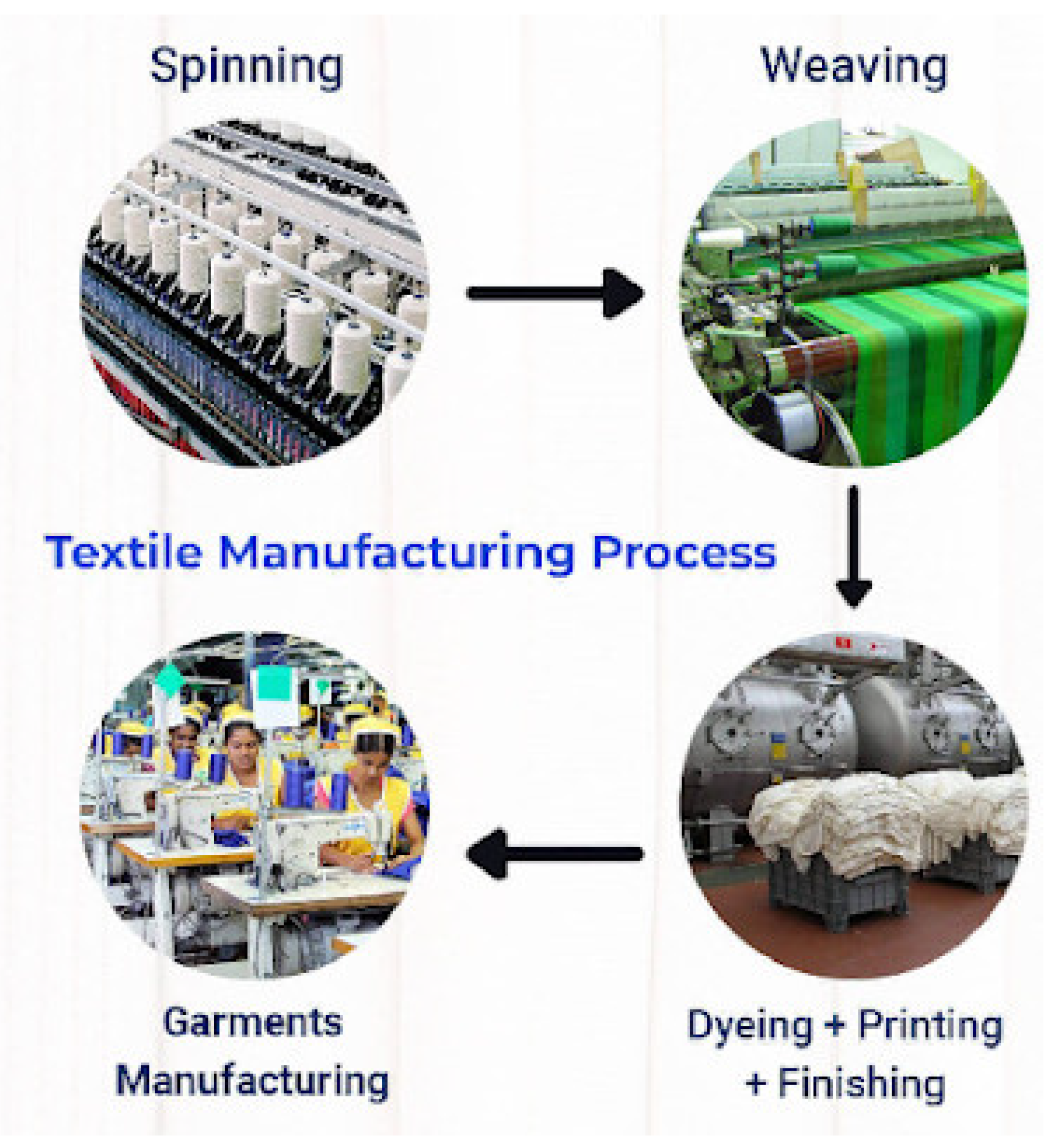
2. Textile Fibers and Textile Production
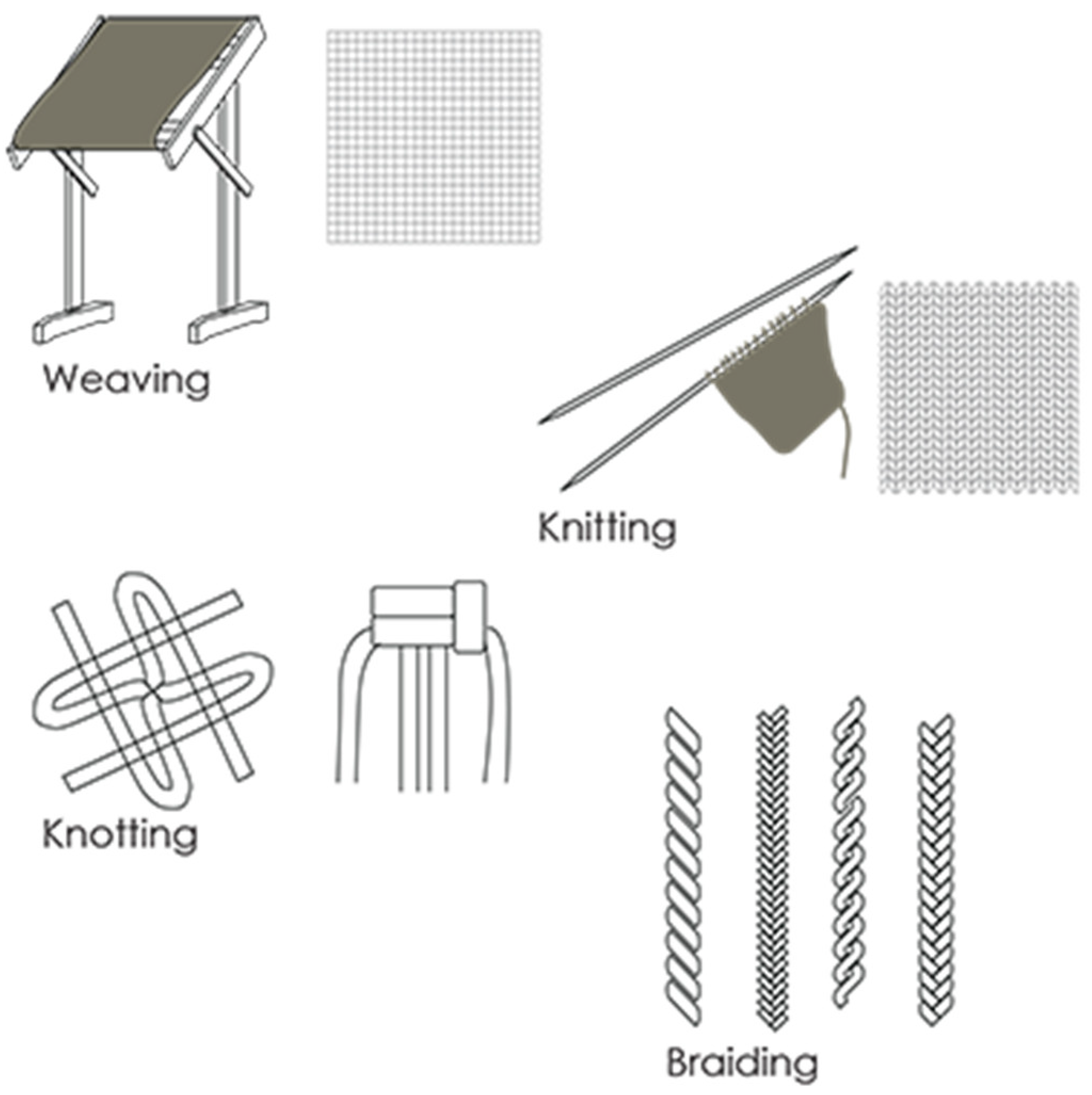
- Weaving: This is a technique for weaving cloth in which long strands are woven over and under one another in a herringbone pattern, parallel to each other.
- Knitting: Knitting is a traditional type of textile production completed by hand with a needle or crochet hook, but many industries today use large knitting machines.
- Braiding: This method of producing textiles involves taking two comparable materials and twisting them into knots according to a predetermined pattern [40].
- Embroidery: Embroidery is important in the textile industry for adding aesthetic value, texture, and customization to fabrics. It involves decorating materials with needle and thread or yarn, either by hand or with machines [40].
- Washing: Everything to clean the fabric from the residues of the previous steps and steps of treatment, most of the time with water and detergents or solvents.
- De-sizing: Removing the sizing chemicals from the warp yarns in fabrics using enzymes, so that they are ready for finishing.
- Scouring: Fatty waxes and greases are removed from natural fibers such as cotton seed and husk using a detergent, base, or solvent.
- Bleaching: Usually done with bleaches; bleaching whitens fabrics to improve absorbency and make coloring easier.
- Mercerizing: Cellulosic fibers are treated to improve their strength, luster, and dyestuff affinity by causing them to swell (e.g., with bases); this can help lower the dyestuff level.
- Carbonizing: vegetable residues such as seed pods are removed from the wool fibers and the contents of the fiber are ‘blackened’ by the application of heat and chemicals (often based on acids) [43].
3. Textile Recycling Methods
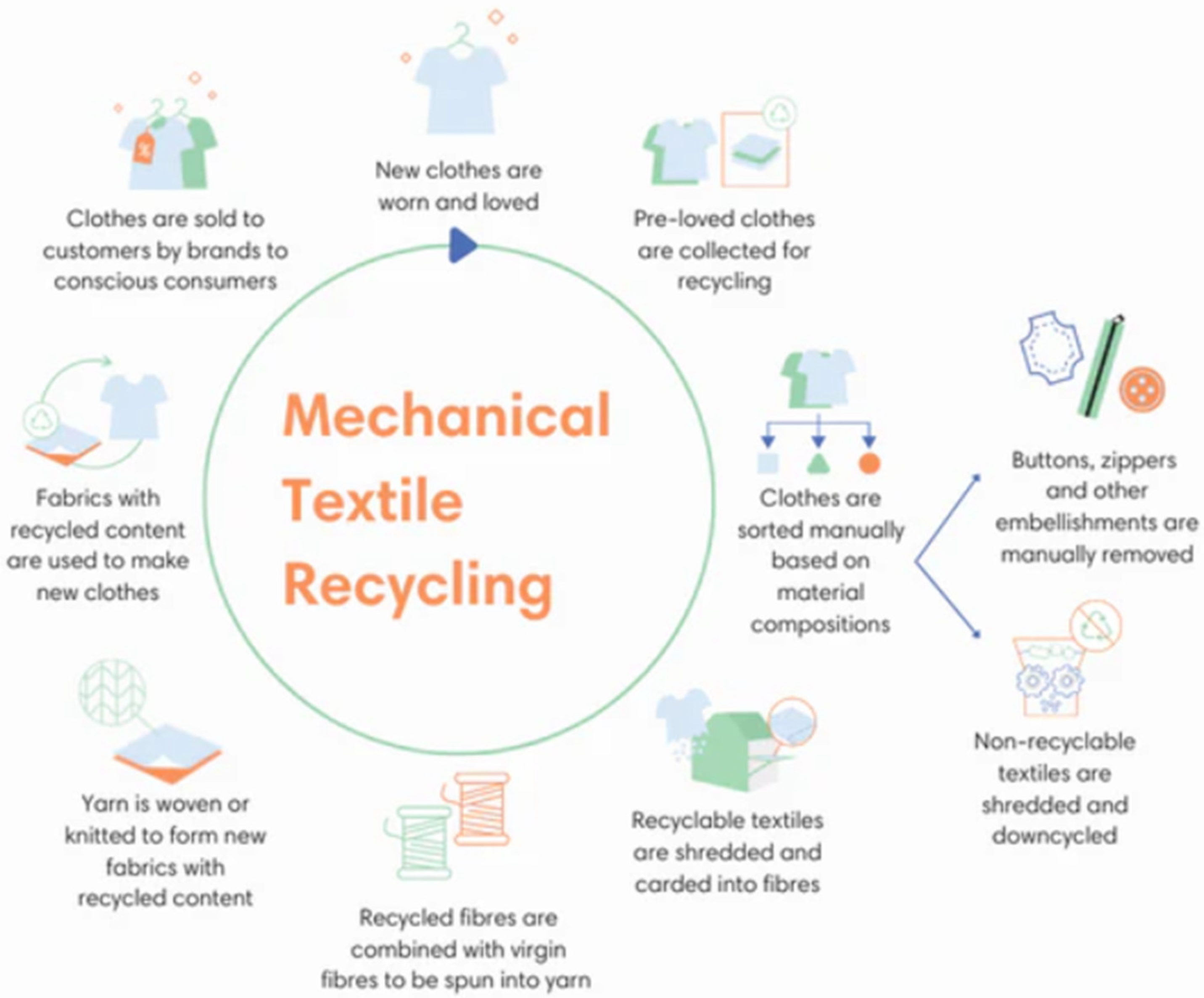
3.1. Mechanical Recycling of Textiles
- Cut fabrics: Cut fabrics are pieces of fabric. If cut fabrics are present, the material needs to go through the tearing machine one or numerous times to produce single fibers. These fibers need blending with virgin fibers to re-spin into yarns because of the decline in fiber quality.
- Spinnable fibers: The output fraction consisting of long fibers of sufficient quality to be spun is known as the spinnable fiber fraction.
- Fluff: Although this product still comprises fiber, it cannot be utilized in a spinning process because the fibers are either too short or too twisted. Typically, fluff material is used to make filler items like insulation for the building sector or specialized non-wovens for the automotive sector.
- Filler materials: During certain procedures (such as milling), the fibers break down, resulting in tiny particles rather than fibers. These particles can be employed as reinforcements or fillers in plastics and composites, depending on their size, shape, and composition [48].
3.2. Chemical Processes for Textile Recycling
3.3. Biological/Enzyme-Based Processes for Textile Recycling
4. Separating Cotton from Polyester in Blended Textiles
- 50% polyester, 50% cotton: Commonly used to make T-shirts, sweatshirts, and bed linens.
- 60% polyester, 40% cotton: Used in sportswear, work uniforms, or casual fashion.
- 65% polyester, 35% cotton: Suitable for casual wear, workwear, or uniforms.
- 35% polyester, 65% cotton: Suitable for various applications from casual wear to home textiles.
- 20% polyester, 80% cotton: Used in sleepwear, casual wear, or summer clothing.
4.1. Dissolution of Cotton
4.2. Dissolution of Polyester
4.3. Hydrolysis of Cotton or Polyester
4.4. Hydrothermal Treatment
4.5. Enzymatic Hydrolysis of Cotton or Polyester
5. Separating Elastane from Other Fibers
5.1. Selective Dissolution of Elastane
5.2. Elastane Degradation by Aminolysis or Solvolysis
6. Recycling of Blended Textiles in Industry
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Kiskira, K.; Zacharopoulos, N.; Chronis, I.; Coelho, F.; Togiani, A.; Kalkanis, K.; Priniotakis, G. A Review of sustainability standards and ecolabeling in the textile industry. Sustainability 2023, 15, 11589. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef] [PubMed]
- Textile terms and definitions. J. Text. Inst. Proc. 1947, 38, 615–628. [CrossRef]
- What is Textile? 2016. Available online: https://textilefocus.com/what-is-textile/ (accessed on 11 July 2024).
- A New Textiles Economy: Redesigning Fashion’s Future. 2017. Available online: http://www.ellenmacarthurfoundation.org/publications (accessed on 11 July 2024).
- Christis, M.; Vercalsteren, A.; Arnold, M.; Nicolau, M.; Lafond, E.; Mortensen, L.; Coscieme, L.; Manshoven, S. Textiles and the Environment in a Circular Economy. 2019. Available online: https://www.eionet.europa.eu/etcs/etc-wmge/products (accessed on 11 July 2024).
- Niinimaki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The environmental price of fast fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M.; Kulikowska, D. The growing problem of textile waste generation—The current state of textile waste management. Energies 2024, 17, 1528. [Google Scholar] [CrossRef]
- Muthu, S.S.; Li, Y.; Hu, J.-Y.; Mok, P.-Y. Recyclability Potential Index (RPI): The concept and quantification of RPI for textile fibres. Ecol. Indic. 2012, 18, 58–62. [Google Scholar] [CrossRef]
- Wagaw, T.; Babu, K.M. Textile Waste Recycling: A Need for a Stringent Paradigm Shift. AATCC J. Res. 2023, 10, 376–385. [Google Scholar] [CrossRef]
- Lu, L.; Fan, W.; Meng, X.; Xue, L.; Ge, S.; Wang, C.; Foong, S.Y.; Tan, C.S.Y.; Sonne, C.; Aghbashlo, M.; et al. Current recycling strategies and high-value utilization of waste cotton. Sci. Total Environ. 2023, 856, 158798. [Google Scholar] [CrossRef]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Ding, J.; Zhang, G.; Zeng, X.; Qingbo, Y.; Zhu, B.; Gao, W. Microfiber pollution: An ongoing major environmental issue related to the sustainable development of textile and clothing industry. Environ. Dev. Sustain. 2021, 23, 11240–11256. [Google Scholar] [CrossRef]
- de Oliveira, C.R.S.; da Silva Júnior, A.H.; Mulinari, J.; Ferreira, A.J.S.; da Silva, A. Fibrous microplastics released from textiles: Occurrence, fate, and remediation strategies. J. Contam. Hydrol. 2023, 256, 104169. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Lykaki, M.; Alrajoula, M.T.; Markiewicz, M.; Kraas, C.; Kolbe, S.; Klinkhammer, K.; Rabe, M.; Klauer, R.; Bendt, E.; et al. Microplastics from textile origin—Emission and reduction measures. Green Chem. 2021, 23, 5247–5271. [Google Scholar] [CrossRef]
- Stone, C.; Windsor, F.M.; Munday, M.; Durance, I. Natural or synthetic—How global trends in textile usage threaten freshwater environments. Sci. Total Environ. 2020, 718, 134689. [Google Scholar] [CrossRef] [PubMed]
- Abbate, S.; Centobelli, P.; Cerchione, R.; Nadeem, S.; Riccio, E. Sustainability trends and gaps in the textile, apparel and fashion industries. Environ. Dev. Sustain. 2023, 26, 2837–2864. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, P.; Scheffer, M.; Bos, H. Textiles for Circular Fashion: The Logic behind Recycling Options. Sustainability 2021, 13, 9714. [Google Scholar] [CrossRef]
- Juanga-Labayen, J.P.; Labayen, I.V.; Yuan, Q. A review on textile recycling practices and challenges. Textiles 2022, 2, 174–188. [Google Scholar] [CrossRef]
- Phan, K.; Ugduler, S.; Harinck, L.; Denolf, R.; Roosen, M.; O’Rourke, G.; De Vos, D.; Van Speybroeck, V.; De Clerck, K.; De Meester, S. Analysing the potential of the selective dissolution of elastane from mixed fiber textile waste. Resour. Conserv. Recycl. 2023, 191, 106903. [Google Scholar] [CrossRef]
- Jonsson, C.; Wei, R.; Biundo, A.; Landberg, J.; Bour, L.S.; Pezzotti, F.; Toca, A.; Jacques, L.M.; Bornscheuer, U.T.; Syren, P.-O. Biocatalysis in the recycling landscape for synthetic polymers and plastics towards circular textiles. ChemSusChem 2021, 14, 4028–4040. [Google Scholar] [CrossRef]
- Baloyi, R.B.; Gbadeyan, O.J.; Sithole, B.; Chunilall, V. Recent advances in recycling technologies for waste textile fabrics: A review. Text. Res. J. 2024, 94, 508–529. [Google Scholar] [CrossRef]
- Incineration of Textile Waste. Available online: https://sustainfashion.info/incineration-of-textile-waste/ (accessed on 11 July 2024).
- European Environmental Ageny. What Happens to Our Used Textile Products such as Discarded Clothing or Shoes? 2024. Available online: https://www.eea.europa.eu/en/about/contact-us/faqs/what-happens-to-our-used-textile-products-such-as-discarded-clothing-or-shoes (accessed on 11 July 2024).
- U.S EPA. Textiles: Material-Specific Data. 2023. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/textiles-material-specific-data (accessed on 11 July 2024).
- Ruiz, A. 17 Most Worrying Textile Waste Statistics & Facts. 2024. Available online: https://theroundup.org/textile-waste-statistics/ (accessed on 11 July 2024).
- Ribul, M.; Lanot, A.; Pisapia, C.T.; Purnell, P.; McQueen-Mason, S.J.; Baurley, S. Mechanical, chemical, biological: Moving towards closed-loop bio-based recycling in a circular economy of sustainable textiles. J. Clean. Prod. 2021, 326, 129325. [Google Scholar] [CrossRef]
- Xie, X.; Hong, Y.; Zeng, X.; Dai, X.; Wagner, M. A systematic literature review for the recycling and reuse of wasted clothing. Sustainability 2021, 13, 13732. [Google Scholar] [CrossRef]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Abrishami, S.; Shirali, A.; Sharples, N.; Kartal, G.E.; Macintyre, L.; Doustdar, O. Textile Recycling and Recovery: An Eco-friendly Perspective on Textile and Garment Industries Challenges. Text. Res. J. 2024, 00405175241247806. [Google Scholar] [CrossRef]
- Espinoza, R. Closed Loop Recycling Definition. 2022. Available online: https://blog.idrenvironmental.com/closed-loop-recycling-definition (accessed on 11 July 2024).
- Loo, S.-L.; Yu, E.; Hu, X. Tackling critical challenges in textile circularity: A review on strategies for recycling cellulose and polyester from blended fabrics. J. Environ. Chem. Eng. 2023, 11, 110482. [Google Scholar] [CrossRef]
- What is Elastane Fabric: Properties, How Its Made and Where. 2024. Available online: https://sewport.com/fabrics-directory/elastane-fabric (accessed on 11 July 2024).
- Stubbe, B.; Van Vrekhem, S.; Huysman, S.; Tilkin, R.G.; De Schrijver, I.; Vanneste, M. White Paper on Textile Fibre Recycling Technologies. Sustainability 2024, 16, 618. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A.; Ipsmiller, W. Enzymatic textile recycling—Best practices and outlook. Waste Manag. Res. 2021, 39, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Classification of Textile Fibres. 2023. Available online: https://textileengineering.net/classification-of-textile-fibres/ (accessed on 11 July 2024).
- Coats. Know about Textile Fibres. Available online: https://www.coats.com/en/information-hub/know-about-textile-fibres (accessed on 11 July 2024).
- Hung, O.; Chan, C.; Kan, C.; Yuen, C. An analysis of some physical and chemical properties of CO2 laser-treated cotton-based fabrics. Cellulose 2017, 24, 363–381. [Google Scholar] [CrossRef]
- Samani, N. Complete Guide to Textile Manufacturing. Available online: https://www.deskera.com/blog/textile-manufacturing/ (accessed on 11 July 2024).
- Mdmustafiz. A Journey through the Textile Manufacturing Process. 2023. Available online: https://medium.com/@mdmustafiz898/a-journey-through-the-textile-manufacturing-process-5650d4cf0998 (accessed on 11 July 2024).
- Textiles. Available online: https://www.next.cc/journey/discovery/textiles (accessed on 11 July 2024).
- The Textile Process. Available online: https://textileguide.chemsec.org/find/get-familiar-with-your-textile-production-processes/ (accessed on 11 July 2024).
- Kim, T.; Kim, D.; Park, Y. Recent progress in regenerated fibers for “green” textile products. J. Clean. Prod. 2022, 376, 134226. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A. Textile recycling processes, state of the art and current developments: A mini review. Waste Manag. Res. 2019, 37, 112–119. [Google Scholar] [CrossRef]
- El Darai, T.; Ter-Halle, A.; Blanzat, M.; Despras, G.; Sartor, V.; Bordeau, G.; Lattes, A.; Franceschi, S.; Cassel, S.; Chouini-Lalanne, N.; et al. Chemical recycling of polyester textile wastes: Shifting towards sustainability. Green Chem. 2024, 26, 6857–6885. [Google Scholar] [CrossRef]
- Lubongo, C.; Alexandridis, P. Assessment of performance and challenges in use of commercial automated sorting technology for plastic waste. Recycling 2022, 7, 11. [Google Scholar] [CrossRef]
- European Commission; Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs; Smes; Duhoux, T.; Maes, E.; Hirschnitz-Garbers, M.; Peeters, K.; Asscherickx, L.; Christis, M.; Stubbe, B.; et al. Study on the Technical, Regulatory, Economic and Environmental Effectiveness of Textile Fibres Recycling—Final Report; Publications Office: Luxembourg, 2021. [Google Scholar] [CrossRef]
- Huang, X.; Tan, Y.; Huang, J.; Zhu, G.; Yin, R.; Tao, X.; Tian, X. Industrialization of open- and closed-loop waste textile recycling towards sustainability: A review. J. Clean. Prod. 2024, 436, 140676. [Google Scholar] [CrossRef]
- Damayanti, D.; Wulandari, L.A.; Bagaskoro, A.; Rianjanu, A.; Wu, H.-S. Possibility routes for textile recycling technology. Polymers 2021, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Lubongo, C.; Congdon, T.; McWhinnie, J.; Alexandridis, P. Economic feasibility of plastic waste conversion to fuel using pyrolysis. Sustain. Chem. Pharm. 2022, 27, 100683. [Google Scholar] [CrossRef]
- Achilias, D.; Andriotis, L.; Koutsidis, I.; Louka, D.; Nianias, N.; Siafaka, P.; Tsagkalias, I.s.; Tsintzou, G. Recent Advances in the Chemical Recycling of Polymers (PP, PS, LDPE, HDPE, PVC, PC, Nylon, PMMA). In Material Recycling—Trends and Perspectives; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Alexandridis, P.; Ghasemi, M.; Furlani, E.P.; Tsianou, M. Solvent processing of cellulose for effective bioresource utilization. Curr. Opin. Green Sustain. Chem. 2018, 14, 40–52. [Google Scholar] [CrossRef]
- Ghasemi, M.; Tsianou, M.; Alexandridis, P. Assessment of solvents for cellulose dissolution. Bioresour. Technol. 2017, 228, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Alexandridis, P.; Tsianou, M. Cellulose dissolution: Insights on the contributions of solvent-induced decrystallization and chain disentanglement. Cellulose 2017, 24, 571–590. [Google Scholar] [CrossRef]
- Kaabel, S.; Arciszewski, J.; Borchers, T.H.; Therien, J.P.D.; Friscic, T.; Auclair, K. Solid-State Enzymatic Hydrolysis of Mixed PET/Cotton Textiles. Chemsuschem 2022, 16, e202201613. [Google Scholar] [CrossRef]
- Bichnguyen. Cotton Polyester Blend Explained: Pros, Cons, Applications. 2024. Available online: https://merchize.com/cotton-polyester-blend/ (accessed on 11 July 2024).
- Poly Cotton Fabric Market Outlook (2023 to 2033). 2024. Available online: https://www.futuremarketinsights.com/reports/poly-cotton-fabric-market (accessed on 11 July 2024).
- Wang, S.; Salmon, S. Progress toward circularity of polyester and cotton textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef] [PubMed]
- Przypis, M.; Wawoczny, A.; Gillner, D. Biomass and Cellulose Dissolution—The Important Issue in Renewable Materials Treatment. Appl. Sci. 2023, 13, 1055. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M.J. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Kahoush, M.; Kadi, N. Towards sustainable textile sector: Fractionation and separation of cotton/ polyester fibers from blended textile waste. Sustain. Mater. Technol. 2022, 34, e00513. [Google Scholar] [CrossRef]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Maattanen, M.; Sixta, H. Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Wu, H.; Wang, B.; Li, T.; Wu, Y.; Yang, R.; Gao, H.; Nie, Y. Efficient recycle of waste poly-cotton and preparation of cellulose and polyester fibers using the system of ionic liquid and dimethyl sulfoxide. J. Mol. Liq. 2023, 388, 122757. [Google Scholar] [CrossRef]
- Yang, K.; Wang, M.; Wang, X.; Shan, J.; Zhang, J.; Tian, G.; Yang, D.; Ma, J. Polyester/cotton-blended textile waste fiber separation and regeneration via a green chemistry approach. ACS Sustain. Chem. Eng. 2024, 12, 4530–4538. [Google Scholar] [CrossRef]
- Sun, X.; Lu, C.; Zhang, W.; Tian, D.; Zhang, X. Acetone-soluble cellulose acetate extracted from waste blended fabrics via ionic liquid catalyzed acetylation. Carbohydr. Polym. 2013, 98, 405–411. [Google Scholar] [CrossRef]
- Rosson, L.; Wang, X.; Byrne, N. Investigating how the dye colour is impacted when chemically separating polyester-cotton blends. J. Text. Inst. 2024, 115, 656–666. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Lv, X.D.; Ni, H.G. Solvent-based separation and recycling of waste plastics: A review. Chemosphere 2018, 209, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J. Closed-Loop Recycling of Polymers Using Solvents. Johns. Matthey Technol. Rev. 2019, 64, 4–15. [Google Scholar] [CrossRef]
- Chaudhari, U.S.; Kulas, D.G.; Peralta, A.; Hossain, T.; Johnson, A.T.; Hartley, D.S.; Handler, R.M.; Reck, B.K.; Thompson, V.S.; Watkins, D.W.; et al. Solvent based dissolution–precipitation of waste polyethylene terephthalate: Economic and environmental performance metrics. RSC Sustain. 2023, 1, 1849–1860. [Google Scholar] [CrossRef]
- Yousef, S.; Tatariants, M.; Tichonovas, M.; Sarwar, Z.; Jonuškienė, I.; Kliucininkas, L. A new strategy for using textile waste as a sustainable source of recovered cotton. Resour. Conserv. Recycl. 2019, 145, 359–369. [Google Scholar] [CrossRef]
- Mu, B.; Yu, X.; Shao, Y.; McBride, L.; Hidalgo, H.; Yang, Y. Complete recycling of polymers and dyes from polyester/cotton blended textiles via cost-effective and destruction-minimized dissolution, swelling, precipitation, and separation. Resour. Conserv. Recycl. 2023, 199, 107275. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Z.; Yang, A.; Li, J.; Wang, L.; Chen, H.; Zheng, X.; Liu, Y. Nanocellulose extracted from waste polyester/cotton fabric by chemical-mechanical separation technology. J. Phys. Conf. Ser. 2021, 1790, 012074. [Google Scholar] [CrossRef]
- Bengtsson, J.; Peterson, A.; Idstrom, A.; de la Motte, H.; Jedvert, K. Chemical Recycling of a Textile Blend from Polyester and Viscose, Part II: Mechanism and Reactivity during Alkaline Hydrolysis of Textile Polyester. Sustainability 2022, 14, 6911. [Google Scholar] [CrossRef]
- Peterson, A.; Wallinder, J.; Bengtsson, J.; Idstrom, A.; Bialik, M.; Jedvert, K.; de la Motte, H. Chemical Recycling of a Textile Blend from Polyester and Viscose, Part I: Process Description, Characterization, and Utilization of the Recycled Cellulose. Sustainability 2022, 14, 7272. [Google Scholar] [CrossRef]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z.; Zhang, M.; Zhang, B.; Dai, J. Separation and characterization of waste cotton/polyester blend fabric with hydrothermal method. Fibers Polym. 2018, 19, 742–750. [Google Scholar] [CrossRef]
- Matsumura, M.; Inagaki, J.; Yamada, R.; Tashiro, N.; Ito, K.; Sasaki, M. Material separation from polyester/cotton blended fabrics using hydrothermal treatment. ACS Omega 2024, 9, 13125–13133. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; von Haugwitz, G.; Pfaff, L.; Mican, J.; Badenhorst, C.P.S.; Liu, W.D.; Weber, G.; Austin, H.P.; Bednar, D.; Damborsky, J.; et al. Mechanism-Based Design of Efficient PET Hydrolases. ACS Catal. 2022, 12, 3382–3396. [Google Scholar] [CrossRef] [PubMed]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manag. 2020, 102, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Hu, Y.Z.; Du, C.Y.; Lin, C.S.K. Recovery of glucose and polyester from textile waste by enzymatic hydrolysis. Waste Biomass Valorization 2019, 10, 3763–3772. [Google Scholar] [CrossRef]
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective conversion of waste polyester–cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem. Eng. J. 2014, 253, 40–45. [Google Scholar] [CrossRef]
- Boondaeng, A.; Keabpimai, J.; Srichola, P.; Vaithanomsat, P.; Trakunjae, C.; Niyomvong, N. Optimization of Textile Waste Blends of Cotton and PET by Enzymatic Hydrolysis with Reusable Chemical Pretreatment. Polymers 2023, 15, 1964. [Google Scholar] [CrossRef]
- Shen, F.; Xiao, W.; Lin, L.; Yang, G.; Zhang, Y.; Deng, S. Enzymatic saccharification coupling with polyester recovery from cotton-based waste textiles by phosphoric acid pretreatment. Bioresour. Technol. 2013, 130, 248–255. [Google Scholar] [CrossRef]
- Steiner, K.; Leitner, V.; Zeppetzauer, F.; Ostner, D.; Burgstaller, C.; Rennhofer, H.; Bartl, A.; Ribitsch, D.; Guebitz, G.M. Optimising chemo-enzymatic separation of polyester cellulose blends. Resour. Conserv. Recycl. 2024, 202, 107369. [Google Scholar] [CrossRef]
- Vonbruel, L.; Cordin, M.; Manian, A.P.; Bechtold, T.; Pham, T. Solvent blends for selective elastane dissolution and recovery from mixed polyamide fabrics. Resour. Conserv. Recycl. 2024, 200, 107302. [Google Scholar] [CrossRef]
- Boschmeier, E.; Archodoulaki, V.-M.; Schwaighofer, A.; Lendl, B.; Ipsmiller, W.; Bartl, A. New separation process for elastane from polyester/elastane and polyamide/elastane textile waste. Resour. Conserv. Recycl. 2023, 198, 107215. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.B.; Donslund, B.S.; Henriksen, M.L.; Kristensen, S.K.; Skrydstrup, T. Selective chemical disassembly of elastane fibres and polyurethane coatings in textiles. Green Chem. 2023, 25, 10622–10629. [Google Scholar] [CrossRef]
- Villar, L.; Schlapp-Hackl, I.; Sanchez, P.B.; Hummel, M. High-quality cellulosic fibers engineered from cotton-elastane textile waste. Biomacromolecules 2024, 25, 1942–1949. [Google Scholar] [CrossRef]
- De Smet, D.; Verjans, J.; Vanneste, M. Selective Solvolysis of Bio-Based PU-Coated Fabric. Polymers 2022, 14, 5452. [Google Scholar] [CrossRef]
- The Future of Sustainable Textiles: Chemistry is Closing the Loop on Fashion Waste; C&E News. 2022. Available online: https://cen.acs.org/sections/discovery-reports/sustainable-textile-clothing-fabric-recycling-microfibers-dyes-leather-PHA-ionic-liquids.html (accessed on 11 July 2024).
- Decarbonizing Supply Chains with Circular Materials. Available online: https://www.ambercycle.com/technology (accessed on 11 July 2024).
- Recovering Polyester and Cotton from Textiles. Available online: https://www.blocktexx.com (accessed on 11 July 2024).
- Available online: https://circ.earth/ (accessed on 11 July 2024).
- Barla, F.G.; Showalter, T.; Su, H.-C.; Jones, J.; Bobe, I. Methods for recycling cotton and polyester fibers from waste textiles. U.S. Patent 12,006,403 B2, 11 June 2024. [Google Scholar]
- Worn Again Technologies. Available online: https://wornagain.co.uk/ (accessed on 11 July 2024).
- Rejuvenating Fibers for a Circular World. Available online: https://purfi.com/ (accessed on 11 July 2024).
- Towards a Circular Future of Textiles. Available online: https://textilechange.com/ (accessed on 11 July 2024).
- OnceMore® Process. Available online: https://www.sodra.com (accessed on 11 July 2024).
- Closing The Loop in Textile-to-Textile Recycling. Available online: https://www.phoenxt.com/ (accessed on 11 July 2024).
- Eeden: We Start Upcycling. Available online: https://eeden.world/technology/ (accessed on 11 July 2024).
















| Polymer | Polysaccharides: Cellulose | Polyester | Polyamide | Polyurethane | Polyolefin | Polyacrylic |
|---|---|---|---|---|---|---|
| Linkage |  | 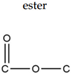 |  | 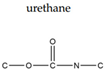 |  |  |
| Fiber Example | Cotton, Linen, Viscose | PET | Wool, Silk, Nylon | Elastane | Polypropylene, Polyethylene | Acryl, Modacryl |
| # | Method | Starting Material and Composition | Process/Reaction | Process/Reaction Conditions | Products | Analysis | Any Other Component Present | Issue/ Problem | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Dissolution of Cotton | Textiles blended with 40/60 polyester and viscose and 50/50 polyester and cotton. | N-methylmorpholine-N-oxide (NMMO), was utilized in this procedure to separate cellulose (cotton and viscose) from blended textiles. | 85% w/w NMMO solution in water was mixed with 15 g of the textile pieces at 120 °C in an oil bath for 2 h under atmospheric conditions. | The products were cellulose solution and undissolved polyester. The cellulose was then either hydrolyzed by cellulase enzymes followed by fermentation to ethanol, or digested directly to produce biogas. | Gas chromatography was used to measure the amount of methane produced during anaerobic digestion. Within 6 days of digestion, 53–62% of the theoretical yield of methane was obtained. | After 2 h treatment, non-cellulosic fibers, and other impurities, e.g., buttons and zippers remained as the solid phase while cellulose is dissolved in the liquid phase. | [63] | |
| 2 | Dissolution of Cotton | White post-consumer textiles. The composition of the poly-cotton blend was 50:50. | [DBNH] [OAc], (1,5-diazabicyclo[4.3.0]non-5-enium acetate) an amidine-based Ionic Liquid, was used as a selective cellulose solvent to separate cotton from polyester. | Using a vertical kneader system, the cotton polyester blend was combined with [DBNH] [OAc] (for 1 h, at 80 °C). | The products were cellulose solution and undissolved polyester. Hydraulic pressure filtering separated the undissolved polyester fraction from the cellulose solution. After solidification, by storing at 8 °C for a couple of days, the resultant cellulose was spun through dry-jet wet spinning. | The molar mass distributions (MMD) of the polyester and cellulose fractions were ascertained using size exclusion chromatography.PET degrades visibly in this method as evidenced by a decline in its MMD (<51%). The linear density, elongation at break, and tenacity of every spun fiber were measured. Linear densities ranging from 0.75 to 2.95 dtex, (dtex or deci-tex is a unit of linear density, which is grams per 10,000 m of yarn) and elongations of 7 to 9%. | PET degraded visibly in this method once it was dispersed in [DBNH] [OAc], as evidenced by a decline in its MMD (<51%) and tensile characteristics (<52%). | [65] | |
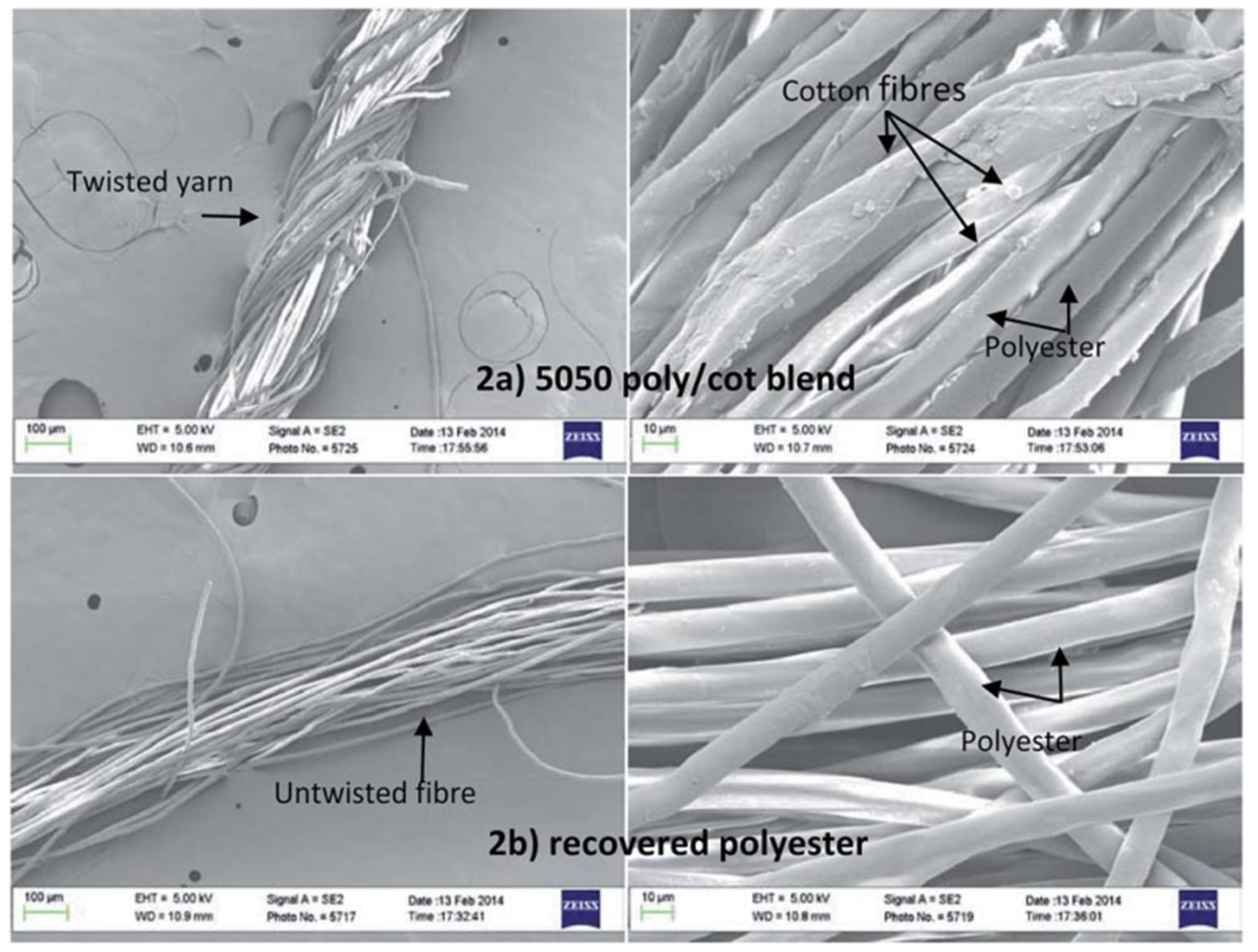
| 3 | Dissolution of Cotton | The cotton polyester blended yarn consisted of 50 wt% cotton and 50 wt% polyester. | To recover cotton from the cotton-polyester blend, cellulose-dissolving ionic liquid 1-allyl-3-methylimidazolium chloride (AMIMCl) and 1-butyl-3- methylimidazolium acetate (BMIMAc) was used to selectively dissolve the cotton component. | Dissolution in AMIMCl at 80 °C for 6 h. | The products were cellulose solution and undissolved polyester. 100% of the cotton from the blend dissolved. Undissolved polyester was removed by filtering. Fibers and/or films can be made from the cotton/AMIMCI solution. | SEM was used to examine the morphology of the materials. Before separation, both cotton and polyester fibers can be observed. After the separation, only polyester fibers are observed in the SEM image. Following the cellulose’s dissolution, a small quantity (less than 2%) of cotton may still be present in the recovered polyester, according to the 13 C Nuclear magnetic resonance NMR spectra and FTIR spectroscopy. On a thermogravimetric analyzer, the treated sample underwent thermogravimetric analyses (TGA) to characterize the structure of the recovered polyester and cotton. The TGA curves showed little to no difference between the sample and recovered material for both cotton and polyester. | The 13C NMR spectrum and FTIR indicated that a small amount (less than 2%) of cotton may remain with the recovered polyester. | [66] | |
| 4 | Dissolution of Cotton | Experiments were conducted using the long-worn lab suits as raw materials consisting of 35% cotton and 65% polyester. | Cotton can be selectively dissolved from waste poly-cotton fabrics using 1-allyl-3-methylimidazole chloride ([Amim]Cl)/Dimethyl sulfoxide (DMSO) and 1-ethyl-3-methylimidazolium diethyl phosphate ([Emim] DEP)/DMSO system as solvents. | ([Amim]Cl) and ([Emim] DEP) as solvent, DMSO as a cosolvent. 0.4 g of waste poly-cotton fabrics combined with solvent (10 g ILs, 10 g DMSO) and dissolved for 5 h at 80 °C. To eliminate the cotton that remained on the regenerated polyester, it was treated for one hour at 50 °C with diluted sulfuric acid. After washing with water, it was dried for 48 h at 105 °C. It was put in a melt-spinning machine. | Wet spinning was used to immediately use the cellulose solution that was produced by the procedure. Melt-spinning was used to prepare the polyester into regenerated polyester fibers. | The cellulose’s relative viscosity was determined, and the degree of polymerization DP of the material was calculated. DP of the sample fabric’s cellulose portion was 1087. DP of the regenerated cellulose using [Amim]Cl/DMSO and [Emim]DEP/DMSO was decreased by 24.4% and 2.9%, respectively. The breaking strength of the regenerated fiber was found to be 1.7 cN/dtex, and the elongation at break was 16.7%. The surface and cross-section of the regenerated cellulose fibers were captured with SEM which showed that the regenerated cellulose fibers were cylindrical with a dense and smooth surface. The FT-IR characteristic peaks of regenerated cellulose and regenerated cellulose fibers were basically similar, indicating that no chemical reaction occurred during normal regeneration and wet spinning process. The dissolution of cotton from waste poly-cotton fabrics utilizing the [Emim]DEP/DMSO system as the solvent was further validated by XRD analysis. The XRD patterns revealed that the waste poly-cotton fabrics initially exhibited diffraction peaks corresponding to both cellulose I-type structure and polyester crystal structure. After the cotton was dissolved and separated, the crystallization peak positions of the regenerated cellulose shifted, indicating a transformation from type I to type II cellulose structure. | The degree of Polymerization of the regenerated cellulose decreased by 25.5% while using [Amim]Cl/ DMSO and 5.0% while using [Emim]DEP/DMSO. | [67] | |
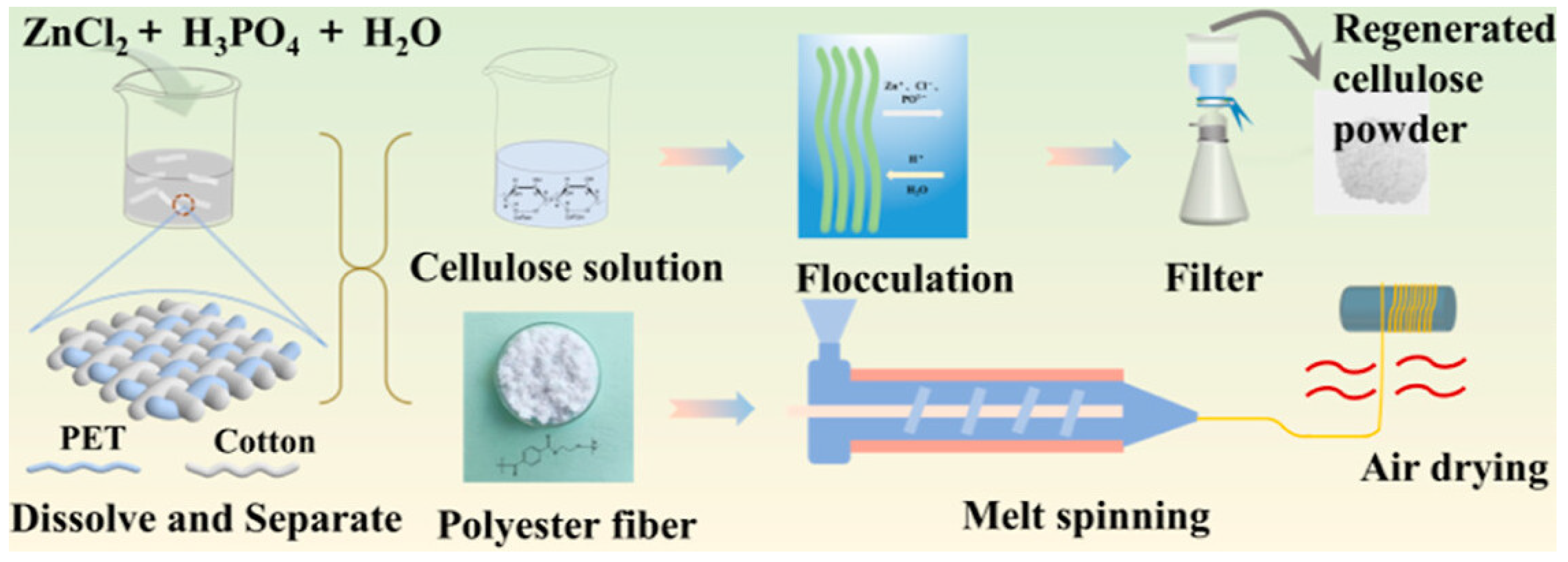
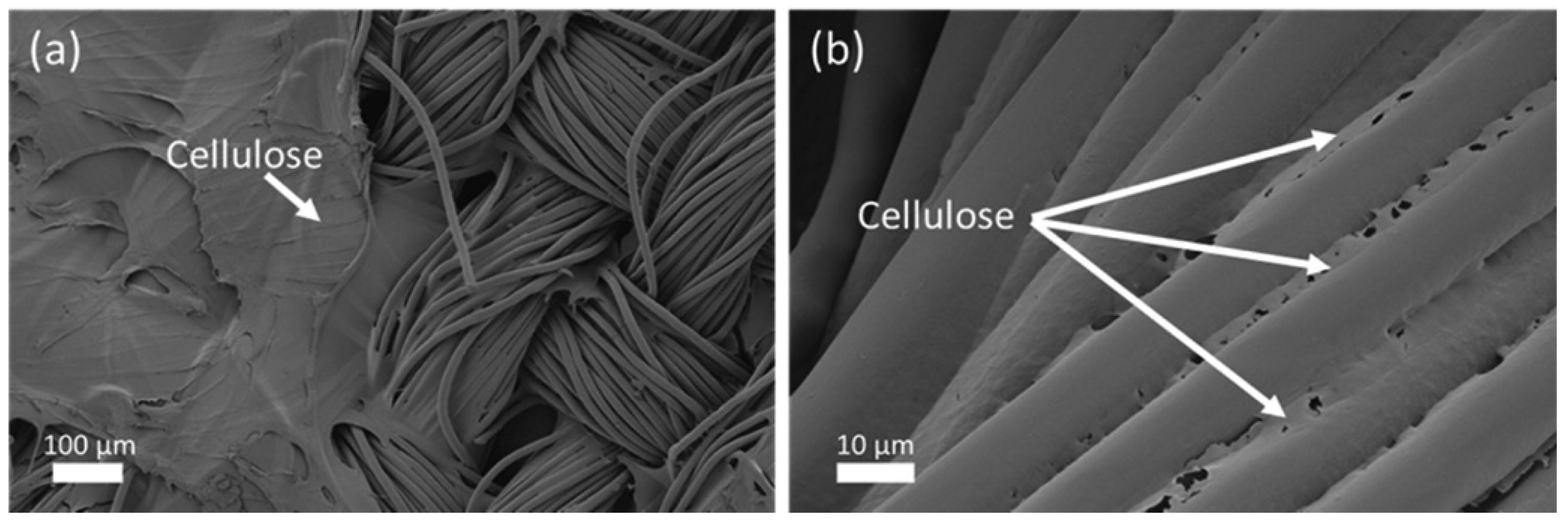
| 5 | Dissolution of Cotton | Polyester-cotton blended fabric with a composition of 65% polyester, 35% cotton. | Selective dissolution of cotton from a poly-cotton blend using a deep eutectic solvent (DES) based on metal salt hydrates. | Synthesis of the metal-salt-hydrate-based DES: For 30 min, ZnCl2, H2O, and H3PO4 in the molar ratio of 1:3:0.5 were magnetically agitated to create a homogeneous ternary solvent. Dissolution: The combined fabric was put into DES. To begin the dissolving separation, the mixture was agitated at a speed of 600 rpm with a cellulose-to-solvent ratio of 5:100. The separated polyester fiber was used as the raw material for the melt-spinning process, which produced the recycled polyester fiber. To create regenerated cellulose fibers, cellulose was extracted from the DES solution system using the coagulation process and then dissolved in a NaOH/urea/H2O solvent for wet spinning. | Cellulose fiber, Polyester fiber | The FTIR spectra of the blended fabric as well as separated polyester were obtained. The FTIR spectra of regenerated PET is very similar to that of original PET indicating the stability of the PET structure before and after separation. The cotton fabric’s XRD diffractogram displayed the standard cellulose I pattern. The regenerated cellulose that came from the DES showed peaks that corresponded to cellulose II patterns. These results pointed to a conversion of cellulose I into cellulose II, which was accompanied by a fast dissociation and reformation of intra- and intermolecular hydrogen bonds between cellulose molecules. | [68] | ||
| 6 | Dissolution of Cotton | Waste polyester/cotton blended fabrics (WBF), with a composition of 65/35 wt%. | Acetylation of cellulose using a Bronsted acidic ionic liquid (IL) N-methyl-imidazolium bisulfate, [Hmim]HSO4, as a novel catalyst. | Using [Hmim]HSO4, an acidic IL N-methyl-imidazolium bisulfate, as the heterogeneous catalyst, acetylation of cellulose was performed at atmospheric pressure. 4.63 g of the pulverized WBFs powders, 20.42 g of acetic anhydride, and 0.18–1.08 g of the [Hmim]HSO4 were mixed and heated at 100 °C for 12 h. The reaction mixture was then added to 100 milliliters of ethanol. The cellulose acetate (CA) and PET-containing material were filtered, washed three times with ethanol, and then dried for 12 h at 60 °C in a vacuum oven. A portion of the sample was refluxed for 12 h using the Soxhlet extraction method and acetone as the solvent to extract the acetone-soluble CA. The acetone-soluble CA product was then obtained by drying the filtrate for 12 h at 60 °C in a vacuum oven. Using DMF as the solvent, the solid portion of the sample was refluxed for 12 h using the same procedure. This led to the extraction of DMF-soluble CA and regeneration of PET. | Acetone-soluble CA and PET. With 84.5% of the cellulose in the WBFs converted, the highest yield of acetone-soluble CA was 49.3%; in the meantime, almost 96% of the PET was recovered. | The degrees of substitution (DS) values of the cellulose acetate (CA) products were determined by 1 H NMR spectroscopy. The acquired CA and the commercial CA sample’s FTIR spectra were gathered. When the spectra of the produced CA were compared to the spectrum of commercial cellulose, they clearly demonstrated acetylation due to the existence of two significant ester bonds at 1752 cm−1, which were given to C=O ester stretching, and 1235 cm−1, which was assigned to the –CO– stretching of the acetyl group. XRD patterns were collected for obtained CA and commercial CA. Comparable structural features were indicated by the similar XRD signals of the obtained CA and commercial CA. The obtained CA’s XRD pattern demonstrated a drop in peak intensities that matched to the crystalline cellulose I structure. The replacement of acetyl groups for hydroxyl groups during the acetylation process was the cause of this decrease in crystallinity. | [69] | ||
| 7 | Dissolution of Cotton | Polycotton fabric (polyester cotton ratio was 80:20 and 50:50) | The separation was done by dissolution of cotton using a co-solvent system of ionic liquid and dimethyl sulfoxide | Fabric samples were treated with an aqueous solution containing 0.5 wt% sulfuric acid to reduce the degree of polymerization (DP) of the cotton component. Subsequently, the samples were treated with a solvent system of 80% DMSO and 20% ionic liquid. After 24 h, the solution was filtered. The cellulose dope filtrate was collected for spinning, while the solid polyester material was washed, dried, and rinsed with DMSO to remove residual cellulose solution before being air-dried. | Regenerated cellulose fiber and intact polyester. | The morphologies of the fabrics and recovered materials were examined by SEM. Which confirmed the removal of cotton from the polycotton blend. | [70] | ||
| 8 | Dissolution of Polyester | Black 100% cotton jeans and blue 80/20 cotton/polyester jeans. | Three-step process: (a) Textile dye leaching using Nitric Acid. (b) Dissolution process using Dimethyl Sulfoxide (DMSO) to dissolve the polyester and remaining organic part from textile dyes. (c) Bleaching using sodium hypochlorite and diluted hydrochloric acid for recovered cotton purification. | Dyes from blue and black samples were dissolved using 1.0 M and 1.5 M HNO3, with an average treatment duration of 20 min. 1 g samples of two different types of jeans were treated with 10–80 milliliters of solvent at a constant temperature of 50 °C. The black sample could be fully separated optimally in 7 h with 40 mL solvent, while the blue sample needed 9 h and 60 mL solvent. Under soundwave treatment for 2 h at 40 °C, the cotton fibers were bleached with sodium hypochlorite and diluted HCl to remove any leftover contaminants. | Dissolved polyester in the spent solvent, cotton fiber. | FTIR spectroscopy was used to examine the chemical structure of the fiber samples both before and after the treatment. It showed that all dyes and contaminating elements were successfully removed by leaching, dissolving, and bleaching and that the recovered fiber was made of highly pure cotton. Thermogravimetric/Derivative Thermogravimetric analysis (TGA-DTG) was used to investigate the thermal degradation and stability trends of the precipitated polyester. The decomposition methods of the black and blue samples were similar and observed within the 80–365 °C range. | [74] | ||
| 9 | Dissolution of Polyester | Colored 50/50 polyester/cotton blended fabric. | Using dimethyl sulfoxide (DMSO), the polyester component and dispersion dyes were extracted from blends of polyester and cotton. Following the breakdown of dye-cellulose linkages, the remaining colored cotton was swollen to remove its dyes. Regenerated fibers were created from colorless polyester and cotton. | One part polyester/cotton and three parts DMSO were combined at 150 °C. In about five minutes, the dissolution took place. After cooling down recycled polyester and dispersed dyes in DMSO, the polyester precipitated. The dispersed dyes were extracted by washing the material with a small amount of heated dimethyl sulfoxide. Following the separation of polyester and dispersion dyes, cellulose molecules were swelled by adding solvents containing varying ratios of DMSO to water to the cotton fibers that retained their color. Since reactive dyes and cellulose had covalent connections, the covalent bindings between the dye and cellulose were broken by adding 0.07–0.6 weight percent of NaOH to the DMSO/water solvent. To completely separate the color from the cotton, the material was swollen three times. Every cycle lasted 10 min at 90 °C. Each cycle’s weight ratios for the solution to cotton were 3. At 270 °C, the dried polyester was wet spun into recycled polyester fibers. By stirring at 110 °C for two hours, a solution comprising 5% color-removed cellulose, 87 wt% NMMO and 13 wt% water was created. Through dry-jet wet spinning, the cellulose solution was extruded into fibers. | Colorless polyester and cotton which were regenerated into fibers. | Morphologies of the regenerated polyester and cellulose fibers were characterized by a field-emission scanning electron microscope. The structures of fibers were determined by X-ray diffraction. The recycled and virgin fibers had similar crystallization behavior. The cellulose’s FTIR spectra were determined to be identical both before and after the separating procedure. The polyester’s FTIRs before and after the separation procedure were identical. | Dyes were extracted as a dye solution. | [75] | |
| 10 | Hydrolysis | The cotton polyester blended bed sheets comprised 48 wt% cotton and 52 wt% polyester. | PET was degraded to terephthalic acid (TPA) and ethylene glycol (EG) using NaOH | 5–15 wt% NaOH in water and temperature in the range between 70 and 90 °C for the hydrolysis of PET, for 40 min. | Three product streams were generated from the process. First is the cotton; second, the TPA; and third, the filtrate containing EG and the process chemicals. Solid Cotton residue (Separated by filtering), TPA was precipitated, and EG was in the filtrate. | NMR spectroscopy was used to characterize the polycotton bed sheets prior to separation. The degraded PET was analyzed using NMR spectroscopy, which revealed that the recovered TPA was free of any impurities. Following PET hydrolysis, the solid (cotton) residue’s ATR FT-IR spectra were compared to a sample of pure cotton. This demonstrated that cellulose I had somewhat changed into cellulose II. | Under the highly alkaline conditions used in the PET-removing procedure, undesired cellulose degradation reactions may also occur in the cellulose portion of a polycotton sample. These processes cause cellulose chains to break, which lowers the cellulose’s DP. | [77] | |
| 11 | Hydrolysis | Polyester-cotton blended fabric. Composition is not mentioned in the study. | Acid treatment with Sulfuric acid then a grinder was used to crush it to separate the polyester from cotton fiber. | The waste polyester/cotton mixed fabric was split into 2 × 2 cm pieces and subjected to varying sulfuric acid concentrations over varying periods of time. After being treated, the blended polyester/cotton cloth was washed and dried. Using a grinder, the cotton fiber powder and the polyester fiber ball stained with the powder were produced. Subsequently, a certain volume of powdered cotton fiber was weighed and swelled using a NaOH solution. The pH was then brought to a neutral level. After using 64% sulfuric acid for a predetermined amount of time, the reaction was completed by adding 10 times as much deionized water. After being diluted, the mixture was dialyzed to neutrality and centrifuged. After a specific number of homogenizations using a high-pressure homogenizer, the treated solution’s concentration was blended to 0.5% and freeze-dried. | The separated products were polyester as a fibrous mass and cotton powder. Nanocellulose from the cotton was obtained by further processing. | The separated cotton fibers’ retention of their cellulose structure was validated by the FTIR analysis, which also showed signs of lignin or hemicellulose removal post treatment. XRD was used to examine the crystallinity of the separated cotton fiber which indicated that the crystallinity of cellulose fiber was very high, and it was the structure of cellulose type I. The morphology of the separated polyester and cotton fibers was investigated using SEM. Following acid hydrolysis and mechanical agitation, the cotton fiber had surface damage, fractures, and a marked reduction in length. The surface of the treated polyester fiber remained intact, and it was stained with a little amount of powdered cotton fiber. | [78] | ||
| 12 | Hydrolysis | Garments with a blended fabric containing 70% viscose and 30% polyester | Alkaline hydrolysis of viscose/PET | The aqueous NaOH (5 wt%) was heated to 90 °C before adding the oven-dried (2 h at 105 °C) sample to the reaction vessel. Hydrolysis was performed for a selected time (60–1440 min). After the reaction, the solid residue was separated from the reaction solution via filtration. | The reaction yielded a solid cellulose residue, and the PET monomers terephthalic acid (TPA) and ethylene glycol (EG). | NMR was utilized to ascertain the purity of TPA derived from PET depolymerization, using a commercial TPA as the reference standard. The spectrum of the precipitate showed a distinct singlet at 7.58 ppm which was assigned to pure TPA. | The alkaline treatment reduces cellulose’s intrinsic viscosity by up to 35%, hence it would not be appropriate for traditional fiber-to-fiber recycling. | [79,80] | |
| 13 | Hydrothermal Treatment | A blue 65/35 cotton/polyester blend fabric served as the waste textile sample. | To recover cotton from the cotton-polyester blend, the cotton component was hydrolyzed and turned into cellulose powder or oligosaccharide using diluted hydrochloric acid as a novel hydrothermal treatment catalyst for cellulose. | 1.5 wt% dilute hydrochloric acid at 150 °C, 3 h of reaction time. | Cellulose powder, polyester fiber. | Using techniques such as SEM, FTIE, XRD, and high-performance liquid chromatography, the morphology and structure of the hydrothermal products—both solid and liquid—were characterized and compared to untreated polyester and cotton. The results indicate that after three hours of hydrolysis, the polyester fiber preserved its fiber properties while the cotton fiber entirely degraded. The hydrolysis did not alter the crystalline structure of cellulose, as seen by the essentially similar XRD patterns of cotton and cellulose powder. | The fate of the blue dye was not reported in this study. | [81] | |
| 14 | Hydrothermal Treatment | White shirts with a composition of 66% cotton and 34% polyester. | Hydrothermal treatment which separated polyester and cotton from the blend. | After being cut into pieces measuring around 5 by 5 cm2, the sample cloth was added to a reactor that held 300 mL of pure water. The cloth was treated for 10–180 min after being heated to the appropriate temperature (180–250 °C). The reactor was then allowed to naturally cool to 40 °C. After the reactant fabric was taken out, filtering was used to recover the solid residue in the water. | Cotton as mesh fabric. Polyester as a solid powder. | Using SEM, the surface conditions of the treated mesh fabric were compared to those of the raw material. Following treatment, SEM images demonstrate that the cotton fiber condition is preserved. It is also evident that the fibers have suffered some little damage from the 230 °C treatment. It wass hypothesized that the cotton fibers have gradually deteriorated due to the subcritical water treatment. FTIR examination verified the minute fragments and powdery material obtained after treatment to be PET, and the mesh fabric to be cotton. | [82] | ||
| 15 | Enzymatic Hydrolysis | Textile waste of cotton and polyester (PET) blend by 60/40. | Using cellulase and β-glucosidase, textile waste was hydrolyzed enzymatically to extract glucose and polyester. | Textile fabrics were enzymatically hydrolyzed at 50 °C for 96 h. The maximum glucose recovery of 98.3% was obtained with 20 FPU/g of cellulase dosage and 10 U/g of β-glucosidase dosage at 3% (w/v) substrate loading, temperature of 50 °C and pH 5 | Glucose, Glucose yield was 98.3%. The recovered PET fiber can be reused by melt-spinning to new PET fiber. | Glucose concentration was determined by HPLC. SEM analysis before and after enzymatic hydrolysis showed significant changes in textile morphology. Prior to hydrolysis, cotton and polyester fibers formed a compact structure. After hydrolysis, the structure loosened with fewer fibers, and small holes appeared due to the digestion of cellulose fibers. PET fibers remained intact. | [86] | ||
| 16 | Enzymatic Hydrolysis | Clothing: with a composition of 65% PET, 35% Cotton. And 80% PET, 20% Cotton. | Using a commercial cutinase from Humicola insolens (HiC) under moist-solid reaction conditions, PET in mixed PET/cotton textiles could be directly and selectively depolymerized to terephthalic acid (TPA). This process was easily combined with cotton depolymerization through simultaneous or sequential application of the Cellic CTec2 cellulases blend, yielding glucose. | After cutting the textile samples into squares measuring 0.7 × 0.7 cm, they were ball milled for 5 or 30 min (30 Hz) in 15 mL stainless-steel milling jars with HiC (0.65% w/w) and/or CTec2 (0.7% w/w) enzymes present. This was followed by seven days of static incubation at 55 °C. | TPA (with a maximum yield of 30 ± 2%) and glucose (with a maximum yield of 83 ± 4%) were obtained through the mechanoenzymatic hydrolysis of the PET/cotton blended textile. Additionally, ethylene glycol (yield unknown) and a small reaction product, up to 0.5% yield of MHET were produced by the hydrolysis of PET. | Cotton hydrolysis products were assessed using a commercially available glucose test, while PET hydrolysis products were quantified by HPLC. | [56] | ||
| 17 | Enzymatic Hydrolysis | A white 40/60 polyester/cotton blended fabric. | Alkali pretreatment and enzymatic hydrolysis followed by saccharification and fermentation. | Waste textiles were pretreated using aqueous alkaline mixes of NaOH (12 wt%), NaOH/urea (7/12 wt%), NaOH/thiourea (9.5/4.5 wt%), and NaOH/urea/thiourea (8/8/6.5). 5 g of waste textile and 95 g of an alkaline solution were combined for the pretreatments, which lasted one hour at various temperatures of 20 °C, 0 °C, 23 °C, and 100 °C. Using 30 FPU cellulase and 60 IU b-glucosidase per gram of cellulose, waste textiles were treated to a 72 h enzymatic hydrolysis at 45 °C and pH 4.8 (in 50 mM sodium citrate buffer supplemented with 0.5 g/L sodium azide) with 3% (w/v) solid (substrates) loading. | Polyester and Ethanol. When saccharification and fermentation were carried out simultaneously on the textile, the greatest yield of ethanol production was 70%. The recovered polyester accounted for 98%. | The characteristics of recovered polyester were evaluated by FTIR, DSC, and viscosity analyses. The FTIR result revealed that after the polyester was treated with NaOH, the trans/gauche ratio dropped, indicating a reduction in the crystalline area. | [87] | ||
| 18 | Enzymatic Hydrolysis | Textile waste blends of cotton and PET, with compositions of 35/65 and 60/40. | Sample waste fabrics were cut into little pieces (about 0.5 × 0.5 cm2) and soaked in freezing alkali/urea for three separate modification methods: autoclaving, freezing, and alkaline pretreatment. Then the sample was subsequently exposed to enzymatic hydrolysis. | After adding the mineral solution to achieve the appropriate initial moisture level, the textile waste was autoclaved for 15 min at 121 °C as part of the autoclave pretreatment. Textile waste was mixed with urea (12% w/v) and NaOH (7% w/v) for the freezing alkali/urea soaking process. The mixture was then frozen at −20 °C for six hours. The textile waste was autoclaved for 15 min at 121 °C or soaked for 3 h at 80 °C in a 15% NaOH solution for alkaline pretreatment. Enzymatic hydrolysis was used to regenerate cellulose from cotton/PET textile waste mixtures. In this study, commercial cellulase (Cellic CTec2, 185 FPU/mL) was employed. Two grams of regenerated cellulose were added, at a substrate-enzyme dose of 25 FPU/g, to a citric buffer (100 mL, 50 mM, pH 4.8). For 96 h, hydrolysis was carried out at 50 °C and 200 rpm. | Glucose and PET as a solid mass. | Utilizing a high-performance liquid chromatography column (HPLC), the amount of glucose was determined. After processing with NaOH, SEM analysis showed notable changes in the surface morphology of textile sample. At first, the fabric had a surface that was a little uneven and harsh. However, after treatment, the cotton/PET digestion by NaOH caused a partial breakdown of the textile’s structure into rough fibers. The textile samples were examined using FTIR both before and after processing. PET and cellulose polymer-corresponding absorption bands were visible in the spectra. Significant alterations in the cellulose polymer bands were found upon analysis of the pretreated textile waste, demonstrating the pretreatment process’s efficacy in changing the polymer structure. | [88] | ||
| 19 | Enzymatic Hydrolysis | Used jeans, composition not mentioned in the study. | Phosphoric acid pretreatment and subsequent enzymatic hydrolysis of cotton-based waste textiles to recover sugar and polyester. Two enzymes were used for enzymatic hydrolysis: cellulase from Trichoderma reesei and cellobiase from Aspergillus niger. | Phosphoric acid pretreatment: 85% phosphoric acid, at 50 °C, for 7 h, and a ratio of fabric and acid of 1:15. Enzymatic hydrolysis In 150 mL flasks with a 50 mL work volume, the regenerated cellulose was enzymatically hydrolyzed in 50 mM sodium citrate buffer (pH 4.8). With a cellulose loading of 7.5 FPU/g regenerated cellulose and a cellobiase loading of 15 CBU/g regenerated cellulose, the substrate consistency for enzymatic hydrolysis was maintained at 1.0% (w/v). For 96 h, the hydrolysis was carried out at 50 °C in an air-bath shaker at 130 rpm. | Polyester in solid form. At the optimized conditions (85% phosphoric acid, 50 °C, 7 h, and a ratio of 1:15), 100% polyester recovery with a maximum sugar recovery of 79.2% was accomplished. | The glucose content was determined by HPLC. Surface morphology was analyzed using SEM. Distinct cotton and polyester fibers were visible in the original waste textiles sample. The cellulose fibers changed significantly and became rougher after pretreatment. The majority of cotton fibers remained intact after enzymatic hydrolysis without pretreatment, underscoring the necessity of pretreatment for effective sugar recovery. Cotton fibers were effectively extracted from polyester using phosphoric acid pretreatment, resulting in 100% polyester recovery without changing the surface of the polyester as seen on the SEM images. | [89] | ||
| 20 | Enzymatic Hydrolysis | Blended textiles (65% polyester, 35% cotton) | NaOH/urea solution pretreatment is followed by cellulase-based enzymatic hydrolysis. It was found that using concentrations of NaOH varying from 20.7% to 26.6% with either 0% urea or a mixture of 13.9% NaOH and 12% urea was efficient and almost entirely eliminated the cellulose in the blended textile. | 400 mL of NaOH/urea solution was used for pretreatment and 4 g of textile substrate were weighed in a beaker for every experiment. Using sodium azide (0.02%) and a 50 mM citric acid buffer at pH 5.0, hydrolysis was carried out in a 1-L flask. In a 500 mL buffer, the textile substrate was added with a solid load of 0.8% (w/v). The activity of the enzyme dose was adjusted to 0.68 filter paper units (FPU) per g of cellulose. For 24 h, hydrolysis was carried out in a heating incubator at 50 °C and 70 rpm orbital shaking. | Polyester as solid residue, and Glucose | To find the released glucose, high performance anion exchange chromatography (HPAEC) was employed. After being hydrolyzed by enzymes, the quality of the recovered synthetic fibers was assessed using Fourier transformed infrared spectroscopy (FTIR). FTIR analysis compared untreated textile blend, pure polyester, and regenerated polyester fibers. It revealed decreased peaks characteristic of cellulose and increased peaks characteristic of PET. | [90] |
| # | Method | Starting Material and Composition | Process/Reaction | Process/Reaction Conditions | Products | Analysis | Any Other Component Present | Issue/Problem | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Selective dissolution of elastane | Polyester-elastane blend and Polyamaide-elastane blend. (PET/elastane: 85/15), (Polyamide/ elastane: 88/12) | The selective dissolution of elastane from blended textile can be done using tetrahydrofurfuryl alcohol THFA and γ-valerolactone. (GVL). | 4 h and 100 °C, tetrahydrofurfuryl alcohol THFA and γ-valerolactone (GVL) as solvents. | Elastane solution. PET fiber and polyamide fiber in the solid form. | Thermogravimetric analysis with Fourier transform infrared spectroscopy (TGA-FTIR) was used to determine the maximal solubilities (4.9 mg/g THFA and 4.3 mg/g γ-valerolactone). The quality of the extracted elastane fibers was assessed by liquid 1H-NMR. Before solvent treatment, liquid 1H-NMR of the pure elastane fiber revealed that it exclusively included methylene diisocyanate derived carbamates. THFA solvent treatment, however, caused the carbamate bond to break, which indicated elastane fiber partially depolymerized. | [21] | ||
| 2 | Selective dissolution of elastane | Polyester/elastane, and polyamide/elastane textile waste samples Green-colored polyester/elastane and black-colored polyamide/elastane post-industrial textile waste samples. The textile compositions were 82% PET/18% EL and 92% PA/8% EL. | Dissolution of elastane. | 1 g sample in 200 mL solvent, dimethyl sulfoxide (DMSO), at 120 °C for 30 min | Elastane solution. By filtering the solution, washing, and drying the filtride, polyester or polyamide components are recovered. | Polymer degradation behavior was analyzed using TGA. In textiles that have not been treated, elastane (EL) degradation peaks could be seen at approximately 300 °C. Because of the high elastane content in the PET/EL textile, an earlier degradation start (412 °C) was seen. The successful separation of elastane was confirmed by this comparison. There appeared to be no deterioration in the polymer matrix since the degradation peak associated with the PET reference (442 °C) coincided with that of the recovered material. Comparably, limited variation between degradation peaks (around 423 °C) for the black PA/EL textile indicated that the polymer matrix had not changed. Morphology of the samples was analyzed using scanning electron microscopy (SEM). The PET/EL fabric’s rich collocation of polyester and elastane fibers was apparent in the SEM images. Following the elastane separation, recovered PET showed no signs of elastane-related residues being adhered to the fibers. | The solvent enriched with colorant and elastane particles during the treatment; therefore it could not be used indefinitely. Elastane could dissolve in DMSO up to an 18 g/L concentration. DMSO could not be used any more beyond this point because elastane particles collected in the treated solvent. | [92] | |
| 3 | Selective dissolution of elastane | Pre-consumer-waste fibers and fabrics. One PA6/elastane blend with 6.3% elastane. Two PA66/elastane blends (with 23% and 32% elastane). | Using a solvent blend (THF: DMSO in a 70%:30% by volume ratio), elastane in a mixed polyamide fabrics can be selectively dissolved. | 1 g fabric in 20 mL solvent, dissolution at 25 °C for 1 h, THF:DMSO in a 70%:30% by volume ratio. | Precipitated Elastane, solid polyamide in the spent solvent. | SEM micrographs of the untreated and solvent treated sample show that, most of the elastane was dissolved in a first cycle, and the washing process in non-solvent precipitated the elastane left on the fabric surface. DSC and FTIR analysis also indicated that elastane was removed from the sample. | [91] | ||
| 4 | Elastane degradation | A white pre-consumer textile blend, composed of 95% (w/w) of cotton and 5% (w/w) of elastane. | Selective Elastane Degradation by Aminolysis. | 4 h at 80 °C. 8 g of the ground fabric was added to 160 mL of a solvent mixture containing a cleaving agent (DETA) and an elastane solvent (DMF or DMSO) in a 1:1 volume ratio. | The solid and liquid phases were separated by filtration. The liquid phase contained the solvent mixture and the degraded elastane products, by adding water to the liquid phase, the elastane products were precipitated. Cellulosic material was dissolved in [DBNH] [OAc] and turned into new fibers via dry-jet wet spinning. | TGA was used to study thermal behavior. Both the recovered elastane and pure elastane showed similar thermal behavior. The recovered elastane underwent FTIR and NMR tests to understand more about its chemical composition. The distinctive polyurethane peaks were visible in the FTIR spectra of the sample elastane and the recovered elastane. | [95] | ||
| 5 | Elastane degradation | Polyester fabric coated with polyurethane. | Selective degradation of PU elastomers by cleavage of C–O and C–N bonds in 70% ZnCl2 aqueous solution. This catalyst-assisted solvolysis did not degrade the polyether bonds and PU was converted to the amine form of the used isocyanate and the original polyether polyol. | 10 g PU coated polyester was added to ZnCl2 solution (70 wt% in water, 250 g), heated to 140 °C and for 2 h. The degradation products were cooled to room temperature and then agitated for five minutes in 250 mL water following the reaction. After the mixture was filtered, an insoluble residue (fabric and insoluble degradation products of PU) and a water mixture were obtained. The fabric was filtered off after the residue was added to 200 mL ethyl acetate. The ethyl acetate mixture was washed three times with brine, then the organic phase was evaporated with a rotary evaporator. The water mixture was evaporated and diethyl ether was added. The precipitated product was separated via filtration and dried overnight at 70 °C at atmospheric pressure. | Polyester fabric, polyol, and amine | The thermal, chemical, and mechanical characteristics of the virgin PET and recycled PET were analyzed through tensile strength tests, IR, TGA, and GPC. The virgin polyester fabric’s tensile force and elongation were 360 N and 33.5%, respectively. The tensile strength of recycled polyester fabric was 350 N, and its elongation was 34%. Therefore, the mechanical characteristics of the polyester fabric were unaffected by the heat treatment in the presence of ZnCl2. The IR spectra of virgin polyester and recycled polyester showed significant similarities which indicates that the PET recovered after the degradation of polyurethane in ZnCl2 solution was not degraded. TGA Analysis showed that the polyester did not degrade during solvolysis, while the PU was converted into polyol and amine. As analyzed in GPC, when compared to virgin PET, the recycled PET’s molecular weight did not decrease, indicating that it did not break down during solvolysis. | [96] | ||
| 6 | Elastane degradation | Fabric sample consisting of 27% Elastane and 73% Nylon. | Solvolysis using tert-amyl alcohol in the presence of KOH. | 1.02 g fabric sample consisting of 27% elastane and 73% Nylon was added to tert-amyl alcohol (5 mL) and KOH (0.19 wt%) at 225 °C for 4.5 h. | The products were 50.9 mg 4,4′-MDA, 266 mg polyTHF in the liquid phase and 698 mg leftover fabric. | FTIR analysis was performed on sample and treated fabric sample. The results suggested that the elastane had been effectively removed from the polyamide matrix. The polyamide remained unaltered, while the elastane fibers in the fabric were depolymerized. For pure elastane, DSC showed a crystalline peak at 20 °C. Both a wide crystalline phase for the polyamide at 225 °C and a crystalline peak for the elastane at 20 °C were seen in the untreated sample. For the treated fabric at 255 °C, the polyamide could form a crystalline peak, and there was no evidence of elastane. This suggests that the elastane has been successfully removed and that the polyamide fiber was intact with no symptoms of contamination or deterioration. | [94] |
| # | Company | Founded | Location | Process | Output | Scale |
|---|---|---|---|---|---|---|
| 1 | Ambercycle | 2015 | Los Angeles, CA, USA | Biological recycling process | PET pellets and fiber | Pilot plant |
| 2 | BlockTexx | 2018 | Loganholme, Australia | Chemical Process | PET and Cellulose | Commercial scale plant |
| 3 | Circ | 2011 | Danville, Virginia | Hydrothermal process | Cellulose, terephthalic acid TPA, and ethylene glycol EG. | Commercial scale plant |
| 4 | Worn Again Technologies | 2005 | Nottingham, England | Solvent-based dissolution | PET and Cellulose | Pilot plant |
| 5 | Purfi | 2018 | Waregem, Belgium | D’Elastane™ technology | PET fiber | Not mentioned |
| 6 | Textile Change | 2019 | Vejle, Denmark | Chemical Process | PET and Cellulose | Pilot plant |
| 7 | Sodra (OnceMore®) | 2022 | Växjö, Sweden | Chemical Process | Cotton fiber | Commercial scale plant |
| 8 | Phoenxt | 2018 | Blomberg, Germany | Solvent-based process | PET, Cellulose | Not mentioned |
| 9 | Eeden | 2022 | Münster, Germany | Chemical Process | Cotton fiber, terephthalic acid TPA, and ethylene glycol EG. | Not mentioned |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, K.; Tsianou, M.; Alexandridis, P. Recycling of Blended Fabrics for a Circular Economy of Textiles: Separation of Cotton, Polyester, and Elastane Fibers. Sustainability 2024, 16, 6206. https://doi.org/10.3390/su16146206
Choudhury K, Tsianou M, Alexandridis P. Recycling of Blended Fabrics for a Circular Economy of Textiles: Separation of Cotton, Polyester, and Elastane Fibers. Sustainability. 2024; 16(14):6206. https://doi.org/10.3390/su16146206
Chicago/Turabian StyleChoudhury, Khaliquzzaman, Marina Tsianou, and Paschalis Alexandridis. 2024. "Recycling of Blended Fabrics for a Circular Economy of Textiles: Separation of Cotton, Polyester, and Elastane Fibers" Sustainability 16, no. 14: 6206. https://doi.org/10.3390/su16146206
APA StyleChoudhury, K., Tsianou, M., & Alexandridis, P. (2024). Recycling of Blended Fabrics for a Circular Economy of Textiles: Separation of Cotton, Polyester, and Elastane Fibers. Sustainability, 16(14), 6206. https://doi.org/10.3390/su16146206







